Natural killer cells and autoimmunity
Natural Killer Cells
PDF icon Download Natural Killer Cells.pdf (419.94 KB)
Philipp Eissmann, Imperial College, London, UK
Natural Killer (NK) Cells are lymphocytes in the same family as T and B cells, coming from a common progenitor. However, as cells of the innate immune system, NK cells are classified as group I Innate Lymphocytes (ILCs) and respond quickly to a wide variety of pathological challenges. NK cells are best known for killing virally infected cells, and detecting and controlling early signs of cancer. As well as protecting against disease, specialized NK cells are also found in the placenta and may play an important role in pregnancy.
NK cells were first noticed for their ability to kill tumour cells without any priming or prior activation (in contrast to cytotoxic T cells, which need priming by antigen presenting cells). They are named for this ‘natural’ killing. Additionally, NK cells secrete cytokines such as IFNγ and TNFα, which act on other immune cells like Macrophage and Dendritic cells to enhance the immune response.
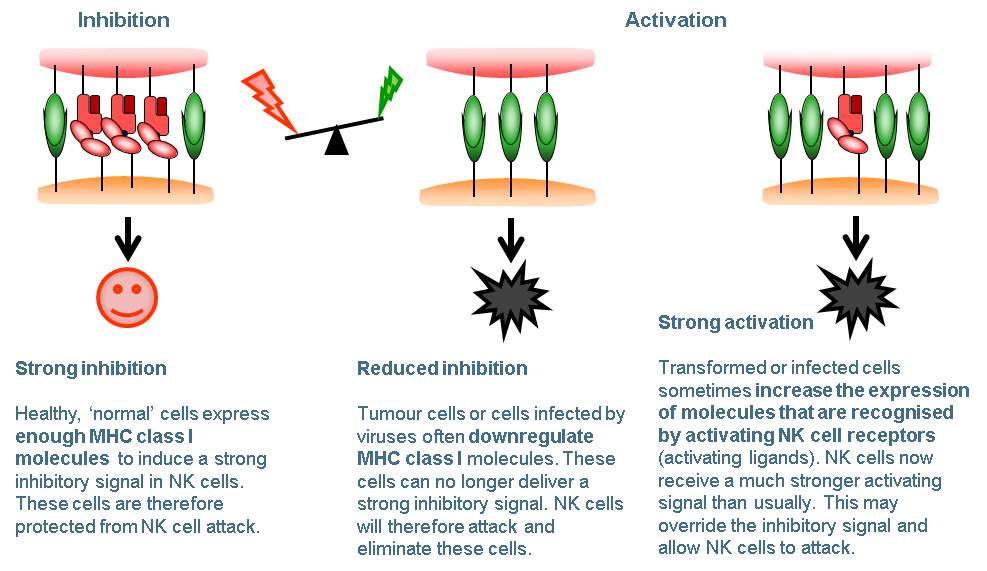
While on patrol NK cells constantly contact other cells. Whether or not the NK cell kills these cells depends on a balance of signals from activating receptors and inhibitory receptors on the NK cell surface. Activating receptors recognise molecules that are expressed on the surface of cancer cells and infected cells, and ‘switch on’ the NK cell. Inhibitory receptors act as a check on NK cell killing. Most normal healthy cells express MHC I receptors which mark these cells as ‘self’. Inhibitory receptors on the surface of the NK cell recognise cognate MHC I, and this ‘switches off’ the NK cell, preventing it from killing. Cancer cells and infected cells often lose their MHC I, leaving them vulnerable to NK cell killing. Once the decision is made to kill, the NK cell releases cytotoxic granules containing perforin and granzymes, which leads to lysis of the target cell.
Natural killer cells - figure 1
The genes for both MHC I and the NK cell inhibitory receptors which recognise them vary a lot between individuals. The versions (or alleles) of these genes a person carries have been linked to their ability to fight HIV infection and their risk of some autoimmune diseases. NK cell varieties also change with age and are affected by chronic viral infections such as cytomegalovirus (CMV).
