肝窦内皮细胞和肝再生
Liver sinusoidal endothelial cells and liver regeneration
摘要
肝窦内皮细胞(LSECs)长期以来被认为有助于肝损伤后的肝再生。在正常肝脏中,肝细胞因子(HGF)的主要细胞来源是肝星状细胞,但在肝损伤后,HGF表达被认为在增殖的LSEC中显着增加。然而,新出现的数据表明即使在受伤后,HGF在LSEC表达也不会大幅增加。相反,富含HGF的LSEC(BM SPC)的骨髓祖细胞在损伤后被募集到肝脏。本综述从肝脏再生的BM SPCs而不是成熟的LSEC驱动肝脏再生的角度来检查肝脏再生。
介绍
HGF是一种细胞因子,在组织再生,刺激细胞生长,细胞运动和形态发生中起关键作用。 20多年前,Jacquelyn Maher首次假设肝窦内皮细胞(LSECs)有助于肝脏再生,证明肝星状细胞是正常肝脏中HGF的主要来源,但在肝脏损伤后,HGF表达在LSECs中显着增加。 (1)。随后显示bFGF刺激肝脏中的LSEC增殖但不刺激肝细胞增殖,并且bFGF的抑制抑制LSEC增殖但不抑制肝细胞增殖。该结果与bFGF或VEGF在部分肝切除术后增加肝脏重量的观察相结合,而bFGF抑制损害肝脏再生,这与内皮细胞指导肝脏再生的概念一致(2)。 VEGF在体外不刺激肝细胞增殖,但肝细胞与肝内皮细胞的体内研究或共培养研究已证明VEGF通过VEGFR1途径刺激肝细胞的增殖(3)。这些研究还表明通过VEGFR1途径在窦内衬细胞或分离的肝内皮细胞中上调HGF基因表达(3)。因此,这些结果表明VEGF通过VEGFR1刺激的肝脏内皮细胞释放HGF促进肝细胞增殖(3)。最后,最近的一篇论文表明,VEGFR2诱导型基因消融小鼠的肝再生受损是由于LSECs中血管分泌因子(包括HGF)的表达减少(4)。然而,与其他内皮细胞一样,LSEC表达非常少的HGF(1,5)。相反,LSECs(BM SPCs)的骨髓祖细胞,其在损伤后和部分肝切除术后被募集到肝脏(5),富含HGF。 BM SPC与LSEC的大小相同,共享表面标记(5);因此,LSEC分离方法还可以恢复移植到肝脏中的BM SPCs以及LSEC。因此,体外研究可能错误地将移植的BM SPCs的特性归因于成熟的LSEC。目前关于LSECs和肝脏再生的许多研究结果与BM SPCs募集到肝脏而不是成熟LSECs推动肝脏再生的假设是一致的,而成熟LSEC驱动肝脏再生的替代假设不能解释几个已知的观察结果。本综述从文献中检验文献,认为已经募集到肝脏的BM SPCs而不是成熟的LSEC是肝脏再生的主要驱动因素(图11)。
图1
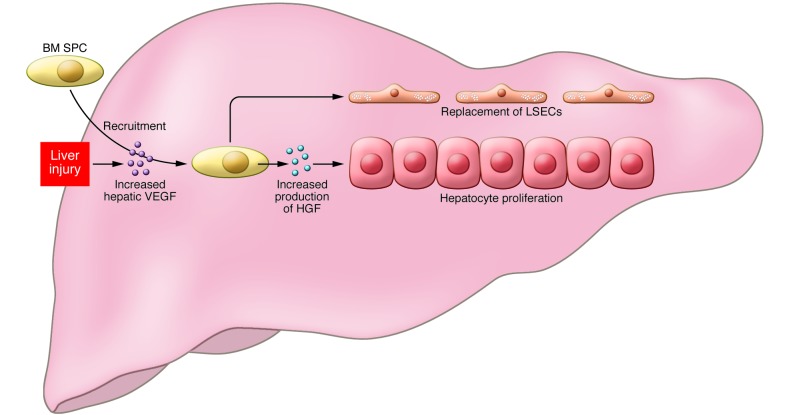
BM SPCs和肝脏再生。
示意图描绘了肝细胞和BM SPC(骨髓祖细胞)对肝再生的贡献。肝损伤(Liver injury)诱导肝脏VEGF表达增加,其驱动富含HGF的BM SPC的募集(Recruitment)并通过驻留的SPC和LSEC促进HGF的表达。 HGF反过来刺激肝再生中肝细胞的增殖(Hepatocyte proliferation)。此外,SPC取代了在受伤期间丢失的LSEC。
LSEC及其祖先
LSEC是形态学和功能上独特的内皮细胞。 LSEC是小细胞,在分离时直径约为6.5μm,伸展成一层非常薄的肝窦内层。 LSEC是唯一将非隔膜窗孔与缺乏基底膜相结合的哺乳动物内皮细胞。窗孔在筛板中聚集在一起,并且窗孔的大小和数量在LSEC中与肝脏的门静脉,中叶和小叶中心区域不同(6-10)。 LSEC在功能上也是独一无二的。受体介导的内吞作用的高活性为LSEC提供了一种高速率,高容量的系统来清除循环中的胶体和可溶性废物大分子(11,12)。内吞作用的三种主要受体是甘露糖受体,清道夫受体和Fcγ受体IIb2(12)。 LSEC是一些肝毒性药物和毒素损伤的最初目标(13-20),并且易受缺血再灌注损伤(21-24)。
如已经描述的其他血管床中的内皮细胞和周细胞(参考文献25-29是许多此类报道中的一些),LSEC与相邻的周细胞,肝星状细胞(30,31)之间存在串扰。肝星状细胞的活化导致纤维化。健康的LSEC可以阻止肝星状细胞的活化,并使活化的肝星状细胞失活(31)。 LSEC在肝纤维化之前发展出改变的表型,称为毛细血管化。毛细管化的LSEC失去了预防肝星状细胞活化和使活化的肝星状细胞失活的能力。在肝纤维化的大鼠模型中,LSEC的毛细血管化的逆转使活化的肝星状细胞失活并加速纤维化的逆转,而在正在进行的损伤期间毛细血管化的逆转阻止了肝硬化的进展(32)。在健康的肝脏中,肝细胞和肝星状细胞通过释放VEGF维持LSEC的表型(30)。
与其他内皮细胞一样,LSEC表达CD31(也称为PECAM)。然而,与大多数内皮细胞不同,正常LSEC的CD31表达局限于细胞质,而不是细胞 - 细胞连接处的细胞表面(30)。与其他内皮细胞形成鲜明对比的是,LSEC是迄今为止报道的唯一表达CD45(10,33,34)的内皮细胞,CD45是一种典型的造血细胞标记物。 CD45在门静脉LSEC上高表达,在中叶LSEC上表达较弱,而在小叶中心LSEC上则不存在(10)。
