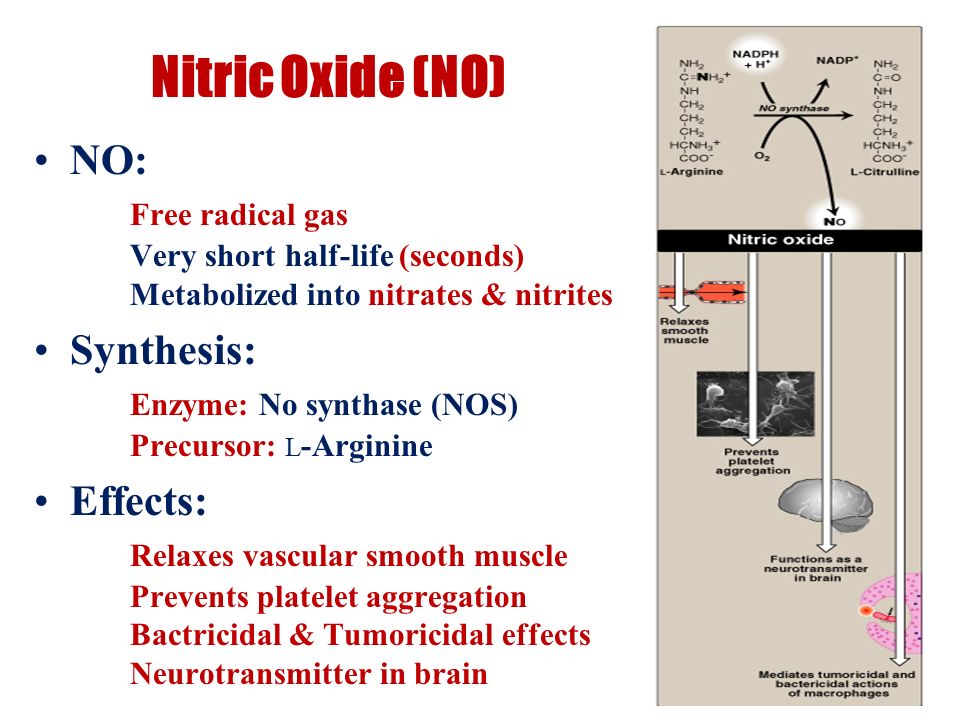
维生素C能够治疗尿路感染-酸化尿液,把亚硝酸盐还原为杀菌的一氧化氮
Effects of pH, Nitrite, and Ascorbic Acid on Nonenzymatic Nitric Oxide Generation and Bacterial Growth in Urine
pH、亚硝酸盐、抗坏血酸对尿中一氧化氮和细菌生长的影响
在尿道感染过程中,尿中的细菌可能会产生亚硝酸盐。亚硝酸盐的酸化导致一氧化氮(NO)和其他活性氮氧化物的形成,这些物质对多种微生物有毒。
我们研究了在含有亚硝酸盐和还原剂维生素C的轻度酸化的人尿中NO的形成和细菌的生长。从健康受试者收集的尿液被置于封闭的注射器中,在不同pH值下添加不同数量的亚硝酸盐和/或抗坏血酸。用化学发光技术在顶空气体中测量NO生成。一个类似的装置也被用来研究尿液中三种细菌的生长。轻度酸化的含亚硝酸盐的尿液产生大量的NO,这种生产因维生素C的添加而大大增强。酸化尿液中添加亚硝酸盐可显著降低大肠杆菌、铜绿假单胞菌和腐生葡萄球菌的生长。抗坏血酸增强了这种抑制作用。总之,当亚硝酸盐存在时,三种常见的泌尿病原体的生长在轻度酸化尿液中被显著抑制。酸化亚硝酸盐的抑菌作用可能与一氧化氮等有毒活性氮中间体的释放有关。这些结果可能有助于解释众所周知的尿酸化的有益作用,例如维生素C治疗和预防尿路感染的作用。
Effects of pH, Nitrite, and Ascorbic Acid on Nonenzymatic Nitric Oxide Generation and Bacterial Growth in Urine
https://doi.org/10.1006/niox.2001.0371Get rights and content
Abstract
Nitrite may be generated by bacteria in urine during urinary tract infections. Acidification of nitrite results in the formation of nitric oxide (NO) and other reactive nitrogen oxides, which are toxic to a variety of microorganisms.
