Reactive Oxygen Species (ROS)
First printed in R&D Systems' 1997 Catalog.
Overview
Aerobic organisms, which derive their energy from the reduction of oxygen, are susceptible to the damaging actions of the small amounts of •O2-, •OH and H2O2 that inevitably form during the metabolism of oxygen, especially in the reduction of oxygen by the electron transfer system of mitochondria. These three species, together with unstable intermediates in the peroxidation of lipids, are referred to as Reactive Oxygen Species (ROS). Many diseases (Table 1) are linked to damage from ROS as a result of an imbalance between radical-generating and radical-scavenging systems - a condition called oxidative stress. The discovery by McCord and Fridovich1 of the superoxide dismutase (SOD) activity of erythrocuprein together with the finding that almost all mammalian cells contain SOD suggests the physiological importance of at least the central ROS, superoxide.
A partial list of conditions that likely involve Oxidative Stress
Alzheimer Disease
Myocardial Infarction
Atherosclerosis
Parkinson Disease Autoimmune Diseases
Radiation Injury
Emphysema
Sunburn
Sources of Oxygen Radicals
Cells generate energy aerobically by reducing molecular oxygen (O2) to water. The cytochrome c oxidase-catalyzed reaction involves transfer of four electrons (e) to oxygen, in principle without intermediates, but, in fact, partially reduced oxygen species are produced. Other enzymes, especially flavin enzymes, also generate partially reduced oxygen species. One to two percent of total oxygen consumption may, in fact, be converted to superoxide anion radical (•O2-). Other sources of ROS include radiation (e.g., UV light), toxic chemicals (e.g., paraquat) and drugs (e.g., adriamycin, bleomycin).
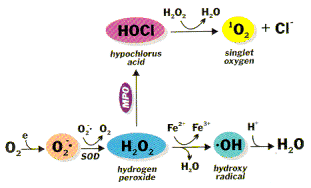
Figure 1. Generation of reactive oxygen species, where MPO is myeloperoxidase and SOD is superoxide dismutase.
Formation of superoxide anion radical leads to a cascade of other ROS2, 3 (Fig. 1). Superoxide dismutates to hydrogen peroxide (H2O2) and oxygen. This reaction is spontaneous and fast, but the SOD-catalyzed reaction is four orders of magnitude faster. Clearly, O2- is more toxic than H2O2 and its rapid removal is important. H2O2 is reduced by three general mechanisms. 1) It is the substrate for two enzymes, catalase and glutathione (GSH) peroxidase,4 that catalyze the conversion of H2O2 to H2O + O2; this presumably is a detoxification mechanism. 2) H2O2 is converted by myeloperoxidase (MPO) in neutrophils to hypochlorous acid (HOC1). This appears to be a mechanism for a physiological toxic agent, since HOCl is a strong oxidant that acts as a bactericidal agent in phagocytic cells. Reaction of HOCl with H2O2 yields singlet oxygen (1O2) and water. The biological significance of singlet oxygen is unclear. 3) H2O2 is converted in a spontaneous reaction catalyzed by Fe2+ (Fenton reaction) to the highly reactive hydroxyl radical (•OH). The hydroxyl radical reacts instantaneously with any biological molecule (RH) from which it can abstract a hydrogen atom. The resulting free radical (R•) is more stable and hence longer-lived than the hydroxyl radical.
Mechanism of Damage
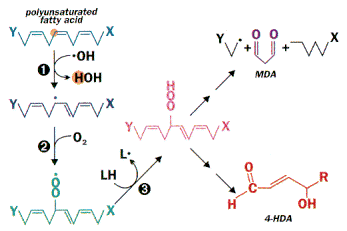
Peroxidation of unsaturated lipids
Figure 2. Peroxidation of unsaturated lipids. The variety of lipids and the random nature of free radical reactions leads to many products. These include 4-hydroxyalkenals (4-HDA) and, when there are three or more unsaturated bonds, malondialdehyde (MDA). These can serve as targets for the measurement of fatty acid peroxidation. The initiating event can be reaction with another radical, uv light or radiation. Since a radical is also produced in the process, it is a chain reaction.
Reactive oxygen species, in particular the hydroxyl radical, can react with all biological macromolecules (lipids, proteins, nucleic acids and carbohydrates). The initial reaction generates a second radical, which in turn can react with a second macromolecule to continue the chain reaction. Among the more susceptible targets are polyunsaturated fatty acids. Abstraction of a hydrogen atom from a polyunsaturated fatty acid initiates the process of lipid peroxidation (Fig. 2). In step #3 of Fig. 2, a hydrogen atom is abstracted from a second lipid, leading to a new ROS. Numerous products are formed, presenting special analytical problems. The choice is between simple, non-specific assays for classes of lipid peroxidation products (e.g., thiobarbituric acid reaction for aldehydes), more specific but less sensitive assays (e.g., uv absorbance by conjugated dienes) or specific, highly sensitive methods that require expensive instrumentation (e.g., mass spectral analysis of hydroxy fatty acids). A sensitive and specific colorimetric assay based on measurement of malondialdehyde and 4-hydroxyalkenals is a frequent compromise.5
ROS modify both the structure and function of proteins. Metal-catalyzed protein oxidation results in addition of carbonyl groups or cross-linking or fragmentation of proteins. Lipid (peroxidation) aldehydes can react with sulfhydryl (cysteine) or basic amino acids (histidine, lysine). Similarly, modification of individual nucleotide bases, single-strand breaks and cross-linking are the typical effects of reactive oxygen species on nucleic acids.
Defense Mechanisms
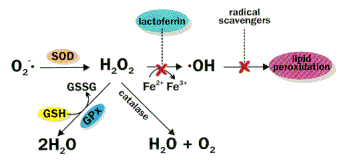
Mammalian cells possess elaborate defense mechanisms to detoxify radicals (Fig. 3). The key metabolic steps are SOD catalysis of the dismutation of superoxide to hydrogen peroxide and oxygen and the conversion of H2O2 to 2H2O by glutathione peroxidase or to O2 + H2O by catalase. Since the reaction catalyzed by glutathione peroxidase requires GSH as substrate and depends in part on the ratio of GSSG:GSH, the concentrations of these reactants and their ratio, which is a reflection of the redox state of the cell, are important to ROS detoxification. Similarly, the concentration of redox-active metals, such as iron, catalyze formation of some ROS. This is minimized by keeping the concentrations of these metal ions very low due to binding to storage and transport proteins (e.g., ferritin, transferrin, lactoferrin), thereby minimizing •OH formation. Finally, radical-scavenging antioxidants (e.g., vitamin E) interrupt the chain reactions by capturing the radical; the vitamin E radical is relatively stable, and it can be enzymatically converted back to its non-radical form. Radical scavengers thus terminate the chain reaction of radical damage.
The potential significance of these ROS defense mechanisms is apparent from considerations of the whole body and sub-cellular distribution of the different components. Vitamin E, the enzymes (SOD, catalase and GSH-peroxidase) and substrates (GSH) tend to be in higher concentration in locations where ROS damage is more likely (e.g., in more highly oxygenated locations) and potentially more damaging.3
Defense mechanism against damage by ROS
Figure 3. Defense mechanism against damage by ROS. Superoxide dismutase (SOD) plus catalase or glutathione peroxidase (GPx) eliminate many damaging oxygen species. Lactoferrin (binds iron) and radical scavengers such as vitamin E, further limit damage.
Reactive Oxygen Species (ROS): R&D Systems https://www.rndsystems.com/cn/resources/articles/reactive-oxygen-species-ros
.png)