维生素C对癌干细胞(CSCs)和糖酵解的靶向作用
Vitamin C: Targeting metabolism and glycolysis in CSCs
NADH自荧光: 癌干细胞(CSCs)的一种新的生物标志物
NADH自荧光是在活细胞中观察到的自动荧光的主要形式。
诺贝尔奖得主莱纳斯·鲍林(Linus Pauling)是第一批描述和临床测试维生素C,作为无毒抗癌药有效的人之一[30]。
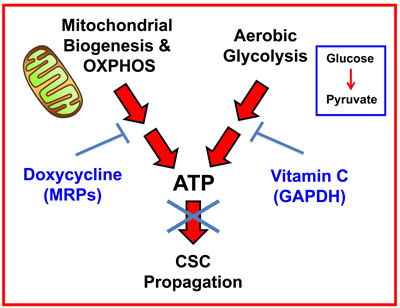
最近,卢·坎特利的研究小组重新研究了维生素C对癌细胞的作用机制[17]。有趣的是,他们发现维生素C有两种作用机制。首先,它是一种强力的 助氧化剂,它能有效地消耗还原性谷胱甘肽(GSH)池,从而导致细胞氧化应激和癌细胞凋亡。此外,它还表现为糖酵解的抑制剂,通过靶向GAPDH的活性,一种关键的糖酵解酶。 图1
图1:维生素C靶向GAPDH-一种糖酵解酶
然而,它对CSC活动的影响以前没有评估过。在这里,我们证明了维生素C也可以用来靶向CSC,因为它是能量代谢的抑制剂,它可以进入线粒体TCA循环和OXPHOS。其他3种糖酵解抑制剂也获得了类似的结果,即2-DG、水飞蓟素和stiripentol。重要的是,stiripentol是一种临床批准的药物,但它的使用主要局限于治疗儿童癫痫发作,而不是治疗癌症[19]。
因此,维生素C可能被证明是新的临床试验的有效药物,目的在于测试其降低癌症患者CSC活性的能力,作为一种更常规的治疗方法,以防止肿瘤复发、进一步的疾病进展和转移。
有趣的是,一项基于乳腺癌的临床研究已经表明,在化疗后6个月内使用维生素C可以显著降低肿瘤的复发和患者的死亡率[31,32]。然而,其潜在临床效益的机制仍然模糊不清。同样地,维生素C治疗可以抑制小鼠体内动物模型的肿瘤生长(33)。
Vitamin C Targeting metabolism and glycolysis in CSCs
NADH auto-auorescence: A new biomarker for CSCs
NADH auto-fluorescence is the dominant form of auto-fluorescence observed in living cells.
The Noble Prize winner, Linus Pauling, was among the first to describe and clinically test the efficacy of Vitamin C, as a relatively non-toxic anti-cancer agent [30]. More recently, Lew Cantley’s group has revisited the mechanism(s) by which Vitamin C targets cancer cells [17]. Interestingly, they showed that Vitamin C has two mechanisms of action. First, it is a potent pro-oxidant, that actively depletes the reduced glutathione pool, leading to cellular oxidative stress and apoptosis in cancer cells. Moreover, it also behaves as an inhibitor of glycolysis, by targeting the activity of GAPDH, a key glycolytic enzyme. However, its effects on CSC activity was not previously evaluated. Here, we show that Vitamin C can also be used to target the CSC population, as it is an inhibitor of energy metabolism that feeds into the mitochondrial TCA cycle and OXPHOS. Similar results were also obtained with 3 other glycolysis inhibitors, namely 2-DG, silibinin and stiripentol. Importantly, stiripentol is a clinically-approved drug, but its use is mainly restricted to the treatment of epileptic seizures in children, and not for cancer therapy [19]. Thus, Vitamin C may prove to be promising agent for new clinical trials, aimed at testing its ability to reduce CSC activity in cancer patients, as an add-on to more conventional therapies, to prevent tumor recurrence, further disease progression and metastasis. Interestingly, a breast cancer based clinical study has already shown that the use of Vitamin C, concurrent with or within 6 months of chemotherapy, significantly reduces both tumor recurrence and patient mortality [31,32]. However, the mechanism underlying its potential clinical benefit remained obscure. Similarly, Vitamin C treatment inhibits tumor growth in murine animal models in vivo(33).