We have studied NO formation and bacterial growth in mildly acidified human urine containing nitrite and the reducing agent vitamin C. Urine collected from healthy subjects was incubated in closed syringes at different pH values with varying amounts of nitrite and/or ascorbic acid added. NO generation was measured in headspace gas using a chemiluminescence technique. A similar setup was also used to study the growth of three strains of bacteria in urine. Mildly acidified nitrite-containing urine generated large amounts of NO and this production was greatly potentiated by ascorbic acid. The growth of Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus saprophyticus was markedly reduced by the addition of nitrite to acidified urine. This inhibition was enhanced by ascorbic acid. In conclusion, we show that the growth of three common urinary pathogens is markedly inhibited in mildly acidified urine when nitrite is present. The bacteriostatic effect of acidified nitrite is likely related to the release of NO and other toxic reactive nitrogen intermediates. These results may help to explain the well-known beneficial effects of urinary acidification with, e.g., vitamin C in treatment and prevention of urinary tract infection.
https://www.sciencedirect.com/science/article/pii/S1089860301903714
Antimicrob Agents Chemother. 1996 Jun; 40(6): 1422–1425.
Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense.
R S Dykhuizen, R Frazer, C Duncan, C C Smith, M Golden, N Benjamin, and C Leifert
Infection Unit, Aberdeen Royal Infirmary, United Kingdom.
ABSTRACT
Dietary intake of nitrate generates salivary nitrite, which is acidified in the stomach, leading to a number of reactive intermediates of nitrogen, among which are the potentially carcinogenic N-nitrosamines. Acidified nitrite, however, also has antimicrobial activity which coincides with the formation of nitric oxide. The present study examines the antimicrobial effect in vitro of acidified nitrite on Salmonella enteritidis, Salmonella typhimurium, Yersinia enterocolitica, Shigella sonnei, and Escherichia coli O157. First-order regression plots showed a linear inverse relationship of log-transformed proton and nitrite concentrations with MICs and MBCs after 30 min, 2 h, and 24 h of exposure (P < 0.001 for all antibacterial activities). Susceptibility to the acidified nitrate solutions ranked as follows: Y. enterocolitica > S. enteritidis > S. typhimurium = Shigella sonnei > E. coli O157 (P < 0.05). Addition of SCN-, but not that of CI-, increased the antibacterial activity (paired t testing, P < 0.001). Generation of salivary nitrite from dietary nitrate may provide significant protection against gut pathogens in humans.Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense.
Nitrogen oxides and nitrous acid are recognized in organic chemistry as noxious compounds and atmospheric pollutants and represent a significant population health risk (21). In humans, ingested nitrate (NO3 2) is absorbed from the gastrointestinal tract into the bloodstream and concentrated in the salivary glands by an active transport system shared with iodide and thiocyanate, increasing concentrations up to 10 times that in plasma (23, 25). Salivary nitrate is then rapidly reduced to nitrite (NO2 2) by nitrate reductase expressed by microorganisms in the mouth (11). N-Nitrosamines are formed from nitrite and secondary amines in the stomach (15, 20), and concerns about the endogenous formation of these potentially carcinogenic compounds has led to calls for restriction of nitrate and nitrite in food products and drinking water (24). We have recently suggested that the production of salivary
nitrite serves a useful purpose as a host defense mechanism against swallowed pathogens via the formation of bacteriocidal compounds in the stomach (1). It has been shown that expelled stomach air contains a high concentration of the antimicrobial
gas nitric oxide (NO˙) which is enhanced by dietary nitrate intake (16). We proposed that the salivary generation of nitrite is accomplished by a symbiotic relationship involving nitratereducing bacteria on the tongue surface, which is designed to provide a host defense against microbial pathogens in the mouth and lower gut via chemical NO˙ production (6). Patients with infective gastroenteritis have increased plasma nitrate levels compared with those in healthy controls (7), septicemic patients (19), and patients with inflammatory bowel disease (7a). During infective gastroenteritis, salivary generation of nitrite might be greatly enhanced, resulting in increased gastric NO˙ production. To investigate the role of salivary nitrite in the bacteriocidal function of the stomach, we studied the effect of acidified nitrite on microorganisms involved in the etiology of infective gastroenteritis. Five microorganisms were tested by using acidification with hydrochloric acid and various concentrations of nitrite characteristic of concentrations found in saliva. We also studied the effects of other anions, including thiocyanate (which is also concentrated in saliva) and chloride (which is secreted into the gastric lumen) in combination with nitrite solutions acidified with sulfuric acid.
