慢性炎症是解开癌症之谜的关键吗?
Is Chronic Inflammation the Key to Unlocking the Mysteries of Cancer?
了解慢性炎症,会导致心脏病、阿尔茨海默病和各种其他疾病,可能是解开癌症之谜的关键
编者注:这个故事最初是在2007年7月的《科学美国人》杂志上发表的,现在有两项新的研究表明,血管生成抑制剂可能会使肿瘤变得更大,而不是更小。
5亿多年前,一组专门的酶和蛋白质进化来保护我们的原始祖先免受外部世界的攻击。如果一个微生物突破了一些坎布里亚时代的动物群的外壳,早期古老的免疫系统的成员会对这些闯入者进行野蛮而协调的攻击,在细胞壁上开孔,吐出化学毒素,或者仅仅是吞咽和消化整个敌人。一旦入侵者被派遣,免疫营就会开修复受损的细胞,或者如果被攻击的细胞被严重损坏,就会让它们休息。
这种炎性免疫反应非常有效,在漫长的进化过程中,它的许多方面都得到了保护。我们知道这是正确的,因为研究发现,我们有许多相同的免疫基因,因为低等果蝇、脊椎动物和无脊椎动物在超过5亿年前的共同祖先中分化。
多年来,免疫学研究人员对这一先天免疫系统的关注相对较少,基本上认为它是一组生化武器,可以穿透任何生物的皮肤或外壳,穿透最微小的缝隙。他们对更先进的适应性免疫系统给予了极大的关注,这种免疫系统可以将抗体和其他武器集结起来,识别并攻击入侵者,而这一特性在未驯服的先天系统中缺乏特异性。
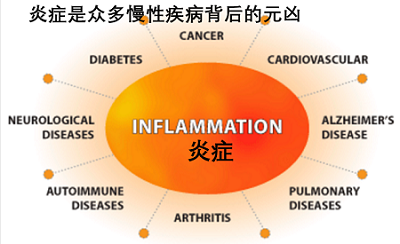
在过去的15年里,先天免疫备受关注。炎症,它的标志特征,已经被公认为几乎所有慢性疾病的潜在因素,除了像风湿性关节炎和克罗恩病这样的明显的罪魁祸首,还包括糖尿病和抑郁症,以及心脏病和中风等主要杀手。在这个十年里,与第三个主要的杀手癌症有联系的可能性受到了严格的审查。“炎症和癌症之间的联系已经转移到研究领域的中心阶段,”麻省理工学院的怀特海德生物医学研究所的罗伯特·A·温伯格(Robert a . Weinberg)指出,他在他的主要教科书《癌症生物学》(Garland Science, 2006)中强调了这一变化的重点。
这种转变认识到免疫炎性状态是肿瘤发展中期的关键中介体。癌症开始于一系列的基因改变,促使一组细胞过度复制,然后侵袭周围组织,这是真正的恶性肿瘤开始的地方。最终,一些肿瘤细胞可能会分裂,并在遥远的地方建立新的增生(转移)。这一点已经被理解了很长时间。但癌症生物学家和免疫学家已经开始意识到从病变组织演变到完全的癌变组织需要修复组织创伤的正常细胞的参与,把它们劫持到癌变组织成为癌变的同谋和帮凶。正如一些研究人员所描述的恶变状态:基因损伤是点燃火焰的火柴,而炎症则是滋养它的燃料。
在重写教科书的过程中,肿瘤不仅仅是一团异常细胞;它还包括一个支持系统,一个肿瘤微环境,它包含多种不同的免疫细胞类型和交叉的化学信号,以及一个血管网络。肿瘤的存在是一个非法器官的状态,它的存在不是为了输送血液或排出体内的毒素,而是只服务于自身的目的。
这种新观点意味着,根除体内每一个癌细胞可能是不必要的。相反,抗炎癌症治疗可以防止癌前细胞癌变,或阻止现有肿瘤扩散到身体的远端部位。癌症患者也许能够存活下来,就像新药物让艾滋病患者活得更久一样。“我不认为治愈是必然的目标。加州大学旧金山分校的癌症生物学家Lisa M. Coussens评论道。“如果你能控制疾病,度过你的自然寿命,那将是一个巨大的胜利。”
多个防御线
理解炎症和癌症之间的联系需要了解身体对入侵者的反应,以及当炎症状态持续太久时,正常的治疗是如何被转化为促进癌症的。当你踩到一颗钉子后,入侵你脚底的细菌会受到来自一系列蛋白质和白细胞的欢迎,这些蛋白质和白细胞类似于电影《爬行动物2》的中心选角。
举一个例子:大约20个补体蛋白质,这样命名,是因为它们与其他身体防御机制相辅相成,
用化学方法把入侵者分离为原生质团。在补体系统用黏液网住这个区域的同时,教科书中描述为“专业的吞噬细胞-专业进食细胞”接着工作。
