高脂肪饮食会导致产生胰岛素的Beta细胞自杀: 给那些有糖尿病和没有糖尿病的人上一课
How Fat Kills Insulin-Producing Beta Cells: A Lesson for Those With and Without Diabetes
By Cyrus Khambatta
关于作者
Cyrus Khambatta. 在加州大学伯克利分校获得营养生物化学博士学位,并获得斯坦福大学机械工程学士学位。他在22岁时被诊断出患有1型糖尿病,在此后的11年里,他一直在研究胰岛细胞衰竭和胰岛素抵抗。被誉为热爱和热衷于营养和锻炼,Khambatta和他的客户通过MangoMan营养疗法和健身指导,教授逆转糖尿病的根源——胰岛素抵抗而不是治疗高血糖症状的原则,实现对血糖波动的高度控制。
Cyrus Khambatta 的自我介绍
在22岁时被诊断为1型糖尿病,我花了10年的时间在博士水平上学习营养学的基础知识。我的目标是与糖尿病前期、1型和2型糖尿病患者分享我的实用营养知识。糖尿病是一个获得良好健康的机会。如果掌握了正确的系统,逆转胰岛素抵抗的影响可能是一个有趣和愉快的过程。这就是为什么我花了10年的时间开发了一种能最大限度降低血糖波动和胰岛素抵抗的可靠的系统。
大量的科学证据表明,高脂肪饮食是在你的肝脏和肌肉中诱导胰岛素抵抗的最有效的方法。
这项研究清楚地表明,增加膳食脂肪的摄入对胰岛素敏感性有直接的负面影响,如果长期进食大量的脂肪(1-26),就会发展成慢性胰岛素抵抗和糖尿病。有关这个话题的更多信息,请阅读引起胰岛素抵抗的原因和1型糖尿病、2型糖尿病和糖尿病前期胰岛素抵抗的3个原因。很多人问我关于脂肪能破坏胰岛素生成细胞的具体机制。让我们详细探讨一下Beta细胞的压力。
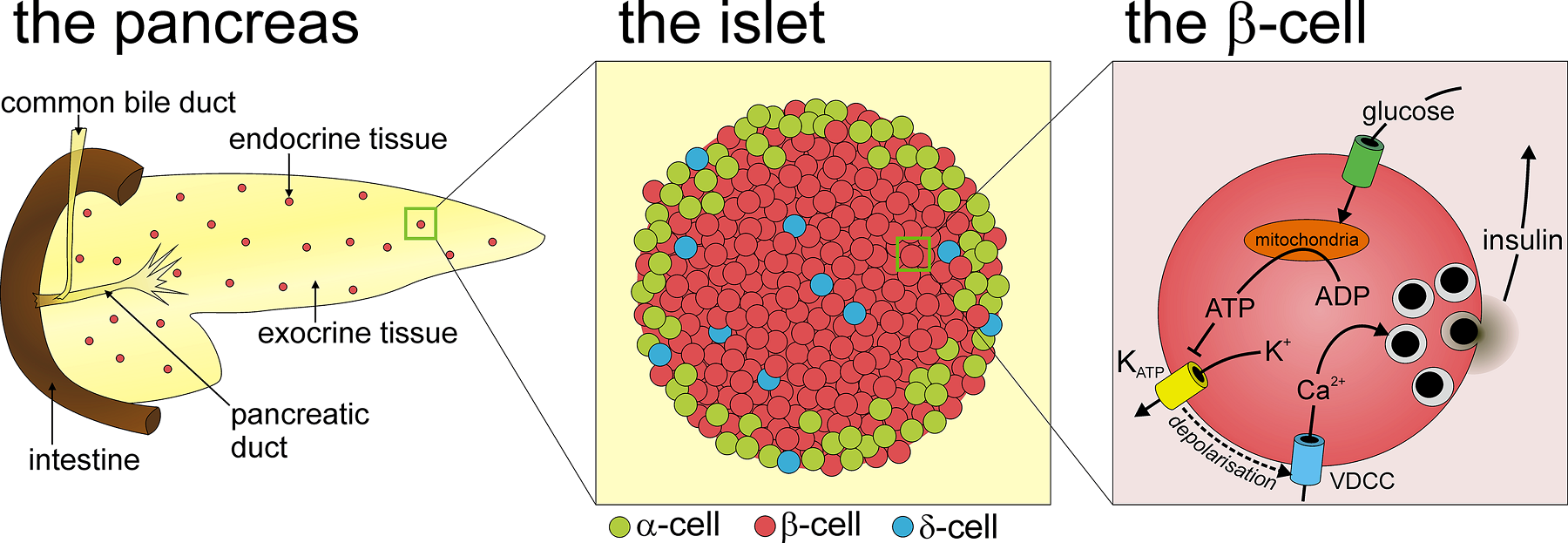
什么是Beta细胞,为什么它们很重要?
