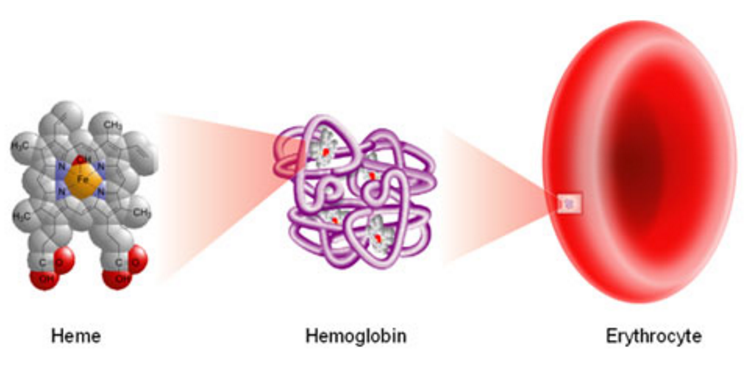
内源性光敏剂-血红素 、血卟啉、胆绿素和胆红素的光动力作用
The photodynamic action of endogenous photosensitizers-
Hemin, Porphyrin and Bilirubin
---叶绿素把光能转化为化学能,产生葡萄糖和氧气、血红素把氧气输送到组织细胞---
癌症的光动力疗法(PDT),PDT控制
Photodynamic therapy (PDT) of cancer. PDT control.
BioSpec开发的设备已成功应用于各种生物医学和临床应用,包括荧光肿瘤诊断和光动力治疗控制,光学活检和血氧饱和度评估:
光动力疗法(PDT)的癌症。 PDT控制。
使用光敏剂对肿瘤疾病进行医疗护理可以诊断和治疗腔内器官和皮肤的癌性肿瘤。世界上已经完成了10000多例成功的光动力疗法病例。我们的设备适用于使用血卟啉,ALA诱导的PP IX,酞菁,二氢卟酚等作为光敏剂。诊断基于以下事实:上述药物选择性地累积在恶性肿瘤中(例如,癌组织中一些酞菁的积累比正常组织中的高15倍)。在这种情况下,通过强荧光确定肿瘤的扩散和定位。通过对散射激光线的归一化来计算生物组织的光学性质的差异,用于激发荧光。
实施例1.皮肤肿瘤(患者A)和各种正常组织的荧光光谱的临床观察。静脉注射光敏剂(血卟啉)剂量为2mg / kg,累积时间为24小时。
为了治疗,用激光照射肿瘤,其波长对应于之前施用的光敏剂的最大吸收。光动力学处理的控制在于测量照射之前,期间和之后的荧光强度。
实施例2.使用荧光测量方法的具有5-ALA效率控制的PDT。 (病人Y.,胃癌)。
Photodynamic therapy (PDT) of cancer. PDT control.
Equipment developed by BioSpec has been successfully applied in various biomedical and clinical applications including fluorescent tumor diagnostics and photodynamic therapy control, optical biopsy and blood oxygen saturation evaluation:
Photodynamic therapy (PDT) of cancer. PDT control.
Medical care of oncological diseases with the use of photosensitizers allows both diagnostics and treatment of cancerous tumors of intracavity organs and skin. More than 10000 successful cases of photodynamic therapy were carried out in the world. Our equipment is adapted to the use of Hematoporphyrin, ALA induced PP IX, phthalocyanines, chlorins, etc. as photosensitizers. The diagnostics is based on the fact that above mentioned drugs are selectively accumulated in malignant tumors (for example, accumulation of some phthalocyanines in cancerous tissues is 15 times higher than those in normal tissues). In this case spreading and localization of tumor is determined by intensive fluorescence. Calculation of difference in optical properties of biological tissues is carried out by normalization to the scattered laser line, used for excitation of fluorescence.
Example 1. Clinical observations of fluorescent spectra of skin tumor (patient A.) and various normal tissues. The photosensitizer (hematoporphyrin) has been injected intravenously in dose 2 mg/kg, accumulation time 24 hours.
For treatment the tumor is irradiated by laser light with a wavelength corresponding to the absorption maximum of the photosensitizer administered before. Control of photodynamic treatment consists in measuring of fluorescence intensity before, during and after irradiating.
Example 2. PDT with 5-ALA efficiency control using fluorescent measurements method. (Patient Y., cancer of stomach).
You may see in Example 2 that fluorescence intensity after PDT is declining to values adequate to normal contiguous tissues. Therefore further irradiating is pointless.
BioSpec - Application Examples http://www.biospec.ru/_Application_Examples_e.html
The photodynamic action of bilirubin on erythrocytes*
Erythrocyte suspensions exposed to bilirubin in concentrations that frequently occur during neonatal life are hemolyzed when irradiated with fluorescent light. The hemolysis is preceded by membrane damage that is reflected by a loss of erythrocyte potassium and a reduction in membrane ATPase activity. The initiation of the cation loss requires the simultaneous presence of molecular oxygen and light and therefore involves a photodynamic action of bilirubin. The development of anemia associated with phototherapy may be a consequence of an in vivo photosensitized hemolysis.
SOURCE:
Author links open overlay panelM.D.Gerard B.OdellabB.S.Ralph S.BrownabM.D.Arthur E.Kopelmanab
Show more
https://doi.org/10.1016/S0022-3476(72)80173-2Get rights and content
The photodynamic action of bilirubin on erythrocytes - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0022347672801732
The mechanism of bilirubin-photosensitized DNA strand breakage in human cells exposed to phototherapy light.
Abstract
Exposure of normal human fibroblasts to visible light (420-490 nm) in the presence of exogenously added 1-100 micrograms/ml bilirubin enhanced the level of DNA strand breakage compared with cells irradiated in the absence of added bilirubin. Treatment of cells in the dark with an irradiated bilirubin solution also induced DNA strand breaks. However, strand breakage was not detected in cells treated with an irradiated bilirubin solution that had been incubated with catalase (H2O2: H2O2 oxidoreductase EC 1.11.1.6). Examination of irradiated bilirubin solutions demonstrated the presence of hydrogen peroxide although, apparently, not at concentrations sufficient to account for the level of DNA strand breakage detected. Hence, irradiation of bilirubin results in the generation of hydrogen peroxide and possibly other peroxides that can cause DNA damage.
SOURCE:
Mutat Res. 1983 Dec;112(6):397-406.
Rosenstein BS, Ducore JM, Cummings SW.
PMID: 6656800
[Indexed for MEDLINE]
The mechanism of bilirubin-photosensitized DNA strand breakage in human cells exposed to phototherapy light. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/6656800
Bilirubin metabolism: Applied physiology
Article in Current Paediatrics 16(1):70-74 · February 2006 with 3,540 Reads
DOI: 10.1016/j.cupe.2005.10.002
Bilirubin is the breakdown product of the haem moiety of haemoglobin and other haemoproteins. Because of internal hydrogen bonding, bilirubin is water-insoluble and requires enzyme-mediated glucuronidation in the liver for biliary excretion. In normal circumstances, plasma bilirubin is mostly unconjugated and is tightly bound to circulating albumin. It is taken up by hepatocytes by facilitated diffusion, stored in hepatocytes bound to glutathione-S-transferases and conjugated to glucuronides by microsomal UGT1A1. Bilirubin glucuronides are actively transported into the bile canaliculi by the ATP-utilizing pump MRP2. Bilirubin is degraded in the intestine by bacteria into urobilinogens, which are partly excreted in the urine. Increased production, reduced uptake and low glucuronidation capacity can increase plasma unconjugated bilirubin levels. In cases of inherited or acquired deficiencies of bilirubin storage or excretion, both conjugated and unconjugated bilirubin accumulate in the plasma. Conjugated bilirubin is less tightly bound to albumin and is excreted in the urine. The capacities of the various steps of bilirubin throughput are finely balanced, and the expression of the gene products mediating these steps is coordinated by nuclear receptors.
Bilirubin metabolism: Applied physiology | Request PDF https://www.researchgate.net/publication/239312492_Bilirubin_metabolism_Applied_physiology
Pediatr Res. 1984 Jan;18(1):3-6.
Enhancement by bilirubin of DNA damage induced in human cells exposed to phototherapy light.
Rosenstein BS, Ducore JM.
Abstract
Normal human fibroblasts were exposed to light from three types of lamps commonly used for phototherapy treatment of neonatal hyperbilirubinemia. These irradiations were performed in either the presence or absence of exogenously added bilirubin. Using the alkaline elution assay, we found that each phototherapy lamp induced strand breaks in the DNA of exposed cells. However, the cross sections for DNA strand breakage were increased 30-40-fold when cells were irradiated in the presence of 100 micrograms/ml bilirubin. Hence, bilirubin acts as a photosensitizing agent enhancing the level of DNA damage in cells exposed to phototherapy light.