缺乏餐桌礼仪,这些巨大的巨噬细胞和中性粒细胞继续吞噬“未被邀请的客人”。攻击旅的其他成员包括自然杀伤细胞、肥大细胞和嗜酸性粒细胞。修复不仅仅是对入侵者发起进攻。与凝血有关的血小板会从注入血管的内层转移到皮肤的破裂处。酶直接修复细胞外基质,即细胞固定化的蛋白质基质。一种痂形成,皮肤长回来,整个炎症过程结束。然而,有时炎症不会停止。任何慢性炎症的组织(不仅仅是皮肤),因为病原体、毒素或基因损伤会都会导致疾病,从心脏病到癌症。
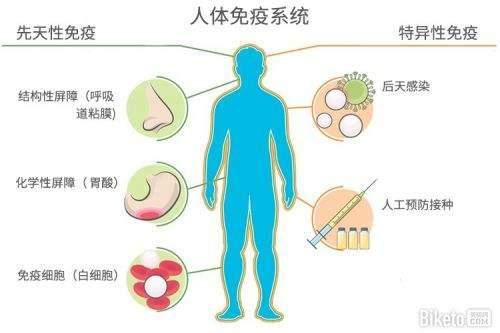
除了第一层防御外,脊椎动物还装备了额外的武器。自适应系统学习入侵者的特定分子特征,然后将其作为杀伤目标。其中的主角是B细胞,它产生抗体分子,能够中和病原体,或者标记它们以便歼灭,此外,T细胞,它会促使感染细胞自杀或分泌化学物质,直接影响其他免疫系统的活动。
近年来,大量的证据表明,慢性炎症在某些类型的肿瘤的发展过程中起着重要的作用。癌症和炎症之间的联系一直被怀疑。在1863年,著名的德国病理学家Rudolf Virchow注意到在恶性组织中存在所谓的淋巴细胞浸润(白细胞)。早在1978年,人类临床研究所和米兰大学的Alberto Mantovani观察到,先天免疫细胞倾向于聚集在一些肿瘤周围。哈佛医学院的癌症生物学家Harold F. Dvorak在1986年说,肿瘤是“不能愈合的伤口”。然而,现状却在别处。甚至在十年前,许多生物学家仍然坚持认为免疫系统不仅可以消灭病原体,而且可以清除癌变前的异常细胞。但是仔细观察肿瘤周围的微环境发现了意想不到的事情。
狩猎的鸽子
在1990年代末,伦敦大学的QUEEN MARY 癌症研究所的Frances Balkwill一直在研究称为肿瘤坏死因子(TNF)的细胞因子(激素样免疫信号分子), 这样命名,是因为大剂量直接注入肿瘤时,这种因子可以杀死癌细胞。但持续性低水平的TNF在肿瘤徘徊时,它的作用就大不相同了。Balkwill的实验室关闭了老鼠的TNF基因,使老鼠无法产生这种蛋白质:令他们吃惊的是,老鼠并没有感染肿瘤。她回忆说:“那真的把我们当成了鸽子中的猫。”所有研究TNF的人都被吓坏了。他们原认为这种细胞因子是一种治疗癌症的方法实际上是一种内生肿瘤启动子。
基因敲除小鼠的现成可用性,可以测试选择性关闭基因的效果,有助于突出癌症-炎症的联系。Coussens和她的同事Douglas Hanahan和Zena Werb在1999年报道说,用激活的癌症基因改造的小鼠,但是没有肥大细胞(另一种固有免疫细胞)发展出了没有进展到完全恶性肿瘤的癌前组织。2001年,杰弗里·w·波拉德(Jeffrey W. Pollard)和他在阿尔伯特·爱因斯坦医学院(Albert Einstein College of Medicine)的同事们对老鼠进行了描述,这些老鼠的基因被改造成易于患上乳腺癌的肿瘤,但它们产生的癌前组织并没有完全变恶性,除非它获得巨噬细胞的帮助。
修改后的图片并没有完全推翻旧的。事实上,它揭示了免疫系统是一把双刃剑。分子和细胞的网络,仅次于大脑的复杂性,仍然是一个悖论:有时它会促进癌症;其他时候它会阻碍疾病。某些类型的天然免疫细胞,如自然杀伤细胞,实际上可以防止肿瘤生长。当微环境被“极化”为炎性状态时,其他免疫细胞可能培养恶性肿瘤; 如果没有炎症,它们可能会把它弄掉。此外,炎症会在许多器官中产生肿瘤,但不是全部,它与血源性癌症的联系尚不明确。
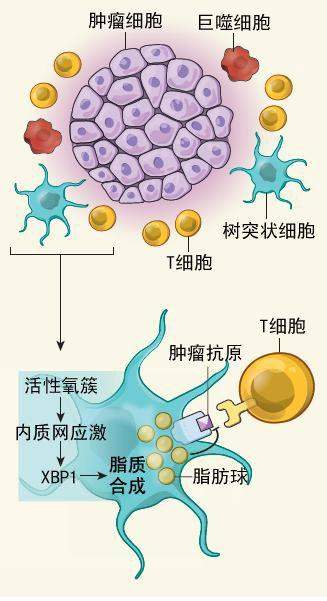
在寻找罪魁祸首时,研究人员常常将显微镜聚焦在巨噬细胞上,而巨噬细胞在肿瘤微环境中的白细胞中占据着重要的位置。巨噬细胞能够杀死肿瘤细胞或向免疫系统的T细胞发出警报,而这是有缺陷的。但是,波拉德和其他研究人员的研究已经详细说明了巨噬细胞是如何被癌细胞“重新教育”来完成他们的任务的。它们成为细胞因子和生长因子的工厂,促进肿瘤的发展。
当肿瘤细胞向将成为巨噬细胞的细胞发出救助信号,吸引它们进入肿瘤时,巨噬细胞开始转变为叛徒。在肿瘤内部,增生的细胞生长得如此之快,以至于它们开始因缺氧而死亡。缺氧和来自肿瘤细胞的信息的结合引发了一个过程,即新到的巨噬细胞作为肿瘤推动者的坏身份。癌症生物学家将这些聚集在肿瘤周围的叛逆者定义为肿瘤相关巨噬细胞(TAM)。
