建立一个新的家园——癌细胞如何扩散到新的器官 (肿瘤微环境系列 5)
一些次生器官欢迎进入的肿瘤细胞。
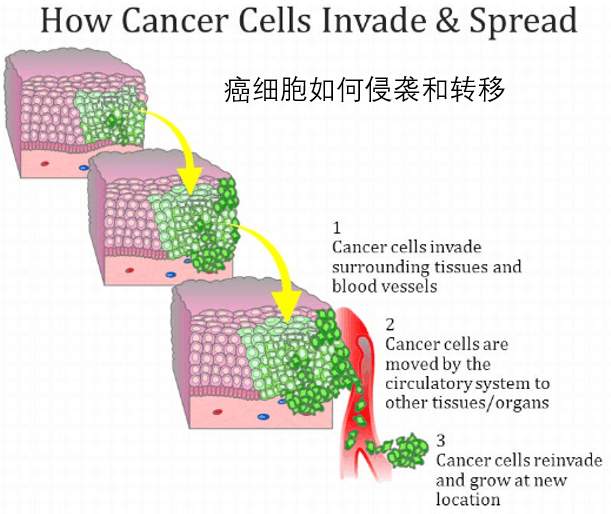
在本系列的前一篇文章中,我们讨论了肿瘤微环境是如何帮助肿瘤细胞离开原发肿瘤并在血流中起程的。
绝大多数这些漂浮的细胞最终到达了一个次要的器官,在那里他们需要安定下来并定居——或者死亡。
但是,在一个次要器官中定居对癌症细胞来说是一个巨大的挑战——如果没有“友好”当地人的帮助,他们就无法克服这种挑战。
在这里,我们将看到原发肿瘤和次级器官是如何形成“转移龛metastatic niche”的,这是一种受欢迎的、可繁殖的微环境,肿瘤细胞可以在那里定居、生长和繁殖。
那些有洞察力的旅行者
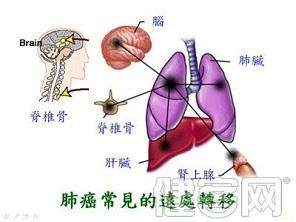
医生们早就知道肿瘤会扩散到特定的器官。例如,乳腺癌通常会扩散到肺部、肝脏和大脑,而前列腺癌通常会导致肝脏和骨骼。
但是是什么让肿瘤如此的挑剔呢?
早在1889年,大多数医生认为,在随机选择的次级位点上,癌细胞只会在它们碰巧被洗掉的地方扎营。但这一理论并没有完全正确地与一位年轻的英国外科医生斯蒂芬·佩吉特(Stephen Paget)的名字相提并论。所以他做了任何有自尊心的科学家都会做的事——他做了一些研究。
肿瘤细胞对它们最终的归宿有选择性
佩吉特查看了700多名乳腺癌患者的详细尸检记录。他注意到,乳腺肿瘤更容易扩散到肝脏——这是不能用血液流动来解释的——因为脾脏(与肝脏有相似血流的器官)几乎从未见过转移。
佩吉特在一篇论文中提出了他的观点,他提出“种子”(一种转移瘤细胞)只能生长在合适的“土壤”中。或者换句话说:对第二器官的选择没有任何随机的——一些器官只是比其他器官更适合。
那么是什么使得这些特殊的器官如此特别呢?简而言之,我们并不知道——但在这个领域的研究发现了一些有趣的发现。
找到当地的热点
令人难以置信的是,一些研究表明,生长肿瘤会将化学信息传递到肺——这是一个常见的转移部位——有效地“提前打电话”,以确保第一个肿瘤细胞到达的准备工作已经到位。研究人员还发现,在被称为骨髓细胞的细胞的帮助下,肺的反应是启动炎症,而游走的肿瘤细胞是无法抗拒的。
骨髓细胞也与局部组织合作,为即将到来的癌细胞提供舒适的口袋。
这些发生在肿瘤细胞之前的次级器官的早期变化产生了科学家所说的“前转移龛”——生物学中一个相对较新的概念。这与佩吉特挑战的随机转移模型相去甚远,因为它是基于一种观点,即原发肿瘤控制其后代的去向。
但是,当转移性龛开始工作时,会发生什么呢?