干细胞被定义为不对称分裂的细胞,产生一个作为干细胞的子细胞和一个作为祖细胞或特化细胞的子细胞(图2).2)。根据定义,干细胞可以自我更新并无限期地增加到更多相同类型的细胞。祖细胞,也称为转运扩增细胞,比干细胞更具谱系,产生特化细胞,并且只能复制有限次数。已经鉴定了两组肝窦内皮细胞祖细胞(SPC):BM SPC和驻留或肝内SPC。 BM SPCs对正常的LSEC转换没有贡献,但在损伤或部分肝切除术后被募集到肝脏(5,33,35)。驻留SPC不是来自成年啮齿动物的骨髓(5)。募集到肝脏的常驻SPCs和BM SPCs与成熟的LSEC一起被分离,“污染”LSEC分离株(5)。啮齿动物驻留SPCs的频率存在显着的变异性,从Sprague-Dawley大鼠的所有LSEC的1%到Fischer大鼠的7%不等(5)。在肝脏内,已经基于其作为标记保留细胞的特征鉴定了推定的驻留LSEC干细胞。自我更新,谱系特异性分化和连续再增殖的确定功能特征尚未得到证实,因此这一名称仍然很脆弱。然而,具有驻留SPC的表面标志物的标记保留细胞的存在表明,驻留的SPC来源于该推定的驻留LSEC干细胞,特别是考虑到驻留的SPC不是来自成年啮齿动物的骨髓。在Sprague-Dawley大鼠中,0.1%的LSEC是推定的驻留LSEC干细胞(5)。与骨髓中有核细胞中造血干细胞的0.01%频率相比,推定的常驻LSEC干细胞的0.1%频率较高(36)。虽然常驻LSEC干细胞和常驻SPC的逻辑功能是在正常周转中产生LSEC,但这种功能尚未得到证实。
图2
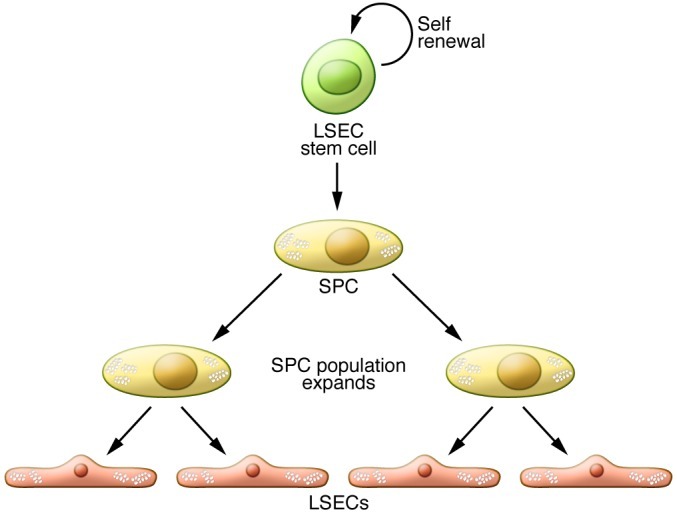
干细胞和祖细胞。
干细胞自我更新并产生祖细胞。祖细胞或转运扩增细胞比干细胞更具谱系,产生额外的祖细胞或特化细胞,并且只能复制有限次数。两个肝脏SPC群体可以产生LSEC:BM SPC,其在损伤或部分肝切除术后被募集到肝脏,以及驻留或肝内SPC,其有助于正常的LSEC转换。 SPC来源于干细胞,SPC群体在产生成熟LSEC之前扩大。
常驻和BM SPC均为祖细胞标记物CD133阳性,并且与LSEC一样,它们表达内皮细胞标记物CD31和造血细胞标记物CD45(5)。常驻SPC对VEGFR1和VEGFR2呈阳性。虽然尚未检查BM SPC中的VEGFR表达,但鉴于LSEC对VEGFR1和VEGFR2是阳性的,BM SPC也可能是这样。驻留SPC具有与成熟LSEC无法区分的开窗模式,而骨髓中的SPC是非开窗的,但在募集到肝脏并植入肝脏后形成正常的LSEC开窗模式(5)。驻留和BM SPC上CD45的存在使它们与更通用的内皮祖细胞(EPC)区别开来,其根据定义CD45阴性。
在部分肝切除术或中毒性损伤后,BM SPC的增殖增加超过两倍,并且BM SPC向循环的动员增加2至4倍(5,35)。骨髓来源的LSEC数量随着时间的推移而增加:在部分肝切除术后第3天获得的LSEC 25%是来自骨髓,在中毒剂量有毒物质二甲基亚硝胺的第5天和第14天后分别40%和70%的LSEC是来自骨髓(5,35)。研究已经排除了骨髓来源细胞募集后LSEC中的融合(33),因此这种结果是真正的植入而不是骨髓细胞与现有LSEC的融合。 BM SPCs在肝脏移植后形成在筛板中组织的窗孔(5,35)。在部分肝切除术后,植入肝脏的BM SPCs比常驻SPCs增殖更多,并且是LSEC部分中增加的HGF的主要来源(5)。
BM SPCs是肝再生所必需的关键证据来自骨髓照射实验。在大鼠中,从踝关节到臀部的两个后肢含有约40%的总骨髓,并且该区域的照射导致外周白细胞计数抑制几乎40%(5)。当这些受照射的大鼠进行部分肝切除术时,肝再生受损40%,并且第5天肝细胞增殖最小表明肝再生不仅仅延迟。在部分肝切除术后第1天将常驻SPC或骨髓输注到受照射的大鼠中显著促进肝细胞增殖并使肝重量的恢复完全正常化。该结果表明,由于BM SPC的丧失,骨髓照射后肝脏再生受损。在本研究中,部分肝切除术后第5天肝脏再生的抑制与骨髓抑制和肝细胞增殖的缺乏相称,表明LSEC和/或常驻SPC和其他HGF来源不能弥补BM SPC刺激肝再生的损失。值得注意的是,在部分肝切除术后第3天(而不是第1天)输注常驻SPC或骨髓几乎没有益处,这表明SPC输注必须在第二轮肝细胞增殖之前发生,这在第2天在大鼠中完成。 LSEC部分内的增殖在部分肝切除术后第1天增加,在第3天达到峰值(5,37,38)。这表明BM SPC的早期流入通过促进肝细胞增殖来刺激肝再生,而来自第3天的LSEC和/或SPC的增殖是再生肝中血管生成所必需的。成熟的LSEC是否也在增殖,或者是否所有的增殖都归因于驻留和BM SPC仍有待确定。
如果输注驻留的SPC可以在骨髓照射后挽救肝再生,则预期驻留的SPC可能有助于肝再生。然而,如上所述,肝再生的抑制与骨髓照射的量成比例,表明驻留的SPC不能补偿BM SPC的损失。注意到输注常驻SPCs刺激肝细胞增殖,但原位驻留SPC对肝脏再生没有显著贡献,这表明这些细胞只要处于肝干细胞生态位中就会保持相对静止状态。