From www.impactjournals.com/oncotarget
SCIENCE. 2015年12月11日;350(6266):1391-6。doi: 10.1126 / science.aaa5004。Epub 2015 11月5日
维生素C通过靶向GAPDH选择性地杀死KRAS和BRAF突变的结直肠癌细胞
摘要
超过一半的人类结肠直肠癌(CRCs)携带KRAS或BRAF突变,并且往往难以被批准的靶向治疗。我们发现培养的带有KRAS或BRAF突变的人CRC细胞在暴露于高水平的维生素C时被选择性地杀死。这种效果是由于通过GLUT1葡萄糖转运体增加了对氧化型维生素C、脱氢抗坏血酸(DHA)的吸收。DHA摄入增加会导致氧化应激,因为细胞内的DHA被还原为维生素C,消耗谷胱甘肽。因此,活性氧积累和失活甘油醛3-磷酸脱氢酶(GAPDH)。在高糖酵解KRAS或BRAF突变细胞中GAPDH的抑制导致能量危机和细胞死亡,而在KRAS和BRAF野生型细胞中则未见。高剂量维生素C损害Apc/Kras(G12D)突变小鼠的肿瘤生长。这些结果为探索维生素C对KRAS或BRAF突变的CRCs的治疗作用提供了理论基础。
美国科学促进会版权所有
Science. 2015 Dec 11;350(6266):1391-6. doi: 10.1126/science.aaa5004. Epub 2015 Nov 5.
Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH.
Abstract
More than half of human colorectal cancers (CRCs) carry either KRAS or BRAF mutations and are often refractory to approved targeted therapies. We found that cultured human CRC cells harboring KRAS or BRAF mutations are selectively killed when exposed to high levels of vitamin C. This effect is due to increased uptake of the oxidized form of vitamin C, dehydroascorbate (DHA), via the GLUT1 glucose transporter. Increased DHA uptake causes oxidative stress as intracellular DHA is reduced to vitamin C, depleting glutathione. Thus, reactive oxygen species accumulate and inactivate glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Inhibition of GAPDH in highly glycolytic KRAS or BRAF mutant cells leads to an energetic crisis and cell death not seen in KRAS and BRAF wild-type cells. High-dose vitamin C impairs tumor growth in Apc/Kras(G12D) mutant mice. These results provide a mechanistic rationale for exploring the therapeutic use of vitamin C for CRCs with KRAS or BRAF mutations.
Copyright © 2015, American Association for the Advancement of Science.
维生素C以氧化的形式通过葡萄糖转运体穿过血脑屏障
大脑中的维生素C浓度是血液中的10倍。在这两种组织中,维生素主要以抗坏血酸的形式存在。我们确定了维生素C的化学形式,很容易穿过血脑屏障,以及这个过程的机制。在我们的研究中,抗坏血酸不能通过血脑屏障。相反,维生素C的氧化形式,脱氢抗坏血酸(氧化抗坏血酸),很容易进入大脑,并以抗坏血酸的形式保留在脑组织中。d-葡萄糖抑制去氢抗坏血酸进入大脑,而l-葡萄糖不抑制。促进性葡萄糖转运蛋白GLUT1在血脑屏障的内皮细胞上表达,负责葡萄糖进入大脑。这项研究提供的证据表明,GLUT1还能将脱水抗坏血酸输送到大脑。这一发现将GLUT1运输脱氢抗坏血酸定义为大脑获取维生素C的机制,并指出抗坏血酸的氧化可能是大脑积累维生素的重要调节步骤。这些结果提示增加中枢神经系统的抗氧化能力。
Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters.
Vitamin C concentrations in the brain exceed those in blood by 10-fold. In both tissues, the vitamin is present primarily in the reduced form, ascorbic acid. We identified the chemical form of vitamin C that readily crosses the blood-brain barrier, and the mechanism of this process. Ascorbic acid was not able to cross the blood-brain barrier in our studies. In contrast, the oxidized form of vitamin C, dehydroascorbic acid (oxidized ascorbic acid), readily entered the brain and was retained in the brain tissue in the form of ascorbic acid. Transport of dehydroascorbic acid into the brain was inhibited by d-glucose, but not by l-glucose. The facilitative glucose transporter, GLUT1, is expressed on endothelial cells at the blood-brain barrier, and is responsible for glucose entry into the brain. This study provides evidence showing that GLUT1 also transports dehydroascorbic acid into the brain. The findings define the transport of dehydroascorbic acid by GLUT1 as a mechanism by which the brain acquires vitamin C, and point to the oxidation of ascorbic acid as a potentially important regulatory step in accumulation of the vitamin by the brain. These results have implications for increasing antioxidant potential in the central nervous system.
Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC508490/
去极化控制后敏化和肿瘤选择性杀伤癌细胞:与活性氧的串扰
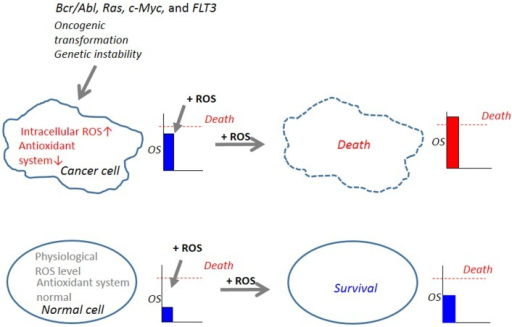
F2:肿瘤细胞中ROS的异常增加是肿瘤选择性杀伤的靶点。细胞氧化应激(OS)水平是由活性氧生成机制(促氧化系统)和活性氧清除机制(抗氧化系统)之间的平衡来调节的。在正常细胞中,抗氧化系统正常,产生低水平的生理活性氧,活性氧是细胞内信号的第二信使,是正常细胞功能所必需的。由于在Bcr/Abl、Ras、c-Myc和FLT3等致瘤转化的控制下,它们的代谢活跃,遗传不稳定,导致活性氧生成增加,抗氧化系统下降,因此癌细胞比正常细胞有更多的活性氧。当使用外源性ROS诱导剂(+ROS)增加OS的等效水平时,癌细胞中的OS水平可以很容易地超过细胞死亡的阈值,而正常细胞中的OS水平则不会。因此,预期癌细胞比正常细胞更容易受到由产生ros的试剂引起的细胞损伤,这种脆弱性可以被利用来选择性地杀死这些细胞。
Depolarization Controls TRAIL-Sensitization and Tumor-Selective Killing of Cancer Cells: Crosstalk with ROS.
F2: Abnormal increases in ROS in cancer cells serves as a target for tumor-selective killing. Cellular oxidative stress (OS) level is regulated by the balance between the machinery for ROS generation (prooxidant system) and the machinery for ROS scavenging (antioxidant system). In normal cells, the antioxidant system is normal and low physiological levels of ROS, which can function as second messengers in intracellular signaling and are required for normal cell function, are generated. Owing to their active metabolism and genetic instability under the control of oncogenic transformation such as Bcr/Abl, Ras, c-Myc, and FLT3, which causes increased ROS generation and decreased antioxidant systems, cancer cells harbor an excess OS over normal cells. When equivalent levels of OS are added by the administration of exogenous ROS-inducing agents (+ROS), OS levels in cancer cells can readily over the threshold of cell death, while OS levels in normal cells do not. Hence, cancer cells are expected to be more vulnerable than normal cells to cell damage induced by ROS-generating agents and this vulnerability can be exploited to selectively kill these cells.
ROS的稳态和代谢:癌细胞中的一种危险的毒素
ROS homeostasis and metabolism: a dangerous liason in cancer cells
E Panieri和M M Santoro
细胞死亡与疾病卷7,pagee2253 (2016)
摘要
肿瘤细胞含有促进活性氧持续产生和增加的基因改变。虽然这种氧化应激条件对正常细胞有害,但它们通过多种方式促进肿瘤的生长,导致DNA损伤和基因组不稳定,并最终通过重新编程癌细胞的新陈代谢。本文综述了肿瘤在氧化应激条件下产生氧化还原辅助因子的代谢依赖机制。特别地,我们描述了线粒体如何在调节氧化还原稳态和肿瘤细胞内代谢的相互作用中起关键作用。最后,我们将讨论直接或间接阻断代谢的药物的潜在治疗用途。
事实
解除控制的氧化还原内稳态是癌细胞的一个特征
增加ROS水平能够促进肿瘤生长和恶性进展
增强恶性细胞的抗氧化能力是其共同特征
肿瘤中特殊代谢途径的改变是常见的
通过代谢抑制使抗氧化防御(NADPH和GSH)失效,可以使肿瘤对化疗和其他抗肿瘤治疗敏感
开放式的问题
什么是氧化还原敏感的转导因子,特别促进癌细胞的信号事件?
由于新陈代谢可以通过NADPH和GSH合成来支持细胞内氧化还原稳态,那么有哪些癌症特异性的途径/改变可以被选择性地用于治疗目的?
抗氧化机制的抑制在多大程度上可用于增强化疗/放疗,而不会对正常细胞产生副作用?
是否有可能建立动物模型,以便在癌症进展过程中实时检测具有高时空分辨率的代谢/氧化还原中间产物?
fingure 2
活性氧的来源和清道夫控制氧化还原的稳态在正常细胞和癌细胞。(a)正常细胞持续产生和消除活性氧,以维持良好的氧化还原平衡。与活性氧诱导剂和抗氧化抑制剂共同处理会破坏氧化还原的稳态,导致氧化应激和不同水平的细胞死亡。(b)癌细胞表现出较高的稳态活性氧水平,与增加的抗氧化能力相平衡。预氧化处理和抗氧化抑制的联合使用预计会导致严重的氧化应激和严重的细胞毒性
ROS稳态与代谢:癌细胞|细胞死亡与疾病的危险环节
https://www.nature.com/articles/cddis2016105

.png)