基质细胞氧化 - 肿瘤细胞获得维生素C并被维生素C杀死的机制
Stromal Cell Oxidation-A Mechanism by Which Tumors Obtain Vitamin C and killed by Vitamin C
Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, New York 10021
图示4:提出的维生素C在肿瘤细胞(TUMOR CELL) 中的转运和积累机制。抗坏血酸(Ascorbic Acid) 是血液中维生素C的主要形式,在肿瘤微环境中它通过超氧阴离子(Superoxide Anion) 被氧化成脱氢抗坏血酸(DHA)。因此,氧化(Oxidation)是肿瘤转运维生素C的调节步骤。然后,脱氢抗坏血酸通过肿瘤细胞表面的GLUT1转运。然后肿瘤细胞内的脱氢抗坏血酸(DHA)被还原为抗坏血酸(Ascorbic Acid),由于它不能通过GLUT1转运,因此抗坏血酸被捕获在肿瘤细胞中。
摘要
人类肿瘤可能含有高浓度的抗坏血酸,但人们对它们如何获得维生素知之甚少。某些特化细胞可以直接通过抗坏血酸钠协同转运蛋白(SVCT)运输抗坏血酸,但在大多数细胞中,维生素C以脱氢抗坏血酸的形式通过促进葡萄糖转运蛋白(GLUT)进入,然后在细胞内还原并保留为抗坏血酸。
研究了已建立的造血细胞和上皮细胞异种移植物的小鼠注射抗坏血酸和脱氢抗坏血酸的积累。大多数造血细胞和上皮肿瘤细胞系只能在体外运输氧化形式的维生素C(脱氢抗坏血酸,DHA);然而,当在小鼠中作为异种移植物生长时,它们在施用放射性标记的抗坏血酸后迅速积累维生素C.通过用D-葡萄糖(D-glucose)而不是L-葡萄糖的竞争性抑制证明了GLUT参与异种移植肿瘤对维生素C的摄取。
由于恶性细胞不能直接转运抗坏血酸,我们推断抗坏血酸在肿瘤微环境中被氧化成脱氢抗坏血酸(DHA)。注射抗坏血酸的动物中维生素C的肿瘤积累通过共同施用超氧化物歧化酶(superoxide dismutase)而被抑制,这意味着超氧阴离子在抗坏血酸的氧化中起作用。
尽管上皮癌细胞系在培养物中不能产生超氧阴离子,但是切碎的异种移植肿瘤确实存在。我们的研究表明,GLUTs对脱氢抗坏血酸的转运是肿瘤获得维生素C的一种手段,表明由肿瘤基质中的细胞产生的超氧阴离子氧化抗坏血酸作为产生可运输形式的维生素的机制。
介绍
膳食中的维生素C对人类和其他灵长类动物至关重要,因为我们不能像大多数其他动物一样在肝脏合成这种维生素(1)。虽然人们对维生素C和维生素C缺乏症的了解很多,但关于癌症中维生素的生理学知识却很少(2)。鉴于维生素C在维持正常免疫过程和宿主防御方面的充分证据,人们普遍认为补充维生素C可“增强”免疫系统(3,4)。服用维生素C的癌症患者通常认为它可以增强对抗癌症的免疫防御。这些概念很少关注癌症本身的营养需求。癌细胞在体外容易摄取维生素C(5,6),并且研究表明肿瘤中的维生素C浓度高于邻近的正常组织(7)。然而,肿瘤在体内累积维生素C的机制尚不清楚。
我们以前发现脱氢抗坏血酸,氧化形式的维生素C,在体外通过促进性葡萄糖转运蛋白(GLUTs) 3转运(8)。非洲爪蟾卵母细胞中GLUT1,GLUT2和GLUT4的表达赋予了摄取脱氢抗坏血酸的能力,脱氢抗坏血酸在还原为抗坏血酸后在细胞内保留。我们还确定GLUT参与正常人中性粒细胞和髓样白血病细胞系HL60的运输和积累维生素C(8,9,10)。在这些细胞中,脱氢抗坏血酸通过细胞膜转运并以还原形式抗坏血酸积累,抗坏血酸不能通过GLUT双向转运(8,9,10)。 GLUT1在血脑屏障中体内转运脱氢抗坏血酸也是大脑获得维生素C的机制(11)。抗坏血酸,即血液中维生素C的形式,不易穿过血脑屏障,而脱氢抗坏血酸穿过血脑屏障并作为抗坏血酸积聚在脑中。