BM SPCs对肝损伤恢复的贡献也已在两种中毒性肝炎模型中得到证实(33,35)。对野百合碱诱导的窦房结综合征(SOS;也称为肝静脉闭塞性疾病)模型的研究表明,野百合碱可破坏LSECs(15,20,33,39)和BM SPCs(33)。在损伤的恢复阶段,约25%的LSEC来自骨髓,表明BM SPC显著修复LSEC。然而,在正弦剥脱和小叶中心出血性坏死的高度,野百合碱使骨髓中SPCs的数量减少50%,并使循环BM SPCs的数量减少95%。骨髓照射引起亚毒性剂量的野百合碱的严重SOS,而骨髓的输注完全阻止了给予一剂量的野百合碱的大鼠的SOS,否则会引起严重的SOS。因此,BM SPCs修复LSEC损伤是SOS的关键决定因素。换句话说,SOS是由于LSEC的损伤和BM SPC缺乏修复的组合。结合我们现在所知的BM SPCs对肝再生的影响,在SOS中BM SPC的修复减少不仅会阻止裸露的正弦曲线的再增殖,还会损害肝再生所需的肝细胞增殖。这可能解释了在造血细胞移植(骨髓移植)之前从高剂量清髓化疗开发SOS的患者的高病例致死率。 BM SPC也在中毒剂量的二甲基亚硝胺后修复LSEC损伤,并且BM SPC的输注减轻了这种损伤。
调节骨髓SPCs的募集
肝脏VEGF(也称为VEGFA)响应于许多形式的肝损伤和部分肝切除术后增加(35,37,38,40-43)。用反义寡核苷酸敲低肝脏VEGF会加剧对肝脏的毒性损伤(35),而输注VEGF可以改善中毒性损伤(44-46)并增加肝细胞增殖(38)和部分肝切除术后肝脏重量(3,4)。在毒性损伤和部分肝切除术后,肝脏VEGF已经显示出调节BM SPC向肝脏募集的每个步骤:BM SPC的增殖,BM SPC向循环的动员,BM SPC在肝脏中的植入以及BM的分化。 SPCs到有孔的LSECs衬里正弦曲线(35)(图(图3).3)。因此,肝脏VEGF是BM SPC募集的中枢调节剂,并且对肝脏再生至关重要。发现诱导型VEGFR2缺陷的Vegfr2flox / flox小鼠在部分肝切除术后肝脏再生受损(4)与两种可能的假设一致:肝脏VEGF将BM SPCs募集到肝脏从而促进肝脏再生,并且这些小鼠缺乏VEGF- BM SPCs刺激HGF释放(3)(见下文)。
图3
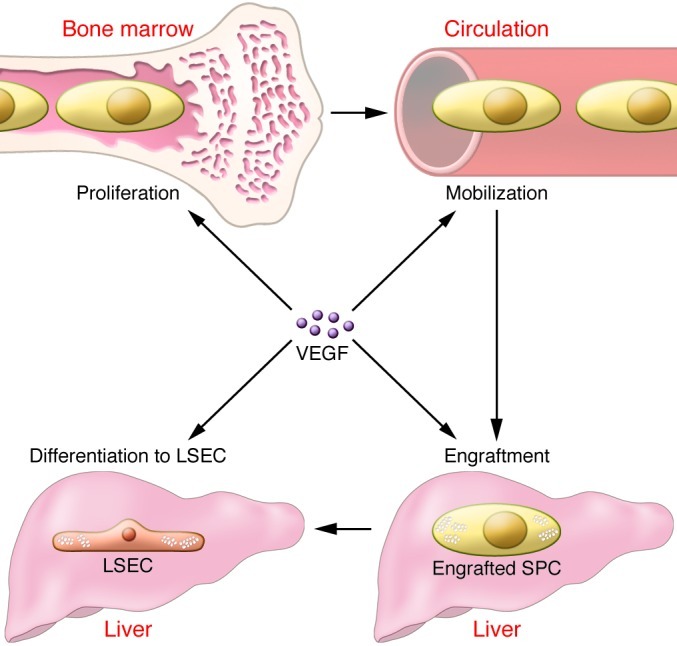
肝脏VEGF是从骨髓到肝脏募集SPC的中心调节剂。
在不同形式的肝损伤后,肝脏VEGF增加。 VEGF的增加对于BM SPC的增殖(Proliferation)增加,BM SPC向循环的动员(mobilization),BM SPC在肝脏中的植入(Engraftment)以及在肝损伤后分化为LSECs(Differentiation to LSEC)是必需的。
肝脏有丝分裂原
关于肝再生的几篇优秀综述提供了对控制肝再生的信号通路的深入讨论(例如,参考文献47,48)。本节仅提及与当前评论相关的一些概念。多种信号传导途径有助于肝再生,并且这些途径可以由完整或不完全的有丝分裂原(肝有丝分裂原)驱动。完整的有丝分裂原在体外肝细胞培养中是促有丝分裂的,并且当在体内注射时能够诱导肝脏增大和肝细胞DNA合成(48)。相反,辅助有丝分裂原在体外不具有促有丝分裂作用,并且不会在体内引起肝细胞DNA合成和肝脏扩大(48)。消融辅助有丝分裂原的信号通路延迟肝再生而不是消除它(48)。最近报道了Wnt2是来自LSEC级分的有丝分裂原(4)。
虽然本综述着重于LSEC及其祖细胞的HGF,但HGF的几种来源可能有助于肝脏再生。肝星状细胞在正常肝脏中的HGF表达高于其他肝细胞,但在肝损伤的四氯化碳模型中表达没有增加(1)。部分肝切除术后1小时内发生的肝脏HGF增加归因于从细胞外和细胞外糖胺聚糖中释放HGF(48)。由于时间的原因,第一轮肝细胞增殖的刺激归因于这种HGF库(48)。部分肝切除术后3小时,肝脏中HGF蛋白表达增加(49),肺(50),肾和脾中HGF基因表达增加(51)。各种肝内和肝外HGF库对肝再生的各自贡献仍有待确定。
DNA结合抑制剂1(Id1)是显性失活的螺旋 - 环 - 螺旋蛋白,其可以与转录因子的基本HLD家族的成员形成异二聚体。 Id1通过增强HIF-1α(52-54)的稳定性和活性来激活VEGF,相反,VEGF / VEGFR2途径可激活Id1(4)。在Id1 - / - 小鼠中的研究已经证明受损的动员和骨髓EPC的募集,导致肿瘤血管生成和肿瘤生长受损(55)。部分肝切除术后的肝再生在Id1 - / - 小鼠中受损(4),表明BM SPC的动员和募集受损,这与EPC发现类似(4)。 Id1 - / - 小鼠在部分肝切除术后HGF和Wnt2的表达降低(4),但不清楚Id1是否是VEGF / VEGFR2刺激的Wnt2和HGF表达的转录因子,或者Wnt2和HGF的肝脏表达是否降低。仅仅是由于表达Wnt2和HGF的BM SPCs的募集减少。虽然这项研究使用肝细胞与人肝脏内皮细胞的体外两周共培养模型来检查是否需要Id1表达来刺激肝细胞增殖(4),但这些结果的解释是困难的,因为LSEC仅在标准co中存活。 - 培养约4天。
