¡¡
Natural killer cells and autoimmunity.
French AR, Yokoyama WM - Arthritis research & therapy (2003)Affiliation: Howard Hughes Medical Institute, Division of Rheumatology, Department of Medicine, Washington University School of Medicine, St Louis, MO, USA. yokoyama@im.wustl.edu
Abstract
Autoimmune diseases are often characterized as clinical syndromes caused by the inappropriate activation of T or B cells resulting in systemic or organ-specific damage. However, studies support a role for the innate immune system, and in particular natural killer (NK) cells, in stimulating or suppressing autoimmunity. This review focuses on recent research elucidating a potential immunoregulatory role for NK cells in modulating T and B cell-mediated autoimmunity.
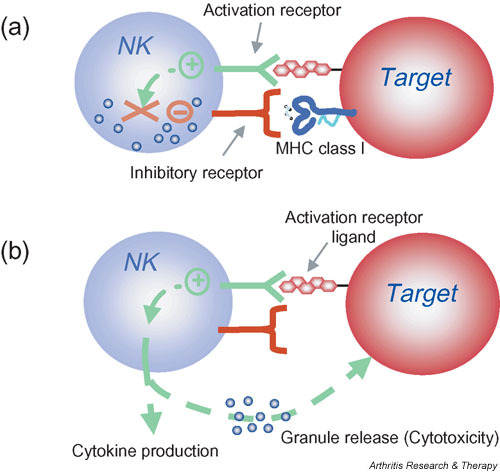
Natural killer (NK) cell activation is controlled by the integration of signals from activation and inhibitory receptors. (a) Inhibitory NK cell receptors recognize self MHC class I and restrain NK cell activation. (b) When unimpeded by the inhibitory receptors, binding of NK cell activation receptors to their ligands on target cells results in NK cell stimulation. In the absence or downregulation of self MHC class I on the target cells, these stimulatory signals are no longer suppressed, resulting in NK cell responses including cytokine production and granule release leading to cytotoxicity. Note that this model indicates that NK cells do not kill by default; that is, when MHC class I inhibition is absent, the NK cell must still be stimulated through activation receptors. Moreover, whether or not an individual NK cell is activated by a target is determined by this complex balance of receptors with opposing function and expression of the corresponding ligands. In general, however, inhibition dominates over activation. Finally, NK cells can be directly stimulated by cytokines such as interleukin-12 that trigger the production of other cytokines by NK cells (not shown). These direct cytokine-mediated responses are not affected by MHC class I expression.
© Copyright Policy
F1: Natural killer (NK) cell activation is controlled by the integration of signals from activation and inhibitory receptors. (a) Inhibitory NK cell receptors recognize self MHC class I and restrain NK cell activation. (b) When unimpeded by the inhibitory receptors, binding of NK cell activation receptors to their ligands on target cells results in NK cell stimulation. In the absence or downregulation of self MHC class I on the target cells, these stimulatory signals are no longer suppressed, resulting in NK cell responses including cytokine production and granule release leading to cytotoxicity. Note that this model indicates that NK cells do not kill by default; that is, when MHC class I inhibition is absent, the NK cell must still be stimulated through activation receptors. Moreover, whether or not an individual NK cell is activated by a target is determined by this complex balance of receptors with opposing function and expression of the corresponding ligands. In general, however, inhibition dominates over activation. Finally, NK cells can be directly stimulated by cytokines such as interleukin-12 that trigger the production of other cytokines by NK cells (not shown). These direct cytokine-mediated responses are not affected by MHC class I expression.Although NK cells are prepared to kill abnormal cells and rapidly release cytokines, they are normally restrained by inhibitory receptors that recognize target-cell-expressed MHC class I molecules and allow NK cells to survey tissues for normal MHC class I expression (Fig. 1). When MHC class I molecules are downregulated or absent, NK cells are released from the inhibitory influence of these receptors and kill target cells more efficiently ('missing self' hypothesis) [14]. It has been proposed that NK cells all express at least one inhibitory receptor that recognizes self MHC to provide NK cell tolerance and to prevent inappropriate NK cell responses directed at self [15,16]. However, release from inhibitory receptor effects does not automatically lead to NK cell activation against cellular targets. NK cells also express different combinations of various activation receptors, allowing them to respond to ligands on potential target cells [17-20]. The ligands for activation receptors are often closely related to the ligands for inhibitory receptors. In other cases, the activation receptor ligands may be upregulated in response to stress or infection, as illustrated by the upregulation of the NKG2D ligand MHC class I-related chain A (MICA) during infection [21-23].