现在,生物学家已经能够将炎症与单个信号分子的水平联系起来,为与癌变的联系提供了更有力的证据。例如,核因子-Kappa B (NF-KB)是一种复杂的蛋白质,它可以作为启动炎症基因和控制细胞死亡的主开关。随着生物学途径的发展,NF-KB是世界闻名的,已经被科技明星发现并获得专利,用于药物开发,其中包括诺贝尔奖得主戴维·巴尔的摩和菲利普·A·利特,后来成为数百万美元专利诉讼的对象。
2004年耶路撒冷希伯来大学的Yinon Ben-Neriah 、Eli Pikarsky和他们的同事报道, 通过遗传改造敲除NF-KB 或者促炎的TNF信号被关闭后,让被遗传设计为肝炎的小鼠(可以导致成肝癌)患上癌前损伤不会发展为肝癌。在后者,中和抗体阻断肿瘤坏死因子,防止其绑定癌变前的肝细胞上的受体;受体的丢失阻止了TNF触发一个分子级联,从而启动NF-KB主开关。阻断NF-KB促使癌前肝细胞开始凋亡,或程序性细胞死亡。在一项相关的研究中,迈克尔·卡琳和他在加州大学圣地亚哥分校的合作者们发现,抑制NF-KB基因的小鼠被设计成结肠炎,这可能导致结肠癌,也促进了细胞凋亡。而在炎性细胞(如巨噬细胞)中关闭通路,也会阻止肿瘤的发展。
迄今为止,最清楚的证据表明,癌症和炎症之间存在关联,这表明炎症促使癌前组织转化为恶性肿瘤。但是生物反应也可能参与到引发疾病和转移的过程中。感染幽门螺杆菌的细菌会引起炎症,这大大增加了胃癌的风险,而丙型肝炎病毒可能会导致肝癌,这里指出两种癌症。病原体也可能产生自由基,从而破坏DNA。尽管炎症可能从一开始就涉及DNA的改变,但很少有研究表明炎症是启动DNA改变的火花。
关于转移的作用的案例比较强烈,最近的研究也证实了这一假设。卡琳的研究小组在4月5日的《自然》杂志上报道说,炎症,而不是癌症细胞的基因变化,会刺激被设计成前列腺癌的老鼠的转移。研究表明,在前列腺肿瘤附近的炎性细胞产生的细胞因子诱导肿瘤细胞减少阻止转移的蛋白质的产生。卡琳指出,这一结果可能解释了一种令人费解的发现,即切割肿瘤,比如前列腺穿刺活检,有时似乎会促进转移。如果他是正确的,干预产生的炎症可能导致转移。与此同时,Pollard的研究小组在对小鼠进行的一项研究中发现,巨噬细胞伴随乳腺肿瘤细胞向血管迁移,并将它们运送到遥远的地方,同时向其伴侣发送化学信息。
先天免疫系统在探索炎症可能导致癌症的过程中得到了最广泛的关注。就像先天免疫系统一样,免疫系统(T细胞和B细胞产生的抗体针对入侵细胞的特定分子)导致了病理或可能与之对抗。几十年来,人们一直在探索增强T细胞对抗癌症的免疫疗法,尽管结果往往令人失望。
此外,一种正在浮出的图像已经开始揭示先天和适应性免疫细胞之间的错综复杂的对话,它们可能参与恶性疾病的传播。研究癌症疫苗的研究人员在设计他们的治疗时,可能需要考虑这些交互作用,以确保它们有效。一项研究表明,卵巢肿瘤产生一种信号分子,用来吸引调节性T细胞,这是一种自适应免疫细胞的亚类,负责使其他T细胞安静下来[参见Lisa Melton的《抑制抑制因子》;《科学美国人》,2002年12月)。
与此同时,Coussens和她在U.C.S.F.的同事在2005年发表在《癌症细胞》上的一项研究中发现,被设计为容易患皮肤癌的小鼠体内的抗体生成B细胞的移除可以防止组织改变和血管生成,这是疾病进展的前提条件。作为病原体攻击者,B细胞产生的抗体,通过血液循环,标志病毒和细菌已被先天性免疫细胞歼灭。然而,作为对癌前组织信号的反应,抗体诱导先天系统在癌症发展中合作。一个公开的研究问题是这个过程是如何开始的。一种可能性是,癌细胞可能会向先天免疫细胞(可能是树突状细胞)发送信息,然后激活B细胞。信号可能与TOLL样受体有关,这种受体在先天免疫信息传递中已经成为重要的中介(参见Luke A. J. O ' neil的“免疫早期预警系统”;《科学美国人》,2005年1月)。
癌症阻滞剂
癌症更像是一个器官,而不仅仅是细胞核中有DNA突变的细胞,这也解释了为什么以前化疗的一些方法取得了有限的成功。波拉德说:“人们采集了细胞,然后在培养基中将它们转化,并将它们植入动物体内。”“它们会长成小球。”他们在那里做一些事情。但它们不是复杂的组织,而自然发生的肿瘤是一种非常复杂的组织。