PMID: 6701032
[Indexed for MEDLINE]
Enhancement by bilirubin of DNA damage induced in human cells exposed to phototherapy light. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/6701032
J Photochem Photobiol B. 1990 Nov;7(2-4):337-46.
Single-strand breaks in the DNA of human cells exposed to visible light from phototherapy lamps in the presence and absence of bilirubin.
Christensen T1, Reitan JB, Kinn G.
Author information
Abstract
Clinical evidence indicates that phototherapy of hyperbilirubinaemia in newborn infants is a safe and efficient form of therapy. The short-term side effects are not serious and seem to be well controlled. There are few long-term follow-up studies of phototherapy-treated infants. Therefore one cannot completely exclude the possibility that side effects can be found in future studies. With this background we undertook the present study of possible genotoxic effects of phototherapy. Human cells of the established glioblastoma cell line TMG-1 were used. The cells were exposed to visible light in the presence of different concentrations of bilirubin or in the absence of bilirubin. DNA was unwound in alkaline solution and the induction of strand breaks was assayed by a method taking advantage of the fluorescence from the dye Hoechst 33258. Blue light induced single-strand breaks in the DNA of cells in culture in the absence of bilirubin. During irradiation of bilirubin solutions with blue and green phototherapy light, long-lived toxic photoproducts were formed under in vitro conditions. At high and clinically relevant bilirubin concentrations, the effects of blue and green light were relatively similar. At low concentrations, there was a smaller effect of green light as expected from the absorption spectrum of bilirubin. It remains to be seen whether the genotoxic effect observed in the present studies can occur in vivo.
PMID: 2128329
[Indexed for MEDLINE]
Single-strand breaks in the DNA of human cells exposed to visible light from phototherapy lamps in the presence and absence of bilirubin. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/2128329
Photochem Photobiol. 1995 Dec;62(6):980-3.
Bilirubin phototoxicity to human cells by green light phototherapy in vitro.
Böhm F1, Drygalla F, Charlesworth P, Böhm K, Truscott TG, Jokiel K.
Author information
1
Humboldt University, Department of Dermatology (Charité), Berlin, Germany.
Abstract
Phototherapy of newborn infants with blue or green light is the most common treatment of neonatal hyperbilirubinemia. Using bilirubin bound to human lymphoid and basal skin cells we obtained the green light dose dependency of the bilirubin phototoxicity to these cell types. Cells (3-5 x 10(6)/mL) were incubated with bilirubin complexed to human serum albumin (final concentrations 340 microM bilirubin, 150 microM albumin). Under these conditions all cells showed maximum binding of bilirubin. Irradiation with broadband green light (lambda max = 512 nm) over 24 h led to a light dose-dependent population of cells, which contained no bilirubin on the cell membrane as determined by Nomarski interference microscopy. The light-induced mechanism of the disappearance of bilirubin caused lethal membrane damage to the cells (trypan blue exclusion test). The cell kill rate increased with the irradiation dose and with the fraction of cells with no bilirubin. When 90% of lymphoid cells were bilirubin free, 46% of them were dead (using 480 J cm-1 green light). Similar results were obtained with basal skin cells. In addition, bilirubin-induced damage of cell membrane and nuclear membrane was also shown by transmission electron microscopy. Bilirubin (340 microM) in the dark led to 5% of the cells being killed. Basal skin cells bind 2.5 times more bilirubin molecules than lymphoid cells and showed a different bilirubin disappearance. Irradiation of bilirubin in carbon tetrachloride with 514.5 nm laser light showed generation of singlet oxygen via its luminescence at 1270 nm. These results demonstrate that green light phototherapy of hyperbilirubinemia may cause both skin and immune system damage.
PMID: 8570745
[Indexed for MEDLINE]
Bilirubin phototoxicity to human cells by green light phototherapy in vitro. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/8570745
Cells, bilirubin and light: formation of bilirubin photoproducts and cellular damage at defined wavelengths.
Acta Paediatr. 1994 Jan;83(1):7-12.
Christensen T1, Kinn G, Granli T, Amundsen I.
Author information
1
Radiation Medicine Department, Norwegian Radiation Protection Authority, Osterås.
Abstract
Cultured cells from one human and one murine cell line were treated with bilirubin and irradiated with visible light of different wavelengths, either from phototherapy lamps or from a Xenon/Mercury lamp equipped with a monochromator. Bilirubin bound to human serum albumin was also irradiated with light. After irradiation, the bilirubin and its photoisomers were extracted and analysed with High Pressure Liquid Chromatography. The formation of single strand breaks in the DNA of treated cells was studied using a fluorescence marker. Cytotoxicity in the mouse skin cell line was measured by loss of the ability to form visible colonies in vitro. Green light exposure favours the production of lumirubin, while blue light causes more DNA damage and cytotoxicity. Green light may be more efficient and safer than shorter wavelength exposure when treating jaundiced newborns with phototherapy.
PMID: 8193477
[Indexed for MEDLINE]
Cells, bilirubin and light: formation of bilirubin photoproducts and cellular damage at defined wavelengths. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/8193477
Bilirubin-induced cell death during continuous and intermittent phototherapy and in the dark.
Abstract
AIM:
To compare continuous and intermittent light exposure in the presence of bilirubin with respect to cellular damage. Furthermore, it was of interest to characterize the nature of cellular toxicity of bilirubin in the dark.
METHOD:
A murine lymphoma cell line, L5178Y-R (LY-R), was exposed to solutions of bilirubin (160 microM) supplemented with human serum albumin (200 microM) and irradiated with phototherapy light (Philips 20W/52) at a constant total dose of approximately 500 kJ/m2. The irradiation was given either as intermittent or continuous treatment with light of variable irradiance. The three lower irradiance levels were clinically relevant. Cells treated with bilirubin were also kept in the dark for various periods of time. Cell toxicity was determined by measuring apoptosis and necrosis. Apoptosis was measured by terminal deoxynucleotide transferase and propidium iodide staining assay, while trypan blue assay was used for detection of necrosis.
RESULTS:
There was no difference (n = 6, p > 0.05) between continuous and intermittent irradiation in the induction of early and late apoptotic cell death. Necrosis was more pronounced after intermittent treatment. Bilirubin dark toxicity was observed and classified as both apoptotic and necrotic.
CONCLUSION:
Continuous and intermittent light exposure caused the same degree of apoptotic cell death, while the cells underwent more necrotic death after intermittent exposure. Bilirubin was cytotoxic in the dark by both cell death mechanisms.
Acta Paediatr. 2005 Oct;94(10):1437-42.
Roll EB1.
Author information
1
Norwegian Radiation Protection Authority, Østerås, Norway. ebr@niom.no
Comment in
Phototherapy: old questions, new answers. [Acta Paediatr. 2005]
PMID: 16263630 DOI: 10.1080/08035250510032646
[Indexed for MEDLINE]
Bilirubin-induced cell death during continuous and intermittent phototherapy and in the dark. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/16263630
过氧化氢,超氧化物和羟基自由基参与血卟啉衍生物对肿瘤细胞的光毒作用
Hydrogen Peroxide, Superoxide, and Hydroxyl Radicals are Involved in the Phototoxic Action of Hematoporphyrin Derivative against Tumor Cells
血卟啉衍生物
是单体和聚集卟啉的复杂混合物,具有光敏活性。全身给药后,血卟啉衍生物在肿瘤细胞中积累,一旦被红色激光(630nm)激活,在氧气存在下,产生单线态氧和其他活性氧自由基,导致局部自由基介导的肿瘤细胞死亡。
https://www.cancer.gov/publications/dictionaries/cancer-drug/def/hematoporphyrin-derivative
本研究旨在评估除单线态氧(1O2)外的活性氧(ROS)参与光动力疗法(PDT)与血卟啉衍生物(HPD)的抗肿瘤作用以及确定光激发HPD的能力。蛋白质过氧化物的形成目前被认为是一种新的ROS形式。
对Ehrlich腹水癌(EAC)细胞进行研究,所述细胞在磷酸盐缓冲盐水中加载HPD,然后在相同缓冲液中在630nm下用红光照射。
实验表明,H2O2和氧自由基可介导HPD-PDT的杀瘤作用;我们发现EAC细胞对HPD的光敏作用导致形成大量的H2O2,超氧化物(O2-)和羟基(OH)基团,其与1O2一起参与体外细胞的光活化。
我们的数据显示,在经受HPD-PDT的EAC细胞中,产生H 2 O 2,O 2 - 和OH。可能主要通过以下方式介导:(i)黄嘌呤氧化酶(XOD)活性的增加,这很可能是由于黄嘌呤脱氢酶(XDH)通过Ca2 +依赖性蛋白水解过程转化为XOD以及SH组氧化。 XDH; (ii)一些细胞成分(蛋白质)的光氧化作用。
我们研究的另一个有趣发现是,在接受HPD-PDT的肿瘤细胞中,Fenton样反应可能在OH的产生中发挥重要作用,并且细胞结合的Cu / Zn-超氧化物歧化酶以及过氧化氢酶可以保护肿瘤细胞对抗HPD的光毒作用。
此外,我们清楚地证明了光激发HPD在肿瘤细胞中产生蛋白质过氧化物的能力。
Hydrogen Peroxide, Superoxide, and Hydroxyl Radicals are Involved in the Phototoxic Action of Hematoporphyrin Derivative against Tumor Cells
hematoporphyrin derivative
A complex mixture of monomeric and aggregated porphyrins with photosensitizing activity. Upon systemic administration, hematoporphyrin derivatives accumulate in tumor cells and, once activated by red laser light (630 nm), in the presence of oxygen, produce singlet oxygen and other reactive oxygen radicals, resulting in local radical-mediated tumor cell death.
https://www.cancer.gov/publications/dictionaries/cancer-drug/def/hematoporphyrin-derivative
This study was aimed to estimate the participation of reactive oxygen species (ROS), other than singlet oxygen (1O2), in the antitumor effect of photodynamic therapy (PDT) with hematoporphyrin derivative (HPD) as well as to determine the ability of photoexcited HPD to the formation of protein peroxides that currently are regarded as a new form of ROS.
