医药中的光敏剂
PHOTOSENSITIZERS in MEDICINE
克里斯蒂安伯格
放射生物学系
挪威镭医院
Montebello,N-0310挪威
Kristian.Berg@rr-research.no
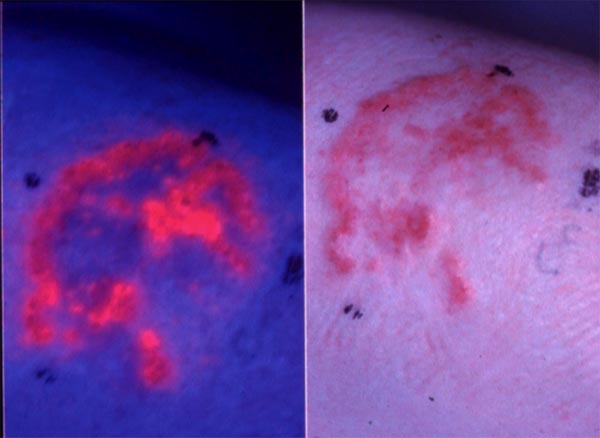
下图(左)显示了基底细胞癌。医生想要治疗这种皮肤癌,但不确定恶性组织的程度。病变的哪个部位是癌变的?边界在哪里?医生可以使用光敏剂来回答这些问题。他们可以用光敏剂(如5-氨基乙酰丙酸)处理该区域,然后使用蓝光观察它,这会导致光敏剂发出荧光(右图)。粉红色/红色荧光区域定义了恶性病变的程度。在本单元中,您将了解有关光敏剂的更多信息以及使其适用于此类应用的特性。
光敏剂已经发展了数百万年,主要用于防御微生物和食草攻击。已经发现这些化合物中的一些具有治疗性质,并且这些化合物和它们的衍生物以及新的化学物已经被开发用于治疗和诊断目的。其他生物分子在经过微小改动后有可能成为光敏剂,而一些人造化合物具有大多数不需要的光敏剂特性。这里将描述光敏剂的一些基本特性,然后概述最重要的已知光敏剂组。
光敏剂的基本特性综述
光化学的第一定律指出“只有分子吸收的光才能在该分子中产生光化学变化”。当光遇到分子时,它可以被分散或吸收。据说吸收了一定量光的分子被激发,可被可见光吸收激发的分子被称为发色团。分子的吸收光谱反映了其在基态与其第一和更高激发态的任何振动和旋转水平之间转变的概率。吸收光谱的特征在于特定波长下的摩尔吸收系数(以前称为消光系数),由Beer-Lambert定律描述:
其中是摩尔吸光系数,I0是入射光的注量率,I是透射光的注量率,d是样品的厚度,c是摩尔浓度,OD是光密度。吸收可以报告为%透射率(100×I / I0)或吸光度(log10(I / I0)。吸光度峰值(Imax)的波长和光谱形状取决于吸收分子的局部浓度,pH,溶剂的极性,与其他分子的结合,聚集和温度。
吸收光量子的分子被激发到第一或更高的激发态(参见基本光物理和光化学模块)。较高的激发态非常快地(ps)消散到第一激发单重态,寿命大约为ns。该激发态可以通过释放热(非辐射衰变)而失活,所述热释放为等于或长于激发光的波长的荧光,或者通过经历系统间交叉(ISC)。由于该状态的寿命短,光化学也可能发生,但通常概率很低。
例外是分子内修饰,例如在新生儿中高胆红素血症(黄疸)的光治疗中引起光致异构化的修饰,如在胆红素中。 ISC通常是被禁止的(选择规则),但具有共轭双键系统( - 电子系统)的大环分子是例外并且允许长寿命(μs-ms)三重态。三重态可以以两种方式反应,或者是涉及从一个分子到另一个分子的电子或氢原子转移的I型机制,或涉及能量转移到分子氧的II型机制(参见基本光敏化的模块)。两种机制可以同时发生,并且相对重要性取决于周围环境和底物分子的性质。由于在I型反应中光敏剂和基板之间的电子转移,当基板浓度高时更可能发生这些反应。缺氧条件也有利于I型反应。释放的热量可用于选择性地损伤靶组织,该过程称为光热致敏。
光敏剂的荧光
大多数光敏剂从第一激发单重态发射一些能量作为荧光。由于发射的光通常比吸收的光的能量低,所以发射的光通常具有更高的波长。光的能量与波长成反比。
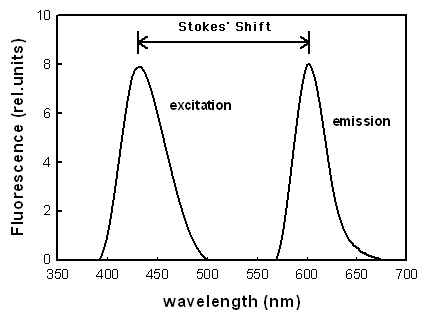
当评估光敏剂的荧光性质,荧光激发和发射光谱时,通常测量两种不同的光谱(图1)。当在一个波长处检测到发射时,获得荧光激发光谱,并且改变激发波长。对于处于其单体形式(未聚集)的光敏剂,荧光激发光谱和吸收光谱的形状相同。聚集的光敏剂通常显示低荧光或无荧光。当激发波长保持恒定时,获得荧光发射光谱,并且改变荧光检测的波长。激发峰和发射峰之间的差异称为斯托克转换(Stoke's shift)。
图1.染料的荧光激发和发射光谱的示意图。