EGF是由十二指肠Brunner腺体产生的完整促分裂原(56); EGF分泌到门脉循环中被认为有助于肝脏再生。鉴于EPC表达EGF(57),还应在驻留和BM SPC中检查EGF表达。如果EGF在SPCs中大量表达,研究将需要检查SPC衍生的EGF在促进肝再生中的作用。
以上部分研究了肝脏VEGF在募集表达HGF和可能还有Wnt2的BM SPC中的作用。然而,用肝内皮细胞进行的体外研究已经证明VEGF可以刺激分离的肝脏内皮细胞(可能含有LSEC和常驻SPCs)产生HGF,从而促进体外肝细胞增殖(3)。在体外研究中VEGF诱导的HGF增加是否来自常驻SPC,成熟LSEC或两者都是未知的(5)。此外,尚不清楚肝损伤后LSEC组分中HGF表达增加的主要原因是肝脏VEGF刺激募集的高表达HGF的BM SPCs,高肝脏VEGF表达刺激移植的BM中HGF的表达。 SPC,或两者的组合。
临床意义和问题
研究表明,大鼠的肝脏再生与骨髓的抑制成比例地被抑制(5)。这些发现的临床意义很明显。如果这些观察结果在人类中得到证实,那么全球骨髓抑制或BM SPCs的选择性抑制将损害肝脏再生,这是临床医生的一个重要考虑因素。然而,这一观察提出了许多临床问题。可以抑制慢性肝病中选择的骨髓群,但BM SPCs是否可以被抑制?各种肝脏疾病和肝毒性损伤对BM SPCs和募集BM SPCs的信号通路有何影响?正常肝脏中的肝再生来自肝细胞增殖(58),但在慢性肝病中,肝再生需要肝细胞祖细胞的增殖。 BM SPCs在依赖肝细胞祖细胞的肝再生中发挥作用吗?再生医学能创造出可以用于治疗肝脏再生的BM SPCs吗?如果促进BM SPC募集的肝脏信号传导在肝脏疾病中减少,那么外源性BM SPCs是肝脏并移植的吗? VEGF施用是昂贵的并且可能因保水而变得复杂;招募BM SPCs的信号通路中的其他步骤是否更适合治疗?
结论
BM SPCs在部分肝切除术和中毒性肝损伤后被募集到肝脏。在这些模型中,BM SPC向肝脏的募集对于肝细胞增殖和肝脏重量的恢复是必需的。 BM SPC是LSEC部分中增加的HGF的主要来源。然而,考虑到损伤后肝内和肝外HGF增加的其他来源,HGF可能无法解释BM SPCs的重要贡献。虽然BM SPCs的HGF可能很重要,但未来的研究需要明确为什么BM SPC募集对肝脏再生至关重要。
VEGF / VEGFR2途径对于肝再生是必需的。 Id1是该途径中必需的转录因子,但其作用需要进一步定义。 VEGF是BM SPC募集的中枢调节剂:损伤后肝脏VEGF的增加导致BM SPC的增殖,BM SPC向循环的动员,肝脏中的植入以及BM SPC向LSEC的分化。 VEGF还刺激LSEC部分细胞中增加的HGF表达,即成熟LSEC和/或LSEC祖细胞。
肝再生对于切除或损伤后肝脏肿块的急性恢复和慢性损伤期间肝脏肿块的维持都是必不可少的。更好地了解BM SPCs对肝再生的贡献以及BM SPC向肝脏募集的调节应该使其成为转化研究的肥沃领域。
Liver sinusoidal endothelial cells and liver regeneration
Abstract
Liver sinusoidal endothelial cells (LSECs) have long been noted to contribute to liver regeneration after liver injury. In normal liver, the major cellular source of HGF is the hepatic stellate cell, but after liver injury, HGF expression has been thought to increase markedly in proliferating LSECs. However, emerging data suggest that even after injury, LSEC expression of HGF does not increase greatly. In contrast, bone marrow progenitor cells of LSECs (BM SPCs), which are rich in HGF, are recruited to the liver after injury. This Review examines liver regeneration from the perspective that BM SPCs that have been recruited to the liver, rather than mature LSECs, drive liver regeneration.