Studies were performed on Ehrlich ascites carcinoma (EAC) cells, which were loaded with HPD in phosphate-buffered saline and then irradiated with red light at 630 run in the same buffer.
Experiments indicated that H2O2 and oxygen radicals could mediate the tumoricidal action of HPD-PDT; we found that photosensitization of EAC cells with HPD leads to the formation of significant amounts of H2O2, superoxide (O2-.), and hydroxyl (OH.) radicals, which along with 1O2 were involved in photoinactivation of the cells in vitro.
Our data showed that in EAC cells subjected to HPD-PDT, the generation H2O2, O2-., and OH. could be largely mediated by: (i) an increase in the activity of xanthine oxidase (XOD), due most probably to the conversion of xanthine dehydrogenase (XDH) to XOD via a Ca2+-dependent proteolytic process as well as oxidation of SH groups in XDH; and (ii) photooxidation of some cellular constituents (proteins).
Another interesting finding of our studies is that in tumor cells subjected to HPD-PDT the Fenton-like reactions could play an important role in the generation of OH., and that cell-bound Cu/Zn-superoxide dismutase as well as catalase can protect tumor cells against the phototoxic action of HPD.
In addition, we clearly demonstrated the ability of photoexcited HPD to the generation of protein peroxides in tumor cells.
Article (PDF Available) in Journal of Environmental Pathology Toxicology and Oncology 25(1-2):51-77 · February 2006 with 72 Reads
DOI: 10.1615/JEnvironPatholToxicolOncol.v25.i1-2.40 · Source: PubMed
Chekulayeva LV1, Shevchuk IN, Chekulayev VA, Ilmarinen K.
Department of Chemistry, Tallinn University of Technology, Akadeemia tee 15,12618 Tallinn, Estonia.
Studies suggest that 1O2 is the main agent responsible for the generation of protein peroxides in EAC cells treated with HPD-PDT, although other ROS (H2O2, O2-., and OH.) were also implicated in this process. However, further work is needed to clarify the significance of these peroxides in the antitumor effect of PDT with HPD.
Comparative study of photodegradation of three hematoporphyrin derivative: Photofrin®, Photogem®, and Photosan®
Article in Laser Physics Letters 4(10):743 - 748 · June 2007 with 80 Reads
DOI: 10.1002/lapl.200710058
We report the photodegradation of the three different photosensitizers derived from hematoporphyrin. In this paper we use the term phototransformation for describing the photodegradation or photobleaching process. This photodegradation alters the fluorescence during illumination. The rate of fluorescence variation was normalized to the solution absorption and the photon energy, resulting in the determination of the necessary number of photons to be absorbed to induce photosensitizer phototransformation. The parameter for rate of the molecules decay, the photon fluence rate and optical properties of the solution, allow us to determine the photosensitizer stability in solution during illumination. The results show that the order of susceptibility for phototransformation rate is: Photofrin® Photogem® < Photosan®. This difference in the phototransformation rate for Photosan® can be explained by the high proportion of aggregates in solution that inhibit the photo-oxidative process that impede the singlet oxygen formation.We hypothesize that there is a correlation between phototransformation rate and photodynamic efficacy witch is governed by singlet oxygen formation responsible by most relevant reaction for the photodynamic induction of cell death. (© 2007 by Astro Ltd., Published exclusively by WILEY-VCH Verlag GmbH & Co. KGaA)
Role of Lipid Peroxidation in Metalloporphyrin-Mediated Phototoxic Reactions in Neonatal Rats
Our group previously demonstrated dose-dependent mortality in neonatal rats treated with tin protoporphyrin and light. We hypothesize that lipid peroxidation may be responsible for the toxic effects of photosensitizing metalloporphyrins. Neonatal rat blood samples with or without metalloporphyrins (40 mM) were exposed to cool white light (20 microW/cm2/nm) for 30 min at 37 degrees C. In the in vivo model, neonatal rat pups were given injections of 40 mumol of either tin protoporphyrin (4 mM), zinc protoporphyrin/kg body weight, or saline and placed over cool white light. The control animals were similarly treated but kept in the dark. After 3 h, the animals were killed, and their tissues were analyzed for malondialdehyde, conjugated dienes, and disappearance of polyunsaturated fatty acids as indices of lipid peroxidation. In all cases, the known photosensitizer tin protoporphyrin was associated with increased conjugated dienes in the liver and disappearance of polyunsaturated fatty acids and increased malondialdehyde in the liver and brain when animals were exposed to light. Zinc protoporphyrin was not associated with increased lipid peroxidation in the light except in the case of blood in vitro where malondialdehyde levels increased. We conclude that lipid peroxidation plays a role in metalloporphyrin-mediated phototoxicity in neonatal rat tissues.
Article in Pediatric Research 33(1):87-91 · February 1993 with 25 Reads
DOI: 10.1203/00006450-199301000-00018 · Source: PubMed
Role of Lipid Peroxidation in Metalloporphyrin-Mediated Phototoxic Reactions in Neonatal Rats | Request PDF https://www.researchgate.net/publication/14765177_Role_of_Lipid_Peroxidation_in_Metalloporphyrin-Mediated_Phototoxic_Reactions_in_Neonatal_Rats
Bilirubin- and light induced cell death in a murine lymphoma cell line☆
Author links open overlay panelTerjeChristensenabEllen B.RollabAlicjaJaworskaaGunnarKinna
Show more
https://doi.org/10.1016/S1011-1344(00)00126-3Get rights and content
Abstract
Cells from the mouse lymphoma cell line L5178Y-R were exposed to blue light from phototherapy lamps in the presence of solutions of 160 μM bilirubin supplemented with serum albumin. HPLC analysis showed that the bilirubin solution was photooxidised as a function of increasing light dose. The cells were stained with trypan blue to score necrosis, and apoptosis was assayed by the terminal deoxynucleotide transferase assay (TdT) or by studying the nuclear structure in cells stained with propidium iodide. A rapidly developing apoptosis was observed after light doses killing 60–80% of the cells as judged from the trypan blue exclusion test. The fraction of apoptotic cells was smaller than the fraction of necrotic cells. Exposure of the cells to fractions of light at a high dose rate was compared to the effect of the same total dose at a lower dose rate given as a single fraction. No large differences were found, however, there was a tendency of a higher degree of necrosis as well as apoptosis in the cells receiving the light in fractions at a high dose rate.
Previous article in issueNext article in issue
Keywords
Phototherapy Newborn Bilirubin Apoptosis Necrosis Blue lightMouse lymphoma cells
Journal of Photochemistry and Photobiology B: Biology
Volume 58, Issues 2–3, November 2000, Pages 170-174
Journal of Photochemistry and Photobiology B: Biology
https://www.sciencedirect.com/science/article/pii/S1011134400001263
Laser Photolysis of Bilirubin
Photodegradation of bilirubin in vitro has been investigated by using monochromatic light supplied by an argon ion laser selecting the 457.9, 488.0 and 514.5 nm wavelengths.
Bilirubin was examined in chloroform, in aqueous solutions and in human serum under different experimental conditions of concentration, laser power and time of irradiance.
Photodecomposition was followed by optical density measurements on the absorption maximum of the electronic band at 460 nm. The rate of degradation of bilirubin was found to be only slightly affected by the wavelength of the exciting lines provided they fall within the absorption band. In particular it was shown that any wavelength, lambda, is equally effective if the corresponding absorbance, A lambda, exceeds a minimum value of 5-10%.
In the aqueous solutions, light with lambda greater than 470 nm has been found to be largely effective in the photodegradation of bilirubin in vitro.