胰腺中的胰岛素分泌细胞是高度专门化的细胞,因为它们是唯一能制造胰岛素的细胞类型。危害胰岛素生产会导致严重的代谢问题,进而导致全身器官功能紊乱,最终导致死亡。正因为如此,在终生保护Beta细胞健康对于长期健康至关重要。
是什么导致了Beta细胞功能紊乱?
正如脂肪从脂肪组织中溢出时,你的肝脏和肌肉会积累脂肪,你的胰腺中的细胞也很容易积聚脂肪。
被称为脂毒性(Lipotoxicity),在你的细胞中积累过多的脂肪会导致严重的细胞功能紊乱(27-38)。
与你的肝脏和肌肉细胞相比,Beta细胞对脂肪酸的损伤特别敏感,因为它们的保护能力有限。
当长时间暴露在高脂肪浓度下时,其抗氧化防御机制不足以保护他们免受功能障碍(33,34)。
第一步: 应激的Beta细胞分泌过量的胰岛素
细胞的行为方式取决于许多因素,包括脂肪浓度、葡萄糖浓度以及它们暴露在高水平的脂肪或葡萄糖的时间(34)。随着脂肪组织的脂肪溢出和全身胰岛素抵抗的水平随着时间的推移而增加,你的胰腺会分泌更多的胰岛素,从而使你的肌肉和肝脏的行为变得正常。实际上,你的细胞在说……
“哇,血液中葡萄糖的含量非常高。”我最好制造更多的胰岛素,这样肝脏和肌肉就没有别的选择,只能摄取葡萄糖了。当血液中的葡萄糖持续升高,就会产生胰岛素!”
因为现在的Beta细胞已经产生超过了生理正常水平的胰岛素,他们进入了细胞应激的状态。随着这个循环的持续,胰岛素抵抗的程度随着时间的推移而增加,你的胰腺分泌的胰岛素也会继续增加。
步骤2:Beta细胞最大化胰岛素生产
在某一时刻,胰岛素的生产不再增加-你的Beta细胞有足够的压力,他们的胰岛素生产最大化。此时,你的Beta细胞不能产生更多的胰岛素。在某些个体中,这个过程可能需要许多年才能形成,而在另一些个体中,这个过程发生得非常快。
在高峰时期产生的胰岛素量在个体之间是高度可变的;一些人的胰岛素产量达到正常水平的150%,而另一些人的胰岛素产量达到正常水平的450%。峰值产生的胰岛素数量取决于Beta细胞的数量和细胞的强度,两者都是个体之间的变量。随着Beta细胞数量的增加和每个细胞的相对强度的增加,更多的胰岛素能够产生。尽管存在这些个体差异,但所有胰岛素抵抗个体之间的共同之处在于,Beta细胞的应激会引发超出生理正常量的过量的胰岛素生产。
第三步: 应激的细胞选择自杀 (Beta Cells Commit Suicide)
在一个应激的细胞的生命中到达某一个点,它选择自杀比活着更有利。在这一点上,细胞将经历一个被称为细胞凋亡的过程(程序性细胞死亡)。这是一个没有回头的点。当大量应激的细胞一起自杀时,胰岛素的产量在短时间内迅速下降。由于大量的死亡,胰岛素的产量下降到正常的生理水平以下。这种状态被称为2型糖尿病。
同样的,在个体之间胰岛素的峰值产生变化,细胞自杀的数量也是一个高度可变的过程。
有些人保留了原来的60%的细胞数量,而其他的则会下降到原来的20%。验尸报告显示,在大多数2型糖尿病患者中,超过一半的Beta细胞被永久杀死(22)。
在这种状态下,只有一小部分的Beta细胞负责分泌足够的胰岛素来满足你的整个身体。正如你所能预测的那样,这项工作是极其困难的,除非你能帮助你的肌肉和肝脏显著降低他们对胰岛素的需求。幸运的是,这些剩余的Beta细胞足够强壮,能够存活,但只要胰岛素抵抗持续存在,它们就有死亡的危险。
20岁以后,你的身体停止制造新的Beta细胞; 因此,Beta细胞死亡被认为是不可逆转的(39)。那么问题就变成了:如果胰岛素抵抗的水平显著降低,剩下的细胞数量是否能够产生足够的胰岛素来满足你整个身体的需求呢? 换句话说,剩下的“士兵”是否足够强大,能够经受住时间的考验?