光敏剂的荧光性质可用于检测癌症。许多光敏剂优先在肿瘤组织中积累,并且它们的荧光特性可能有益于这种病变的检测和诊断(图2)。膀胱内滴注5-氨基乙酰丙酸(ALA) - 己基酯(Hexvix®)(产生基于原卟啉IX(PpIX)的荧光)被批准用于检测膀胱癌,特别是原位癌,这是难以检测的。已发现膀胱癌的荧光引导切除有望用于治疗目的。类似地,全身性5-ALA(Gliolan)已经被批准用于残余神经胶质瘤的术中荧光引导检测,并且用于进一步切除剩余的肿瘤组织。
图2.用5-氨基乙酰丙酸甲酯(5-ALA ME)处理的基底癌发出的荧光。右图显示了基底细胞癌。用5-ALA ME处理相同的病变,3小时后暴露于蓝光。左侧显示了由损伤中的5-ALA ME诱导的原卟啉IX的红色荧光。
PHOTOSENSITIZERS in MEDICINE
Kristian Berg
Department of Radiation Biology
The Norwegian Radium Hospital
Montebello, N-0310 Norway
Kristian.Berg@rr-research.no
The picture below (left) shows a basal cell carcinoma. A physician wants to treat this skin cancer, but is unsure about the extent of the malignant tissue. What part of the lesion is cancerous? Where are the borders? Physicians can use photosensitizers to answer these questions. They can treat the area with a photosensitizer, such as 5-aminolevulinic acid, and then look at it using a blue light, which causes the photosensitizer to fluoresce (right). The pink/red fluorescent areas define the extent of the malignant lesion. In this module you will learn more about photosensitizers and the properties that make them useful for applications like this.
Photosensitizers have evolved over millions of years, mainly for defence against microbial and herbivorous attack. Some of these compounds have been found to have therapeutic properties, and these and derivatives of these compounds as well as new chemical entities, have been developed for therapeutic and diagnostic purposes. Other biomolecules have the potential to become photosensitizers upon minor modifications, and some man-made compounds have photosensitizer properties that are mostly unwanted. Here, some basic properties of photosentizers will be described, followed by an overview of the most important groups of known photosensitizers.