与仅仅杀死癌细胞-现在药物治疗和放射疗法的目标不同,新的方法可以通过减缓炎症补充现有药物。没有巨噬细胞和其它先天性免疫细胞介入,癌前组织可能会收到控制。
从本质上说,癌症可能会成为一种类似于风湿性关节炎的慢性疾病,这是另一种炎症性疾病。“记住,几乎没有人死于原发性癌症,”德克萨斯大学安德森癌症中心(University of Texas m.d. Anderson cancer Center)的教务长雷蒙德·杜布瓦(Raymond DuBois)说,他是癌症的抗炎药物研究人员。“病人几乎总是死于转移。”
一种抗慢性炎症的药物比杀死恶性细胞(不可避免的,包括健康的细胞)更有吸引力,它是现有化疗的结果。单独服用,这样的药物可能是良性的,可以每天使用作为预防高危患者。流行病学和临床研究表明,使用非甾体类抗炎药(非甾体抗炎药)如阿司匹林,可以延缓某些实体肿瘤的发生。 更能选择性地阻断前列腺素的产生的研究仍在进行。前列腺素是由非甾体抗炎药抑制的调控分子。特别是抑制前列腺素E2生产的药物可以抑制炎症和肿瘤生长,同时避免像Vioxx和早期NSAIDs一类的胃肠道问题等药物的心血管副作用[参见Gary Stix的《更好的目标疼痛方法》[参见]。《科学美国人》, 2007年1月)。人们还在考虑使用无所不在的他汀类药物来降低胆固醇的抗炎作用。
一些治疗方案已经存在。药物Avastin抑制血管生成-促进VEGF的产生,尽管肿瘤学家必须在肿瘤微环境中与其它促进血管生长的因子作竞争。为更常见的炎性疾病而开发的药物也可以抗癌——这些药物可能被合并成类似HIV(艾滋病病毒)样的药物鸡尾酒,也包括血管生成抑制剂和杀细胞剂。
TNF的抑制剂已经获得了治疗类风湿关节炎、克罗恩病和其他疾病的批准,目前正在进行实体肿瘤和血癌的临床试验。在类风湿关节炎和B细胞淋巴瘤中抑制B细胞的单克隆抗体Rituxan,可能会阻止形成固体肿瘤的炎症反应。其他的细胞因子和相关分子(IL-6、IL-8和CCL2等)也是潜在的靶标,NF-KB也是。
一些现有的化合物,包括非甾体抗炎药,甚至是在香料姜黄中发现的,至少通过抑制NF-KB来发挥其作用。但是,主要的制药实验室正在研究这种分子链针的高选择性抑制剂,其中许多是针对调节NF-KB活性的酶(如I-KB激酶)。
一个化学木马
一组正在考虑一种激进的治疗方法,一种分子特洛伊木马。英国谢菲尔德大学的Claire Lewis和Munitta Muthana和他们的同事设计了一种药物传递方案,利用巨噬细胞的自然吸引力,使其进入肿瘤缺氧的区域。他们设计了巨噬细胞,将一种治疗性的病毒送到缺氧的肿瘤区域,这些区域对常规治疗如化疗和放疗的反应很差,因为血液供应不足。一旦巨噬细胞到达肿瘤(到目前为止在培养基中生长),每一个巨噬细胞都会释放数千个病毒拷贝,然后感染癌细胞,然后这些细胞中的蛋白质激活每个病毒的治疗基因。然后,这个动作指示细胞-杀死毒素的合成。“巨噬细胞正在迁移到一个地方,做我们想做的事,而不是以正常的方式驱动肿瘤的发展,”Lewis说。
对癌症的抗炎策略的确切轮廓还有待阐明。调整免疫细胞,形成抵御病原体的防御屏障,也有其自身的风险。“这是一个非常复杂的问题,”杜布瓦说。“如果你神奇地关闭了免疫系统,你就会遇到机会感染的问题,就像艾滋病一样。”在其他炎症性疾病中使用TNF阻滞剂与结核和其他感染有关,甚至可能是淋巴瘤。此外,抑制NF-KB通路可能会在某些情况下诱发癌症。抑制NF-KB有时会导致组织损伤,并导致组织异常再生,从而导致癌症。
尽管如此,新一代的抗炎药似乎仍将加入化疗药物的行列。慢性疾病——以及它们潜在的炎症条件——是人口老龄化的标志。波拉德观察到:“我们都有点过度兴奋了。”治疗围绕着肿瘤的闷烧余烬而不是仅仅是突变细胞可以使癌症成为一种与我们共同生存的疾病。
https://s.click.taobao.com/hYsTINw
参考文献
Is Chronic Inflammation the Key to Unlocking the Mysteries of Cancer?