抗坏血酸也可以直接通过一系列Na +依赖性抗坏血酸转运蛋白(SVCT)转运,这些转运蛋白最近已被分子鉴定(12)。我们未发现WBC中或本研究中包括的前列腺,乳腺和造血肿瘤细胞系中Na +依赖性抗坏血酸摄取的证据(9,10)。
该研究表明前列腺,乳腺和造血人类异种移植肿瘤通过GLUT获得氧化形式的维生素C.肿瘤微环境产生超氧阴离子,将抗坏血酸氧化成脱氢抗坏血酸,这是维生素C的可运输形式。运输的脱氢抗坏血酸通过还原成不可转移形式的维生素C,即抗坏血酸而被捕获在肿瘤中。
材料和方法
动物研究
从美国国家癌症研究所 - 弗雷德里克癌症中心获得4至6周龄的裸体无胸腺BALB / c雄性小鼠并皮下注射。以下列浓度肿瘤细胞进入侧腹:HL-60细胞,2.0×107细胞; LNCaP细胞,3.0×106细胞; MDA468细胞,1.0×107细胞; 接种后3~4周,可测定~1.0×1.0×1.0cm的肿瘤和CEM细胞,2.0×107。以重建的基底膜(Matrigel; Collaborative Research,Bedford,MA)以1:1的体积比注射LNCaP细胞。遵循了在研究中正确和人道地使用动物的制度准则。
组织转运和积累研究
携带异种移植肿瘤的小鼠用5μCi的1- [1-14C]抗坏血酸(比活性,6.6mCi / mmol; DuPont-NEN,Boston,MA),[14C]脱氢抗坏血酸或[果糖-1-3H]注入尾静脉。蔗糖(比活度,20.0Ci / mmol; DuPont-NEN)。 [14C]在所有实验中通过将[14 C]抗坏血酸与抗坏血酸氧化酶(每1.0mmol L-抗坏血酸盐1单位,来源于南瓜属物种; Sigma Chemical Co.,St.Louis,MO)一起产生脱氢抗坏血酸。将DTT(0.1mmol / l; Sigma)加入到维生素C制剂中。然后切除器官和肿瘤并在70%甲醇中匀浆。如前所述(11)处理样品用于闪烁光谱测定或HPLC。在甲醇部分上进行HPLC,加入1mmol /升EDTA。在Whatman强阴离子交换Partisil 10 SAX(4.6×25cm)柱(Whatman,Inc.,Clifton,NJ)上分离HPLC样品。使用Whatman型WCS溶剂调节柱,并用System Gold液相色谱仪(Beckman Instruments,Inc.,Fullerton,CA)监测洗脱液,其中二极管阵列检测器和放射性同位素检测器串联布置。通过265nm处的吸光度和放射性监测抗坏血酸。脱氢抗坏血酸在265nm处没有显示吸光度,因此仅通过放射性监测。用2-脱氧-D-葡萄糖(Sigma),L-葡萄糖(Sigma),SOD(超氧化物氧化还原酶,来自人红细胞; Sigma)和过氧化氢酶(H2O2氧化还原酶,来自小鼠肝脏; Sigma)进行共注射实验。
超氧阴离子生成的测量。
测量超氧阴离子产生为的SOD可抑制的细胞色素C还原。 将细胞系和新鲜分离的切碎的肿瘤在37℃下用HBSS预孵育15分钟,用含有细胞色素C的HBSS洗涤一次,并与含有或不含SOD(200单位/ ml)的1mg / ml细胞色素C在HBSS中孵育。 摇床。 将PMA(Sigma)以80nm的终浓度添加至细胞系。 在60分钟时,从细胞中取出培养基并置于冰上以停止反应,立即读取550nm处的吸光度。 细胞色素C的超氧化物特异性还原表示为使用21.1nm-1·cm-1的消光系数,在有或没有SOD的情况下孵育的细胞之间的吸光度差异。切碎肿瘤的细胞计数来自体积测量。
结果
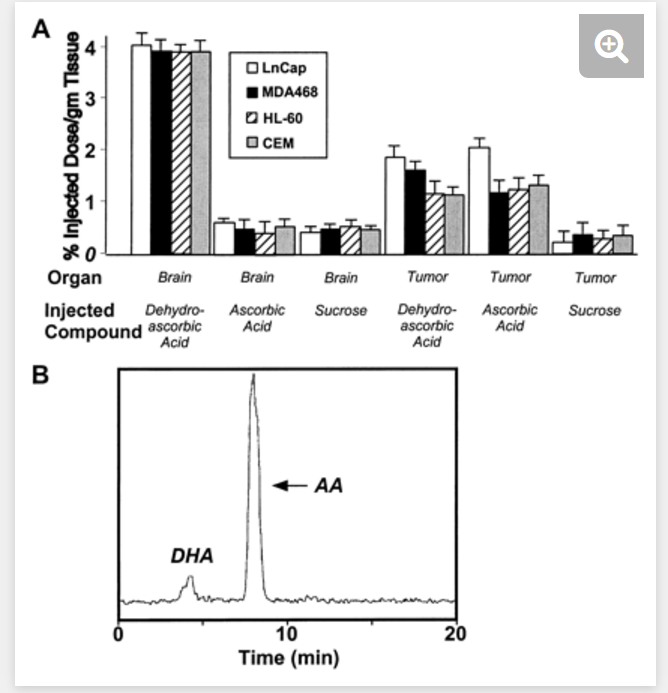