Article in Pediatric Research 15(12):1517-9 · January 1982 with 23 Reads
DOI: 10.1203/00006450-198112000-00013 · Source: PubMed
Laser Photolysis of Bilirubin https://www.researchgate.net/publication/15875774_Laser_Photolysis_of_Bilirubin
Heme Metabolism and Disposition of Bilirubin
The largest repository of heme in the human body is in red blood cells, which have a life span of about 120 days. There is thus a turnover of about 6 g/day of hemoglobin, which presents 2 problems. First, the porphyrin ring is hydrophobic and must be solubilized to be excreted. Second, iron must be conserved for new heme synthesis.
Normally, senescent red blood cells and heme from other sources are engulfed by cells of the reticuloendothelial system (phagocytic macrophages primarily of the spleen but also of the liver, lymph, and bone marrow). The globin is recycled or converted into amino acids, which in turn are recycled or catabolized as required. Heme is oxidized, with the heme ring being opened by the endoplasmic reticulum enzyme, heme oxygenase. The oxidation step requires heme as a substrate, and any hemin (Fe3+) is reduced to heme (Fe2+) prior to oxidation by heme oxygenase. The heme oxygenase-mediated oxidation occurs on a specific carbon producing the linear tetrapyrrole biliverdin, ferric iron (Fe3+), and carbon monoxide (CO).
Porphyrin and Heme Synthesis and Bilirubin Metabolism http://themedicalbiochemistrypage.org/heme-porphyrin.php#regulation
Heme metabolism in macrophages
Heme degradation
Share on FacebookTweet about this on TwitterShare on LinkedInPin on PinterestShare on Google+Share on StumbleUpon
The main source of iron in the body is from turnover of hemoglobin in red blood cells. This is accomplished by macrophages which phagocytize effete RBC in physiologic states or normal RBC in pathophysiologic states (hemolytic anemia). Once taken up by the macrophages, RBC are degraded in a phagolysosome which liberates hemoglobin, consisting of a heme group (porphyrin ring + iron) and globin chains. The globins are broken down to amino acids (aa), which are then used for protein synthesis. The porphyrin ring of heme is oxidized by microsomal heme oxygenase, producing biliverdin and releasing the iron. Biliverdin is reduced by biliverdin reductase to unconjugated bilirubin. The unconjugated bilirubin is released into the plasma, where it binds to albumin and is taken up by hepatocytes (not shown). Once released from the heme group, iron has three fates:
1. The iron can then be exported into plasma through iron channels, where it binds to apotransferrin (the iron transport protein) forming transferrin. In order for this to be accomplished, iron must be converted to the Fe2+ state, which is facilitated by the copper-dependent enzyme, ceruloplasmin. Thus, copper is required for iron release from macrophages or enterocytes (haephestin is copper dependent).
2. Iron can be stored within cells as ferritin, i.e. after the iron is bound to the storage protein, apoferritin. With time, ferritin becomes oxidized and degrades to form hemosiderin. Hemosiderin can be visualized within macrophages as a dusky blue-gray pigment and can be definitively stained with Prussian blue (which turns hemosiderin blue).
3. The iron can be immediately incorporated into proteins that need it, e.g. cytochrome P450.
Heme metabolism in macrophages | eClinpath http://www.eclinpath.com/chemistry/iron-metabolism/heme-metabolism/
Heme degradation
Hyperbilirubinemia
Hyperbilirubinemia in the neonate can be treated with blue fluorescent light phototherapy which converts bilirubin to water soluble isomers.
Jaundice
is the symptom which is observed when there is accumulation of bilirubin in the skin and sclera
Kernicterus
-is toxic bilirubin encephalopathy that is observed in neonates with high levels of unbound bilirubin. All neonates have a tendency toward elevated bilirubin levels because bilirubin glucuronyl transferase is low at birth.
-Kernicterus is caused by unconjugated hyperbilirubinemia either from haemolytic disease or the inability of the liver to conjugate bilirubin.
-Unbound, unconjugated bilirubin crosses the blood-brain barrier and, because it is lipid soluble, it penetrates neuronal and glial membranes.
- Babies with bilirubin encephalopathy are lethargic, hypotonic or hypertonic, and have a high pitched cry, seizures, and may die if bilirubin is not lowered.
Porphyria Cutanea Tarda (PCT)
-caused by deficiency in uroporphyrinogen decarboxylase
-accumulation of uroporphyrin in the urine
-most common porphyria
-caused by alcohol, halogenated hydrocarbons, other toxic substances
-enzyme activity lacking in the liver
but not erythrocytes
-Patients are photosensitive
SOURCE:
Heme and Porphyrin Metabolism Quizlet https://quizlet.com/188026165/heme-and-porphyrin-metabolism-flash-cards/
胆红素:体外具有抗病毒活性的内源性分子
摘要
胆红素-IX-α(BR)是通过血红素加氧酶/胆绿素还原酶(HO / BVR)系统的血红素代谢的最终产物。之前的论文通过直接或间接机制报道了HO副产物胆绿素-IX-α,一氧化碳和铁的杀微生物作用。
在本文中,提供了BR对人单纯疱疹病毒1型(HSV-1)和肠道病毒EV71的杀病毒作用的证据。浓度为1-10μM的胆红素-IX-α与血液和组织中发现的浓度接近,分别显着降低了Hep-2和Vero细胞系中的HSV-1和EV71复制。胆红素-IX-α在病毒感染前2小时,同时和2小时后抑制Hep-2和Vero细胞的病毒感染。此外,BR甚至与饱和浓度的人血清白蛋白复合仍保持其抗病毒活性。此外,10μMBR分别增加了Vero和Hep-2细胞系中一氧化氮的形成和c-Jun N末端激酶的磷酸化,从而暗示了这两种途径在胆汁抗病毒活性机制中的作用。
总之,这些结果支持BR对HSV-1和肠道病毒的体外抗病毒作用,并为进一步的基础和临床研究奠定基础,以了解BR作为内源性抗病毒分子的真正作用。
关键词:胆红素,胆绿素还原酶,肠道病毒,血红素加氧酶,单纯疱疹病毒,一氧化氮
Bilirubin: An Endogenous Molecule with Antiviral Activity in vitro
Abstract
Bilirubin-IX-alpha (BR) is the final product of heme metabolism through the heme oxygenase/biliverdin reductase (HO/BVR) system. Previous papers reported on the microbicidal effects of the HO by-products biliverdin-IX-alpha, carbon monoxide and iron, through either direct or indirect mechanisms. In this paper the evidence of a virucidal effect of BR against human herpes simplex virus type 1 (HSV-1) and the enterovirus EV71 was provided. Bilirubin-IX-alpha, at concentrations 1–10 μM, close to those found in blood and tissues, significantly reduced HSV-1 and EV71 replication in Hep-2 and Vero cell lines, respectively. Bilirubin-IX-alpha inhibited viral infection of Hep-2 and Vero cells when given 2 h before, concomitantly and 2 h after viral infection. Furthermore, BR retained its antiviral activity even complexed with a saturating concentration of human serum-albumin. Moreover, 10 μM BR increased the formation of nitric oxide and the phosphorylation of c-Jun N-terminal kinase in Vero and Hep-2 cell lines, respectively, thus implying a role of these two pathways in the mechanism of antiviral activity of the bile pigment. In conclusion, these results support the antiviral effect of BR against HSV-1 and enterovirus in vitro, and put the basis for further basic and clinical studies to understand the real role of BR as an endogenous antiviral molecule.
Keywords: bilirubin, biliverdin reductase, enterovirus, heme oxygenase, herpes simplex virus, nitric oxide
SOURCE:
Front Pharmacol. 2012; 3: 36.