Understanding chronic inflammation, which contributes to heart disease, Alzheimer's and a variety of other ailments, may be a key to unlocking the mysteries of cancer
By Gary Stix on November 9, 2008
Is Chronic Inflammation the Key to Unlocking the Mysteries of Cancer?
Credit: ESA
Editor's Note: This story, originally printed in the July 2007 issue of Scientific American, is being posted in light of two new studies showing that angiogenesis inhibitors, discussed in this article, may actually make tumors bigger, not smaller.
More than 500 million years ago a set of specialized enzymes and proteins evolved to defend our primitive ancestors against assaults from the outside world. If a microbe breached the shell of some Cambrian-era fauna, the members of this early vintage immune system would stage a savage but coordinated attack on these interlopers—punching holes in cell walls, spitting out chemical toxins or simply swallowing and digesting the enemy whole. Once the invaders were dispatched, the immune battalion would start to heal damaged cells, or if the attacked cells were too badly damaged it would put them to rest.
This inflammatory immune response worked so well that many aspects of it have been preserved during the protracted aeons of evolution. We know this to be true because studies have found that we share many of the same immune genes as the lowly fruit fly—and vertebrates and invertebrates diverged from a common ancestor in excess of half a billion years ago.
ADVERTISEMENT
For years, immunology researchers have paid relatively little attention to this thuggish innate immune system, basically thinking of it as a crew of biochemical bouncers that pummel anything able to penetrate the tiniest opening in a living being’s skin or shell. They lavished their attention, instead, on the more advanced adaptive immune system, which can marshal antibodies and other weaponry that identify and then target an intruder with a specificity lacking in the untamed innate system.
In the past 15 years, innate immunity has come into its own. Inflammation, its hallmark characteristic, has gained recognition as an underlying contributor to virtually every chronic disease—a list that, besides obvious culprits such as rheumatoid arthritis and Crohn’s disease, includes diabetes and depression, along with major killers such as heart disease and stroke. The possibility of a link with a third major killer—cancer—has received intensive scrutiny in this decade. “The connection between inflammation and cancer has moved to center stage in the research arena,” notes Robert A. Weinberg of the Massachusetts Institute of Technology’s Whitehead Institute for Biomedical Research, who has highlighted the changing emphasis in a revision of his leading textbook, The Biology of Cancer (Garland Science, 2006).
This transformation recognizes that the immune inflammatory state serves as a key mediator of the middle stages of tumor development. Cancer begins with a series of genetic changes that prompt a group of cells to overreplicate and then invade surrounding tissue, the point at which true malignancy begins. Eventually some tumor cells may break off and establish new growths (metastases) at distant sites. That much has been understood for a long time. But cancer biologists and immunologists have begun to realize that the progression from diseased tissue to full-blown invasive cancer often requires cells that normally participate in healing cuts and scrapes to be diverted to the environs of the premalignant tissue, where they are hijacked to become co-conspirators that aid and abet carcinogenesis. As some researchers have described the malignant state: genetic damage is the match that lights the fire, and inflammation is the fuel that feeds it.
In this rewriting of the textbooks, a tumor is not just a clump of aberrant cells; it also includes a support system, a tumor microenvironment, which encompasses a multitude of varying immune cell types and crisscrossing chemical signals, along with a network of blood vessels. The tumor assumes the status of an outlaw organ that exists not to pump blood or rid the body of toxins but to serve only its own ends.
This new view implies that rooting out every last cancer cell in the body might not be necessary. Anti-inflammatory cancer therapy instead would prevent premalignant cells from turning fully cancerous or would impede an existing tumor from spreading to distant sites in the body. Cancer sufferers might then be able to survive, in the same way that new drugs have let HIV patients live longer. “I don’t think a cure is necessarily the goal. It doesn’t need to be,” comments Lisa M. Coussens, a cancer biologist at the University of California, San Francisco. “If you can manage the disease and live your natural life span, that’s a huge win.”