将具有异种移植肿瘤的小鼠用[14 C]抗坏血酸,[14 C]脱氢抗坏血酸或[3H]蔗糖注射到尾静脉中,并在注射后1分钟处死。在1分钟后,在异种移植组的脑中发现大约4%的注射脱氢抗坏血酸放射性(表示为每克组织的ID的百分比)(图1A),结果与我们先前的研究一致(11)。注射的抗坏血酸和蔗糖在1分钟时仅在脑匀浆中产生微量放射性,证实抗坏血酸不容易通过血脑屏障。蔗糖不被代谢或运输,因此它被用作血浆体积的标记物(13)。异种移植肿瘤在1分钟时累积注射脱氢抗坏血酸,LNCaP(前列腺)肿瘤中的肿瘤组织为〜2.0%ID / g,MDA468(乳腺)肿瘤为1.6%ID / g,HL-60为1.2%ID / g(髓细胞白血病) )CEM中的肿瘤和1.2%ID / g(T-淋巴细胞白血病;图1A⇓)。蔗糖未被转运到肿瘤中。与大脑不同,当将抗坏血酸注入动物时,异种移植肿瘤容易吸收放射性。 LNCaP肿瘤累积〜2.3%ID / g,MDA-468 1.2%ID / g,HL-60 1.3%ID / g,和CEM 1.3%ID / g肿瘤组织(图1A)⇓。进行来自携带异种移植物的小鼠的肿瘤匀浆的甲醇部分的HPLC分析,以鉴定累积的维生素C的形式。结果表明,在注射脱氢抗坏血酸的动物和注射抗坏血酸的动物中,肿瘤中累积的维生素C比注射脱氢抗坏血酸的动物和注射抗坏血酸的动物> 86%抗坏血酸(图1B)⇓。
维生素C被大多数细胞(包括癌细胞)以氧化形式作为脱氢抗坏血酸通过促进性葡萄糖转运蛋白(GLUTs;参考文献8,9,10)摄取。因此,抗坏血酸必须在细胞周围环境中被氧化为脱氢抗坏血酸,以通过GLUT转运到细胞中,随后它被还原并以抗坏血酸的形式被捕获。因为大多数肿瘤细胞不能运输抗坏血酸,我们假设注射的抗坏血酸在肿瘤微环境中被氧化成脱氢抗坏血酸,然后通过GLUT运输。为了确定抗坏血酸是否以这种方式进入肿瘤细胞,我们用D-和L-葡萄糖进行抑制实验,因为GLUT选择性地转运D-葡萄糖而不是L-葡萄糖。已显示D-脱氧葡萄糖和D-葡萄糖以剂量依赖的方式通过GLUT抑制脑中脱氢抗坏血酸的摄取高达70%(11)。在荷瘤小鼠中,D-脱氧葡萄糖抑制脱氢抗坏血酸和抗坏血酸的转运和积累(图2,A-D)⇓。 5秒时间点用于研究转运,并且维生素C摄取的剂量依赖性抑制大约为35-50%(均注射脱氢抗坏血酸和抗坏血酸)。 1分钟和5分钟的时间点代表运输和积累,并证明了维生素C摄取的最大剂量依赖性40-65%最大抑制(均注射脱氢抗坏血酸和抗坏血酸)。给予L-葡萄糖对异种移植物中维生素C的转运或积累没有影响(数据未显示)。在注射抗坏血酸的小鼠中,脱氧葡萄糖对肿瘤维生素C摄取和积累的抑制表明抗坏血酸转化为脱氢抗坏血酸,从而通过GLUT转运到肿瘤细胞中。
我们假设抗坏血酸在肿瘤微环境中被超氧阴离子氧化。 为了测试这个概念,我们共同注射携带抗坏血酸和SOD,过氧化氢酶或盐水的异种移植物的动物。 接受SOD和放射性标记的抗坏血酸的动物肿瘤维生素C积累减少约50%,而与脱氢抗坏血酸和SOD共同注射的动物中维生素C的肿瘤积累没有变化(图3A)⇓。 对抗坏血酸和过氧化氢酶的共同给药没有影响,表明过氧化物可能在将抗坏血酸氧化成脱氢抗坏血酸方面没有作用(图3A)。
我们测试了肿瘤细胞自身产生超氧阴离子的能力。 超氧阴离子的产生仅用HL-60细胞系证明(图3B),并且其他细胞系不具有产生超氧阴离子的能力。 正如所料,当用PMA活化时,HL-60细胞系增加超氧化物的产生,而其他细胞系显示没有PMA诱导的超氧阴离子产生。与亲本HL-60细胞系相比,即使是切碎的HL-60异种移植肿瘤的超氧阴离子产生也增加了3倍。 因为与细胞系不同的切碎的异种移植肿瘤具有产生超氧阴离子的显着能力,我们得出结论,肿瘤基质中的非肿瘤细胞是产生超氧化物的原因。
讨论