Natural killer (NK) cell activation is controlled by th | Open-i
https://openi.nlm.nih.gov/detailedresult?img=PMC400423_ar1034-1&req=4¡¡
Immunology. 2007 Jun; 121(2): 197¨C206.
Interleukin-12- and interferon-¦Ã-mediated natural killer cell activation by Agaricus blazei Murill
Eri Yuminamochi,1,2,* Taisuke Koike,1,2,* Kazuyoshi Takeda,1 Isao Horiuchi,2 and Ko Okumura1
1Department of Immunology, Juntendo University School of Medicine, Tokyo, Japan
2Japan Applied Microbiology Research Institute Ltd, Yamanashi, Japan
ABSTRACT
Dried fruiting bodies of Agaricus blazei Murill (A. blazei) and its extracts have generally used as complementary and alternative medicines (CAMs).Here, we report that the oral administration of A. blazei augmented cytotoxicity of natural killer (NK) cells in wild-type (WT) C57BL/6, C3H/HeJ, and BALB/c mice.
Augmented cytotoxicity was demonstrated by purified NK cells from treated wild-type (WT) and RAG-2-deficient mice, but not from interferon-¦Ã (IFN-¦Ã) deficient mice. NK cell activation and IFN-¦Ã production was also observed in vitro when dendritic cell (DC)-rich splenocytes of WT mice were coincubation with an extract of A. blazei.
Both parameters were largely inhibited by neutralizing anti-interleukin-12 (IL-12) monoclonal antibody (mAb) and completely inhibited when anti-IL-12 mAb and anti-IL-18 mAb were used in combination. An aqueous extract of the hemicellulase-digested compound of A. blazei particle; (ABPC) induced IFN-¦Ã production more effectively, and this was completely inhibited by anti-IL-12 mAb alone.
NK cell cytotoxicty was augmented with the same extracts, again in an IL-12 and IFN-¦Ã-dependent manner. These results clearly demonstrated that A. blazei and ABPC augmented NK cell activation through IL-12-mediated IFN-¦Ã production.
Keywords: Agaricus blazei, NK cells, IFN-¦Ã, IL-12, cytotoxicityInterleukin-12- and interferon-¦Ã-mediated natural killer cell activation by Agaricus blazei Murill
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2265935/¡¡
Annu Rev Immunol. 1995;13:251-76.
Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity.
Trinchieri G1.
Wistar Institute of Anatomy and Biology, Philadelphia, Pennsylvania 19104-4268, USA.
Abstract
Interleukin-12 (IL-12) is a heterodimeric cytokine produced mostly by phagocytic cells in response to bacteria, bacterial products, and intracellular parasites, and to some degree by B lymphocytes.IL-12 induces cytokine production, primarily of IFN-gamma, from NK and T cells, acts as a growth factor for activated NK and T cells, enhances the cytotoxic activity of NK cells, and favors cytotoxic T lymphocyte generation.
In vivo IL-12 acts primarily at three stages during the innate resistance/adaptive immune response to infection:
1. Early in the infection, IL-12 is produced and induces production from NK and T cells of IFN-gamma, which contributes to phagocytic cell activation and inflammation;
2. IL-12 and IL-12-induced IFN-gamma favor Th1 cell differentiation by priming CD4+ T cells for high IFN-gamma production; and
3. IL-12 contributes to optimal IFN-gamma production and to proliferation of differentiated Th1 cells in response to antigen. The early preference expressed in the immune response depends on the balance between IL-12, which favors Th1 responses, and IL-4, which favors Th2 responses.
Thus, IL-12 represents a functional bridge between the early nonspecific innate resistance and the subsequent antigen-specific adaptive immunity.