Published online 2012 Mar 9. Prepublished online 2012 Jan 27. doi: [10.3389/fphar.2012.00036]
PMCID: PMC3297833
PMID: 22408623
Rosaria Santangelo,1,† Cesare Mancuso,2,*† Simona Marchetti,1 Enrico Di Stasio,3 Giovambattista Pani,4 and Giovanni Fadda1
Author information Article notes Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Bilirubin: An Endogenous Molecule with Antiviral Activity in vitro https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3297833/
血红素加氧酶/胆绿素还原酶(HO / BVR)轴
The heme oxygenase/biliverdin reductase (HO/BVR) axis
血红素加氧酶/胆绿素还原酶(HO / BVR)轴是血红素降解的主要代谢途径。这些酶的联合作用将血红素转化为二价铁(FeII),一氧化碳(CO)和胆绿素-IX-α(BV),后者是胆红素-IX-α的前体(BR; Maines,1997; Mancuso和Barone) ,2009)。多年来,HO / BVR轴的副产品被认为仅仅是废品,但在过去二十年中,许多研究人员将注意力集中在HO / BVR及其产品上,以试图阐明其实际的生物功能。 Stocker等。 (1987b)描述了BR的抗氧化特性,6年后Verma等人。 (1993)提出了CO作为内源性神经调节剂的作用。这些观察结果后面有大量论文,证明了CO作为突触传递,心脏功能和血管张力调节剂的重要作用(Wu和Wang,2005)。胆红素-IX-alpha通过激活重要的促存活信号通路,例如涉及原癌基因Akt或丝裂原活化蛋白激酶家族(MAPK)的途径,以及通过清除活性氧(ROS)和活性氮物种(RNS),帮助维持细胞的氧化还原平衡( ROS和RNS分别为; Stocker等,1987b; Minetti等,1998; Kaur等,2003; Mancuso等,2006b,2008)。这些研究结果表明,HO / BVR轴的上调是改善细胞应激反应的有用机制,正在研究已知增加体外HO活性的物质作为治疗自由基诱导疾病的潜在药物( Mancuso和Barone,2009)。
此外,HO的上调显示出对抗细菌和病毒感染的重要作用。 HO(HO-1)的诱导型同种型的过表达显示出对肠病毒和肝炎病毒的显著抗病毒活性(Zhu等人,2010; Tung等人,2011)。关于涉及该抗病毒活性的HO系统的副产物,BV显示干扰人类丙型肝炎病毒(HCV)和人类疱疹病毒(HHV)-6复制以及降低人类的细胞病变效应。 Huh或MT-4细胞中的免疫缺陷1型病毒(HIV)(Mori等人,1991; Nakagami等人,1992; Lehmann等人,2010; Zhu等人,2010)。此外,CO抑制肠道病毒,大肠杆菌,铜绿假单胞菌以及金黄色葡萄球菌的生长(Nobre等,2007; Tung等,2011; Desmard等,2012),铁几乎完全减少HCV核心mRNA和蛋白质(Hou等人,2009)。
总之,这些结果强调了HO在抗病毒和抗菌防御中的主要作用。也就是说,值得一提的是,迄今为止还没有关于BVR产生的BR潜在抗病毒活性的数据。如前所述,BR被证明是ROS的有效清除剂,而ROS目前被视为免疫应答抗菌活性的效应物(Bogdan等,2000; Fang,2004; Fialkow等,因此,BR的直接抗病毒作用不大可能。另一方面,最近的研究结果证明了BR的细胞保护活性的替代机制,包括刺激一氧化氮(NO)释放和调节嗜铬细胞瘤细胞和大鼠小脑颗粒的原代培养物中的MAPK系统(Mancuso等, 2008)。 NO在抗病毒防御中起重要作用(Croen,1993; Akaike和Maeda,2000),并且它也是某些药物的杀菌活性的中间体(Timmins等,2004; Koide等,2009)。此外,特异性MAPK的上调,例如c-Jun N-末端激酶(JNK),显示出增加对病毒的细胞保护防御(Hrincius等,2010)。
The heme oxygenase/biliverdin reductase (HO/BVR) axis
The heme oxygenase/biliverdin reductase (HO/BVR) axis is the main metabolic pathway by which heme is degraded. The combined action of these enzymes converts heme into ferrous iron (FeII), carbon monoxide (CO), and biliverdin-IX-alpha (BV) which is the precursor of bilirubin-IX-alpha (BR; Maines, 1997; Mancuso and Barone, 2009). For several years, the by-products of the HO/BVR axis were considered mere waste products, but over the past two decades, a number of investigators have focused their attention on both HO/BVR and their products in an attempt to elucidate their true biological functions. Stocker et al. (1987b) described the antioxidant properties of BR, and 6 years later Verma et al. (1993) proposed a role for CO as an endogenous neuromodulator. These observations were followed by numerous papers demonstrating CO’s important role as a regulator of synaptic transmission, cardiac function, and vessel tone (Wu and Wang, 2005). Bilirubin-IX-alpha helps maintain the cell’s redox equilibrium by activating important pro-survival signaling pathways, such as those involving the proto-oncogene Akt or the mitogen-activated protein kinase family (MAPK), and by scavenging reactive oxygen and nitrogen species (ROS and RNS, respectively; Stocker et al., 1987b; Minetti et al., 1998; Kaur et al., 2003; Mancuso et al., 2006b, 2008). These findings suggest that up-regulation of the HO/BVR axis is a useful mechanism for improving cells’ responses to stress, and substances known to increase HO activity in vitro are being explored as potential drugs for the treatment of free radical-induced diseases (Mancuso and Barone, 2009).
In addition, the up-regulation of HO was shown to play an important role against bacterial and viral infections. The overexpression of the inducible isoform of HO (HO-1) displayed a significant antiviral activity against enterovirus and hepatitis viruses (Zhu et al., 2010; Tung et al., 2011). With regard to the by-products of the HO system involved in this antiviral activity, BV was shown to interfere with human hepatitis C virus (HCV) and human herpes virus (HHV)-6 replication as well as to reduce the cytopathic effect of human immunodeficiency type 1 virus (HIV) in Huh or MT-4 cells (Mori et al., 1991; Nakagami et al., 1992; Lehmann et al., 2010; Zhu et al., 2010). In addition, CO inhibited the growth of enterovirus, E. coli, P. aeruginosa as well as S. aureus (Nobre et al., 2007; Tung et al., 2011; Desmard et al., 2012) and iron almost completely decreased HCV core mRNA and protein (Hou et al., 2009). Taken together these results underlined a major role for HO in antiviral and antibacterial defense. That said, it is noteworthy to mention that no data are available to date about the potential antiviral activity of BR produced by BVR. As mentioned before, BR was shown to serve as an efficient scavenger for ROS which, in turn, are currently viewed as effectors of the antimicrobial activity of the immune response (Bogdan et al., 2000; Fang, 2004; Fialkow et al., 2007), therefore a direct antiviral role for BR is unlikely. On the other hand, recent findings demonstrated alternative mechanisms for the cytoprotective activity of BR, including the stimulation of nitric oxide (NO) release and the modulation of the MAPK system in pheochromocytoma cells and primary cultures of rat cerebellar granules (Mancuso et al., 2008). NO plays an important role in the antiviral defense (Croen, 1993; Akaike and Maeda, 2000) and it is also an intermediate in the bactericidal activity of certain drugs (Timmins et al., 2004; Koide et al., 2009). Moreover, the up-regulation of specific MAPK, such as the c-Jun N-terminal kinase (JNK), was shown to increase the cytoprotective defense against viruses (Hrincius et al., 2010).
SOURCE:
Bilirubin: An Endogenous Molecule with Antiviral Activity in vitro https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3297833/
胆红素及其结合物的膜转运蛋白:系统评价
Membrane Transporters for Bilirubin and Its Conjugates: A Systematic Review
胆红素是一种高度疏水的四吡咯,可与血浆白蛋白结合。它在肝脏中与葡糖醛酸结合,水溶性葡糖苷酸在尿液和胆汁中排出。胆红素二葡糖苷酸的膜转运蛋白是众所周知的。然而,仍未定义的是转运蛋白从血液中摄取胆红素进入肝脏,这一过程已知是快速且不受速率限制的。通过考虑在正常成人中,由于血红蛋白和细胞色素的生理学更新,每天产生200-300mg胆红素,可以评价该过程的生物学重要性。
介绍
胆红素(BR)是在血浆中发现的四吡咯色素,作为白蛋白结合的可逆复合物(Jacobsen和Brodersen,1983)。它源于血红素的分解代谢,由血红蛋白(75%)和细胞色素(25%)释放,在正常成人中每天产生约200-300mg(Levitt和Levitt,2014)。肝脏是BR被吸收的器官,通过葡萄糖醛酸结合代谢,并排泄到胆汁中(Sticova和Jirsa,2013)。
在肝脏中,BR的膜转运机制具有独特的特征,取决于BR分子(缀合或未缀合),质膜界面(血管或胆管)和转运的生物能学(至电化学平衡与否)。
考虑到BR在肝血窦的摄取,肝脏在一次通过中有能力承受多达50%的BR负荷(Stollman等,1983),通过一种显示底物饱和和其他有机阴离子竞争抑制的过程,如吲哚菁绿和溴磺基酞(BSP)(Hunton等,1961; Scharschmidt等,1975; Bloomer和Zaccaria,1976)。这是转运蛋白介导过程的证据。考虑到共轭BR从肝实质到胆汁的转运,它是限速的,不受BR抑制(Goresky,1965)和能量依赖性(Nakatani等,1995)。
Bilirubin is a highly-hydrophobic tetrapyrrole which binds to plasma albumin. It is conjugated in the liver to glucuronic acid, and the water-soluble glucuronides are excreted in urine and bile. The membrane transporters of bilirubin diglucuronide are well-known. Still undefined are however the transporters performing the uptake of bilirubin from the blood into the liver, a process known to be fast and not rate-limited. The biological importance of this process may be appraised by considering that in normal adults 200–300 mg of bilirubin are produced daily, as a result of the physiologic turnover of hemoglobin and cellular cytochromes.