我们试图通过在裸体无胸腺小鼠中使用人肿瘤异种移植模型并测量[14C]抗坏血酸的摄取来确定人肿瘤细胞如何在体内摄取维生素C. 注射抗坏血酸后,肿瘤迅速占据了标签,尽管我们之前已经在体外证明细胞不能直接转运抗坏血酸(8,10)。我们推测肿瘤细胞以脱氢抗坏血酸的形式摄取维生素C. 观察到的注射脱氧葡萄糖摄取的抑制证实了这样的观点,即细胞以脱氢抗坏血酸的形式获得通过促进性GLUT运输的维生素C .因为GLUT仅运输氧化形式的维生素C和脱氢抗坏血酸,并且因为细胞没有其他摄取维生素C的机制,所以很明显抗坏血酸在肿瘤微环境中被氧化成脱氢抗坏血酸。大脑同时缺乏14C标记物的摄取表明抗坏血酸在循环中未被氧化。通过SOD抑制注射抗坏血酸的动物中维生素C的摄取指出了超氧阴离子在抗坏血酸氧化中的作用。上皮肿瘤缺乏超氧阴离子的产生和异种移植肿瘤中超氧阴离子的产生,表明基质细胞是超氧阴离子的来源。因此,我们提出抗坏血酸在肿瘤微环境中通过非恶性基质细胞产生超氧阴离子而被氧化。已经通过具有NADPH氧化酶的成纤维细胞,嗜中性粒细胞,单核细胞和内皮细胞证实了组成型超氧阴离子的产生(14,15,16,17)。已显示嗜中性粒细胞和HL-60细胞以超氧化物依赖性方式氧化细胞外抗坏血酸(16,18,19)。某些肿瘤(例如,髓样白血病,本研究中的HL-60异种移植物)可直接氧化抗坏血酸,而其他肿瘤(上皮肿瘤)可依赖非肿瘤基质细胞来实现该功能。图4总结了肿瘤转运和积累维生素C的模型。
抗坏血酸钠协同转运蛋白(SVCT)存在于许多器官中(12),尽管我们在本研究中未发现中性粒细胞,单核细胞,HL-60细胞,T淋巴细胞细胞系或前列腺癌和乳腺癌细胞系中的钠依赖性抗坏血酸摄取 (SVCT)(9,10)。因此,通过GLUT以脱氢抗坏血酸的形式摄取维生素C似乎是维生素C摄取的一般机制。正如我们之前所示,抗坏血酸钠协同转运蛋白可能在某些肿瘤维生素C摄取中起作用(6)。
维生素C在肿瘤细胞中的确切功能尚不清楚。研究表明,与正常组织相比,某些人类肿瘤中维生素C的浓度增加(7),维生素C对体外某些肿瘤细胞的生长很重要(20)。维生素C摄入量增加会提高血清抗坏血酸水平(21),因此可能会增加维生素C的肿瘤浓度。细胞内维生素C浓度的增加可能对肿瘤生长和肿瘤的反应能力产生影响。氧化应激与化疗和放射治疗有关。尽管评估维生素C补充剂在癌症患者中的作用的研究通常表明在生存或肿瘤消退方面没有任何益处(22),但尚不清楚人类肿瘤中高浓度的维生素C是否能提供具有代谢优势的恶性细胞。
https://s.click.taobao.com/MuACULw
https://s.click.taobao.com/U7CCULw
Stromal Cell Oxidation-A Mechanism by Which Tumors Obtain Vitamin C and killed by Vitamin C
A proposed mechanism of vitamin C transport and accumulation in tumors. Ascorbic acid, the predominant form of vitamin C in blood, is oxidized to dehydroascorbic acid in the tumor microenvironment by superoxide anion. Oxidation is, thus, a regulatory step for tumor transport of vitamin C. The dehydroascorbic acid is then transported through GLUT1 at the surface of the tumor cell. The dehydroascorbic acid in the tumor is then reduced to ascorbic acid, which is trapped in the tumor because it cannot be transported through GLUT1.