ADVERTISEMENT
Multiple Lines of Defense
Comprehension of the link between inflammation and cancer requires knowing how the body reacts to invaders—and how normal healing is then subverted into promoting cancer when the inflammatory state lasts too long. After you step on a nail, the bacteria that invade the sole of your foot receive a welcome from an array of proteins and white blood cells that resemble rejects from central casting for the movie Creepshow 2. Just one example: Some 20 complement proteins, so called because they complement other bodily defense mechanisms, chemically spritz pathogens until the invaders explode into a big protoplasmic mess. While the complement system slimes the area, an assemblage known in immunology textbooks as professional phagocytes—literally “expert eating cells”—goes to work.
Lacking table manners, these Pac-Man-like macrophages and neutrophils proceed to engulf and consume the uninvited guests. Other members of the attack brigade include natural killer cells, mast cells and eosinophils. Healing represents more than launching an offensive against invaders. Blood platelets involved with clotting migrate to the break in the skin from an inner layer infused with blood vessels. Enzymes direct the repair of the extracellular matrix, the protein-based mortar in which the cells are immobilized. A scab forms, the skin grows back and the whole process of inflammation ends. Sometimes, though, inflammation does not stop. Any tissue (not just skin) that is chronically inflamed because of the persistent presence of pathogens, toxins or genetic damage helps to spur illness, from heart disease to cancer.
Beyond this first layer of defense, vertebrates are equipped with additional weaponry. The adaptive system learns an invader’s specific molecular signature and then uses it as a target for killing. Among the protagonists are B cells, which produce antibody molecules able to neutralize pathogens or mark them for destruction, and T cells, which prompt infected cells to kill themselves or secrete chemicals that direct the activities of other immune players.
In recent years a body of evidence has accumulated to show that chronic inflammation can play an important role in the progression of some types of tumors from a premalignant state to full-blown disease. A link between cancer and inflammation has long been suspected. In 1863 the prominent German pathologist Rudolf Virchow noted the presence of so-called lymphoreticular infiltrate (white blood cells) in malignant tissue. As early as 1978 Alberto Mantovani of Humanitas Clinical Institute and the University of Milan had observed that innate immune cells tend to congregate around some tumors. Cancer biologist Harold F. Dvorak of Harvard Medical School remarked in 1986 that tumors are “wounds that do not heal.” The status quo, though, lay elsewhere. Even a decade ago many biologists still hewed to the idea that the immune system serves not only to eliminate pathogens but to ferret out cells that are the abnormal precursors of cancer. But a closer look at the microenvironment surrounding tumors found the unexpected.
Hunting Pigeons
In the late 1990s Frances Balkwill of the Institute of Cancer at Queen Mary, University of London, had been doing research on a cytokine (a hormonelike immune signaling molecule) known as tumor necrosis factor (TNF), which was named for its ability to kill cancer cells when administered directly into a tumor at high levels. But when TNF lingers as a chronic, low-level presence in the tumor, it acts very differently. Balkwill’s lab turned off the TNF gene in mice so that the rodents could not produce the protein: to their surprise, the mice did not contract tumors. “That really put us as the cat among pigeons,” she recalls. “All the people who were working on TNF as an anticancer agent were horrified. This cytokine they thought was a treatment for cancer was actually working as an endogenous tumor promoter.”
ADVERTISEMENT
The ready availability of knockout mice, in which the effects of selectively switching off genes could be tested, helped to highlight the cancer-inflammation link. Coussens and her U.C.S.F. colleagues Douglas Hanahan and Zena Werb reported in 1999 that mice engineered with activated cancer genes but without mast cells (another type of innate immune cell) developed premalignant tissue that did not progress to full malignancy. In 2001 Jeffrey W. Pollard and his co-workers at the Albert Einstein College of Medicine described mice that were genetically engineered to be susceptible to breast cancer tumors but that produced precancerous tissue that did not turn fully malignant unless it enlisted the assistance of macrophages.
The altered picture does not completely overturn the old one. In fact, it reveals that the immune system functions as a double-edged sword. The network of molecules and cells, second in complexity only to the brain, remains a paradox: sometimes it promotes cancer; other times it hinders disease. Some types of innate immune cells, such as natural killer cells, can actually protect against tumor growth. Others may nurture a malignancy only when the microenvironment is “polarized” into an inflammatory state; when not, they may blot it out. Inflammation, moreover, produces tumors in many organs, but not all—and its link to blood-borne cancers is not well characterized.
When looking for culprits, researchers have often focused their microscopes on macrophages, which occupy a meaningful spot among the white blood cells in the tumor microenvironment. The macrophages are capable of killing tumor cells or sending out an alarm to T cells of the adaptive immune system that something is amiss. But work by Pollard and other researchers has detailed how macrophages are “reeducated” by cancer cells to do their bidding. They become factories for cytokines and growth factors that nurture tumor development.