Because of their ability to kill tumour cells, NK cells are an attractive target for cancer immunotherapies. Some therapeutic monoclonal antibodies rely on NK cell killing. Researchers are also developing treatments to activate NK cells using small molecules or cytokines, and even testing genetically modified living NK cells as therapies
Natural Killer Cells | British Society for Immunology
https://www.immunology.org/public-information/bitesized-immunology/cells/natural-killer-cells
重症患者有明显特征!北京地坛医院研究称,年龄≥50岁且淋巴细胞明显降低的新冠肺炎,应尽快收入重症监护室
中国循环杂志
02-13 23:17
2月12日,首都医科大学附属北京地坛医院研究中性粒细胞/淋巴细胞比值人员在医学生物类论文预印本平台medRvix发表的一项研究提示,中性粒细胞/淋巴细胞比值(NLR)有助于早期发现重症新型冠状病毒肺炎(新冠肺炎)患者。
研究者发现,年龄≥50岁且NLR≥3.13的患者进展为重症的可能性大,应尽快转入重症监护病房治疗。
在年龄≥50岁的患者中,NLR≥3.13者一半会进展为重症,而NLR<3.13的患者中仅9.1%进展为重症。
研究者认为,对于新冠肺炎患者,可以根据NLR和年龄进行危险分层和管理。
年龄<50岁且NLR<3.13的患者进展为重症的风险为0,可以在社区医院或家中隔离。
年龄<50岁、NLR≥3.13的患者进展为重症的风险较低,需要住普通隔离病房治疗。
年龄≥50岁、NLR<3.13的患者进展为重症的风险是中等的,应入院隔离治疗,并进行呼吸监测和支持治疗。
年龄≥50岁、NLR≥3.13的患者进展为重症的风险较高,应该积极转入重症监护病房,给予有创呼吸系统支持。
研究者认为,在有大量病例的情况下,这种危险分层和管理方法有助于缓解医疗资源短缺,降低重症患者的死亡率。
该研究前瞻性纳入北京地坛医院于2020年1月13日至1月31日收治的61例新冠肺炎患者,采用LASSO COX回归分析重症患者的预测因素。
分析结果显示,NLR是新冠肺炎患者进展为重症的独立危险因素,且其预测准确性较高(c指数为0.807)。
来源:Neutrophil-to-Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage. medRvix, Posted February 12, 2020.
Neutrophil in Viral Infections, Friend or Foe?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3557572
Polymorphonuclear leukocytes or neutrophils are the first immune cells to the site of injury and microbial infection. Neutrophils are crucial players in controlling bacterial and fungal infections, and in particular secondary infections, by phagocytosis, degranulation and neutrophil extracellular traps (NETs).
Cited by: 61
Publish Year: 2013
Author: Brandon D. Drescher, Fengwei Bai
A Role for Neutrophils in Viral Respiratory Disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5427094
May 12, 2017 · Thus, there is evidence that the role of neutrophils in viral infections of the respiratory system is not limited to inflammation, but likely includes recovery from infection and the initiation of adaptive immunity (74, 138, 284).
Cited by: 29
Publish Year: 2017
Author: Jeremy V. Camp, Colleen B. Jonsson
CIK(cytokine-induced killer),中文名:[自体细胞免疫疗法]多种细胞因子诱导的杀伤细胞
CIK(cytokine-induced killer),中文名:[自体细胞免疫疗法]多种细胞因子诱导的杀伤细胞。由于该种细胞同时表达CD3+和CD56+两种膜蛋白分子,故又被称为NK细胞样T淋巴细胞,兼具有T淋巴细胞强大的抗瘤活性和NK细胞的非MHC限制性杀瘤优点。因此,应用CIK细胞被认为是新一代抗肿瘤过继细胞免疫治疗的首选方案。CIK细胞中的效应细胞CD3+和CD56+细胞在正常人外周血中较少,仅1%-5%。