Introduction
HGF is a cytokine that plays a crucial role in tissue regeneration, stimulating cell growth, cell motility, and morphogenesis. More than 20 years ago, Jacquelyn Maher first hypothesized that liver sinusoidal endothelial cells (LSECs) contribute to liver regeneration, demonstrating that hepatic stellate cells serve as the major source of HGF in normal liver but that after liver injury, HGF expression increased markedly in LSECs (1). It was shown subsequently that bFGF stimulates LSEC proliferation in the liver but not hepatocyte proliferation, and that inhibition of bFGF inhibits LSEC proliferation but not hepatocyte proliferation. This result, coupled with the observation that bFGF or VEGF increases liver weight after partial hepatectomy, whereas bFGF inhibition impairs liver regeneration, is consistent with the concept that endothelial cells direct liver regeneration (2). VEGF does not stimulate hepatocyte proliferation in vitro, but in vivo studies or co-culture studies of hepatocytes with liver endothelial cells have demonstrated that VEGF stimulates proliferation of hepatocytes through the VEGFR1 pathway (3). These studies also demonstrated upregulation of HGF gene expression through the VEGFR1 pathway in sinusoidal lining cells or isolated liver endothelial cells (3). Thus these results suggested that VEGF promotes hepatocyte proliferation through VEGFR1-stimulated release of HGF from liver endothelial cells (3). Finally, a recent paper has suggested that impaired liver regeneration in mice with inducible genetic ablation of VEGFR2 is due to diminished expression of angiocrine factors, including HGF, from LSECs (4). However, like other endothelial cells, LSECs express very little HGF (1, 5). In contrast, bone marrow progenitor cells of LSECs (BM SPCs), which are recruited to the liver after injury and after partial hepatectomy (5), are rich in HGF. BM SPCs are the same size as LSECs and share surface markers (5); therefore, LSEC isolation methods can also recover BM SPCs engrafted in the liver along with LSECs. Thus, in vitro studies could mistakenly attribute the properties of engrafted BM SPCs to mature LSECs. Many current findings on LSECs and liver regeneration are consistent with the hypothesis that BM SPCs recruited to the liver, rather than mature LSECs, drive liver regeneration, whereas the alternate hypothesis, that mature LSECs drive liver regeneration, cannot account for several known observations. This Review examines the literature from the perspective that BM SPCs that have been recruited to the liver, rather than mature LSECs, are the major drivers of liver regeneration (Figure (Figure11).
Figure 1
BM SPCs and liver regeneration.
Schematic depicting the contributions of liver cells and BM SPCs to liver regeneration. Liver injury induces increased hepatic VEGF expression, which drives recruitment of HGF-rich BM SPCs and promotes expression of HGF by resident SPCs and LSECs. HGF, in turn stimulates the proliferation of hepatocytes in liver regeneration. In addition, SPCs replace LSECs that were lost during injury.
LSECs and their progenitors
LSECs are unique endothelial cells, both morphologically and functionally. LSECs are small cells, with a diameter of around 6.5 μm when isolated, that are stretched out into a very thin layer lining the hepatic sinusoids. LSECs are the only mammalian endothelial cells that combine non-diaphragmed fenestrae with the lack of a basement membrane. The fenestrae are clustered together in sieve plates, and the size and number of fenestrae differ in LSECs from the periportal, midlobular and centrilobular regions of the liver (6–10). LSECs are also functionally unique. The high activity of receptor-mediated endocytosis provides LSECs with a high-rate, high-capacity system to clear colloids and soluble waste macromolecules from the circulation (11, 12). The three main receptors for endocytosis are the mannose receptor, the scavenger receptor, and the Fcγ receptor IIb2 (12). LSECs are the initial target of injury for some hepatotoxic drugs and toxins (13–20) and are susceptible to ischemia-reperfusion injury (21–24).
As has been described for endothelial cells and pericytes in other vascular beds (refs. 25–29 are a few of many such reports), crosstalk exists between LSECs and the neighboring pericytes, the hepatic stellate cells (30, 31). Activation of hepatic stellate cells leads to fibrosis. Healthy LSECs prevent the activation of hepatic stellate cells and inactivate activated hepatic stellate cells (31). LSECs develop an altered phenotype preceding hepatic fibrosis that is called capillarization. Capillarized LSECs lose the ability to prevent hepatic stellate cell activation and inactivate activated hepatic stellate cells. In a rat model of hepatic fibrosis, reversal of capillarization of LSECs inactivates activated hepatic stellate cells and accelerates reversal of fibrosis, whereas reversal of capillarization during an ongoing insult prevents progression of cirrhosis (32). In healthy liver, hepatocytes and hepatic stellate cells maintain the phenotype of LSECs through release of VEGF (30).
Like other endothelial cells, LSECs express CD31 (also known as PECAM). However, unlike most endothelial cells, the CD31 expression of normal LSECs is restricted to the cytoplasm rather than to the cell surface at cell-cell junctions (30). Also in stark contrast to other endothelial cells, LSECs are the only endothelial cells reported to date that express CD45 (10, 33, 34), a classic hematopoietic cell marker. CD45 is highly expressed on periportal LSECs, less strongly expressed on midlobular LSECs, and absent on centrilobular LSECs (10).
Stem cells are defined as cells that divide asymmetrically, producing one daughter cell that is a stem cell and one daughter cell that is either a progenitor cell or a specialized cell (Figure (Figure2).2). By definition, stem cells can self-renew and give rise indefinitely to more cells of the same type. Progenitor cells, also known as transit-amplifying cells, are more lineage committed than stem cells, give rise to specialized cells, and can only replicate a limited number of times. Two populations of liver sinusoidal endothelial cell progenitor cells (SPCs) have been identified: BM SPCs and resident or intrahepatic SPCs. BM SPCs do not contribute to normal LSEC turnover, but are recruited to the liver after injury or partial hepatectomy (5, 33, 35). Resident SPCs are not derived from the bone marrow in the adult rodent (5). Resident SPCs and BM SPCs recruited to the liver are isolated along with mature LSECs, “contaminating” LSEC isolates (5). Marked variability exists in the frequency of resident SPCs in rodents, varying from 1% of all LSECs in Sprague-Dawley rats to 7% in Fischer rats (5). Within the liver, a putative resident LSEC stem cell has been identified based on its characteristics as a label-retaining cell. The definitive functional characteristics of self-renewal, lineage-specific differentiation, and serial repopulation have not been demonstrated, so this designation remains tenuous. However, the presence of a label-retaining cell with the surface markers of the resident SPC suggests that resident SPCs derive from this putative resident LSEC stem cell, especially given that resident SPCs do not come from the bone marrow in the adult rodent. In the Sprague-Dawley rat, 0.1% of all LSECs are putative resident LSEC stem cells (5). The 0.1% frequency of the putative resident LSEC stem cell is high when compared with the 0.01% frequency of hematopoietic stem cells among the nucleated cells in the bone marrow (36). Although the logical function for the resident LSEC stem cell a3nd resident SPC would be to give rise to LSECs in normal turnover, such functionality has yet to be demonstrated.
