小心: 太咸会损伤血管内壁-导致心血管疾病
原标题:食用盐会损伤内皮糖蛋白层-你可能从未听说过的最重要的心血管疾病风险因素
DIETARY SALT IMPAIRS THE ENDOTHELIAL GLYCOCALYX: THE MOST IMPORTANT CARDIOVASCULAR DISEASE RISK FACTOR YOU MAY NEVER HAVE HEARD ABOUT
---在中国,心肌梗塞和中风发病率最高的地区是山东、河南和湖北,是人均食盐最多的省份;在美国发表的一个研究报告发现,进食过多的食盐和40%的总死亡人数有关。译者---
By Loren Cordain, PhD, Professor Emeritus 2017年2月5日
科学家们经常有一些重要的生理学发现,逃过大众、健康专家、甚至其他科学家的视线。这就是内皮糖蛋白层(EGL)的情况:在所有血管的内表面形成的一层纤弱的结构。
早在1940年代就从血流中推测内皮糖蛋白层(Endothelial Glycocalyx)(EGL)的存在,但由于其纤弱的结构,直到1966年当它第一次被发现与电子显微镜使用特殊的染色过程,它从来未被证实, (1)。在接下来的30年里,科学家们推测内皮糖蛋白层的功能,但直到1996年,两位研究者,Vink和Duling(2),提供了一个答案。他们的结论是,内皮糖蛋白层可能代表了血液和毛细血管壁之间真正的活跃界面.”
50年前,一种纤弱的血管结构首次被视觉化,随后科学家又花了30年时间,才将许多人认为是次要的功能分配给它。但是内皮糖蛋白层的功能真的那么微不足道吗?
事实上,内皮糖蛋白层(EGL)非常重要,它的功能障碍在动脉粥样硬化的几乎每一环节都起着关键的作用——导致心脏病和中风的过程。如你所见,饮食中的钠可以直接导致这种功能紊乱并引发疾病。
可视化内皮糖蛋白层
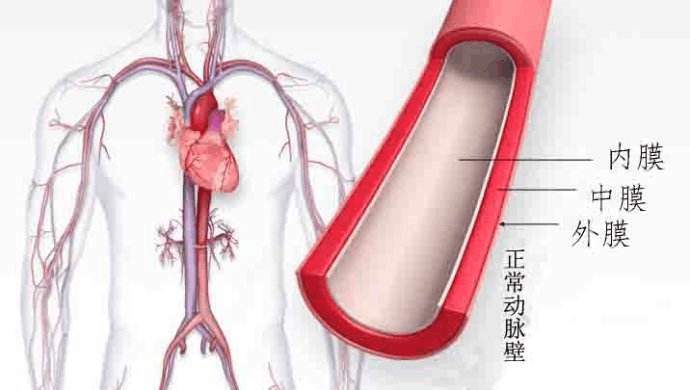
我倾向于像许多人一样,是一个视觉学习者。让我们来看看动脉的横截面,这是许多心脏病学家在1996年之前想象的。下面的图1显示了动脉内壁的内层是内皮细胞。然而,看不到的是内皮糖蛋白层。这种对血管结构的认知一直持续到1994年,当时,一组科学家开发了一种新的染色程序(alcian blue),以便更好地观察内皮细胞的糖蛋白层。
图1 经典的动脉横截面图。
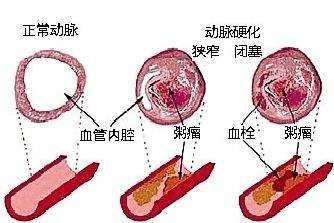
缺乏内皮糖蛋白层的血管的普遍印象使研究心血管疾病的科学家们对动脉粥样硬化过程的一个不完美的认识,动脉粥样硬化心脏病和中风的基础。图2显示了在没有内皮糖蛋白层的血管中动脉粥样硬化的普遍观点。
图2 动脉粥样硬化的经典形象化,无内皮糖蛋白层。
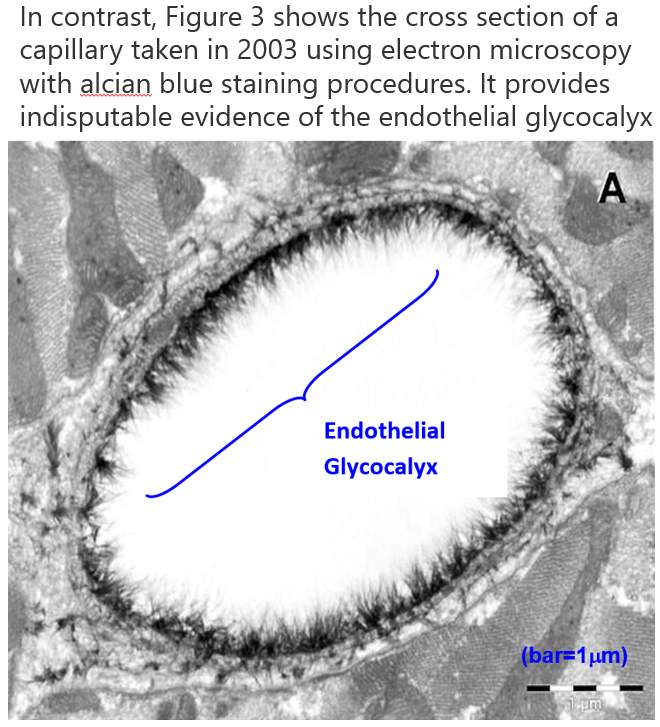
相比之下,图3显示了在2003年使用电子显微镜使用alcian blue染色程序的毛细血管的横截面。它提供了无可争辩的证据,证实了内皮细胞的内皮糖蛋白(3)。
图3 用电子显微镜观察一名alcian蓝染色大鼠左心室心肌毛细血管显示内皮糖蛋白的情况(3)。
内皮糖蛋白层(EGL)的功能
1996年标志着一个重要的拐点,心血管生理学家认识到, 内皮糖蛋白层(Endothelial Glycocalyx) 担任“血液和毛细管壁之间的真正活跃的接口”(2)。在接下来的20年里, 一个巨大的科学文献表明, 这个纤弱的结构对保持健康,幸福和远离心血管疾病(CVD)非常重要(1-51)。
那么,这些纤弱的内皮糖蛋白层究竟对我们的整体健康和健康有什么重要意义呢? 早期,很明显内皮细胞糖蛋白层是一种屏障结构,以防止循环的红细胞与血管内壁1-4、9、12)的内皮细胞接触。糖蛋白层的进一步功能已被确认,包括防止循环的白细胞(白细胞)和血小板(血液凝血成分)附着在组成血管内壁的内皮细胞(4-6、12、15、17、18、26、33)。糖蛋白层还对血管的渗透性(6,12)进行调节,包括低密度脂蛋白胆固醇(LDL胆固醇)(20、22、28)和血液流动剪切应力信息(湍流或非紊流)传递给内皮细胞(1、7、21、23)。