定义
所谓CIK细胞,就是将人的外周血单个核细胞在体外用多种细胞因子[如抗CD3单抗(CD3McAb)、白细胞介素-2(IL-2)、干扰素-γ、 (IFN -γ)和IL—1α等]共同培养一段时间后获得的,具有非主要组织相容性抗原(MHC)限制性杀瘤活性的一群异质细胞。 [1]
特点
CIK细胞中的效应细胞CD3+CD56+细胞在正常人外周血中极其罕见,仅1%~5%,在体外经多因子培养28~30天,CD3+CD56+细胞迅速增多,较培养前升幅可达1000倍以上。 [2] 实验证明,扩增出的CD3+CD56+细胞来源于CD3+CD56-T细胞,而CD3-CD56+NK细胞。同时发现在CD3+CD56-的T 细胞中,除CD4+CD8-T细胞外,其余三种T 细胞亚群(CD4-CD8+、CD4-CD8-、CD4+CD8+)均可通过体外多因子培养而获得CD56分子的表达,而由于CD4+CD8+细胞和CD4-CD8-细胞在正常人外周血中含量极低而间接提示此CD3+CD56+细胞绝大多数来源于外周血中CD4-CD8+T细胞。而由于CD4-CD8-T细胞在培养1个月后有近56%的T 细胞同时表达CD56和CD3,表明其也是CIK细胞的重要来源。比较CD3+CD56+CIK细胞中表达CD8+和CD8-,的两群细胞其杀瘤活性没有显著性差异,提示CIK细胞的细胞毒性与CD3CD56表达成相关趋势,而与CD8的表达未表现出相关性。
1.CIK细胞增殖速度快,抗肿瘤活性细胞可大量增殖,且细胞活性也大大增强。
2.CIK细胞具有识别肿瘤的机制,对正常的细胞无毒性作用。
3.杀瘤谱广,可用于白血病、淋巴瘤、肺癌、胃癌、肠癌等多种肿瘤的治疗,对多重耐药肿瘤细胞同样敏感。
4.是典型的个性化生物治疗模式。将这类细胞回输后,还能使机体免疫能力提高,产生特异的抗病毒作用,从而对肿瘤治疗施以双重的作用。
5.由于CIK细胞是活化的自体细胞,用起来非常安全。
杀伤原理
CIK细胞能够通过三种途径杀灭肿瘤细胞和病毒感染细胞:
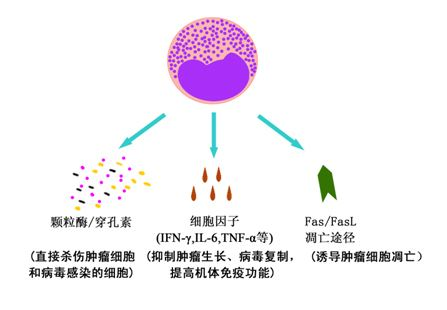
①CIK细胞对肿瘤细胞和病毒感染细胞的直接杀伤:CIK细胞可以通过不同的机制识别肿瘤细胞,释放颗粒酶/穿孔素等毒性颗粒,导致肿瘤细胞裂解。
②CIK细胞释放的大量炎性细胞因子具有抑瘤杀瘤活性:体外培养的CIK细胞可以分泌多种细胞因子,如IFN-γ、TNF-α、IL-2等,不仅对肿瘤细胞有直接抑制作用,还可通过调节机体免疫系统反应性间接杀伤肿瘤细胞。
③CIK细胞能够诱导肿瘤细胞的凋亡:CIK细胞在培养过程中表达FasL(Ⅱ型跨膜糖蛋白)通过与肿瘤细胞膜表达的Fas(Ⅰ型跨膜糖蛋白)结合,诱导肿瘤细胞凋亡。
CIK细胞发挥作用的三种途径
CIK细胞发挥作用的三种途径
杀瘤特点
应用LAK细胞是当今较为普及的肿瘤过继免疫治疗方案,广泛使用于黑色素瘤、肾细胞癌、非霍奇金淋巴瘤、肺癌和结肠癌。因LAK细胞扩增数量有限,杀瘤活性也较TIL等T淋巴细胞为低,故虽然杀瘤谱广,但效果局限。相比较而言,TIL 细胞本身较LAK细胞具有更强大的抗瘤效力,但由于杀瘤谱窄,制备困难,及在收集过程中可能导致的功能改变而限制了其临床应用价值。与上述两种效应细胞过继免疫治疗相比,CIK细胞具有独特的优势,列举如下。
1. 增殖速度快
CIK细胞在培养过程中加入IFN-γ、IL-1α、抗CD3、McAb、IL-2等多因子后,细胞增殖速度迅速加快,远超过LAK细胞。在培养第22天增殖曲线达顶峰,约增加100倍,其中CD3+CD56+细胞不仅绝对数量增加1000倍以上,且所占百分比也大幅上升,培养至28~30天时达平台期,细胞毒活性亦达峰值,而LAK细胞培养前后数量没有明显增加。
2. 杀瘤活性高
CIK细胞是以CD3+CD56+T细胞为主的异质细胞群,大量体内外实验证实CIK细胞较以NK细胞为主的LAK细胞具备更强大的杀瘤活性,而且其体内杀瘤细胞毒性的维持不必依赖大剂量外源性IL-2的持续给予。体外实验中,任欢和Lu等均发现在体外等数量的CIK细胞比LAK细胞对肿瘤细胞系的杀伤能力稍高或相近,但因CIK细胞在培养过程中CD3+CD56+效应细胞增长迅速,故CIK细胞的总杀伤单位(TLU)为LAK 细胞的73倍甚至更高,其杀伤效率显著高于LAK细胞。肿瘤克隆抑制实验显示,CIK细胞的瘤细胞抑制Log指数为2.5~3.5,较LAK细胞的瘤细胞抑制指数高2个Log。体内实验发现,对于在严重联合免疫缺陷小鼠身上构建的人类B 细胞淋巴瘤SU-DHL4模型,CIK细胞在清除荷瘤鼠体内肿瘤病灶,抑制转移,延长生存期等方面的作用均明显优于LAK细胞。
3. 杀瘤谱广
CIK细胞虽然以CD3+CD56+ T细胞为主要效应细胞,但却没有T 淋巴细胞杀伤时的MHC 限制性,故对于多种肿瘤细胞系(包括NK敏感的K562和NK不敏感的Hela、HL60、人T 细胞急淋白血病细胞系OCRF-CEM,人淋巴瘤细胞系OCI-LY8、LAM53,人结肠癌细胞系HT-29、CR75,人肾癌细胞系A704)和新鲜肿瘤组织均表现出强大的杀伤活性。
4. 对多重耐药肿瘤细胞同样敏感
Wolf用阿霉素和长春新碱诱导出多重耐药细胞系K562/DOX和CCRF-CEM-VBL,发现CIK细胞对化疗药物敏感的亲本细胞和不敏感的转化细胞均具有强大的杀伤活性,两者比较无差别。
5. 杀瘤活性不受CsA、FK506等免疫抑制剂的影响
Mehta观察到免疫抑制剂CsA和FK506虽然可以抑制抗CD3单抗介导的CIK细胞脱颗粒过程,却不影响靶细胞诱导的CIK细胞脱颗粒,并且CIK细胞对靶细胞的杀伤活性不会因此降低。
6. 对正常骨髓造血前体细胞毒性很小