对你来说幸运的是,答案几乎总是肯定的。即使是在Beta细胞数量明显减少的情况下,其余的Beta细胞群也常常能够产生足够的胰岛素来组织所有的组织。但是为了做到这一点,你必须降低你的全身胰岛素抵抗的水平,减少你摄入的膳食脂肪,否则其余的细胞仍然有压力,并将继续自杀。
下面是一个总结细胞死亡过程的图片:
图示:胰岛素β细胞自杀 (Beta Cells Commit Suicide)
第四步:逆转胰岛素抵抗,以免为时太晚!
一种低脂肪、植物性的全食物(A low-fat, plant-based whole foods )方法是降低全身胰岛素抵抗和长久保持Beta细胞功能的最有效方法。
https://s.click.taobao.com/oCymNMw
https://s.click.taobao.com/lBxmNMw
How Fat Kills Insulin-Producing Beta Cells: A Lesson for Those With and Without Diabetes
By Cyrus Khambatta
ABOUT THE AUTHOR
Cyrus Khambatt
Diagnosed with type 1 diabetes at the age of 22, I have spent over a decade learning the fundamentals of nutrition at the doctorate level. My goal is to share my knowledge of practical nutrition and fitness with people with prediabetes, type 1 and type 2 diabetes. Diabetes is an OPPORTUNITY to attain excellent health. Reversing the effects of insulin resistance can be a fun and enjoyable process if the right system is in place. That's why I've spent over 10 years developing a rock solid system that can minimize blood glucose variability and insulin resistance.
An overwhelming amount of scientific evidence shows that a high-fat diet is the single most effective method at inducing insulin resistance in both your liver and muscle. This research clearly demonstrates that increasing dietary fat intake has an immediate negative effect on insulin sensitivity, which can then develop into a chronic state of insulin resistance and diabetes if the quantity of dietary fat remains high (1–26). For more information on this topic, read What Causes Insulin Resistance? Lipid Overload and The 3 Causes of Insulin Resistance in Type 1 Diabetes, Type 2 Diabetes and Prediabetes. Many people ask me about the specific mechanism how fat can destroy insulin-producing beta cells. Let’s explore beta cell stress in detail.
What are Beta Cells and Why are They Important?
The insulin producing beta cells in your pancreas are highly specialized cells because they are the only cell type that can make insulin. Jeopardizing insulin production results in severe metabolic problems that can then lead to whole-body organ dysfunction and eventually death. Because of this, protecting beta cell health throughout life is crucial for long-term health.
What Causes Beta Cell Dysfunction?
In the same way that your liver and muscle accumulate fat when fat spills out of your adipose tissue, the beta cells in your pancreas are also highly susceptible to fat accumulation.
Known as lipotoxicity, the accumulation of excess fat in your beta cells leads to severe beta cell dysfunction (27–38).
In comparison with cells in your liver and muscle, beta cells are particularly sensitive to damage caused by fatty acids because they have a limited ability to protect themselves against damage.
When exposed to high fat concentrations for long periods of time, their antioxidant self-defense mechanisms are inadequate to protect them against dysfunction (33,34).
Step 1: Stressed Beta Cells Make Excess Insulin
The way that beta cells behave depends on a number of factors, including fat concentration, glucose concentration and the amount of time that they are exposed to high levels of either fat or glucose (34). As fat spills over from adipose tissue and the level of whole-body insulin resistance increases over time, your pancreas responds by making more insulin, to overpower your muscle and liver into behaving properly. In effect, your beta cells are saying…
“Wow the amount of glucose in the blood is incredibly high. I better make more insulin so that the liver and muscle will have no choice but to take it up. When the going gets tough, the tough make insulin!”