Review of Basic Properties of Photosentizers
The first law of photochemistry states that "only light absorbed by a molecule can produce photochemical change in that molecule". When light encounters a molecule, it can either be scattered or absorbed. A molecule that has absorbed a quantum of light is said to be excited, and molecules that can be excited by the absorption of visible light are named chromophores . The absorption spectrum of a molecule reflects its probability of transition between the ground state and any of the vibrational and rotational levels of its first and higher excited states. The absorption spectrum, characterized by the molar absorption coefficient (previously known as the extinction coefficient) at a particular wavelength, is described by Beer-Lambert's law:
where is the molar absorption coefficient, I0 is the fluence rate of the incoming light, I is the fluence rate of the transmitted light, d is the thickness of the sample, c is the molar concentration, and OD is the optical density. Absorption can be reported either as % transmittance (100 x I/I0 ) or as absorbance (log10 (I/I0). The wavelength of the peak of absorbance (Imax), and the spectral shape are dependent on the local concentration of absorbing molecules, pH, polarity of the solvent, binding to other molecules, aggregation and temperature.
A molecule that has absorbed a light quantum is excited to the first or higher excited states (see the modules on Basic Photophysics and Photochemistry). The higher excited states are dissipated very fast (ps) down to the first excited singlet state with a lifetime of the order of ns. This excited state can be deactivated by the release of heat (nonradiative decay), emitted as fluorescence of a wavelength equal to or longer than that of the excitation light, or by undergoing intersystem crossing (ISC). Photochemistry may also occur, but usually with a low probability, due to the short lifetime of this state.
Exceptions are intramolecular modifications, e.g., those that cause photoisomerization, as in bilirubin, upon the light treatment of hyperbilirubinemia (jaundice) in newborns. ISC is usually forbidden (selection rules), but macrocyclic molecules with conjugated double bond systems (-electron system) are exceptions and allow for long-lived (µs-ms) triplet states. The triplet state may react in two ways, either a Type I mechanisms involving electron or hydrogen atom transfer from one molecule to the other, or Type II mechanisms involving energy transfer to molecular oxygen (see modules on Basic Photosensitization). Both mechanisms may occur simultaneously, and the relative importance depends on the surroundings and the nature of the substrate molecules. Due to the transfer of electrons between the photosensitizer and substrate in Type I reactions, these reactions are more likely to occur when the substrate concentration is high. Hypoxic conditions also favours Type I reactions. The heat released may be used to selectively damage target tissues, and this process is named photothermal sensitization.
The Fluorescence of Photosensitizers
Most photosensitizers emit some of the energy from the first excited singlet state as fluorescence. Since the emitted light is usually less energetic than the absorbed light, the emitted light is usually of higher wavelength. The energy of light is inversely proportional to the wavelength.
Two different spectra are usually measured when the fluorescence properties of photosensitizers are evaluated, the fluorescence excitation and emission spectra (Figure 1). The fluorescence excitation spectra are obtained when the emission is detected at one wavelength, and the excitation wavelength is varied. For photosensitizers that are in their monomeric form (not aggregated), the fluorescence excitation spectra and absorption spectra are identical in shape. Aggregated photosensitizers usually show low or no fluorescence. A fluorescence emission spectrum is obtained when the excitation wavelength is kept constant, and the wavelength for the detection of fluorescence is varied. The difference between the excitation and emission peak is called the Stoke's shift.
Figure 1. Schematic illustration of fluorescence excitation and emission spectra of dyes.
The fluorescence properties of photosensitizers may be utilized for the detection of cancers. Many photosensitizers preferentially accumulate in neoplastic tissues, and their fluorescence properties may be beneficial in the detection and diagnosis of such lesions (Figure 2). Intravesical instillation of 5-aminolevulinic acid(ALA)-hexyl ester (Hexvix®) (resulting in protoporphyrin IX(PpIX)-based fluorescence) is approved for the detection of bladder cancer, in particular carcinoma in situ, which is difficult to detect. The fluorescence guided resection of bladder cancer has been found promising for treatment purposes. Similarly, systemic 5-ALA (Gliolan) has been approved for intra-operative fluorescence guided detection of residual glioma, and is used for the further resection of remaining tumor tissue.
Figure 2. Fluorescence emitted from basal carcinomas treated with 5-aminolevulinic acid methyl ester (5-ALA ME). A basal cell carcinoma is shown in the right figure. The same lesion is treated with 5-ALA ME and 3 hrs later exposed to blue light. Red fluorescence from protoporphyrin IX induced by 5-ALA ME in the lesion is shown on the left side.
Photosensitizers in Medicine http://photobiology.info/Berg.html
.png)