Seheffold通过CFU-GM形成实验检测CIK细胞对骨髓造血前体细胞的影响,发现CIK细胞对K562细胞的杀伤强度高达3级,但对GM-CFU仅有不足1级的抑制。
Holye也证实CIK细胞对正常髓系克隆生成几乎没有影响,只是对红系的生成显示出轻度抑制,这可能与CIK细胞自身分泌较高水平的IFN-γ有关。
7. 能抵抗肿瘤细胞引发的效应细胞Fas-FasL凋亡
已证明过继免疫治疗失败的一个重要原因是过继效应细胞被肿瘤细胞表面表达的某些蛋白(主要是FasL)诱导凋亡,而CIK细胞虽然在Fas被占据后会引起少量细胞凋亡,但对其杀瘤细胞毒性没有明显影响。Verneris的实验提示CIK细胞内有抗凋亡基因表达,并检出多种保护基因,如cFLIP、Bcl-2、Bcl-X1、DAD1和survivin的转录水平上调。同时发现CIK细胞具备合成FasL的能力,CIK细胞培养上清中可以检测到具生物学活性的水溶性FasL,表明CIK细胞可以对抗体内FasL 阳性肿瘤所引发的效应细胞活性下降甚至消失。
影响因素
1.外源性细胞因子的补充
CIK细胞的体外扩增需要外源性细胞因子,如IL-2、IL-7、IL-12等的辅助,这些因子控制着人免疫系统内各种抗原特异性细胞的扩增及其生物学活性。外源性IL-2、IL-7、IL-12可以显著促进淋巴细胞的生长,尤其存在有IL-2和IL-7条件下CIK细胞的增殖率为高,而外源性,IL-2、IL-7、IL-12对CIK细胞的细胞毒活性没有影响。外源性IL-2和IL-7的刺激会降低CIK细胞表面相应受体的表达量,而CD28分子在IL-7存在条件下较IL-2时表达更高。IL-12会降低CIK细胞表面ICAM-1的表达,IL-7则会提高CD56的表达。与IL-2相比,IL-7可明显增加CD4+细胞的比例。虽然外源性IL-2、IL-7和IL-12培养过程中均可出现少量凋亡细胞,但有研究显示外源性IL-12的添加会增加CIK细胞中坏死细胞的比例。
抗CD3McAb不仅在CIK细胞培养过程中起着重要作用,在提高CIK细胞对白血病及淋巴瘤的杀伤敏感性上同样具有促进作用。Lefterov将抗CD3McAb与靶细胞预先共同孵育可增加CIK细胞对其的杀伤敏感性,而且这种增强作用可以被抗FcR的抗体(如抗CD36、抗CD32)所部分阻断,间接证明抗CD3McAb引发的杀伤活性增高与FcR介导的抗体结合有关。
2.多种细胞因子基因的转染
由于CIK细胞扩增对外源性细胞因子有依赖,因此通过基因转移方法将相关基因转入CIK细胞,不仅可减少外源性细胞因子的使用量,还可提高CIK细胞自身的抗瘤活性。
IL-7:Fitke利用改进的腺病毒转基因系统将人IL-7基因转染CIK细胞,发现转染后细胞可以生成较高浓度IL-7,最多者可达1 100pg/106cell/24h。合成的IL-7具有明显的生物学活性,可以促进转染CIK细胞的增殖,显著高于未转染细胞。外源IL-7基因的表达同时改变了CIK细胞对其他细胞因子的分泌,其中尤以TNF-α分泌显著升高,这一现象在未转染CIK体外添加IL-7时并未观测到。虽然转染后CIK细胞表面各种与细胞杀伤活性相关的表面抗原,如ICAM-1 等与未转染CIK细胞相比无明显变化,但转染后CIK细胞在对多种肿瘤细胞系如肾癌、恶性黑色素瘤以及结肠癌的杀伤能力较未转染CIK细胞有明显增强。
IL-2:Lu等发现CIK细胞培养过程中CD56分子的表达是IL-2依赖性的,但单独IL-2的存在却会降低培养后CIK细胞的表型变化幅度。尽管有实验指出CIK细胞的体内治疗并不需要IL-2体外持续供给,但Zoll等的研究结果表明,体外培养中IL-2对CIK细胞的增殖和杀伤功能有促进作用。Schmidt-Wolf将包含人IL-2基因片段的重组质粒用电穿孔法导入CIK细胞治疗转移性实体瘤,发现转染后细胞可分泌较高水平的IL-2(330-1 800pg/106 cell/24h,平均达836pg/106 cell/24h )。虽然转染前后CIK细胞表面各种膜蛋白表达并无显著变化,但体外检测转染后CIK 细胞在增殖率和细胞杀伤活性上均高于未转染细胞。
制备流程
抽取患者的外周血,在37℃,5%的二氧化碳培养箱中孵育2h,使用高速离心机分离,收集悬浮细胞,在体外(模拟人体内环境),加入多种细胞因子如CD3单抗,IFN-R,IL-2等培养,每隔2-3天换一次培养液,第7、11、13、15天收集CIK细胞,CD3+和CD56+细胞迅速增多,较培养前升幅可达1000倍以上。
CIK细胞制备流程
CIK细胞制备流程
发展历程
1985年美国外科医生首次发现大剂量的IL-2在体外可以将淋巴细胞培养成具有很强肿瘤杀伤作用的细胞,称为LAK细胞。以后发现在培养液中加入CD3单克隆抗体可以将这些细胞的肿瘤杀伤活性提高十几倍,扩增的数量也大幅提高,这种细胞称为CD3AK。在这个基础上再在培养液中加入INF-r、IL-1等细胞因子,得到表达CD3\CD8\CD56阳性的异质细胞群,这些细胞称为CIK,相比LAK细胞增殖倍数更多,杀毒活力更强。
1991年美国斯坦福大学Schmidt Wolf等人首次报道了CIK细胞。
临床应用编辑
早在1996年国内已有CIK细胞治疗技术临床应用的研究论文发表,国内已有超过百家的医疗单位开展了该项治疗技术的相关临床应用与研究。
根据国际国内细胞治疗临床应用的进展状况,2009年5月1日卫生部颁发执行的《医疗技术临床管理应用办法》将“自体免疫细胞治疗技术”列为三类医疗技术。
肝癌患者接受手术联合CIK治疗后1年、3年、5年的无病生存期比较,具体如图:
适应症