David B. Agus, Juan C. Vera and David W. Golde
Abstract
Human tumors may contain high concentrations of ascorbic acid, but little is known about how they acquire the vitamin. Certain specialized cells can transport ascorbic acid directly through a sodium ascorbate cotransporter(SVCT), but in most cells, vitamin C enters through the facilitative glucose transporters (GLUTs) in the form of dehydroascorbic acid, which is then reduced intracellularly and retained as ascorbic acid.
Mice with established hematopoietic and epithelial cell xenografts were studied for the accumulation of injected ascorbic acid and dehydroascorbic acid. Most hematopoietic and epithelial tumor cell lines can only transport vitamin C in the oxidized form (dehydroascorbic acid) in vitro; however, when grown as xenografts in mice, they rapidly accumulated vitamin C after administration of radiolabeled ascorbic acid. The involvement of the GLUTs in vitamin C uptake by the xenografted tumors was demonstrated by competitive inhibition with d-glucose but not l-glucose.
Because the malignant cells were not capable of directly transporting ascorbic acid, we reasoned that the ascorbic acid was oxidized to dehydroascorbic acid in the tumor microenvironment. Tumor accumulation of vitamin C in animals injected with ascorbic acid was inhibited by coadministration of superoxide dismutase, implying a role for superoxide anion in the oxidation of ascorbic acid.
Whereas the epithelial cancer cell lines could not generate superoxide anion in culture, the minced xenograft tumors did. Our studies show the transport of dehydroascorbic acid by GLUTs is a means by which tumors acquire vitamin C and indicate the oxidation of ascorbic acid by superoxide anion produced by cells in the tumor stroma as a mechanism for generating the transportable form of the vitamin.
Introduction
Dietary vitamin C is critical for humans and other primates because we cannot synthesize the vitamin in the liver as do most other animals (1) . Whereas much is known about vitamin C and vitamin C deficiency states, there is little information regarding the physiology of the vitamin in cancer (2) . Given the well-documented role of vitamin C in the maintenance of normal immune processes and host defense, it is popularly believed that supplemental vitamin C “strengthens” the immune system (3 , 4) . Patients with cancer who take vitamin C generally believe that it can enhance immune defense against the cancer. These notions give little attention to the nutritional needs of the cancer itself. Cancer cells readily take up vitamin C in vitro (5 , 6) , and studies have demonstrated high vitamin C concentrations in neoplasms compared with the adjacent normal tissue (7) . The mechanism by which cancers accumulate vitamin C in vivo, however, is unknown.
We previously found that dehydroascorbic acid, the oxidized form of vitamin C, is transported in vitro through the facilitative GLUTs 3 (8) . Expression of GLUT1, GLUT2, and GLUT4 in Xenopus oocytes conferred the ability to take up dehydroascorbic acid which was retained intracellularly after reduction to ascorbic acid. We also established that GLUTs are involved in the transport and accumulation of vitamin C by normal human neutrophils and the myeloid leukemia cell line, HL60 (8, 9, 10) . In these cells, dehydroascorbic acid is transported across the cell membrane and accumulated in the reduced form, ascorbic acid, which is not transportable through the bidirectional GLUTs (8, 9, 10) . The in vivo transport of dehydroascorbic acid by GLUT1 at the blood-brain barrier is also a mechanism by which the brain acquires vitamin C (11) . Ascorbic acid, the form of vitamin C in the bloodstream, does not readily cross the blood-brain barrier, whereas dehydroascorbic acid crosses the blood-brain barrier and accumulates in the brain as ascorbic acid.
Ascorbic acid may also be transported directly through a family of Na+-dependent ascorbic acid transporters that have recently been molecularly characterized (12) . We have found no evidence of Na+-dependent ascorbic acid uptake in WBCs or in the prostate, breast, and hematopoietic tumor cell lines included in this study (9 , 10) .
This study demonstrates that prostate, breast, and hematopoietic human xenograft tumors obtain vitamin C in the oxidized form through the GLUTs. The tumor microenvironment generates superoxide anions that oxidize ascorbic acid to dehydroascorbic acid, the transportable form of the vitamin. The transported dehydroascorbic acid is trapped in the tumors by reduction to the nontransportable form of vitamin C, ascorbic acid.
Materials and Methods
Animal Studies.
Four- to 6-week old nude athymic BALB/c male mice were obtained from the National Cancer Institute-Frederick Cancer Center and injected s.c. into the flank with tumor cells at the following concentrations: HL-60 cells, 2.0 × 107 cells; LNCaP cells, 3.0 × 106 cells; MDA468 cells, 1.0 × 107 cells; and CEM cells, 2.0 × 107. Three to 4 weeks after inoculation, ∼1.0 × 1.0 × 1.0 cm tumors could be measured. The LNCaP cells were injected in a 1:1 volume ratio with reconstituted basement membrane (Matrigel; Collaborative Research, Bedford, MA). Institutional guidelines for the proper and humane use of animals in research were followed.
