¡¡
Bilirubin inhibits the up-regulation of inducible nitric oxide synthase by scavenging reactive oxygen species generated by the toll-like receptor 4-dependent activation of NADPH oxidase
¡¡
Highlights
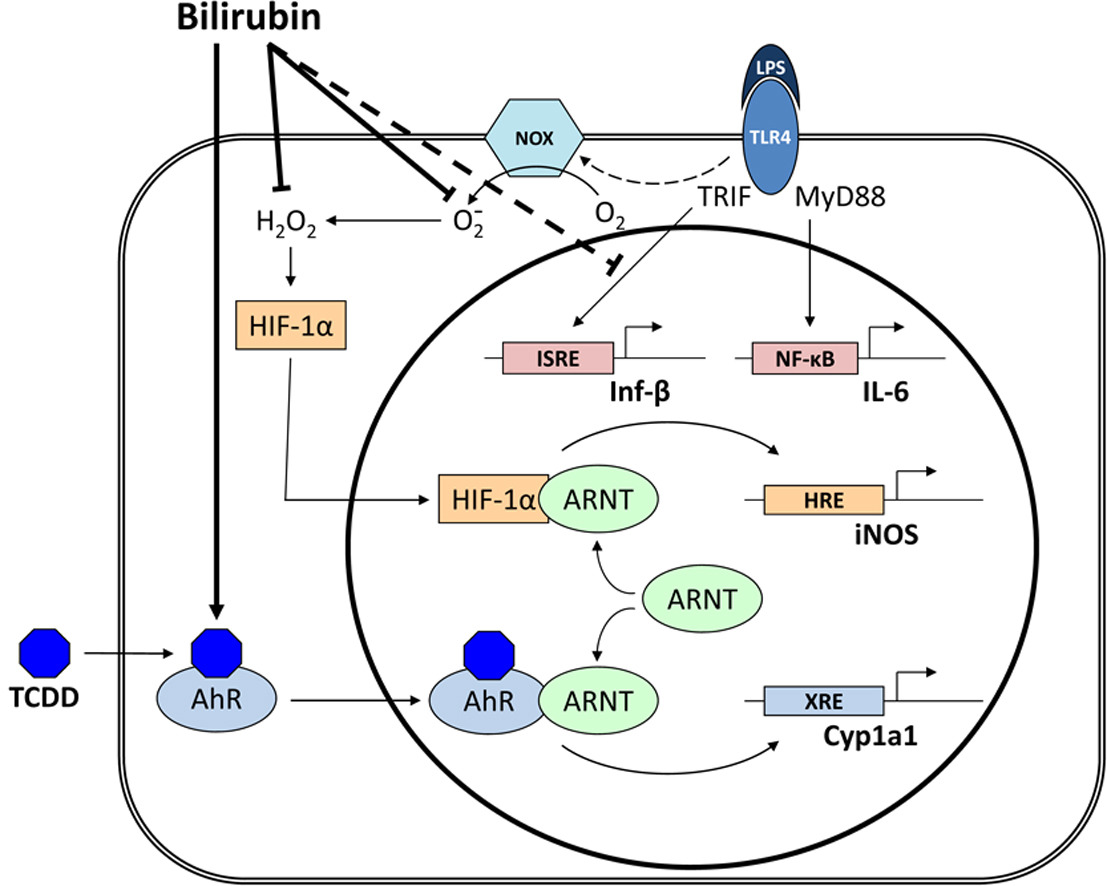
¡ñ Bilirubin blocks TLR4 signaling by scavenging NADPH oxidase-derived reactive oxygen species.
¡ñ Bilirubin specifically inhibits the TRIF-dependent TLR4 signaling pathway.
¡ñ LPS activation of inducible nitric oxide synthase is mediated by HIF-1¦Á.
¡ñ Macrophages exhibit reciprocal regulation of HIF-1¦Á and aryl hydrocarbon receptor pathways.
¡ñ Potential mechanisms underlying the anti-inflammatory effects of bilirubin are delineated.
Abstract
It has been previously shown that bilirubin prevents the up-regulation of inducible nitric oxide synthase (iNOS) in response to LPS.