Turning the macrophages into traitors begins when tumor cells send out help signals that attract cells that become macrophages once they reach the tumors. Inside the tumors, proliferating cells grow so quickly that they begin to die for lack of oxygen. A combination of hypoxia and messages from the tumor cells initiates a process whereby the newly arrived macrophages assume their bad-body identity as tumor promoters. Cancer biologists give the name tumor-associated macrophages to these mutineers that congregate in and around the tumor.
Biologists have now been able to follow the inflammation link down to the level of individual signaling molecules, providing harder evidence for a connection to carcinogenesis. For example, nuclear factor-kappa B (NF-KB) is a complex of proteins that acts as a master switch for turning inflammation genes on and for controlling cell death. As biological pathways go, NF-KB’s is world-famous, having been discovered and patented for use in drug development by scientific stars that include Nobelists David Baltimore and Phillip A. Sharp and having subsequently become the object of multimillion-dollar patent litigation.
ADVERTISEMENT
In 2004 Yinon Ben-Neriah and Eli Pikarsky of the Hebrew University of Jerusalem and their colleagues reported that mice engineered to develop hepatitis (which can cause liver cancer) contracted precancerous lesions that did not progress to full malignancy when NF-KB was curtailed through a genetic alteration or when the proinflammatory TNF signaling molecule was shut off. In the latter group, a neutralizing antibody blocked TNF and prevented it from binding to a receptor on the premalignant liver cells; loss of the receptor prevented the TNF from triggering a molecular cascade that turns on the NF-KB master switch. Blocking NF-KB prompted the precancerous liver cells to initiate apoptosis, or programmed cell death. In a related finding that year, Michael Karin and his collaborators at the University of California, San Diego, found that inhibiting NF-KB in mice engineered to develop colitis, which can lead to colon cancer, also promoted apoptosis. And shutting down the pathway in inflammatory cells, such as macrophages, deterred tumor development as well.
So far the clearest evidence of a link between cancer and inflammation is the data demonstrating that inflammation encourages the conversion of precancerous tissue to full malignancy for many cancers. But the biological response may also be involved in initiating the disease and in advancing metastasis. Infections with Helicobacter pylori bacteria induce inflammation that greatly increases the risk of gastric cancer, and the hepatitis C virus can bring on liver cancer, to name just two cancers. Pathogens may also generate free radicals, which can damage DNA. But although inflammation may be involved from the outset, few studies have shown yet that an inflammatory condition actually alters DNA to provide the initiating spark.
The case for a role in metastasis is stronger—and recent studies lend credence to this hypothesis. Karin’s group reported in the April 5 Nature that inflammation, not genetic changes in cancer cells, spurs metastasis in mice engineered to acquire prostate cancer. The research suggests that a cytokine produced by inflammatory cells near a prostate tumor induces tumor cells to decrease production of a protein that blocks metastasis. This result, Karin notes, may explain the puzzling observation that cutting into tumors, such as for a prostate biopsy, sometimes seems to encourage metastasis. If he is correct, the inflammation generated by the intervention could be at fault. Around the same time, Pollard’s group reported in Cancer Research on a study in mice that observed that macrophages accompany breast tumor cells in their migration toward blood vessels that will transport them to remote sites, all the while sending chemical messages to their partners.
The innate immune system has received the most attention in explorations of how inflammation might cause cancer. As with innate immunity, the adaptive immune system—the T cells and antibodies produced by B cells that target specific molecules on invading cells—contributes to pathology or may also fight against it. For decades, immunotherapies designed to enhance T cell responses against cancer have been explored, though often with disappointing results.
Furthermore, an emerging picture has begun to reveal an intricate cross talk between innate and adaptive immune cells that may participate in the promoting of malignant disease. Researchers working on cancer vaccines may need to take account of these interactions in designing their treatments if they are ever to prove effective. One study showed that ovarian tumors produce a signaling molecule that serves to attract regulatory T cells, a subclass of adaptive immune cells responsible for quieting other T cells [see “Subduing Suppressors,” by Lisa Melton; Scientific American, December 2002].
ADVERTISEMENT
Meanwhile Coussens and her colleagues at U.C.S.F. found in a 2005 study published in Cancer Cell that the removal of antibody-making B cells from mice engineered to be prone to skin cancer prevented the tissue changes and angiogenesis that are prerequisites for disease progression. In their normal role as pathogen fighters, B cell–produced antibodies circulate through the bloodstream and mark viruses and bacteria for destruction by innate immune cells. In response to a signal from precancerous tissue, however, the antibodies induce the innate system to collaborate in cancer development. An open research question is how this process starts. One possibility suggests that a cancer cell may send a message to innate immune cells, perhaps dendritic cells, that then activate B cells. Signaling may involve toll-like receptors, which have emerged as prominent intermediaries in innate immune messaging [see “Immunity’s Early Warning System,” by Luke A. J. O’Neill; Scientific American, January 2005].