CIK细胞治疗属于过继细胞免疫疗法 [3] ,由于CIK细胞溶瘤作用是非MHC限制性的,即不受肿瘤组织类型的限制,因此对任何一种肿瘤均有杀伤作用,但对高抗原表达的癌症疗效最好,如:髓性白血病、黑色素瘤、肾细胞癌、转移性肾癌、非何杰金氏淋巴瘤等。其它癌症如肺癌、结肠癌、乳腺癌、肝癌、胃癌、大肠癌等,CIK细胞治疗亦有较好的疗效。CIK细胞治疗适用于任何一期的癌症患者,但对早期肿瘤患者或经过手术及放化疗后肿瘤负荷较小的患者效果好。它对于手术、放化疗或造血干细胞移植后患者体内微小残留病灶的清除,防止癌细胞扩散和复发,提高患者自身免疫力,减少毒性反应等方面具有重要作用。某些不适合手术、不能耐受放化疗的中晚期肿瘤患者,CIK细胞治疗可以提高其生活质量,延长带瘤生存时间。
CIK 细胞通过直接杀伤、分泌多种细胞因子等直接抑制肿瘤细胞生长,诱导肿瘤细胞凋亡起到治疗作用。大多数执行杀伤功能的细胞在回输后立即执行其功能,半衰期约2周至一个月。回输的细胞中包括一部分记忆细胞,可存活几年至几十年,当遇到相应刺激后,迅速在体内活化,杀伤靶细胞。所以CIK细胞治疗的疗程间隔原则上为一个月,建议首先间隔一个月连续做三个疗程,以后每半年做一个疗程。Natural killer cells and autoimmunity.
French AR, Yokoyama WM - Arthritis research & therapy (2003)Affiliation: Howard Hughes Medical Institute, Division of Rheumatology, Department of Medicine, Washington University School of Medicine, St Louis, MO, USA. yokoyama@im.wustl.edu
Abstract
Autoimmune diseases are often characterized as clinical syndromes caused by the inappropriate activation of T or B cells resulting in systemic or organ-specific damage. However, studies support a role for the innate immune system, and in particular natural killer (NK) cells, in stimulating or suppressing autoimmunity. This review focuses on recent research elucidating a potential immunoregulatory role for NK cells in modulating T and B cell-mediated autoimmunity.
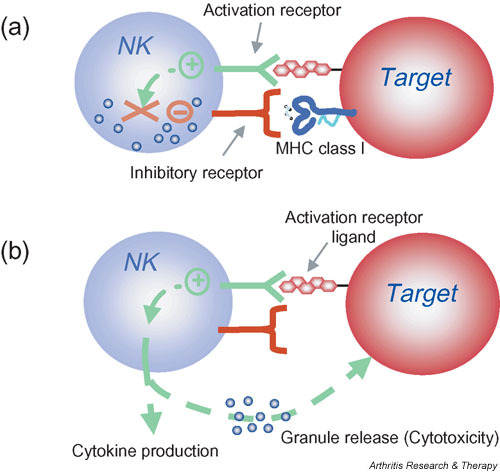
Natural killer (NK) cell activation is controlled by the integration of signals from activation and inhibitory receptors. (a) Inhibitory NK cell receptors recognize self MHC class I and restrain NK cell activation. (b) When unimpeded by the inhibitory receptors, binding of NK cell activation receptors to their ligands on target cells results in NK cell stimulation. In the absence or downregulation of self MHC class I on the target cells, these stimulatory signals are no longer suppressed, resulting in NK cell responses including cytokine production and granule release leading to cytotoxicity. Note that this model indicates that NK cells do not kill by default; that is, when MHC class I inhibition is absent, the NK cell must still be stimulated through activation receptors. Moreover, whether or not an individual NK cell is activated by a target is determined by this complex balance of receptors with opposing function and expression of the corresponding ligands. In general, however, inhibition dominates over activation. Finally, NK cells can be directly stimulated by cytokines such as interleukin-12 that trigger the production of other cytokines by NK cells (not shown). These direct cytokine-mediated responses are not affected by MHC class I expression.