MATERIALS AND METHODS
Production of standardized bacterial inocula. Patient isolates of Salmonella enteritidis, Salmonella typhimurium, Shigella sonnei, Yersinia enterocolitica, and Escherichia coli O157 were tested. All experiments used early-log-phase cultures. Flasks (125 ml) containing 75 ml of nutrient broth (Oxoid CM1) were inoculated and incubated on a shaker (New Brunswick Scientific Co., Edison, N.J.) for 18 h at 378C. The optical density was adjusted by dilution with fresh nutrient broth to produce a density of 2 3 107 CFU ml21
.
Determination of the bacteriostatic activity of acidified nitrite. The experiments were carried out on disposable, flat-bottom microwell plates (96 wells of 300 ml). Nitrite solutions to give a final concentrations of 0, 0.05, 0.1, 0.2, 0.5, 1, 2, and 10 mmol of nitrite per ml in the microwells and nutrient broth solutions acidified by hydrochloric acid to give final pHs of 5.4, 4.8, 4.2, 3.7, 3.0, and 2.1 were prepared. The microwells were filled with nitrite solution (60 ml), bacterial suspension (60 ml), and acidified nutrient broth (120 ml). The plates were sealed and incubated for 24 h at 378C on the shaker. The inhibitory effect of acidified nitrite on bacterial growth was determined by measurement of the optical density (570 nm) of the wells using a microwell plate reader (MRX Microplate Reader; Dynatech Products Ltd., Guernsey, Channel Islands, Great Britain). To determine the effect of Cl2 and SCN2 on the antimicrobial activity of acidified nitrite, the experiment was repeated with S. enteritidis using acidification by sulfuric acid (H2SO4) with 10 mM NaCl, Na2SO4, or NaSCN in the microwells. All experiments were carried out in triplicate. Determination of the bacteriocidal activity of acidified nitrite. After 30 min, 2 h, and 24 h of exposure to acidified nitrite, 20 ml of the bacterial suspensions was transferred to 180 ml of a recovery medium (nutrient broth; pH 5 7.0). From this first transfer, a further 20 ml was transferred to recovery medium to accomplish neutralization of acid, dilution of nitrite concentration, and reduction of the original inoculum size to a final number of 10,000 microorganisms. Recovery media were incubated on the shaker for 24 h at 378C before assessment of microbial growth with the microwell plate reader. Interpretation of results and statistical analysis. The MIC of NO2 2 at thedifferent pH settings was defined as the lowest NO2 2 concentration at which no growth of microorganisms had taken place after 24 h. The MBC was defined as the lowest NO2 2 concentration at which no growth was detected after transfer into recovery media. MICs and MBCs of nitrite (in micromoles per milliliter) were log transformed for statistical analysis. Differences in susceptibility of mi-croorganisms to nitrite acidified with HCl were assessed by analysis of variance and paired t testing for means of the MIC, MBC after 30 min exposure time
(MBC0.5h), MBC2h, and MBC24h at the six pH values applied in the experiments. The same method was used to assess the differences in susceptibility of S. enteritidis to nitrite acidified with sulfuric acid with 10 mM Na2SO4, NaCl, or NaSCN present in the solution. Paired t testing was also applied to compare the mean concentrations of nitrite required to accomplish bacteriostasis, and bacterial killing after 30 min, 2 h, and 24 h of exposure. The slopes of the regression curves of acidified nitrite for the different microorganisms and antibacterial activities were assessed by regression analysis.
RESULTS
The means of the nitrite concentrations showing antimicrobial activity at pHs 2.1, 3, 3.7, 4.2, 4.8, and 5.4 are summarized in Table 1. Y. enterocolitica, S. enteritidis, and S. typhimurium were all killed at pH 2.1 after 30 min of exposure. Shigella sonnei and E. coli O157 survived, unless nitrite was present in the solution (0.20 and 1 mmol/ml, respectively [Table 1]). A linear relationship between log[NO2
2] and pH was present for MIC, MBC0.5h, MBC2h, and MBC24h between pHs 2.1 and 4.8 (Fig. 1). The cumulative R for the regression lines was significant for all antimicrobial activities (P , 0.001). Regression analysis showed a significantly steeper slope for the MIC
regression line compared with those for MBC0.5h (P 5 0.007) and MBC2h (P 5 0.032). At a pH of $4.8, the nitrite required to achieve bacterial killing after short exposure times (MBC0.5h and MBC2h) was frequently .10 mmol/ml and the linear relationship between
log[NO2] and pH was lost. There was a significant difference between MBC0.5h, MBC2h, MBC24h, and MIC at all pH settings (paired t testing for means, P , 0.001). Analysis of variance showed significant differences in susceptibility to acidified nitrite between individual organisms (P , 0.001). At pH 2.1, E. coli O157 was significantly less susceptible compared with all other microorganisms (paired t testing, P , 0.001), and throughout the pH range, analysis of regression showed the slope of its regression line to be significantly lower (P , 0.001). The susceptibilities of S. typhimurium and Shigella sonnei were not significantly different. S. enteritidis was more susceptible than S. typhimurium (paired t testing P , 0.001), and Y. enterocolitica was most susceptible of all microorganisms tested (P 5 0.047 compared with S. enteritidis and P , 0.001 compared with all other microorganisms). Adding 10 mmol of NaSCN per liter to the microwell resulted in a significant reduction of the amount of acidified nitrite required to accomplish activity (Table 2) (paired t testing, P , 0.001). Addition of NaCl or Na2SO4 resulted inidentical antibacterial activities.