Tissue Transport and Accumulation Studies.
Mice bearing xenograft tumors were injected into the tail vein with 5 μCi of l-[1-14C]ascorbic acid (specific activity, 6.6 mCi/mmol; DuPont-NEN, Boston, MA), [14C]dehydroascorbic acid, or [fructose-1-3H]sucrose (specific activity, 20.0 Ci/mmol; DuPont-NEN). [14C]Dehydroascorbic acid was generated in all experiments by incubating [14C]ascorbic acid with ascorbate oxidase (1 unit per 1.0 mmol of l-ascorbate, derived from Cucurbita species; Sigma Chemical Co., St. Louis, MO). DTT (0.1 mmol/liter; Sigma) was added to the vitamin C preparations. The organs and tumors were then dissected out and homogenized in 70% methanol. Samples were processed for scintillation spectrometry or HPLC as described previously (11) . HPLC was performed on the methanol fraction with the addition of 1 mmol/liter EDTA. HPLC samples were separated on a Whatman strong anion exchange Partisil 10 SAX (4.6 × 25 cm) column (Whatman, Inc., Clifton, NJ). A Whatman-type WCS solvent-conditioning column was used, and the eluates were monitored with a System Gold liquid chromatograph (Beckman Instruments, Inc., Fullerton, CA) with a diode array detector and radioisotope detector arranged in series. Ascorbic acid was monitored by absorbance at 265 nm and by radioactivity. Dehydroascorbic acid shows no absorbance at 265 nm, and so it was monitored only by radioactivity. Coinjection experiments were performed with 2-deoxy-d-glucose (Sigma), l-glucose (Sigma), SOD (superoxide oxidoreductase, from human erythrocytes; Sigma), and catalase (H2O2 oxidoreductase, from mouse liver; Sigma).
Measurement of Superoxide Anion Production.
Superoxide anion production was measured as the SOD-inhibitable reduction of cytochrome c. Cell lines and freshly isolated minced tumors were preincubated with HBSS for 15 min at 37°C, washed once with HBSS containing cytochrome c, and incubated with 1 mg/ml cytochrome c with or without SOD (200 units/ml) in HBSS on a shaking table. PMA (Sigma) was added to the cell lines at a final concentration of 80 nm. At 60 min, the medium was removed from the cells and placed on ice to stop the reaction, and the absorbance at 550 nm was immediately read. Superoxide-specific reduction of cytochrome c was expressed as the difference in absorbance between cells incubated with or without SOD by use of an extinction coefficient of 21.1 nm−1·cm−1. Cell counts of minced tumor were derived from volumetric measurements.
Results
Mice with xenograft tumors were injected into the tail vein with [14C]ascorbic acid, [14C]dehydroascorbic acid, or [3H]sucrose and sacrificed 1 min after injection. Approximately 4% of the injected dehydroascorbic acid radioactivity (expressed as percentage of ID per gram of tissue) was found in the brain of the xenograft groups after 1 min (Fig. 1A) ⇓ , a result consistent with our previous work (11) . Injected ascorbic acid and sucrose yielded only trace radioactivity in the brain homogenate at 1 min, confirming that ascorbic acid did not readily pass the blood-brain barrier. Sucrose is not metabolized or transported, and therefore it is used as a marker of plasma volume (13) . The xenograft tumors accumulated injected dehydroascorbic acid at 1 min with ∼2.0%ID/g tumor tissue in LNCaP (prostate) tumors, 1.6%ID/g in MDA468 (breast) tumors, 1.2%ID/g in HL-60 (myeloid leukemia) tumors, and 1.2%ID/g in CEM (T-lymphocyte leukemia; Fig. 1A ⇓ ). Sucrose was not transported into the tumors. Distinct from the brain, the xenograft tumors readily took up radioactivity when ascorbic acid was injected into the animals. LNCaP tumors accumulated ∼2.3%ID/g, MDA-468 1.2%ID/g, HL-60 1.3%ID/g, and CEM 1.3%ID/g tumor tissue (Fig. 1A) ⇓ . HPLC analysis of the methanol fraction of the tumor homogenate from the xenograft-bearing mice was performed to identify the form of vitamin C accumulated. The results show that the vitamin C accumulated in the tumors was >86% ascorbic acid in animals injected with dehydroascorbic acid as well as those injected with ascorbic acid (Fig. 1B) ⇓ .

Fig. 1.