Introduction
Bilirubin (BR) is a tetrapyrrolic pigment found in plasma as an albumin-bound reversible complex (Jacobsen and Brodersen, 1983). It derives from catabolism of heme, released by both hemoglobin (75%) and cytochromes (25%), with a daily production of about 200–300 mg in a normal adult (Levitt and Levitt, 2014). The liver is the organ where BR is taken up, metabolized by glucurono-conjugation, and excreted into the bile (Sticova and Jirsa, 2013).
In the liver, the membrane transport mechanisms of BR have distinctive features, depending on the BR molecule (conjugated or not), the plasma membrane interface (vascular or biliary), and the bioenergetics of transport (to electrochemical equilibrium or not).
Considering sinusoidal uptake of BR, the liver has the capacity to take up as much as 50% of a BR load in a single pass (Stollman et al., 1983) by a process that shows substrate saturation and competitive inhibition by other organic anions, like indocyanine green and bromosulfophthalein (BSP) (Hunton et al., 1961; Scharschmidt et al., 1975; Bloomer and Zaccaria, 1976). This is evidence for a transporter-mediated process. Considering transport of conjugated BR from the liver parenchyma to the bile, it is rate-limiting, not inhibited by BR (Goresky, 1965), and energy-dependent (Nakatani et al., 1995).
https://www.frontiersin.org/articles/10.3389/fphar.2017.00887/full
The fluorescence of bilirubin upon interaction with human erythrocyte ghosts
Bilirubin fluorescence increased upon interaction with bovine serum albumin and human erythrocyte ghosts. When bound to albumin, the emission maximum occurred at 534 +/- 3 nm with maximum excitation at 462 +/- 3 nm. Upon interaction with human erythrocyte ghosts, bilirubin fluorescence increased in a biphasic manner. A rapid initial increase was followed by a slower process that required at least 40 min to reach maximum enhancement at 25 degrees C. At equilibrium, bilirubin fluorescence in erythrocyte ghosts was heterogeneous. With 470 nm excitation, maximum emission occurred in the range of 520 to 535 nm. However, decreasing the excitation to 450 nm or below, produced a red shift in the emission difference spectra. These results suggest that bilirubin exists in different states or sites when associated with plasma membranes.
Article in Biochimica et Biophysica Acta 719(1):65-73 · November 1982 with 18 Reads
DOI: 10.1016/0304-4165(82)90308-7 · Source: PubMed
The fluorescence of bilirubin upon interaction with human erythrocyte ghosts | Request PDF https://www.researchgate.net/publication/16026717_The_fluorescence_of_bilirubin_upon_interaction_with_human_erythrocyte_ghosts
Nano-Encapsulation of Bilirubin in Pluronic F127–Chitosan Improves Uptake in β Cells and Increases Islet Viability and Function after Hypoxic Stress
... We attribute this to bilirubin, an endogenous fluorophore formed from hemoglobin degradation over time. [4,5] As its excitation occurs at 470 nm and emission at approximately 520 nm, its autofluorescence can be captured by spectralis OCT with BluePeak system (Heidelberg Engineering, Germany) which use an excitation wavelength of 488 nm and detect emission wavelength of 500-700 nm. [2,5] Finally, with clearing of heme, the hyperautofluorescence resolved. ...
Nano-Encapsulation of Bilirubin in Pluronic F127–Chitosan Improves Uptake in β Cells and Increases Islet Viability and Function after Hypoxic Stress
Pancreatic islet transplantation is the only curative, noninvasive treatment for type 1 diabetes mellitus; however, high rates of cell death in the immediate postimplantation period have limited the success of this procedure. Bilirubin, an endogenous antioxidant, can improve the survival of murine pancreatic allografts during hypoxic stress but has poor solubility in aqueous solutions. We hypothesized that nano-encapsulation of bilirubin in pluronic 127–chitosan nanoparticle bilirubin (nBR) would improve uptake by murine pancreatic islet cells and improve their viability following hypoxic stress. Nano-bilirubin was synthesized, and drug release characteristics were studied in vitro. Cellular uptake of nBR was compared to free bilirubin (fBR) in an insulinoma cell line (INS-R3) model using confocal-like structured illumination microscopy. Next, C57BL/6 mouse islets were treated with concentrations of 0 to 20 μM of nBR, fBR, or empty nanoparticle (eNP), prior to incubation under standard or hypoxic conditions. Islet viability and function were compared between treatment groups. Release of bilirubin was greatest from nBR suspended in protein-rich solution. Increased, selective uptake of nBR by INS-R3 cells was demonstrated. Cell death after hypoxic stress was significantly decreased in murine islets treated with 5 μM nBR (18.5% ± 14.1) compared to untreated islets (33.5% ± 17.5%; P = 0.019), with reduction in central necrosis. Treatment group had a significant effect on glucose stimulation index [SI], (P = 0.0137) and islets treated with 5 μM nBR had the highest SI overall. Delivery of bilirubin using pluronic F127–chitosan NP improves uptake by murine islets compared to fBR and offers dose-dependent protective effects following hypoxic stress.
(PDF) Nano-Encapsulation of Bilirubin in Pluronic F127–Chitosan Improves Uptake in β Cells and Increases Islet Viability and Function after Hypoxic Stress https://www.researchgate.net/publication/321895675_Nano-Encapsulation_of_Bilirubin_in_Pluronic_F127-Chitosan_Improves_Uptake_in_b_Cells_and_Increases_Islet_Viability_and_Function_after_Hypoxic_Stress
A Bilirubin-Inducible Fluorescent Protein from Eel Muscle
Highlights
• A novel eel fluorescent protein UnaG belongs to the fatty-acid-binding protein family
• UnaG fluorescence is induced by a noncovalent ligand, bilirubin, a heme metabolite
• The HoloUnaG crystal structure at 1.2 Å revealed a biplanar coordination of bilirubin
• UnaG enabled the establishment of a human bilirubin assay with clinical application
The fluorescent protein toolbox has revolutionized experimental biology. Despite this advance, no fluorescent proteins have been identified from vertebrates, nor has chromogenic ligand-inducible activation or clinical utility been demonstrated. Here, we report the cloning and characterization of UnaG, a fluorescent protein from Japanese eel. UnaG belongs to the fatty-acid-binding protein (FABP) family, and expression in eel is restricted to small-diameter muscle fibers. On heterologous expression in cell lines or mouse brain, UnaG produces oxygen-independent green fluorescence. Remarkably, UnaG fluorescence is triggered by an endogenous ligand, bilirubin, a membrane-permeable heme metabolite and clinical health biomarker. The holoUnaG structure at 1.2 Å revealed a biplanar coordination of bilirubin by reversible π-conjugation, and we used this high-affinity and high-specificity interaction to establish a fluorescence-based human bilirubin assay with promising clinical utility. UnaG will be the prototype for a versatile class of ligand-activated fluorescent proteins, with applications in research, medicine, and bioengineering.
A Bilirubin-Inducible Fluorescent Protein from Eel Muscle: Cell https://www.cell.com/cell/fulltext/S0092-8674(13)00644-2
Fluorescence turn on detection of bilirubin using Fe (III) modulated BSA stabilized copper nanocluster; A mechanistic perception
Highlights
•
BSA-CuNCs were synthesized via microwave assisted method.
•
BSA-CuNCs can be quenched by Fe3+.
•
BSA-CuNCs/ Fe3+ act as a turn on platform for bilirubin.
•
The designed probe shows LOD of 6.62 nM.
Abstract
Hyperbilirubinemia is the condition when bilirubin exceeds normal concentration in body (19.80 mg/mL in newborns and 1.19 × 10−2 mg/mL in adults). Bilirubin encephalopathy in newborns may cause irreversible neurological disorders. Current methods for detection of bilirubin suffer from compromising accuracy. In the present work, bovine serum albumin stabilized copper nanocluster (BSA-CuNCs) was synthesized via a one pot microwave assisted method as a turn on detector for bilirubin. The synthesized BSA-CuNCs having size less than 4 nm, exhibited bright blue emission at 405 nm. Interestingly, no observable change in fluorescence emission was noticed over a wide pH range (1–11) or at high ionic conditions. However, the interaction of Fe3+ with BSA-CuNCs induces quenching of fluorescence. Moreover, the fluorescence can be regained by the addition of bilirubin over other possible coexisting biomolecules. A good linearity was observed for BSA-CuNCs based turn on probe with a Limit of Detection (LoD) 6.62 nM. Furthermore, real sample analyses were carried out with human serum and urine which showed good recovery percentage.