Because the beta cells are now over producing insulin beyond their physiologically normal level, they enter a state of cellular stress. As this cycle continues and the degree of insulin resistance increases over time, the amount of insulin produced by your pancreas also continues to increase.
Insulin sensitivity insulin resistance
Step 2: Beta Cells Maximize Insulin Production
At a certain point, insulin production no longer increases – your beta cells are sufficiently stressed and their production of insulin is maximized. At this point, your beta cells simply cannot make more insulin. In some individuals, this process can take many years to develop, and in others this process occurs very quickly.
The amount of insulin produced at the peak is highly variable between individuals; some people hit peak insulin production at 150% of normal whereas others hit peak insulin production at 450% of normal. The amount of insulin produced at peak depends on both the number of beta cells and the strength of the beta cells, both of which are variable between individuals. More insulin is capable of being produced as both the size of the beta cell population and the relative strength each individual beta cell increases. Despite these individual differences, the common thread between all insulin resistant individuals is that beta cells stress triggers excess insulin production beyond the physiological normal amount.
Step 3: Stressed Beta Cells Commit Suicide
There comes a point in the life of a stressed beta cell where it is more advantageous to commit suicide than it is to stay alive. At this point, beta cells will undergo a process called apoptosis (programmed cell death). This is a point of no return. When a large population of stressed beta cells commit suicide together, insulin production falls rapidly in a short period of time. As a result of this massive die off, insulin production falls to below normal physiological levels. This state is called type 2 diabetes.
In the same way that peak insulin production varied between individuals, the amount of beta cell suicide is also a highly variable process.
Some individuals retain 60% of their original beta cell mass whereas others will drop to as low as 20% of their original beta cell mass. Autopsies have revealed that in the majority of patients with type 2 diabetes, more than half of the beta cell population has been permanently killed off (22).
In this state, only a small population of beta cells are now responsible for secreting enough insulin to satisfy your entire body. As you may be able to predict, this job is extremely difficult unless you help your muscle and liver significantly reduce their requirement for insulin. Fortunately, these remaining beta cells are strong enough to remain alive, however they are at risk for death as long as insulin resistance persists.
After the age of twenty, your body stops making new beta cells; beta cell death is therefore considered irreversible (39). The question then becomes this: if the level of insulin resistance is significantly reduced, can the remaining beta cell population produce enough insulin to meet the demands of your entire body? In other words, are the remaining “soldiers” strong enough to withstand the test of time?
Fortunately for you, the answer is almost always yes. Even when beta cell mass has been significantly compromised, the remaining beta cell population is often capable of producing sufficient insulin for all tissues. But in order to do this, you must reduce your level of whole-body insulin resistance by reducing your intake of dietary fat, otherwise the remaining beta cells remain stressed and will continue to commit suicide.
Below is a picture to summarize the process of beta cell death:
beta cell death insulin
Step 4: Reverse Insulin Resistance Before It’s Too Late!
A low-fat, plant-based whole foods approach is the most powerful method of reducing whole-body insulin resistance and preserving long-term beta cell function. Period. End of story.
If you’re interested in adopting a low-fat, plant-based whole foods diet for increased energy, weight loss, reduced blood glucose and exceptional long-term health, contact me using the widget below and let’s see if a group-based coaching program is right for you.
- Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006 Jun;6(3):177–81.
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997 Jan;46(1):3–10.
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest. 2002 Jun 1;32:14–23.
- Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006 Jun;6(3):177–81.
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest. 2002 Jun 1;32:14–23.
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-Induced Insulin Resistance in Human Muscle Is Associated With Changes in Diacylglycerol, Protein Kinase C, and IκB-α. Diabetes. 2002 Jul 1;51(7):2005–11.
- Savage DB, Petersen KF, Shulman GI. Disordered Lipid Metabolism and the Pathogenesis of Insulin Resistance. Physiol Rev. 2007 Apr 1;87(2):507–20.
- Xiao C, Giacca A, Carpentier A, Lewis GF. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia. 2006 Apr 5;49(6):1371–9.
- Wang P-Y, Kaneko T, Wang Y, Tawata M, Sato A. Impairment of Glucose Tolerance in Normal Adults Following a Lowered Carbohydrate Intake. Tohoku J Exp Med. 1999;189(1):59–70.