PMID: 7612223 DOI: 10.1146/annurev.iy.13.040195.001343Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/7612223¡¡
¡¡
Revisiting Interleukin-12 as a Cancer Immunotherapy Agent | Clinical Cancer Research
https://clincancerres.aacrjournals.org/content/24/12/2716¡¡
Oxidant-induced cell death in lymphocytes ¨C mechanisms of induction and resistance
Fredrik B Thor¨¦n
University of Gothenburg, Germany
Reactive oxygen species (oxidants, oxygen radicals) produced by the phagocytic NADPH oxidase have pivotal roles in immunity. Patients lacking a functional NADPH oxidase suffer from chronic granulomatous disease, which is characterized by recurring bacterial infections and thus manifesting the importance of reactive oxygen species in host defense against bacteria. However, NADPH oxidase-derived radicals also efficiently inhibit lymphocyte-mediated immunity. Oxidant-induced inactivation of lymphocytes is reportedly a control mechanism for autoreactive lymphocytes and hence prevents autoimmunity. In malignant diseases, oxygen radicals have been proposed to contribute to the characteristic state of anergy of cytotoxic lymphocytes, which prevents immune-mediated rejection of the tumor. Studies of the mechanisms of radical-induced inactivation of lymphocytes may therefore be helpful in understanding the pathophysiology of important disease entities. The first paper in this thesis shows that oxidant-induced functional inhibition and cell death in cytotoxic lymphocytes is critically dependent on cooperation between a nuclear enzyme involved in DNA repair, PARP-1, and a mitochondrion-derived protein, AIF. The results presented in Paper II demonstrate that pharmacological inhibition of the PARP-1 enzyme not only prevents oxidant-induced cell death, but also preserves functions of cytotoxic lymphocytes, such as cytotoxicity against malignant cells, cytokine production, and proliferation. Paper III shows that subsets of natural killer (NK) cells display differential sensitivity to oxygen radicals: the cytotoxic CD56dimCD16+ NK cells were found to be highly sensitive to oxidative inactivation and apoptosis, while the immunoregulatory, cytokine-producing CD56brightCD16- NK cells were highly resistant to the toxicity of oxidants. These data were extended in Paper IV, in which the effect of oxygen radical-producing phagocytes on the expression of the activating NK cell receptors, NKp46 and NKG2D, was investigated. The expression of both receptors was efficiently downregulated on CD56dim NK cells, while the expression remained intact on CD56bright cells. Recent data imply that reciprocal interactions between NK cells and dendritic cells (DCs) are important for the development of adaptive immunity. The results presented in Paper V demonstrate that DCs are equipped with an antioxidative system that efficiently protects cytotoxic cells from oxidant-induced inactivation.¡¡
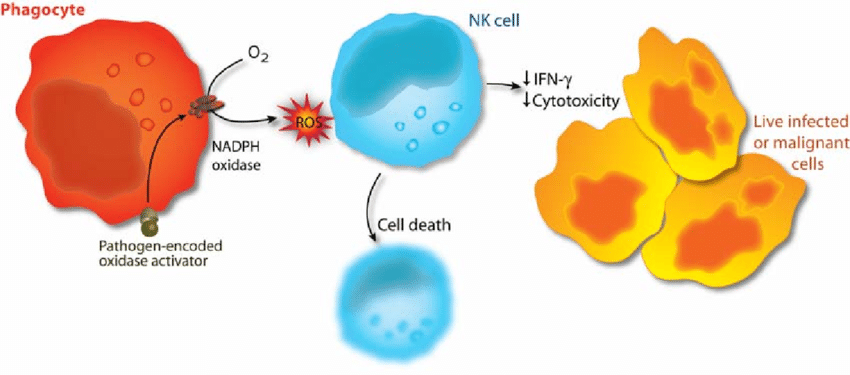
Phagocyte activation as an immunoevasive strategy. In malignant and infected tissues, phagocytes are recruited with ensuing production of oxygen radicals. The release of oxygen radicals from phagocytes inactivates NK cells and other cytotoxic lymphocytes, enabling survival of malignant or virus-infected cells (adapted from 298).
¡¡
¡¡
¡¡
¡¡
.jpg)