最后,糖蛋白层可以调节炎症过程(8,14,19,77)。糖蛋白层是一种动态结构,它会因炎症反应(8,14,19,77)和其他因素而脱落,但也不断地重新合成。糖蛋白层脱落的过程是对 损伤或感染的正常反应,因为它允许白细胞浸润内皮细胞并进入动脉内膜,在那里这些免疫细胞可以开始疗愈,或清除受损或病变的血管组织。
内皮糖蛋白层和动脉粥样硬化
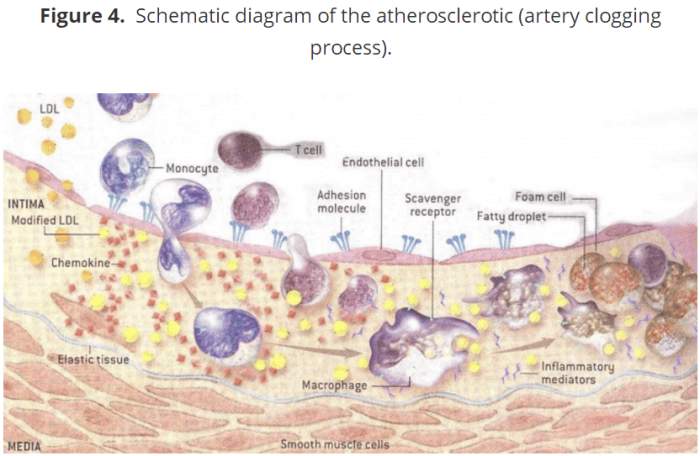
下面的图4是动脉粥样硬化过程的图解视图,它阻塞动脉,导致心脏病发作和中风。在图的顶端,有一个动脉的内部(腔)被描绘成一个已经脱落的糖蛋白层。注意扁平内皮细胞层。
这代表了血源的内在最大障碍。内皮细胞层下面是内皮细胞层,称为内膜,是动脉粥样硬化斑块形成和积累的地方。同时,注意LDL胆固醇分子进入内膜。在正常情况下,它们被一种完整的内皮糖蛋白层限制在循环血流中,这阻碍了它们进入内膜(20,22,28)。
图4 动脉粥样硬化(动脉阻塞过程)的原理图
当动脉内膜形成动脉粥样硬化斑块时,一系列关键的步骤最终会导致心血管疾病:
1) 炎症(8,14,19,77)导致内皮糖蛋白层脱落(14-29),促进炎症。
2) 在动脉粥样硬化斑块形成的湍流血流(1、7、21、23)区域中,糖蛋白层变薄。
3) 糖蛋白层的脱落可以促进LDL胆固醇从血液进入内膜(20,22,28)。
4) 低密度脂蛋白胆固醇(LDL)被氧化,并继续促进糖蛋白层脱落。
5) 白细胞、单核细胞、T细胞、其他白细胞和血小板附着于内皮细胞表面的粘附分子(4-6、12、15、17、18、26、33),然后进入内膜(INITMA)。
6) 进入动脉内膜后,一种称为单核细胞(Monocyte)的特定类型的白细胞被转化为巨噬细胞(Macrophage)。
7) 巨噬细胞消耗氧化的低密度脂蛋白胆固醇和其他元素成为肥大细胞。
8)随着时间的推移和持续的炎症,这些肥大细胞(Foam Cell)成为阻塞动脉的动脉粥样硬化斑块。
9)最终在斑块上形成纤维帽,如果通过持续的炎症过程破裂,会导致心脏病发作和中风。
动脉粥样硬化的过程比这些简单的九个步骤要复杂得多,但这些步骤为你提供足够的信息,以了解高盐饮食如何在几乎每一步都促进动脉粥样硬化。
然而,在我向你展示高盐饮食如何损害内皮糖细胞和促进心血管疾病、心脏病和中风之前,必须知道一个更重要的细节。
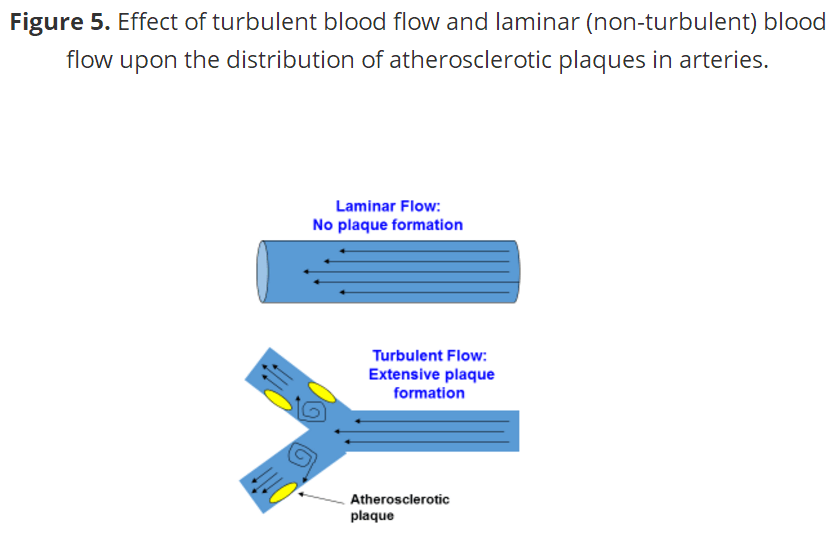
动脉粥样硬化斑块的解剖位置是动脉粥样硬化过程的另一个透露真相的线索。早在1971年,C.G.卡罗和他的同事(52,53)就表明早期的动脉粥样硬化病变几乎完全发生在动脉的 分叉(分支)附近的湍流血流中,很少出现在非紊流(层流)血流中。这一事实已在所有关于动脉粥样硬化的现代研究中得到证实(1、7、21、21、23、53)。图5显示了在湍流流动区域内斑块形成的现象,其中内皮糖蛋白层的厚度和密度降低。目前的证据表明,动脉粥样硬化发生在这些湍流的区域,因为它们比非湍流的区域更难以对血流中的炎症条件作出反应,从而促进了糖蛋白层的脱落(8、14、19、77)。
图5 血流和层流(非紊流)血流对动脉粥样硬化斑块分布的影响。
高盐饮食如何促进动脉粥样硬化和心血管疾病(CVD)
正常情况下,血浆中钠的浓度维持在一个狭窄的范围内,从134到148 mmol/L(54-56)。在没有高血压的人群中增加食用盐可以使正常范围内或超出正常范围内的血浆钠浓度升高(57,58)。
在旧石器饮食(原始人饮食)社区,一种普遍的看法是,在旧石器饮食中加入盐,特别是海盐,对健康和健康几乎没有负面影响(59-68)。然而,数据显示,盐,甚至添加到正常血压 者的饮食中,会升高血浆钠的浓度,对健康有重要而深远的影响,尤其是如果用盐的习惯在整个生命中都是持续的。