可用房间-转移利龛
前转移龛为肿瘤细胞的到来做准备。
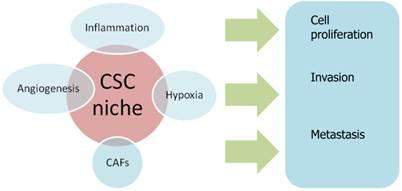
癌细胞龛是癌细胞增殖侵袭和转移的种子
当第一个肿瘤细胞到达的时候,前转移龛就变成了转移龛,它在决定肿瘤细胞命运方面的作用变得比以往任何时候都更加重要。
研究表明,其他的局部细胞类型——如成纤维细胞和内皮细胞——会使粘粘的、胶状的分子帮助肿瘤细胞附着在新环境上。
研究人员还认为骨髓细胞有助于保护肿瘤细胞免受巡逻免疫细胞的侵袭。我们并不完全了解他们是如何做到这一点的,但有证据表明,它们产生的分子会抑制免疫系统——使肿瘤细胞得以休息,并在未被检测到的情况下恢复。
换句话说,当肿瘤细胞到达时,次级器官会努力工作以保持它们的安全。
当地人聊天
但是安全的对接目标器官是很容易的——真正的挑战是学习适应和在陌生环境中茁壮成长。然而,成功再次取决于与当地社区的富有成效的互动。
首先,肿瘤细胞必须抑制自我毁灭的冲动,而这种自我毁灭通常是在异国他处出现的。但是,新环境下的欢迎信号被认为有助于缓和和消除疑虑。
局部组织也是“生长因子”的便利来源——细胞依赖于生长和发育的分子。这些都是无价的,因为肿瘤细胞可以在构建自己的血液供应和形成自己的殖民地的关键任务上发挥作用。
"让好客变成不好客"
研究总是进入未知领域,最好的研究往往会抛出更多的问题而不是答案。
我们还有很多问题要问。我们需要了解目标器官是如何被肿瘤选择的,我们必须破解不同癌症类型的主要肿瘤和靶器官之间的分子间的交流。
因此,我们还有很长的路要走,但仔细分析微环境的作用,应该会填补我们对这个复杂而致命的过程的理解。
潜在的回报是巨大的。在未来,我们甚至可以使次级器官“驱逐”肿瘤细胞停止转移,甚至在它有机会站稳之前。鉴于大约90%的癌症死亡是由转移引起的,这种方法可以挽救成千上万人的生命。
斯蒂芬·佩吉特于1926年逝世,享年71岁。在他去世的时候,科学界还没有完全接受他的观点,但今天,他被认为是癌症生物学中一个“最热门话题”的先驱和父亲。遗憾的是,他没有活着看到他标志性的“土壤和种子”理论结出硕果。
https://s.click.taobao.com/hYsTINw
参考文献
A home from home – how cancer cells spread to new organs
Category: Science blog June 4, 2013 Safia Danovi2 comments
This entry is part 5 of 5 in the series Microenvironment
Welcome_mat_2
Some secondary organs welcome incoming tumour cells
In our previous post in this series, we talked about how the tumour microenvironment helps tumour cells leave a primary tumour and set sail along the bloodstream.
The vast majority of these drifting cells eventually arrive at a secondary organ where they need to settle and establish themselves – or die.
But settling in a secondary organ presents a huge challenge for cancer cells – and it’s one they can’t overcome without help from ‘friendly’ locals.
Here, we’ll see how the primary tumour and the secondary organ conspire to create the ‘metastatic niche’ – a welcoming and fertile microenvironment where tumour cells can settle, grow and colonise.
The discerning traveller
Doctors have known for a long time that tumours tend to spread to specific organs. For example breast cancers often spread to the lungs, liver and brain while prostate cancers usually head for the liver and the bone.
But what makes tumours so picky?
Back in 1889, most doctors believed that secondary sites were chosen at random and cancer cells merely set up camp wherever they happened to wash up. But this theory didn’t quite sit right with a young British surgeon by the name of Stephen Paget. So he did what any self-respecting scientist would do – he did some research.