Vitamin C is transported into tumors independent of the redox state of the vitamin injected. A, mice were injected into the tail vein with [14C]ascorbic acid, [14C]dehydroascorbic acid, or [3H]sucrose and sacrificed 2 min after injection. Columns, means of 12 animals per group; bars, SE. B, HPLC analysis of the methanol-soluble fraction of the xenograft tumor (MDA468) of a mouse injected with 30 μCi of [14C]dehydroascorbic acid and sacrificed at 1 min. Accumulation of vitamin C in the tumor is in the form of ascorbic acid (AA; ∼91%; retention time, ∼7.93 min) in the dehydroascorbic acid (DHA)-injected animal
Vitamin C is taken up in the oxidized form as dehydroascorbic acid by most cells, including cancer cells, via the facilitative hexose transporters (GLUTs; Refs. 8, 9, 10 ). Thus, ascorbic acid must be oxidized in the pericellular milieu to dehydroascorbic acid to be transported through the GLUTs into the cell, where it is subsequently reduced and trapped in the form of ascorbic acid. Because most tumor cells are unable to transport ascorbic acid, we hypothesized that the injected ascorbic acid was oxidized to dehydroascorbic acid in the tumor microenvironment and then transported via the GLUTs. To ascertain whether ascorbic acid entered the tumor cells in this manner, we conducted inhibition experiments with d- and l-glucose because GLUTs selectively transport d-glucose but not l-glucose. d-Deoxyglucose and d-glucose have been shown to inhibit uptake of dehydroascorbic acid in the brain through GLUTs up to 70% in a dose-dependent fashion (11) . In the tumor-bearing mice, d-deoxyglucose inhibited the transport and accumulation of both dehydroascorbic acid and ascorbic acid (Fig. 2, A–D) ⇓ . The 5-s time point was used to study transport, and there was an approximate maximal 35–50% dose-dependent inhibition of vitamin C uptake (both injected dehydroascorbic acid and ascorbic acid). The 1- and 5-min time points represented both transport and accumulation and demonstrated an approximate dose-dependent 40–65% maximal inhibition of vitamin C uptake (both injected dehydroascorbic acid and ascorbic acid). Administration of l-glucose had no effect on vitamin C transport or accumulation in the xenografts (data not shown). The inhibition by deoxyglucose of tumor vitamin C uptake and accumulation, in mice injected with ascorbic acid, indicated that the ascorbic acid was converted to dehydroascorbic acid and thereby transported into the tumor cells by the GLUTs.
Fig. 2.
Specificity of the transport of dehydroascorbic acid through GLUT1 in the tumor xenografts. [14C]Dehydroascorbic acid and [14C]ascorbic acid entered the xenograft tumors (A, LNCaP; B, HL-60; C, CEM; and D, MDA468), and their accumulation was blocked by increasing amounts of d-deoxyglucose, which is transported through GLUT1 (▪, 0 mg of d-deoxyglucose; , 5.3 mg of d-deoxyglucose; Embedded Image, 13.4 mg of d-deoxyglucose). A mouse has a baseline blood glucose concentration of ∼5–7 mm or 2.67 mg of glucose in the entire animal, based on an average blood volume of the mouse. The amount of exogenous glucose administered in this experiment was based on whole mouse blood glucose and subsequent multiples to a maximum tolerable amount. The measured concentrations of both blood glucose and dehydroascorbic acid in the mice change rapidly and widely during the time after injection and it was difficult to obtain meaningful quantitative data regarding blood levels. Serum concentrations of ascorbic acid and dehydroascorbic acid were not affected by increasing concentrations of d-deoxyglucose or l-glucose. All experiments were carried out over the indicated time course. Columns, means of four mice; bars, SE.
We hypothesized that ascorbic acid was oxidized in the tumor microenvironment by superoxide anion. To test this concept, we coinjected animals bearing xenografts with ascorbic acid and SOD, catalase, or saline. The animals receiving SOD and radiolabeled ascorbic acid had an ∼50% reduction in tumor vitamin C accumulation, whereas there was no change in the tumor accumulation of vitamin C in animals coinjected with dehydroascorbic acid and SOD (Fig. 3A) ⇓ . There was no effect of coadministration of ascorbic acid and catalase, indicating that peroxide likely did not play a role in oxidizing ascorbic acid to dehydroascorbic acid (Fig. 3A) ⇓ .
Fig. 3.
Superoxide anions are responsible for the oxidation of vitamin C in the tumor xenografts. A, [14C]dehydroascorbic acid and [14C]ascorbic acid both entered the tumor xenografts and the accumulation of ascorbic acid, by the xenografts, was blocked by coinjected SOD and not by catalase or saline. All experiments were carried out over 1 min. Columns, means of four mice; bars, SE. B, superoxide anions generated by the tumor microenvironment are responsible for the oxidation of vitamin C in the tumor xenografts. All experiments were carried out over 60 min. Columns, means of four replicates; bars, SE.