Figure 2
Stem and progenitor cells.
Stem cells self-renew and give rise to progenitor cells. Progenitor cells or transit-amplifying cells are more lineage committed than stem cells, give rise to additional progenitor cells or to specialized cells, and can only replicate a limited number of times. Two populations of liver SPCs can give rise to LSECs: BM SPCs, which are recruited to the liver after injury or partial hepatectomy, and resident or intrahepatic SPCs, which contribute to normal LSEC turnover. SPCs derive from stem cells, and the SPC population expands before giving rise to the mature LSECs.
Both resident and BM SPCs are positive for the progenitor cell marker CD133 and, like the LSEC, they express the endothelial cell marker CD31 and the hematopoietic cell marker CD45 (5). The resident SPC is positive for VEGFR1 and VEGFR2. While VEGFR expression in BM SPCs has not yet been examined, given that LSECs are positive for VEGFR1 and VEGFR2, BM SPCs may be as well. The resident SPC has a fenestration pattern that is indistinguishable from mature LSECs, whereas SPCs in the bone marrow are non-fenestrated but develop the normal LSEC fenestration pattern after recruitment to and engraftment in the liver (5). The presence of CD45 on resident and BM SPCs distinguishes them from the more generic endothelial progenitor cell (EPC) that is by definition CD45 negative.
After partial hepatectomy or toxic injury, proliferation of BM SPCs increases more than two-fold and mobilization of BM SPCs to the circulation increases two- to four-fold (5, 35). The number of LSECs of bone marrow origin increases over time: 25% of LSECs are bone marrow derived on day 3 after partial hepatectomy, whereas 40% and 70% of LSECs are bone marrow derived on days 5 and 14, respectively, after a toxic dose of dimethylnitrosamine (5, 35). Studies have ruled out fusion in LSECs after recruitment of the bone marrow–derived cells (33), so this result is true engraftment rather than fusion of a bone marrow cell with existing LSECs. BM SPCs develop fenestrae organized in sieve plates after engraftment in the liver (5, 35). After partial hepatectomy, BM SPCs that engraft in the liver proliferate much more than resident SPCs and are the major source of increased HGF in the LSEC fraction (5).
The key evidence that BM SPCs are necessary for liver regeneration comes from experiments with bone marrow irradiation. In rats, the two hind limbs from ankle to hip contain about 40% of the total bone marrow, and irradiation of this area results in suppression of peripheral leukocyte count by almost 40% (5). When these irradiated rats undergo partial hepatectomy, liver regeneration is impaired by 40%, and minimal hepatocyte proliferation on day 5 suggests that liver regeneration is not merely delayed. Infusion of either resident SPCs or bone marrow into the irradiated rats on day 1 after partial hepatectomy markedly promotes hepatocyte proliferation and completely normalizes restoration of liver weight. This result demonstrates that liver regeneration is impaired following bone marrow irradiation due to the loss of BM SPCs. The suppression of liver regeneration proportionate to bone marrow suppression and the lack of hepatocyte proliferation on day 5 after partial hepatectomy in this study indicate that LSECs and/or resident SPCs and other sources of HGF cannot compensate for the loss of BM SPC stimulation of liver regeneration. Of note, infusion of resident SPCs or bone marrow on day 3 (rather than day 1) after partial hepatectomy has little benefit, suggesting that SPC infusion must occur prior to the second round of hepatocyte proliferation, which is completed by day 2 in the rat. Proliferation within the LSEC fraction is increased by day 1 after partial hepatectomy and peaks by day 3 in the rat (5, 37, 38). This suggests that the early influx of BM SPCs stimulates liver regeneration by promoting hepatocyte proliferation, whereas proliferation of LSECs and/or SPCs from day 3 on is required for angiogenesis in the regenerating liver. Whether the mature LSEC also proliferates or whether all of the proliferation is attributable to resident and BM SPCs remains to be determined.
If infusion of resident SPCs can rescue liver regeneration after bone marrow irradiation, resident SPCs might be expected to contribute to liver regeneration. However, as noted above, suppression of liver regeneration is proportionate to the amount of bone marrow irradiated, indicating that resident SPCs do not compensate for the loss of BM SPCs. The finding that infusion of resident SPCs stimulates hepatocyte proliferation, but that resident SPCs in situ do not contribute significantly to liver regeneration, suggests that these cells remain relatively quiescent as long as they are in the liver stem cell niche.
The contribution of BM SPCs to recovery from liver injury has also been demonstrated in two models of toxic hepatitis (33, 35). Studies in the monocrotaline-induced model of sinusoidal obstruction syndrome (SOS; also referred to as hepatic venoocclusive disease) have demonstrated that monocrotaline damages both LSECs (15, 20, 33, 39) and BM SPCs (33). During the recovery phase of injury, around 25% of LSECs are of bone marrow origin, demonstrating marked repair of LSECs by BM SPCs. However, at the height of sinusoidal denudation and centrilobular hemorrhagic necrosis, monocrotaline decreases the number of SPCs in the bone marrow by 50% and reduces the number of circulating BM SPCs by 95%. Bone marrow irradiation elicits severe SOS from a subtoxic dose of monocrotaline, whereas infusion of bone marrow completely prevents SOS in rats given a dose of monocrotaline that would otherwise cause severe SOS. Thus, repair of LSEC injury by BM SPCs is a key determinant of SOS. In other words, SOS is due to a combination of injury to LSECs and lack of repair by BM SPCs. Taken together with what we now know about the effect of BM SPCs on liver regeneration, diminished repair by BM SPCs in SOS will not only prevent repopulation of the denuded sinusoid, but also impair hepatocyte proliferation needed for liver regeneration. This likely explains the high case lethality in patients who develop SOS from high-dose myeloablative chemotherapy prior to hematopoietic cell transplantation (bone marrow transplantation). BM SPCs also repair LSEC injury after a toxic dose of dimethylnitrosamine, and infusion of BM SPC attenuates this injury (35).