© Copyright Policy
F1: Natural killer (NK) cell activation is controlled by the integration of signals from activation and inhibitory receptors. (a) Inhibitory NK cell receptors recognize self MHC class I and restrain NK cell activation. (b) When unimpeded by the inhibitory receptors, binding of NK cell activation receptors to their ligands on target cells results in NK cell stimulation. In the absence or downregulation of self MHC class I on the target cells, these stimulatory signals are no longer suppressed, resulting in NK cell responses including cytokine production and granule release leading to cytotoxicity. Note that this model indicates that NK cells do not kill by default; that is, when MHC class I inhibition is absent, the NK cell must still be stimulated through activation receptors. Moreover, whether or not an individual NK cell is activated by a target is determined by this complex balance of receptors with opposing function and expression of the corresponding ligands. In general, however, inhibition dominates over activation. Finally, NK cells can be directly stimulated by cytokines such as interleukin-12 that trigger the production of other cytokines by NK cells (not shown). These direct cytokine-mediated responses are not affected by MHC class I expression.Although NK cells are prepared to kill abnormal cells and rapidly release cytokines, they are normally restrained by inhibitory receptors that recognize target-cell-expressed MHC class I molecules and allow NK cells to survey tissues for normal MHC class I expression (Fig. 1). When MHC class I molecules are downregulated or absent, NK cells are released from the inhibitory influence of these receptors and kill target cells more efficiently ('missing self' hypothesis) [14]. It has been proposed that NK cells all express at least one inhibitory receptor that recognizes self MHC to provide NK cell tolerance and to prevent inappropriate NK cell responses directed at self [15,16]. However, release from inhibitory receptor effects does not automatically lead to NK cell activation against cellular targets. NK cells also express different combinations of various activation receptors, allowing them to respond to ligands on potential target cells [17-20]. The ligands for activation receptors are often closely related to the ligands for inhibitory receptors. In other cases, the activation receptor ligands may be upregulated in response to stress or infection, as illustrated by the upregulation of the NKG2D ligand MHC class I-related chain A (MICA) during infection [21-23].
Natural killer (NK) cell activation is controlled by th | Open-i
https://openi.nlm.nih.gov/detailedresult?img=PMC400423_ar1034-1&req=4
Immunology. 2007 Jun; 121(2): 197–206.
Interleukin-12- and interferon-γ-mediated natural killer cell activation by Agaricus blazei Murill
Eri Yuminamochi,1,2,* Taisuke Koike,1,2,* Kazuyoshi Takeda,1 Isao Horiuchi,2 and Ko Okumura1
1Department of Immunology, Juntendo University School of Medicine, Tokyo, Japan
2Japan Applied Microbiology Research Institute Ltd, Yamanashi, Japan
ABSTRACT
Dried fruiting bodies of Agaricus blazei Murill (A. blazei) and its extracts have generally used as complementary and alternative medicines (CAMs).Here, we report that the oral administration of A. blazei augmented cytotoxicity of natural killer (NK) cells in wild-type (WT) C57BL/6, C3H/HeJ, and BALB/c mice.
Augmented cytotoxicity was demonstrated by purified NK cells from treated wild-type (WT) and RAG-2-deficient mice, but not from interferon-γ (IFN-γ) deficient mice. NK cell activation and IFN-γ production was also observed in vitro when dendritic cell (DC)-rich splenocytes of WT mice were coincubation with an extract of A. blazei.
Both parameters were largely inhibited by neutralizing anti-interleukin-12 (IL-12) monoclonal antibody (mAb) and completely inhibited when anti-IL-12 mAb and anti-IL-18 mAb were used in combination. An aqueous extract of the hemicellulase-digested compound of A. blazei particle; (ABPC) induced IFN-γ production more effectively, and this was completely inhibited by anti-IL-12 mAb alone.
NK cell cytotoxicty was augmented with the same extracts, again in an IL-12 and IFN-γ-dependent manner. These results clearly demonstrated that A. blazei and ABPC augmented NK cell activation through IL-12-mediated IFN-γ production.
Keywords: Agaricus blazei, NK cells, IFN-γ, IL-12, cytotoxicityInterleukin-12- and interferon-γ-mediated natural killer cell activation by Agaricus blazei Murill
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2265935/
Annu Rev Immunol. 1995;13:251-76.
Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity.
Trinchieri G1.
Wistar Institute of Anatomy and Biology, Philadelphia, Pennsylvania 19104-4268, USA.
Abstract
Interleukin-12 (IL-12) is a heterodimeric cytokine produced mostly by phagocytic cells in response to bacteria, bacterial products, and intracellular parasites, and to some degree by B lymphocytes.IL-12 induces cytokine production, primarily of IFN-gamma, from NK and T cells, acts as a growth factor for activated NK and T cells, enhances the cytotoxic activity of NK cells, and favors cytotoxic T lymphocyte generation.