Fluorescence turn on detection of bilirubin using Fe (III) modulated BSA stabilized copper nanocluster; A mechanistic perception - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0003267018306159
Keywords
Bilirubin BSACuNCs Fluorescence Turn on sensor Bilirubin encephalopathy Quenching
Ultraviolet irradiation induces autofluorescence enhancement via production of reactive oxygen species and photodecomposition in erythrocytes
Ultraviolet (UV) light has a significant influence on human health. In this study, human erythrocytes were exposed to UV light to investigate the effects of UV irradiation (UVI) on autofluorescence. Our results showed that high-dose continuous UVI enhanced erythrocyte autofluorescence, whereas low-dose pulsed UVI alone did not have this effect. Further, we found that H2O2, one type of reactive oxygen species (ROS), accelerated autofluorescence enhancement under both continuous and pulsed UVI. In contrast, continuous and pulsed visible light did not result in erythrocyte autofluorescence enhancement in the presence or absence of H2O2. Moreover, NAD(P)H had little effect on UVI-induced autofluorescence enhancement. From these studies, we conclude that UVI-induced erythrocyte autofluorescence enhancement via both UVI-dependent ROS production and photodecomposition. Finally, we present a theoretical study of this autofluorescence enhancement using a rate equation model. Notably, the results of this theoretical simulation agree well with the experimental data further supporting our conclusion that UVI plays two roles in the autofluorescence enhancement process.
Biochemical and Biophysical Research Communications
XianWuLeitingPanZhenhuaWangXiaoliLiuDanZhaoXinzhengZhangRomano A.RuppJingjunXu
The Key Laboratory of Weak-Light Nonlinear Photonics, Ministry of Education, TEDA Applied Physics School and School of Physics, Nankai University, Tianjin 300457, China
Received 3 May 2010, Available online 13 May 2010.
Ultraviolet irradiation induces autofluorescence enhancement via production of reactive oxygen species and photodecomposition in erythrocytes - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0006291X10009356
Solar and artificial ultraviolet-B induced erythrocytes hemolysis with photosensitizers
Aim of this study was to monitor the solar ultraviolet-B intensity and to compare the phototoxic effect of different intensity of natural and artificial ultraviolet-B on human red blood cells in presence of compounds as riboflavin and chloroquine. Photohemolysis of erythrocytes was studied under natural solar radiation and artificial ultraviolet-B radiation of 312 nm. Monitoring of solar ultraviolet-B radiation was performed in Garhwal region of Uttarakhand, India. Level of solar ultraviolet-B measured show seasonal and altitudinal variations. Monthly average of solar UV-B intensity was minimum in the month of December and January (0.299 mw/cm2) and maximum in the month of July and August (1.027 mw/cm2). Natural solar radiation intensities 0.402 mw/cm2 and 0.824 mw/cm2 of the month of January and June were used in the photohemolysis experiment. Two intensities of artificial UV-B i.e. 0.824 mw/cm2 and a double intensity 1.65 mw/cm2 were also used. Results on human erythrocytes hemolysis indicate that haemolysis was highest i.e. 71% in chloroquine + artificial ultraviolet-B intensity (1.65 mw/cm2) followed by 62% in chloroquine + artificial ultraviolet-B (0.824 mw/cm2) exposed groups and 54% in natural solar radiation intensity 0.824 mw/cm2 + chloroquine. Natural solar UV-B alone caused 17% hemolysis and show dose response relationship. A difference in phototoxicity was observed in natural solar and artificial UV-B of same intensity. Artificial UV-B was found more toxic. Riboflavin was more phototoxic in presence of solar light, while chloroquine was more phototoxic with artificial UV-B.
NISCAIR Online Periodicals Repository
Authors: Kumar, Sunil
Devi, Shoma
Misra, Prashasti
Priyanka
Keywords: Chloroquine;Ozone depletion;Photohemolysis;Riboflavin;Ultraviolet-B
Issue Date: Nov-2009
NOPR: Solar and artificial ultraviolet-B induced erythrocytes hemolysis with photosensitizers http://nopr.niscair.res.in/handle/123456789/6537
http://nopr.niscair.res.in/bitstream/123456789/6537/1/IJEB%2047%2811%29%20906-910.pdf
Hemolysis: bilirubin levels
Definition
3000 mg of bilirubin is formed daily, 75% of which is from heme metabolism and 25% of which is from other proteins (ex. cytochrome P450)
Normal Bilirubin Levels
Total: 0.1-1.9 mg/dL (depending on the source)
Conjugated: 0-0.4 mg/dL (also depending on the source)
What is bilirubin? – bile pigment, a physiological product of heme metabolism – not all jaundice indicates liver dz – metabolized in the liver and excreted into bile ducts • Except for liver diseases, HEMOLYSIS, will also present with jaundice.
Metabolism of bilirubin: Bilirubin–>heme and globin –>iron and biliverdin–>bilirubin –>binds tightly with albumin in the blood stream, and is separated just before being uptaken into liver cells–>conjugates with glucuronic acid –>conj. Bilirubin–>free unconjugated bilirubin in the blood stream and is not soluble in water. After being taken into hepatocytes, it is converted to soluble conjugated form and is able to be excreted into bile ducts.
About 15 ~ 20 % of the urobilinogen is reabsorbed from the intestine into portal veins and finally
90 % of them return to the liver and is re-excreted in the bile, it is called entero-hepatic circulation of bilirubin. The remaining 10 % gets into the systemic circulation and finally exreted in the urine through kidney. Thus the urine urobilinogen increases in the case of hemolytic disease, hepatocellular disease and porto-systemic shunt.
Excretion of bilirubin: bilirubin–> urobilinogen(colorless) by intestinal pathogens–>urobilin (aka, stercobilin, colored)–>excreted via kidneys most of the reabsorbed urobilinogen return to the liver and re-excreted into bile
Subspecialty
General
Related Media
Keyword history
65%/2009
See Also:
Sources
Barash, PG. Clinical Anesthesia, 5th ed. (Philadelphia), p. 1078, 2006
Hemolysis: bilirubin levels https://www.openanesthesia.org/hemolysis_bilirubin_levels/
Hemolytic Jaundice
Hemolytic jaundice, also known as hematogenous jaundice, is one of the most common type of jaundice. It is caused due to the increase of bilirubin from breakdown of the red blood cells in the body.
Hemolytic or hematogenous jaundice is caused due to excess destruction of the erythrocytes or red blood cell. Bile juice is produced by the liver which play a vital role in the digestive system. When the red blood cell disintegrate, they produce bilirubins. These bilirubins are being excreted from the body by the liver. Increase in the bilirubin formation or inefficiency of the liver to maintain the balance can result in hemolytic jaundice. This bilirubin gives the body a pale or yellow color, which can be easily noticed.
Hemolytic jaundice is common among in the newborns. Babies born before 36 or more days before the completion of nine months, are prone to acquire jaundice easily, as at this stage their liver is not completely developed. Till the baby is in the mothers womb, their body's bile is maintained by the mothers body. So, the baby is unable to maintain the balance and gets jaundice one delivered. Let's find out about the causes and symptoms of hemolytic jaundice in the following paragraphs.
Hemolytic Jaundice Causes
Following are some of the causes of hemolyticor hematogenous jaundice.
Infection: both viral and bacterial
Trauma to red cells: cardiac hemolysis
Sickle cell anemia
G6PD deficiency
Congenital spherocytosis
Drug and toxins (acetaminophen, alcohol, estrogen, etc.)
Enlarged spleen (Hodgkin's disease)
Antibodies in serum
Gilbert's syndrome (decreased conjugation of bilirubin, especially with dehydration, fasting, etc.)
Physiologic jaundice of newborn
Hemolytic Jaundice Symptoms
The hematogenous jaundice symptoms are very much identical with the other two types of jaundice. Following are some of the symptoms.
Yellow or discoloration of skin and mucous membrane
White part of the eye turns yellow
Light colored stools
Dark yellow or brown colored urine
Increased bilirubin level
Anemia
Nausea and vomiting
Abdominal pain
Fever
Weakness
Headache
Confusion
Loss of appetite
Swelling of the leg and abdomen
These are the major symptoms of hematogenous jaundice. But in babies you can find following symptoms.
Changes in muscle tone
High pitched crying
Poor feeding
Lethargy
Seizures
As soon as you observe such symptoms you should immediately approach your doctor for medical care. Jaundice is a serious condition and needs strict medical attention. Delayed or careless approach towards this condition can also lead to death. Follow the prescribed tests and exams for the confirmation of this condition.
Hemolytic Jaundice Home Care Tips
Keep your body hydrated by drinking lots of water.
Avoid drinking alcohol.
Strictly follow the diet suggested by your doctor.
Take the medications suggested by the doctor only. Don't take any herbs or other medication without consulting your doctor.
If the conditions worsen then consult your doctor immediately.
In case of newborn babies follow the doctors advice strictly.
Hemolytic Jaundice Precautions
If you follow proper precautionary methods, you can definitely stay away from this life-treatening condition. Following are some of them.
Avoid the intake of contaminated food and water.