在2007年对体外培养的活体内皮细胞进行的实验表明,低于 135 mmol/L时,细胞的硬度不受影响; 但在135 - 145 mmol/L(70)的浓度下,细胞硬度迅速上升。此外,通过在日常饮食中添加盐(54-58),更高的血浆钠浓度(143-150 mmol/L)很容易地在人体中实现,即使大部分仍在正常范围内,仍会导致内皮细胞变硬并减少其一氧化氮(NO)(70,72)的产生,一氧化氮(NO)是一种在全身发现的化合物。在血管中,一氧化氮抑制细胞炎症和白细胞和血小板粘附于内皮细胞(71)。通过这种方式, 产生一氧化氮的正常动脉内皮细胞抑制血液凝块和促进正常的血液流动。一氧化氮的代谢和产生随着年龄和心血管疾病(CVD)(71)而减少。
在2007年(70)进行的体外实验中,其中一个问题是内皮细胞不仅与钠一起孵育,而且还与一种叫做醛固酮的激素一起孵育。因此,对实验的解释是混乱的。是钠,醛固酮,还是两者都导致了内皮细胞的硬化和一氧化氮的减少?在随后的几年里,其他研究人员复制了这些结果(46,72),但问题仍然存在:是什么导致 了内皮功能功能障碍和影响一氧化氮的产生?
这一问题最终于2016年在一个活体(体外)小鼠模型中得到了解答,该模型的基因突变阻止了它合成醛固酮(醛固酮合酶敲除小鼠)(69)。高盐饮食,无醛固酮,增加血浆钠到150 mmol/L,增加内皮细胞的硬度44%,抑制一氧化氮的产生。这些结果与最近的两项随机对照人体试验相一致,这些试验表明高盐饮食(65 mmol Na)增加了血浆钠的浓度,降低了内皮功能,同时增加了动脉硬化(74,75)。
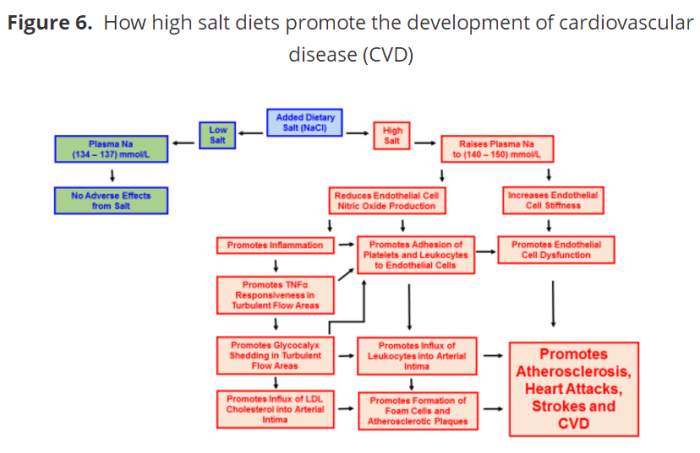
在活的动物模型(体内),高盐饮食喂养老鼠增加动脉内皮细胞,对在湍流动脉区域内的促炎细胞因子(TNFα)的响应性。高盐饮食也增加了白细胞粘附在这些相同的湍流的区域(76)。这两种生理变化都是人类心血管疾病发展的特征。正如我前面所提到的,高盐饮食促进炎症损害一氧化氮的释放(69、70、72),而TNFα直接导致内皮细胞脱落glycocalyx(8日,14日,19日,77)。因此,由高盐饮食引起的炎症所引起的糖蛋白层脱落促进了动脉粥样硬化斑块的形成和 心血管疾病的发展。图6总结了高盐饮食对CVD发展的影响。
在涉及12779名受试者的社区研究中,来自动脉粥样硬化风险的临床数据显示,血浆钠浓度是10年冠心病风险的重要预测指标(77)。这些发现表明,即使在正常生理范围内(134 ~ 148 mmol/L),血浆钠的升高也伴随着心血管的变化,从而促进CVD(77)的发展。
实际的暗示和最终的想法
也许这一最新的、巨大的、有说服力的科学文献中最明显的暗示,就是不要在你在健康的、自然的、新鲜的石器时代食物和食谱中加入盐或海盐。未掺假、天然、新鲜的旧石器(原始人)食材的钠含量很少。在之前的一篇博客中,我已经演示了基本上不可能从天然的、不含盐的石器时代食物中摄取超过2300毫克的钠。从这张表中,我们还可以看到,天然的、没有盐的史前饮食的钾含量通常是钠的5倍多。
高饮食水平的钾可以通过软化血管内皮细胞(78)、增加一氧化氮(78)和防止钠诱导的内皮功能损伤(75)来克服钠的许多不良心血管效应。因此,从实际的角度来说,不可能总是避免食用添加了盐的食物。如果是这样的话,那就试着用富含钾的水果和蔬菜来 中和高盐的食物的影响。我最喜欢的高钾食物有桃子、樱桃、香蕉、枣子、葡萄干、无花果干、山核桃、榛子、西红柿、蒸椰菜和夏季南瓜。
另一种有效的方法是多喝水。对于一个血浆钠浓度为143 mmol/L的人来说,在六到八小时内喝一升水可以将这个值降低到137 mmol/L的低水平,如果不摄取没有额外的高盐食品(77)。
所以,带回家的信息来防止盐引起的CVD是这样的
:1)不要把盐或海盐添加到新鲜,健康的石器时代食品;
2)尽量避免腌制、加工食品。如果你正好沉溺于盐的食物中:
3)多吃富含钾的水果和蔬菜,多喝水。
https://s.click.taobao.com/uuzaKNw
https://s.click.taobao.com/zcxaKNw
参考文献:
DIETARY SALT IMPAIRS THE ENDOTHELIAL GLYCOCALYX: THE MOST IMPORTANT CARDIOVASCULAR DISEASE RISK FACTOR YOU MAY NEVER HAVE HEARD ABOUT