In vivo IL-12 acts primarily at three stages during the innate resistance/adaptive immune response to infection:
1. Early in the infection, IL-12 is produced and induces production from NK and T cells of IFN-gamma, which contributes to phagocytic cell activation and inflammation;
2. IL-12 and IL-12-induced IFN-gamma favor Th1 cell differentiation by priming CD4+ T cells for high IFN-gamma production; and
3. IL-12 contributes to optimal IFN-gamma production and to proliferation of differentiated Th1 cells in response to antigen. The early preference expressed in the immune response depends on the balance between IL-12, which favors Th1 responses, and IL-4, which favors Th2 responses.
Thus, IL-12 represents a functional bridge between the early nonspecific innate resistance and the subsequent antigen-specific adaptive immunity.
PMID: 7612223 DOI: 10.1146/annurev.iy.13.040195.001343Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/7612223
Revisiting Interleukin-12 as a Cancer Immunotherapy Agent | Clinical Cancer Research
https://clincancerres.aacrjournals.org/content/24/12/2716
Oxidant-induced cell death in lymphocytes – mechanisms of induction and resistance
Fredrik B Thorén
University of Gothenburg, Germany
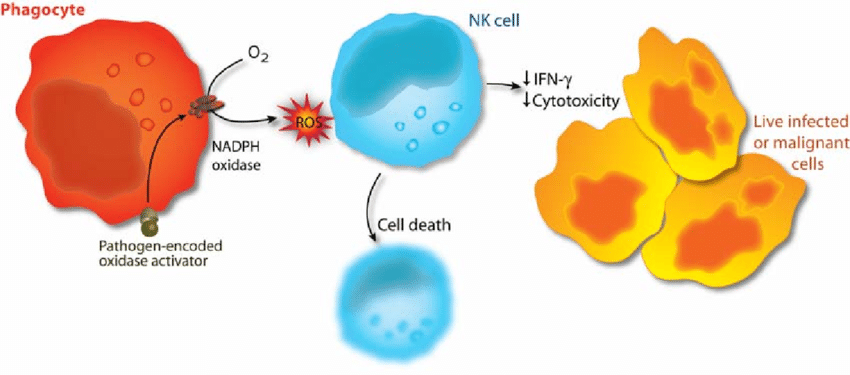
Reactive oxygen species (oxidants, oxygen radicals) produced by the phagocytic NADPH oxidase have pivotal roles in immunity. Patients lacking a functional NADPH oxidase suffer from chronic granulomatous disease, which is characterized by recurring bacterial infections and thus manifesting the importance of reactive oxygen species in host defense against bacteria.However, NADPH oxidase-derived radicals also efficiently inhibit lymphocyte-mediated immunity. Oxidant-induced inactivation of lymphocytes is reportedly a control mechanism for autoreactive lymphocytes and hence prevents autoimmunity. In malignant diseases, oxygen radicals have been proposed to contribute to the characteristic state of anergy of cytotoxic lymphocytes, which prevents immune-mediated rejection of the tumor.
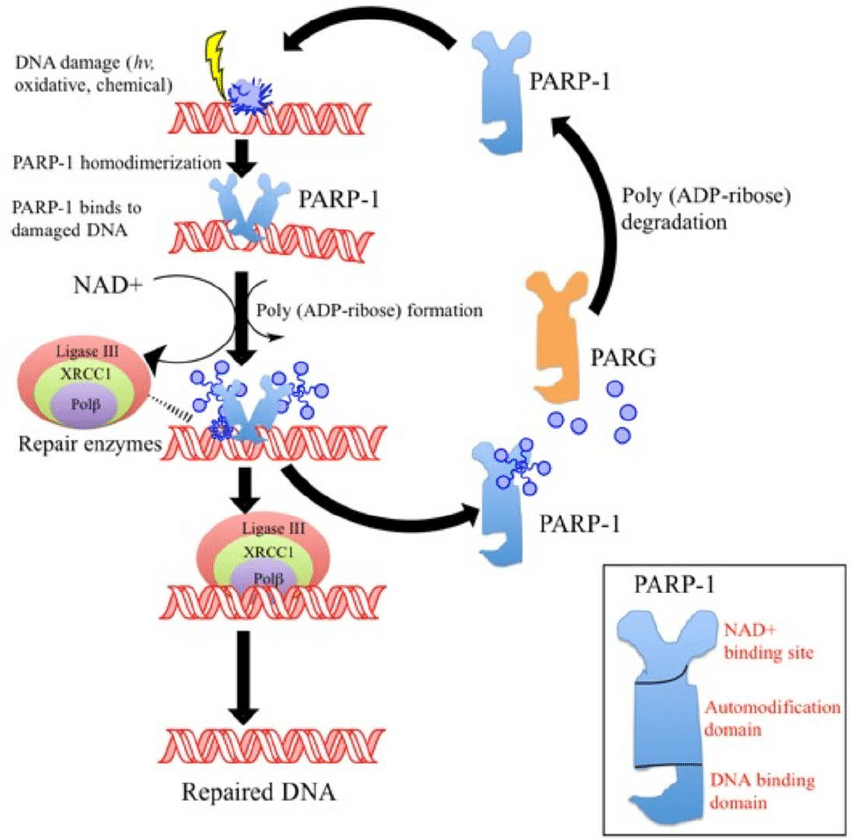
Studies of the mechanisms of radical-induced inactivation of lymphocytes may therefore be helpful in understanding the pathophysiology of important disease entities. The first paper in this thesis shows that oxidant-induced functional inhibition and cell death in cytotoxic lymphocytes is critically dependent on cooperation between a nuclear enzyme involved in DNA repair, PARP-1, and a mitochondrion-derived protein, AIF.