Bluff_signpost
Tumour cells are selective about where they end up.
Paget looked at the detailed autopsy records of more than 700 patients with breast cancer that had spread. He noticed that breast tumours spread to the liver more than any other organ in the body – something that couldn’t be explained by blood flow – because the spleen (an organ neighbouring the liver with a similar blood flow) – almost never saw metastases.
Paget wrote his ideas up in a paper proposing that ‘the seed’ (a metastasising tumour cell) could only grow in suitable ‘soil’. Or in other words: there was nothing random about the choice of secondary organ at all – some organs were simply more hospitable than others.
So what makes these particular organs so special? The short answer is that we don’t really know – but research in this area has unearthed some fascinating findings.
Finding the local hotspots
Incredibly, some research suggests that growing tumours send chemical messages to the lung – a common site of metastasis – effectively ‘phoning ahead’ to ensure that preparations for the arrival of the first tumour cells are in place. The researchers also found that lungs responded – with the help of cells called myeloid cells – by kick-starting inflammation which wandering tumour cells find irresistible.
Myeloid cells also collaborate with local tissue to carve out cosy pockets in which incoming cancer cells can lodge.
These early changes in secondary organs which occur before tumour cells arrive create what scientists call the ‘pre-metastatic niche’ – a relatively new concept in biology. It’s a far cry from the random model of metastasis that Paget challenged because it’s based on the idea that the primary tumour controls where its seedlings go.
But what happens when the pre-metastatic niche opens for business?
Rooms available – the metastatic niche
Vacancies
The pre-metastatic niche prepares for the arrival of tumour cells.
When the first tumour cells arrive, the pre-metastatic niche becomes the metastatic niche and its role in determining tumour cell fate becomes more crucial than ever before.
Research suggests that other local cell types – such as fibroblasts and endothelial cells – make sticky, glue-like molecules to help tumour cells attach to their new surroundings.
Researchers also think myeloid cells help protect tumour cells from patrolling immune cells. We don’t completely understand how they do this, but there’s some evidence that they produce molecules which dampen the immune system – allowing tumour cells to rest and recuperate undetected.
In other words, when tumour cells arrive, secondary organs work hard to keep them there and keep them safe.
Chatting to the locals
But docking safely to the target organ is the easy bit – the real challenge is learning to adapt and thrive in a foreign environment. Yet again, success depends on productive interactions with the local community.
For starters, tumour cells have to quash the urge to self-destruct that usually comes with being alone in a foreign land. But again, welcome signals from its new environment are thought to help soothe and reassure.
Local tissue is also a handy source of ‘growth factors’ – molecules that cells depend on to grow and thrive. These are invaluable as tumour cells get to work on the crucial task of building their own blood supply and forming colonies of their own.
Making the hospitable inhospitable
Rejected
In the future, we could stop metastasis from happening.
Research is always a voyage into the unknown and the best studies often throw up more questions than answers.
We’ve still got big questions to address. We need to understand how target organs are chosen by tumours, and we’ve got to decipher the molecular chatter between the primary tumour and the target organ in different cancer types.
So we’ve got a long way to go but dissecting the role of the microenvironment should fill large holes in our understanding of this complex and deadly process.
And the potential rewards are significant. In the future, we might even be able to make secondary organs ‘deport’ tumour cells – stopping metastasis before it even has a chance to take hold. Given that around nine in ten deaths from cancer are caused by metastasis, this approach could save thousands of lives in the future.
Stephen Paget died in 1926 at the age of 71. At the time of his death, the scientific community had yet to fully embrace his ideas but today, he’s rightfully recognised as a pioneer and the father of one of the ‘hottest topics’ in cancer biology. It’s just a shame that he didn’t live to see his landmark ‘soil and seed’ theory bear fruit.
Safia Danovi
Reference
Psaila, B., & Lyden, D. (2009). The metastatic niche: adapting the foreign soil Nature Reviews Cancer, 9 (4), 285-293 DOI: 10.1038/nrc2621
.png)
.png)
.png)
.png)
.png)