Hepatitis B: The Creation and Destruction of a Virus
Professor Jianming Hu is based at the Pennsylvania State University College of Medicine. His work focuses on the hepatitis B virus, specifically how the protein shell or ‘capsid’ of the virus assembles and disassembles. Both processes offer opportunities as targets for future antiviral therapies as well as improving fundamental understanding of the complex aspects of the hepatitis B viral replication cycle.
Approximately 2 billion people worldwide have been infected with the hepatitis B virus (HBV), with 350 million of them becoming chronically infected. HBV infection causes a wide range of serious illnesses, including acute and chronic hepatitis, cirrhosis and hepatocellular carcinoma: around 1 million fatalities each year are associated with HBV infection.
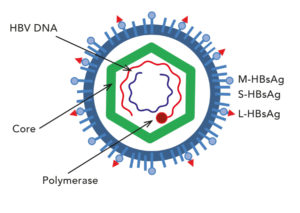
This diagram shows a simplified structure of the hepatitis B virus. CC BY-SA 3.0
HBV is a member of the Hepadnaviridiae family, which also includes related virus species such as duck hepatitis B virus. All hepadnaviruses contain a small, partially double stranded, relaxed circular DNA genome. The virus replicates this DNA genome via an RNA intermediate using a process called reverse transcription. The virus itself is an enveloped virus with the relaxed circular DNA genome housed within an inner protein shell, or nucleocapsid. The HBV capsid shell enclosing the DNA is composed of multiple copies of a single viral protein, HBV core protein (HBc). Normally, there are 240 copies of this protein which make up the viral capsid.
Approximately 2 billion people worldwide have been infected with hepatitis B virus (HBV), with 350 million of them becoming chronically infected.
The primary cell type infected by HBV is liver cells, or hepatocytes. Once in the nucleus of the hepatocyte, the relaxed circular DNA needs to be converted to a different type of DNA – covalently closed circular DNA (CCC DNA) – in order for viral replication to be established and sustained. A prerequisite for CCC DNA formation is the uncoating of the virus in order to expose their DNA. Only viruses with relaxed circular genomes are considered mature viruses as they have the ability to be secreted from infected cells and then internalised by the next host cells. Interestingly, empty capsids containing no viral DNA or RNA are also produced and secreted as enveloped empty virions.
There are two fates of the mature nucleocapsids containing relaxed circular DNA. They can either be enveloped by viral envelope proteins, followed by secretion out of the cell as a complete virus particle, or the nucleocapsid is disassembled to release relaxed circular DNA into the host cell nucleus to form CCC DNA. Immature nucleocapsids are unable to do either of these processes. Structural changes associated with nucleocapsid maturation are likely to play a part in the selective uncoating of mature capsids to allow CCC DNA formation and its preferential envelopment for virion secretion.
A simplified version of hepatitis B replication: the virus enters the cell; capsid disassembly; viral relaxed circular DNA enters nucleus of cell; CCC-DNA created; transcription to RNA occurs; RNA is packaged up into a new capsid; new virus is created; virus leaves cell.
Assembling a capsid…
Laboratory approaches used to investigate HBV capsid assembly usually centre around over-expression of HBc in bacteria. Using this system, it is possible to interfere with the conditions in which the virus must assemble. For example, Professor Hu’s group, as well as many others around the world, have shown that the first 140 amino acids of the HBc protein (the major component of the capsid), the ‘assembly domain’, are sufficient for virus assembly. Amino acids found at the opposite end of the protein in the C-terminal domain, and the linking region between the two domains, are dispensable. In contrast, Professor Hu’s group have recently demonstrated, using systems which mimic physiological conditions in infected human cells, that the C-terminal domain is also required to facilitate capsid assembly. Indeed, Professor Hu’s lab has developed a mammalian-cell free system in which HBc is expressed at physiologically low levels and which assembles into capsids under near physiological conditions. This is in contrast to the traditional systems which rely on non-physiological levels of protein and salts to induce HBc assembly into capsids. In addition, the phosphorylation state of the C-terminal domain which regulates capsid assembly and the packaging of RNA in the cell-free system, is the same as occurs in human cells.