Regulation of recruitment of bone marrow SPCs
Hepatic VEGF (also known as VEGFA) increases in response to many forms of liver injury and after partial hepatectomy (35, 37, 38, 40–43). Knockdown of hepatic VEGF with antisense oligonucleotides can exacerbate toxic injury to the liver (35), whereas infusion of VEGF can ameliorate toxic injury (44–46) and increases hepatocyte proliferation (38) and liver weight after partial hepatectomy (3, 4). After both toxic injury and partial hepatectomy, hepatic VEGF has been shown to regulate each step of BM SPC recruitment to the liver: proliferation of BM SPCs, mobilization of BM SPCs to the circulation, engraftment of BM SPCs in the liver, and differentiation of BM SPCs to fenestrated LSECs lining the sinusoids (35) (Figure (Figure3).3). Thus, hepatic VEGF is a central regulator of BM SPC recruitment and is critical to liver regeneration. The finding that inducible VEGFR2-deficient Vegfr2flox/flox mice have impaired liver regeneration after partial hepatectomy (4) is consistent with two possible hypotheses: that hepatic VEGF recruits BM SPCs to the liver and thereby promotes liver regeneration, and that these mice lack VEGF-stimulated HGF release by BM SPCs (3) (see below).
Figure 3
Hepatic VEGF is a central regulator of recruitment of SPCs from the bone marrow to the liver.
Hepatic VEGF increases after disparate forms of liver injury. The increase in VEGF is necessary for increased proliferation of BM SPCs, mobilization of BM SPCs to the circulation, engraftment of BM SPCs in the liver, and differentiation to LSECs after liver injury.
Hepatic mitogens
Several excellent reviews on liver regeneration provide an in-depth discussion of the signaling pathways that control liver regeneration (for example, refs. 47, 48). This section will only mention a few concepts that are relevant to the current Review. Multiple signaling pathways contribute to liver regeneration, and these pathways can be driven by complete or incomplete mitogens (Hepatic mitogens). Complete mitogens are mitogenic in hepatocyte culture in vitro and able to induce liver enlargement and hepatocyte DNA synthesis when injected in vivo (48). In contrast, auxiliary mitogens are not mitogenic in vitro and do not cause hepatocyte DNA synthesis and liver enlargement in vivo (48). Ablation of the signaling pathways for auxiliary mitogens delays liver regeneration rather than abolishing it (48). Wnt2 has recently been reported as being a mitogen derived from the LSEC fraction (4).
Although this Review focuses on HGF from LSECs and their progenitors, several sources of HGF may contribute to liver regeneration. Hepatic stellate cells have much higher HGF expression in normal liver than do other liver cells, but expression does not increase in the carbon tetrachloride model of liver injury (1). The increase in hepatic HGF that occurs within one hour of partial hepatectomy is attributed to release of HGF from pericellular and extracellular glycosaminoglycans (48). Because of the timing, stimulation of the first round of hepatocyte proliferation is attributed to this pool of HGF (48). Three hours after partial hepatectomy, HGF protein expression increases in the liver (49) and HGF gene expression is increased in lung (50), kidney, and spleen (51). The respective contributions of the various intrahepatic and extrahepatic HGF pools to liver regeneration remain to be defined.
Inhibitor of DNA binding 1 (Id1) is a dominant-negative helix-loop-helix protein that can form heterodimers with members of the basic HLD family of transcription factors. Id1 activates VEGF by enhancing the stability and activity of HIF-1α (52–54), and conversely the VEGF/VEGFR2 pathway may activate Id1 (4). Studies in Id1–/– mice have demonstrated impaired mobilization and recruitment of bone marrow EPCs, leading to impaired tumor angiogenesis and tumor growth (55). Liver regeneration after partial hepatectomy is impaired in Id1–/– mice (4), suggesting an impaired mobilization and recruitment of BM SPCs that is analogous to the EPC findings (4). Id1–/– mice have decreased expression of HGF and Wnt2 after partial hepatectomy (4), but it is unclear whether Id1 is a transcription factor for VEGF/VEGFR2-stimulated expression of Wnt2 and HGF or whether decreased hepatic expression of Wnt2 and HGF are solely due to decreased recruitment of BM SPCs expressing Wnt2 and HGF. While this study used an in vitro two-week co-culture model of hepatocytes with human liver endothelial cells to examine whether Id1 expression is required to stimulate hepatocyte proliferation (4), interpretation of these results is difficult, as LSECs only survive in standard co-culture for about 4 days.
EGF is a complete mitogen that is produced by duodenal Brunner’s glands (56); secretion of EGF into the portal circulation is thought to contribute to liver regeneration. Given that EPCs express EGF (57), EGF expression should also be examined in resident and BM SPCs. If EGF is abundantly expressed in SPCs, studies will need to examine the contribution of SPC-derived EGF in the promotion of liver regeneration.
The section above examines the role of hepatic VEGF in recruiting BM SPCs that express HGF and perhaps also Wnt2. However, in vitro studies with liver endothelial cells have demonstrated that VEGF stimulates production of HGF from isolated liver endothelial cells (presumably containing both LSECs and resident SPCs), which promotes hepatocyte proliferation in vitro (3). Whether the VEGF-induced increase in HGF in the in vitro studies comes from resident SPCs, mature LSECs, or both is not known (5). In addition, it is unclear whether the major reason for increased expression of HGF in the LSEC fraction after liver injury is due to hepatic VEGF-stimulated recruitment of BM SPCs that highly express HGF, high hepatic VEGF expression that stimulates expression of HGF in engrafted BM SPCs, or a combination of both.
Clinical implication and questions
Studies demonstrate that liver regeneration in the rat is suppressed proportionately to the suppression of bone marrow (5). The clinical implication of these findings is clear. If these observations are confirmed in humans, then global bone marrow suppression or selective suppression of BM SPCs will impair liver regeneration, an important consideration for clinicians. However this observation raises numerous clinical questions. Suppression of select bone marrow populations in chronic liver disease can occur, but are BM SPCs one of the populations that can be suppressed? What effect do various liver diseases and hepatotoxic insults have on BM SPCs and on the signaling pathways that recruit BM SPCs? Liver regeneration in normal liver comes from hepatocyte proliferation (58), but in chronic liver disease, liver regeneration requires proliferation of hepatocyte progenitors. Do BM SPCs play a role in liver regeneration that is dependent on hepatocyte progenitors? Can regenerative medicine create BM SPCs that could be used therapeutically to promote liver regeneration? If hepatic signaling that promotes BM SPC recruitment is diminished in liver disease, would exogenous BM SPCs home to the liver and engraft? VEGF administration is costly and can be complicated by water retention; will other steps in the signaling pathway that recruits BM SPCs be more amenable to therapy?