The results presented in Paper II demonstrate that pharmacological inhibition of the PARP-1 enzyme not only prevents oxidant-induced cell death, but also preserves functions of cytotoxic lymphocytes, such as cytotoxicity against malignant cells, cytokine production, and proliferation.
Paper III shows that subsets of natural killer (NK) cells display differential sensitivity to oxygen radicals: the cytotoxic CD56dimCD16+ NK cells were found to be highly sensitive to oxidative inactivation and apoptosis, while the immunoregulatory, cytokine-producing CD56brightCD16- NK cells were highly resistant to the toxicity of oxidants.
These data were extended in Paper IV, in which the effect of oxygen radical-producing phagocytes on the expression of the activating NK cell receptors, NKp46 and NKG2D, was investigated. The expression of both receptors was efficiently downregulated on CD56dim NK cells, while the expression remained intact on CD56bright cells.
Recent data imply that reciprocal interactions between NK cells and dendritic cells (DCs) are important for the development of adaptive immunity. The results presented in Paper V demonstrate that DCs are equipped with an antioxidative system that efficiently protects cytotoxic cells from oxidant-induced inactivation.
Phagocyte activation as an immunoevasive strategy. In malignant and infected tissues, phagocytes are recruited with ensuing production of oxygen radicals. The release of oxygen radicals from phagocytes inactivates NK cells and other cytotoxic lymphocytes, enabling survival of malignant or virus-infected cells (adapted from 298).
DNA repair responses to PARP1
University of Texus, MD Anderson Canver Center
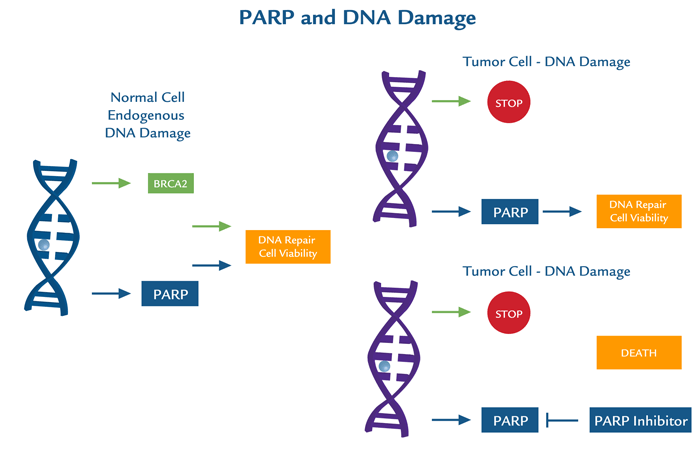
PARP1 encompasses a broad scope of DNA repair responses which makes it an attractive candidate for therapeutic inhibition, either alone or in combination with cancer chemotherapy or radiotherapy. Inhibition of PARP1 has potential for use in cancer treatment particularly by increasing tumor sensitivity to chemotherapeutic agents that damage DNA and by inducing synthetic lethality in tumor cells that are highly dependent on PARP1 due to deficiencies in DNA repair proteins (e.g., BRCA1/2).PARP1 and DNA damage
Other mechanisms by which PARP exerts its effects are through rapid mitochondrial dysfunction with membrane permeability transition, NAD+ depletion, and translocation of apoptosis inducing factor (AIF) from the mitochondria to the nucleus. Inhibition/manipulation of other components of the PARP pathway, including PAR and PARG may therefore, be valuable therapeutic interventions not only for cancer, but for other disease states.
MDAnderson - PARP1 DNA ToolkitIn collaboration with MD Anderson Cancer Center, a highly specific toolkit has been developed and validated to analyse PARP1 in a panel of control and siRNA knockdown cell lysates by multiple immunoassays. This toolkit can specifically and quantitatively distinguish PARP1 from 17 other PARP family members using highly specific antibodies and optimised methods.
Most clinically used PARP inhibitors bind to conserved regions in the PARP domains that permits cross-selectivity with other PARPs containing homologous catalytic domains. Thus, the differences between therapeutic effects and unwanted side effects resulting from pan-PARP inhibition compared to selective inhibition are not well understood. The development and use of more selective agents targeting other domains that make up PARPs, will help answer important questions concerning PARP inhibitors as chemotherapy. A clear understanding of the inhibition profiles of PARP inhibitors will not only enhance the understanding of the biology of individual PARPs, but may provide improved therapeutic options for patients.
Do not hesitate to contact us to test this new toolkit!https://www.tebu-bio.com/blog/2015/10/13/dna-repair-responses-to-parp1/

.jpg)