… and disassembling a capsid
In order for the viral genetic material (genome) inside the virus to do its job, it must be released from the virus particle in which it is packaged. Once the virus has undergone binding to its target cell in the host, the disassembly process can begin. The uncoating, i.e., disassembly of the nucleocapsid, process is subject to host regulation, which therefore influences HBV species tropism, i.e. which species the virus is able to infect and replicate within. The capsid disassembly process is poorly understood. However, the work of Professor Hu’s group has shown that this step may be regulated by the capsid itself, as well as the host.
Previous studies have been hampered by the lack of convenient laboratory or animal model systems. In particular, mouse hepatocytes are incapable of supporting CCC DNA formation, which has prevented the development of this convenient small animal model as a fully-permissive host for HBV infection study. Intriguingly, Professor Hu’s lab was able to show recently that an immortalised mouse liver cell line could support HBV CCC DNA formation, hence suggesting that mouse hepatocytes may be manipulated to produce HBV CCC DNA and thus, it may be possible to develop a mouse model that supports HBV CCC DNA formation.
As HBV can be recognised by the host immune system in some situations through exposed viral DNA, it may provide a situation which can be exploited therapeutically.
Professor Hu and his group have shown using mutants of the HBc protein in human cells, as well as the mouse liver cell line, that efficient CCC DNA conversion is associated with enhanced nucleocapsid uncoating, i.e., release of the relaxed circular DNA. However, exposure of this viral DNA can allow the host to sense the foreign DNA and mount an innate immune response against the virus that is able to modulate HBV gene expression and replication. Indeed, it is already well established that foreign or mis-localised DNA acts as a pathogen-associated molecular pattern which is recognised by pattern-recognition receptors in the host. Therefore, as HBV can be recognised by the host immune system in some situations through exposed viral DNA, it may provide a situation which can be exploited therapeutically to clear persistent viral infections, possibly through induction of viral suicide. DNA sensing may also contribute to the understanding of inflammation caused by HBV-associated diseases. In contrast, it is important to consider the effects that exacerbating the inflammatory response and overriding the regulatory and suppressor aspects of the immune system, albeit temporarily, would have on the host.
Representation of a liver infected with hepatitis B virus.
Other aspects of the HBV replication cycle
The group at Pennsylvania State University also investigate other aspects of the HBV replication cycle, such as the functions and therapeutic targeting of a viral enzyme called reverse transcriptase. Reverse transcriptase is a multifunctional protein which is critical to HBV replication and is the primary target of currently approved therapies for HBV infection. Professor Hu’s work suggests that this essential viral enzyme can be targeted in novels ways for new therapeutic interventions.
Therapeutic interventions
The projects in Professor Hu’s lab not only uncover fundamental details about mechanisms of viral replication, but also offer possible opportunities to develop novel antiviral therapies which target the capsid assembly and disassembly. As technologies continue to improve, it is becoming possible to consider more detailed characterisation of the HBV replication cycle, for example by comparing mature and immature nucleocapsids using high-resolution imaging methods such as cryo-electron microscopy.
How can our understanding of HBV capsid assembly and disassembly be translated from fundamental mouse studies to potential future human antiviral strategies?
Viral capsid assembly and disassembly are complex processes that are essential for viral replication. Our work on HBV capsid assembly indicates that not only the assembly domain but also the C-terminal domain can be targeted to block capsid assembly. Furthermore, our work on nucleocapsid disassembly (uncoating) indicates that this process can be blocked to prevent the release of the viral genome, and thus prevent the formation of the critical CCC DNA species, or enhanced to allow exposure of the viral DNA to trigger host immune response to clear HBV. Manipulation of uncoating may also allow the development of a mouse model of HBV infection to facilitate basic research and drug development.
https://researchfeatures.com/2018/05/30/hepatitis-b-the-creation-and-destruction-of-a-virus/
.png)
.png)
.png)
.png)