Conclusions
BM SPCs are recruited to the liver after partial hepatectomy and toxic liver injury. Recruitment of BM SPCs to the liver is essential for hepatocyte proliferation and restoration of liver weight in these models. BM SPCs are the major source of increased HGF in the LSEC fraction. However, HGF may not account for the essential contribution of BM SPCs, given that there are other sources of increased intrahepatic and extrahepatic HGF after injury. Although HGF from BM SPCs is probably important, future studies will need to define why BM SPC recruitment is essential for liver regeneration.
The VEGF/VEGFR2 pathway is essential for liver regeneration. Id1 is an essential transcription factor in this pathway, but its role needs further definition. VEGF is a central regulator of BM SPC recruitment: the increase in hepatic VEGF after injury leads to proliferation of BM SPCs, mobilization of BM SPCs to the circulation, engraftment in the liver, and differentiation of BM SPCs to LSECs. VEGF also stimulates increased HGF expression in the cells of the LSEC fraction, i.e., mature LSECs and/or the LSEC progenitors.
Liver regeneration is essential to both acute restoration of liver mass after resection or injury and to maintenance of liver mass during chronic injury. A better understanding of the contribution of BM SPCs to liver regeneration and the regulation of BM SPC recruitment to the liver should make this a fertile field for translational research.
J Clin Invest. 2013 May 1; 123(5): 1861–1866.
Laurie D. DeLeve
Author information Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Published online 2013 May 1. doi: [10.1172/JCI66025]
PMCID: PMC3635729
PMID: 23635783
Liver sinusoidal endothelial cells and liver regeneration https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3635729/
Gastroenterology. 2012 Apr;142(4):918-927.e6. doi: 10.1053/j.gastro.2011.12.017. Epub 2011 Dec 16.
Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats.
Xie G1, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD.
Author information
1
Division of Gastrointestinal and Liver Disease, University of Southern California Research Center for Liver Disease, Los Angeles, California 90033, USA.
Abstract
BACKGROUND & AIMS:
Capillarization, characterized by loss of differentiation of liver sinusoidal endothelial cells (LSECs), precedes the onset of hepatic fibrosis. We investigated whether restoration of LSEC differentiation would normalize crosstalk with activated hepatic stellate cells (HSC) and thereby promote quiescence of HSC and regression of fibrosis.
METHODS:
Rat LSECs were cultured with inhibitors and/or agonists and examined by scanning electron microscopy for fenestrae in sieve plates. Cirrhosis was induced in rats using thioacetamide, followed by administration of BAY 60-2770, an activator of soluble guanylate cyclase (sGC). Fibrosis was assessed by Sirius red staining; expression of α-smooth muscle actin was measured by immunoblot analysis.
RESULTS:
Maintenance of LSEC differentiation requires vascular endothelial growth factor-A stimulation of nitric oxide-dependent signaling (via sGC and cyclic guanosine monophosphate) and nitric oxide-independent signaling. In rats with thioacetamide-induced cirrhosis, BAY 60-2770 accelerated the complete reversal of capillarization (restored differentiation of LSECs) without directly affecting activation of HSCs or fibrosis. Restoration of differentiation to LSECs led to quiescence of HSCs and regression of fibrosis in the absence of further exposure to BAY 60-2770. Activation of sGC with BAY 60-2770 prevented progression of cirrhosis, despite continued administration of thioacetamide.
CONCLUSIONS:
The state of LSEC differentiation plays a pivotal role in HSC activation and the fibrotic process.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Division of Gastrointestinal and Liver Disease, University of Southern California Research Center for Liver Disease, Los Angeles, California 90033, USA.
Comment in
Liver sinusoidal endothelial cells in disease--and for therapy? [J Hepatol. 2013]
Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/22178212/
Circ Res. 2018 Aug 3;123(4):477-494. doi: 10.1161/CIRCRESAHA.118.313237.
Emerging Roles of Vascular Endothelium in Metabolic Homeostasis.
Pi X1, Xie L1, Patterson C2.
Author information
1
From the Section of Athero & Lipo, Department of Medicine, Cardiovascular Research Institute, Baylor College of Medicine, Houston, TX (X.P., L.X.).
2
University of Arkansas for Medical Sciences, Little Rock (C.P.).
Abstract
Our understanding of the role of the vascular endothelium has evolved over the past 2 decades, with the recognition that it is a dynamically regulated organ and that it plays a nodal role in a variety of physiological and pathological processes. Endothelial cells (ECs) are not only a barrier between the circulation and peripheral tissues, but also actively regulate vascular tone, blood flow, and platelet function. Dysregulation of ECs contributes to pathological conditions such as vascular inflammation, atherosclerosis, hypertension, cardiomyopathy, retinopathy, neuropathy, and cancer. The close anatomic relationship between vascular endothelium and highly vascularized metabolic organs/tissues suggests that the crosstalk between ECs and these organs is vital for both vascular and metabolic homeostasis. Numerous reports support that hyperlipidemia, hyperglycemia, and other metabolic stresses result in endothelial dysfunction and vascular complications. However, how ECs may regulate metabolic homeostasis remains poorly understood. Emerging data suggest that the vascular endothelium plays an unexpected role in the regulation of metabolic homeostasis and that endothelial dysregulation directly contributes to the development of metabolic disorders. Here, we review recent studies about the pivotal role of ECs in glucose and lipid homeostasis. In particular, we introduce the concept that the endothelium adjusts its barrier function to control the transendothelial transport of fatty acids, lipoproteins, LPLs (lipoprotein lipases), glucose, and insulin. In addition, we summarize reports that ECs communicate with metabolic cells through EC-secreted factors and we discuss how endothelial dysregulation contributes directly to the development of obesity, insulin resistance, dyslipidemia, diabetes mellitus, cognitive defects, and fatty liver disease.
Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/30355249
.png)

.png)