- Martins AR, Nachbar RT, Gorjao R, Vinolo MA, Festuccia WT, Lambertucci RH, et al. Mechanisms underlying skeletal muscle insulin resistance induced by fatty acids: importance of the mitochondrial function. Lipids Health Dis. 2012;11:30.
- Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007 Mar;10(2):142–8.
- Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999 Jun;48(6):1270–4.
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002 Dec 27;277(52):50230–6.
- Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol. 2010 Jan;222(1):187–94.
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000 Jul;106(2):171–6.
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996 Jun 15;97(12):2859–65.
- Silveira LR, Fiamoncini J, Hirabara SM, Procópio J, Cambiaghi TD, Pinheiro CHJ, et al. Updating the effects of fatty acids on skeletal muscle. J Cell Physiol. 2008 Oct;217(1):1–12.
- Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. News Physiol Sci Int J Physiol Prod Jointly Int Union Physiol Sci Am Physiol Soc. 2004 Jun;19:92–6.
- Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008 Nov;295(5):E1009-1017.
- Yamamoto Noguchi CC, Kunikane N, Hashimoto S, Furutani E. Mixed model of dietary fat effect on postprandial glucose-insulin metabolism from carbohydrates in type 1 diabetes. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2015 Aug;2015:8058–61.
- Sumiyoshi M, Sakanaka M, Kimura Y. Chronic Intake of High-Fat and High-Sucrose Diets Differentially Affects Glucose Intolerance in Mice. J Nutr. 2006 Mar 1;136(3):582–7.
- Taylor R. Banting Memorial lecture 2012: reversing the twin cycles of type 2 diabetes. Diabet Med J Br Diabet Assoc. 2013 Mar;30(3):267–75.
- Pańkowska E, Błazik M, Groele L. Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: the conclusion of a randomized study. Diabetes Technol Ther. 2012 Jan;14(1):16–22.
- Smart CEM, Evans M, O’Connell SM, McElduff P, Lopez PE, Jones TW, et al. Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care. 2013 Dec;36(12):3897–902.
- Paterson M, Bell KJ, O’Connell SM, Smart CE, Shafat A, King B. The Role of Dietary Protein and Fat in Glycaemic Control in Type 1 Diabetes: Implications for Intensive Diabetes Management. Curr Diab Rep. 2015 Jul 23;15(9):1–9.
- Neu A, Behret F, Braun R, Herrlich S, Liebrich F, Loesch-Binder M, et al. Higher glucose concentrations following protein- and fat-rich meals – the Tuebingen Grill Study: a pilot study in adolescents with type 1 diabetes. Pediatr Diabetes. 2015 Dec 1;16(8):587–91.
- Haber EP, Ximenes HMA, Procópio J, Carvalho CRO, Curi R, Carpinelli AR. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol. 2003 Jan;194(1):1–12.
- Haber EP, Procópio J, Carvalho CRO, Carpinelli AR, Newsholme P, Curi R. New insights into fatty acid modulation of pancreatic beta-cell function. Int Rev Cytol. 2006;248:1–41.
- Kraegen EW, Cooney GJ, Ye JM, Thompson AL, Furler SM. The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc. 2001;109 Suppl 2:S189-201.
- Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis Int J Program Cell Death. 2009 Dec;14(12):1484–95.
- Manco M, Calvani M, Mingrone G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes Metab. 2004 Nov;6(6):402–13.
- Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002 Feb;143(2):339–42.
- Robertson RP, Harmon J, Tran POT, Poitout V. β-Cell Glucose Toxicity, Lipotoxicity, and Chronic Oxidative Stress in Type 2 Diabetes. Diabetes. 2004 Feb 1;53(suppl 1):S119–24.
- Sharma RB, Alonso LC. Lipotoxicity in the pancreatic beta cell: not just survival and function, but proliferation as well? Curr Diab Rep. 2014 Jun;14(6):492.
- Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2498–502.
- Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985 Mar;28(3):119–21.
- Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001 Feb;50 Suppl 1:S118-121.
- Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995 Aug;44(8):863–70.
- Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010 Feb;53(2):321–30.
https://www.mangomannutrition.com/fat-kills-insulin-producing-beta-cells/
.png)
.png)
.png)
.png)
.png)
.png)