Cancer Blockers
The recognition that cancer is more like an organ than just a clump of cells with DNA mutations in cell nuclei may also explain why some of the previous approaches to chemotherapy have met with limited success. “People have taken cells and then transformed them in culture and stuck them into animals,” Pollard says. “They grow as little balls. They do certain things there. But they are not complex tissues, whereas a naturally occurring tumor is a very complex tissue.”
Instead of just killing cancer cells—the goal of current drug therapies and radiation—new approaches may supplement existing drugs by slowing inflammation. Without the involvement of macrophages and other innate cells, the premalignant tissue would remain in check.
Cancer could, in essence, become a chronic disease akin to rheumatoid arthritis, another inflammatory condition. “Keep in mind almost no one dies of primary cancer,” says Raymond DuBois, provost of the University of Texas M. D. Anderson Cancer Center and a researcher of anti-inflammatory agents for cancer. “A patient almost always dies from a metastasis.”
A pharmaceutical against chronic inflammation represents a more alluring proposition than massacring malignant cells (and, unavoidably, healthy ones), a consequence of existing chemotherapies. Taken alone, such an agent might be benign enough to use every day as a preventive for high-risk patients. Epidemiological and clinical studies have shown some promise for the use of nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin to stave off the onset of some solid tumors. Investigations continue on more selective blocking of the production of prostaglandins, the regulatory molecules that are curtailed by NSAIDs. In particular, drugs that inhibit production of prostaglandin E2 may curb inflammation and tumor growth, while avoiding the cardiovascular side effects of drugs such as Vioxx and the gastrointestinal problems of the earlier class of NSAIDs [see “Better Ways to Target Pain,” by Gary Stix; Scientific American, January 2007]. The anti-inflammatory effects of the ubiquitous statins used to lower cholesterol are also being contemplated.
Some treatment options already exist. The drug Avastin inhibits production of the angiogenesis- promoting VEGF, although oncologists must contend with other molecules in the tumor microenvironment that promote blood vessel growth. Drugs developed for more familiar inflammatory diseases may also fight cancer—and these medicines might be combined into HIV-like drug cocktails, that also include angiogenesis inhibitors and cell-killing agents.
Inhibitors of TNF have received approval for treatment of rheumatoid arthritis, Crohn’s disease and other disorders and are now in clinical trials for both solid tumors and blood cancers. The drug Rituxan, a monoclonal antibody that represses B cells in rheumatoid arthritis and B cell lymphoma, might prevent the inflammatory response that fuels formation of solid tumors. Other cytokines and related molecules (IL-6, IL-8 and CCL2, among others) are also potential targets, as is NF-KB.
Some existing compounds, including NSAIDs and even one found in the spice turmeric, exert at least some of their effects by inhibiting NF-KB. But major pharmaceutical laboratories are investigating highly selective inhibitors of this molecular linchpin, many of them targeted at the enzymes (such as I-KB kinase) that regulate NF-KB activity.
A Chemical Trojan
One group is contemplating a radically ambitious treatment, a molecular Trojan horse of sorts. Claire Lewis and Munitta Muthana of the University of Sheffield in England and their colleagues have designed a drug delivery scheme that takes advantage of the natural attraction of macrophages to the oxygen-starved areas in tumors. They have engineered macrophages to deliver a therapeutic virus to hypoxic tumor regions, which respond poorly to conventional treatments such as chemotherapy and radiation because of an insufficient blood supply. Once the macrophages arrive in a tumor (grown in culture so far), each one releases thousands of copies of the virus, which then infect the cancer cells, after which a protein in those cells activates the therapeutic gene in each virus. This action then directs synthesis of a cell-killing toxin. “The macrophage is migrating into a site and doing what we want it to do rather than driving tumor development in a normal way,” Lewis says.
The exact outlines of an anti-inflammatory strategy against cancer have yet to be elucidated. Tweaking immune cells that form a defensive barrier against pathogens bears its own risks. “It’s a very complicated issue,” DuBois notes. “If you magically shut down the immune system, you will have problems with opportunistic infections, just like with AIDS.” Use of TNF blockers in other inflammatory disorders has been linked to tuberculosis and other infections, even potentially lymphoma. Moreover, inhibiting the NF-KB pathway can paradoxically promote cancer in some instances. Constraining NF-KB can at times lead to tissue damage and a process of abnormal regeneration of that tissue that can foster cancer.
Still, it seems likely that a new generation of anti-inflammatory agents will join the chemotherapeutic arsenal. Chronic diseases—and their underlying inflammatory conditions—are hallmarks of an aging population. “We’re all a little bit overinflamed,” Pollard observes. Treating the smoldering embers that surround the tumor rather than just mutant cells could make cancer a disease we can live with.
https://www.scientificamerican.com/article/chronic-inflammation-cancer/
.png)


.png)
.png)