The present study examines whether this effect is exerted through modulation of Toll-Like Receptor-4 (TLR4) signaling. LPS-stimulated iNOS and NADPH oxidase (Nox) activity in RAW 264.7 murine macrophages was assessed by measuring cellular nitrate and superoxide (O2−) production, respectively. The generation of both nitrate and O2− in response to LPS was suppressed by TLR4 inhibitors, indicating that activation of iNOS and Nox is TLR4-dependent.
While treatment with superoxide dismutase (SOD) and bilirubin effectively abolished LPS-mediated O2− production, hydrogen peroxide and nitrate release were inhibited by bilirubin and PEG-catalase, but not SOD, supporting that iNOS activation is primarily dependent upon intracellular H2O2.
LPS treatment increased nuclear translocation of the redox-sensitive transcription factor Hypoxia Inducible Factor-1¦Á (HIF-1¦Á), an effect that was abolished by bilirubin. Cells transfected with murine iNOS reporter constructs in which the HIF-1¦Á-specific hypoxia response element was disrupted exhibited a blunted response to LPS, supporting that HIF-1¦Á mediates Nox-dependent iNOS expression.
Bilirubin, but not SOD, blocked the cellular production of interferon-¦Â, while interleukin-6 production remained unaffected. These data support that bilirubin inhibits the TLR4-mediated up-regulation of iNOS by preventing activation of HIF-1¦Á through scavenging of Nox-derived reactive oxygen species. Bilirubin also suppresses interferon-¦Â release via a ROS-independent mechanism. These findings characterize potential mechanisms for the anti-inflammatory effects of bilirubin.
¡¡
Redox Biology
Volume 5, August 2015, Pages 398-408
Research Paper
Author links open overlay panel GilaIdelman Darcey L.H. Smith Stephen D.Zucker
Division of Digestive Diseases, University of Cincinnati, Cincinnati, OH 45267-0595, USA
¡¡
https://doi.org/10.1016/j.redox.2015.06.008Get rights and content
Under a Creative Commons license
Bilirubin inhibits the up-regulation of inducible nitric oxide synthase by scavenging reactive oxygen species generated by the toll-like receptor 4-dependent activation of NADPH oxidase - ScienceDirect https://www.sciencedirect.com/science/article/pii/S2213231715000592
Bilirubin inhibits the up-regulation of inducible nitric oxide synthase by scavenging reactive oxygen species generated by the toll-like receptor 4-dependent activation of NADPH oxidase https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4506991/
¡¡
SERUM LIPIDS IN PATIENTS WITH RHEUMATOID ARTHRITIS AND IN PATIENTS WITH OBSTRUCTIVE JAUNDICE
¡¡
This Issue
Article
July 1941
¡¡
A COMPARATIVE STUDY
WALTER D. BLOCK, Ph.D.; OLIVER H. BUCHANAN, M.S.; RICHARD H. FREYBERG, M.D.
Arch Intern Med (Chic). 1941;68(1):18-24. doi:10.1001/archinte.1941.00200070028002
Full
Text
Abstract
In 1933 Hench1 reported the analgesic effect of jaundice in cases of chronic arthritis, fibrositis and sciatic pain and followed this report with several papers on further observations of the phenomenon. Sidel and Abrams,2 Borman,3 and others have confirmed these observations. Hench stated that bilirubin appears to be the most likely agent responsible for the beneficial effect of jaundice on arthritis. This appears true in view of Race's findings4 that the icteric index and the concentration of serum bilirubin are likely to be low among patients who have rheumatoid arthritis. However, Hench suggested the possibility that bile salts, hepatic autolysate, special diet or other factors may be responsible for the phenomenon. Assuming that the responsible agent ("factor X") is a specific chemical substance or a combination of substances and not a nonspecific set of circumstances, Hench further speculated that this phenomenon results from (a) the correction
SERUM LIPIDS IN PATIENTS WITH RHEUMATOID ARTHRITIS AND IN PATIENTS WITH OBSTRUCTIVE JAUNDICE: A COMPARATIVE STUDY | JAMA Internal Medicine | JAMA Network https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/547358
¡¡
Liver abnormalities in rheumatic diseases - Clinics in Liver Disease https://www.liver.theclinics.com/article/S1089-3261(02)00052-1/fulltext
¡¡