Maintain Hygiene.
Avoid taking medicines that can cause hemolysis (medicines which lead to the breakdown of the red blood cells).
Avoid medicines that can damage the liver.
Hemolytic jaundice is totally curable if treated on time. So, never delay or ignore medical assistance, if you observe any sign. Try to get it treated in the initial stage itself.
Hemolytic Jaundice https://healthhearty.com/hemolytic-jaundice
Study of the effects of ultraviolet light on biomembranes. IV. The effect of oxygen on UV induced hemolysis and lipid photoperoxidation in rat erythrocytes and liposomes
— Hemolysis induced by irradiation with ultraviolet (UV) light at 254 nm showed a pronounced oxygen effect: under irradiation in vacuum, the rate of hemolysis was decreased by an order of magnitude. Irradiation at 254 nm in air but not under vacuum caused the peroxidation of erythrocyte membrane lipids. These results suggest that membrane lipid photoperoxidation is one of the causative factors of UV hemolysis. Irradiation at different wavelengths showed that UV-induced lipid photoperoxidation in erythrocyte membranes developed while the antioxidant α-tocopherol was directly photooxidized. It is shown that the process of lipid photolysis in erythrocyte membranes involves sensitization, possibly by protoporphyrin, whose presence in liposomes accelerates the photoperoxidation at 254 and 365 nm of unsaturated fatty acid residues in lecithin. Possible mechanisms of photochemical damage to erythrocyte membranes are discussed.
Article in Photochemistry and Photobiology 21(2):63-9 · March 1975 with 19 Reads
DOI: 10.1111/j.1751-1097.1975.tb06629.x · Source: PubMed
Study of the effects of ultraviolet light on biomembranes. IV. The effect of oxygen on UV induced hemolysis and lipid photoperoxidation in rat erythrocytes and liposomes | Request PDF https://www.researchgate.net/publication/22020916_Study_of_the_effects_of_ultraviolet_light_on_biomembranes_IV_The_effect_of_oxygen_on_UV_induced_hemolysis_and_lipid_photoperoxidation_in_rat_erythrocytes_and_liposomes
Free-radical and cyclooxigenase-catalyzed lipid peroxidation in membranes of blood cells under UV irradiation
the data on the role of lipid peroxidation in the effects of UV irradiation of blood are revewed. Lipid photoperoxidation in blood cells is the result of photochemical transformation of lipid hydroperoxides, both existing and newly formed ones, into free radicals and direct photolysis of antioxidants. Dark lipid autoperoxidation is also induced by UV radiation. Both photoperoxidation and autoperoxidation of lipids are inhibited by low concentrations of anti-radical antioxidants. The cyclooxygenase-catalyzed peroxidation of arachidonic acid in blood cells is stimulated by UV radiation. This process is suppressed by acetylsalicylic acid or indomethacin. The therapeutic activity of the blood that was UV-irradiated and then infused into rats with peritonitis was due to the cyclooxigenase activation.
Free-radical and cyclooxigenase-catalyzed lipid peroxidation in membranes of blood cells under UV irradiation | Request PDF https://www.researchgate.net/publication/288376345_Free-radical_and_cyclooxigenase-catalyzed_lipid_peroxidation_in_membranes_of_blood_cells_under_UV_irradiation
胆红素选择性抑制细胞色素C氧化酶活性并诱导未成熟皮质神经元细胞凋亡:评估甘油脱氧胆酸的保护作用
高水平的未结合胆红素(UCB)可能引发新生儿的脑病,主要发生在早产儿。这种胆红素诱导的神经功能障碍(BIND)的分子机制尚未阐明,目前尚未有被全世界接受的神经保护策略。
在这里,我们表明,UCB,在模仿那些高胆红素血症的新生儿(50μMUCB在100μM的人血清白蛋白的存在下),迅速(在1个小时内)条件下抑制细胞色素C氧化酶的活性和抗坏血酸驱动耗氧量在体外大鼠皮质神经元3天。这是伴随着一个生物能量和氧化的危机,和细胞凋亡,判断的依据是内线粒体膜电位的崩溃,增加糖酵解活性,超氧阴离子自由基的生成,而ATP释放,以及谷胱甘肽氧化还原状态的破坏。此外,抗氧化剂化合物甘油脱氧胆酸(GUDCA)完全消除UCB诱导的细胞色素C氧化酶抑制并显著防止氧化应激,代谢改变和细胞死亡。这些结果表明,与新生儿胆红素诱发的脑病相关的神经毒性是通过能量代谢失调而发生的,并支持GUDCA可用于治疗BIND的观点。
Bilirubin selectively inhibits cytochrome c oxidase activity and induces apoptosis in immature cortical neurons: assessment of the protective effects of glycoursodeoxycholic acid
High levels of unconjugated bilirubin (UCB) may initiate encephalopathy in neonatal life, mainly in pre‐mature infants. The molecular mechanisms of this bilirubin‐induced neurologic dysfunction (BIND) are not yet clarified and no neuroprotective strategy is currently worldwide accepted. Here, we show that UCB, at conditions mimicking those of hyperbilirubinemic newborns (50 μM UCB in the presence of 100 μM human serum albumin), rapidly (within 1 h) inhibited cytochrome c oxidase activity and ascorbate‐driven oxygen consumption in 3 days in vitro rat cortical neurons. This was accompanied by a bioenergetic and oxidative crisis, and apoptotic cell death, as judged by the collapse of the inner‐mitochondrial membrane potential, increased glycolytic activity, superoxide anion radical production, and ATP release, as well as disruption of glutathione redox status. Furthermore, the antioxidant compound glycoursodeoxycholic acid (GUDCA) fully abrogated UCB‐induced cytochrome c oxidase inhibition and significantly prevented oxidative stress, metabolic alterations, and cell demise. These results suggest that the neurotoxicity associated with neonatal bilirubin‐induced encephalopathy occur through a dysregulation of energy metabolism, and supports the notion that GUDCA may be useful in the treatment of BIND.
Ana Rita Vaz Maria Delgado‐Esteban Maria Alexandra Brito Juan P. Bolaños Dora Brites Angeles Almeida
First published: 08 December 2009 https://doi.org/10.1111/j.1471-4159.2009.06429.x Cited by: 32
Bilirubin selectively inhibits cytochrome c oxidase activity and induces apoptosis in immature cortical neurons: assessment of the protective effects of glycoursodeoxycholic acid - Vaz - 2010 - Journal of Neurochemistry - Wiley Online Library https://onlinelibrary.wiley.com/doi/full/10.1111/j.1471-4159.2009.06429.x
Cardiovasc Toxicol. 2012 Mar;12(1):83-9. doi: 10.1007/s12012-011-9142-y.
Bilirubin attenuates bufadienolide-induced ventricular arrhythmias and cardiac dysfunction in guinea-pigs by reducing elevated intracellular Na(+) levels.
Ma H1, Zhang J, Jiang J, Zhou J, Xu H, Zhan Z, Wu Q, Duan J.
Author information
Abstract
Bufadienolides, known ligands of the sodium pump, have been shown to inhibit the proliferation of several cancer cell types. However, their development to date as anticancer agents has been impaired by a narrow therapeutic margin resulting from their potential to induce cardiotoxicity. In the present study, we examined the effects of bilirubin, an endogenous antioxidant, on the cardiotoxicity of bufadienolides (derived from toad venom) in guinea-pigs. The results showed that bufadienolides (8 mg/kg) caused ventricular arrhythmias, conduction block, cardiac dysfunction and death in guinea-pigs. Pretreatment with bilirubin (75 and 150 mg/kg) significantly prevented bufadienolide-induced premature ventricular complexes, ventricular tachycardia, ventricular fibrillation and death. Bilirubin also markedly improved the inhibition of cardiac contraction in bufadienolide-treated guinea-pigs as evidenced by increases in left ventricular systolic pressure and decreases in left ventricular diastolic pressure in vivo. Furthermore, bilirubin significantly reduced the intracellular sodium content ([Na(+)]( i )) in ex vivo bufadienolide-stimulated guinea-pig ventricular myocytes loaded with the sodium indicator Sodium Green. An antitumor study showed that bilirubin did not compromise the ability of bufadienolides to inhibit gastric cancer cell MGC-803 proliferation. These results suggested that bilirubin can attenuate bufadienolide-induced arrhythmias and cardiac dysfunction in guinea-pigs by reducing elevated [Na(+)]( i ) and may improve bufadienolide therapeutic index in cancer treatment.
PMID: 22127853 DOI: 10.1007/s12012-011-9142-y
[Indexed for MEDLINE]
Share on FacebookShare on TwitterShare on Google+
Bilirubin attenuates bufadienolide-induced ventricular arrhythmias and cardiac dysfunction in guinea-pigs by reducing elevated intracellular Na(+) ... - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/22127853
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)

.png)
.png)
.png)
.png)