DISCUSION
Acidification of nitrite will lead to generation of reactive intermediates of nitrogen that have cytotoxic properties (Fig.2). At a given pH value, the quantity of NO˙ generated in vitro is dependent on the nitrite concentration (6). In the solutions used during this experiment, a nitrite concentration of 0.01 mmol/ml at pH 3 generated a peak concentration of nitric oxide of 1 ppm in the headspace and a nitrite concentration of 1.2 mmol/ml generated 10 ppm. Within this range of nitrite concentrations at pH 3, a bacteriocidal effect within 2 h of exposure was accomplished for all microrganisms (Table 1), suggesting that gastric contents generating 1- to 10-ppm NO˙ would have a bacteriocidal effect on these gut pathogens within the transit time of a food bolus through the stomach. In vivo measurements of NO˙ production in the human stomach have shown values between 1 and 180 ppm, depending on dietary nitrate intake (5, 16). Nitric oxide inhibits respiratory chain enzymes through inactivation of iron-sulfur complexes (9) and disrupts DNA replication by inhibiting ribonucleotide reductase (17). Its toxicity has been demonstrated for a rapidly expanding list of microorganisms (3) as well as for tumor cells (18). However, experiments with NO˙ donor compounds have shown little antibac terial activity of NO˙ itself (4), and its toxic effects are mor likely to be accomplished via the formation of peroxynitrite in the presence of superoxide (29), the oxygen-dependent generation of the nitrogen dioxide radical when nitric oxide concentrations are high (2), and/or the formation of still-uncharacterized nitrogen species (28). It seems most likely that the antibacterial activity of acidified nitrite is due to an additive contribution of reactive intermediates of nitrogen (12).
Susceptibilities to the acidified nitrite solutions ranked as follows: Y. enterocolitica . S. enteritidis . S. typhimurium 5 Shigella sonnei. E. coli O157 was different in its response to acidified nitrite compared with the other four microorganisms; in the absence of nitrite it was significantly more resistant to acid, but addition of nitrite seemed to abolish this difference (Table 1). In conclusion, addition of nitrite to acidic solutions achieves killing of gut pathogens where acid alone allows growth to continue. Physiological concentrations of nitrite accomplish killing after exposure times that are comparable with the transfer time of a food bolus through the stomach. Addition of thiocyanate, which is also concentrated in saliva, but not of chloride increased antibacterial activity (Table 2). Generation of salivary nitrite increases greatly after nitrate ingestion, suggesting that ingestion of foods rich in nitrate protects against infective gastroenteritis. The high plasma nitrate levels observed in patients that are suffering from infective gastroenteritis may protect against the fecal-oral route of reinfection via increased generation of salivary nitrite. This mechanism may limit the impact of outbreaks of gastroenteritis, which would be relevant in humans but also would be of particular importance in other mammalian species and animal husbandry.
Health-conscious individuals and government authorities have advocated restriction of dietary nitrates for the last 20 years after ingestion of amines and nitrates had been associated with gastric cancer in animal models. Although the harmful and potentially carcinogenic activity of N-nitrosamines cannot be dismissed, epidemiological evidence for this association has been lacking (8, 13). We submit that the mechanism of chemical host defense which seems to take place in symbiosis with nitrate-reducing bacteria on the surface of our tongues may be of fundamental importance. Rather than a potential carcinogen, we postulate that nitrate may be a useful nutrient, particularly when accompanied by ascorbic acid (26), as is the case with vegetables. A conclusive demonstration of the antimicrobial effect of acidified nitrite in vivo would require a major reinterpretation of the role of dietary nitrate in human
health and animal husbandry.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163343/401422.pdf
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163343/pdf/401422.pdf

.png)
.png)
.png)