We tested the ability of the tumor cells themselves to generate superoxide anion. Production of superoxide anion was only demonstrable with the HL-60 cell line (Fig. 3B) ⇓ , and the other cell lines had no capacity to generate superoxide anion. As expected, the HL-60 cell line increased superoxide generation when activated with PMA, whereas the other cell lines showed no PMA-inducible superoxide anion generation. Even the minced HL-60 xenograft tumors had a 3-fold increase in superoxide anion generation compared with the parent HL-60 cell line. Because minced xenograft tumors, distinct from the cell lines, had a prominent ability to generate superoxide anion, we concluded that nonneoplastic cells in the tumor stroma were responsible for the superoxide generation.
Discussion
We sought to determine how human tumor cells take up vitamin C in vivo by using human tumor xenograft models in nude athymic mice and measuring the uptake of [14C]ascorbic acid. The tumors rapidly took up the label after injection of ascorbic acid, although we had previously shown in vitro that the cells were unable to directly transport ascorbic acid (8 , 10) . We postulated that the tumor cells were taking up vitamin C in the form of dehydroascorbic acid. The observed inhibition of uptake by injected deoxyglucose confirmed the notion that the cells obtained vitamin C in the form of dehydroascorbic acid transported through the facilitative GLUTs. Because the GLUTs only transport the oxidized form of vitamin C and dehydroascorbic acid and because the cells had no other mechanism for taking up vitamin C, it was apparent that the ascorbic acid was oxidized to dehydroascorbic acid in the tumor microenvironment. The simultaneous lack of uptake of 14C label by the brain indicated that the ascorbic acid was not oxidized in the circulation. The inhibition of vitamin C uptake in ascorbic acid-injected animals by SOD pointed to a role for superoxide anion in the oxidation of ascorbic acid. The lack of production of superoxide anion by the epithelial tumors and the presence of superoxide anion generation in xenograft tumors suggested stromal cells as the source of the superoxide anion. We propose, therefore, that ascorbic acid is oxidized in the tumor microenvironment through the production of superoxide anion by nonmalignant stromal cells. Constitutive superoxide anion production has been demonstrated by fibroblasts, neutrophils, monocytes, and endothelial cells that have the NADPH oxidase enzyme (14, 15, 16, 17) . Neutrophils and HL-60 cells have been shown to oxidize extracellular ascorbic acid in a superoxide-dependent fashion (16 , 18 , 19) . Certain tumors (e.g., myeloid leukemia, the HL-60 xenograft in this study) can oxidize ascorbic acid directly, whereas others (epithelial tumors) may rely on nonneoplastic stromal cells for this function. A model for the transport and accumulation of vitamin C by tumors is summarized in Fig. 4 ⇓ .
Fig. 4.
A proposed mechanism of vitamin C transport and accumulation in tumors. Ascorbic acid, the predominant form of vitamin C in blood, is oxidized to dehydroascorbic acid in the tumor microenvironment by superoxide anion. Oxidation is, thus, a regulatory step for tumor transport of vitamin C. The dehydroascorbic acid is then transported through GLUT1 at the surface of the tumor cell. The dehydroascorbic acid in the tumor is then reduced to ascorbic acid, which is trapped in the tumor because it cannot be transported through GLUT1.
A sodium ascorbate cotransporter is present in many organs (12) although we have found no sodium-dependent ascorbic acid uptake in the neutrophils, monocytes, HL-60 cells, T-lymphocyte cell lines, or prostate and breast cancer cell lines included in this study (9 , 10) . Thus, the uptake of vitamin C in the form of dehydroascorbic acid through the GLUTs appears to be a general mechanism for vitamin C uptake. As we have shown previously, the sodium ascorbate cotransporter may have a role in vitamin C uptake of certain tumors (6) .
The precise function of vitamin C in neoplastic cells is not known. Studies have demonstrated increased concentrations of vitamin C in certain human tumors compared with normal tissue (7) , and vitamin C is important for the growth of some neoplastic cells in vitro (20) . Increased vitamin C consumption will raise the serum ascorbic acid level (21) and, therefore, could be expected to increase the tumor concentration of vitamin C. The increased intracellular concentration of vitamin C may have effects on tumor growth and the tumor’s ability to respond to oxidative stress associated with chemotherapy and radiation therapy. Although studies evaluating the role of vitamin C supplementation in cancer patients have generally shown no benefit with respect to survival or tumor regression (22) , it is not known whether high concentrations of vitamin C in human tumors afford the malignant cells with a metabolic advantage.
http://cancerres.aacrjournals.org/content/59/18/4555
.png)
.png)
.png)
.png)
.png)
.png)