Loren Cordain, PhD, Professor Emeritus
Posted on February 5, 2017
Frequently, important physiological discoveries made by scientists who study obscure topics escape the attention of the general public, health professionals, and even other scientists. Such has been the case for the endothelial glycocalyx: a delicate and fragile structure lining the inside surface of all blood vessels.
The endothelial glycocalyx had been inferred from blood flow measurements as far back as the 1940’s, but due to its fragile configuration the structure had never been viewed until 1966 when it was first detected with an electron microscope using special staining procedures (1). Over the next 30 years, scientists speculated about the function of the endothelial glycocalyx, but it was not until 1996 that two researchers, Vink and Duling (2), provided an answer. They concluded that the endothelial glycocalyx “may represent the true active interface between blood and the capillary wall”.
Okay, no big deal. A tiny and fragile blood vessel structure was first visualized 50 years ago and then it took scientists another 30 years to assign what many would consider a minor function to it. But is the function of the endothelial glycocalyx really that minor?
In fact, the glycocalyx is so important that its dysfunction plays a critical role in almost every step of atherosclerosis – the process that leads to heart disease and stroke. And as you’ll see, dietary sodium can directly contribute to this dysfunction and start the disease process.
VISUALIZING THE GLYCOCALYX
I tend to be a visual learner, as do many people. So, let’s take a look at the cross section of an artery, the way many cardiologists visualized it prior to 1996. Figure 1 below shows that the inner-most surface of arteries is lined by cells called the endothelium. However, nowhere in sight is the endothelial glycocalyx. This perception of blood vessel structure was commonly held until about 1994 when a select group of scientists developed a new staining procedure (alcian blue) to better visualize the endothelial glycocalyx (1).
Figure 1. Classical visualization of an artery cross section
Figure 1 Classic Artery Visualization
The widespread impression of blood vessels devoid of an endothelial glycocalyx gave scientists studying cardiovascular disease an imperfect view of the atherosclerotic process which underlies heart attacks and strokes. Figure 2 shows the commonly held perspective of the day for atherosclerosis in a blood vessel without an endothelial glycocalyx.
Figure 2. Classical visualization of atherosclerosis without the endothelial glycocalyx.
Figure 2 Classic Atherosclerosis
In contrast, Figure 3 shows the cross section of a capillary taken in 2003 using electron microscopy with alcian blue staining procedures. It provides indisputable evidence of the endothelial glycocalyx (3).
Figure 3. Electron microscope overview of an alcian blue stained rat left ventricular myocardial capillary showing the endothelial glycocalyx (3).
Figure 3 Electon Micography of Glycocalyx
FUNCTION OF THE ENDOTHELIAL GLYCOCALYX
1996 marked a pivotal year when cardiovascular physiologists recognized that the endothelial glycocalyx served as “the true active interface between blood and the capillary wall” (2). In the ensuing 20 years, an enormous body of scientific literature has emerged showing that this tiny, fragile structure is of utmost importance in maintaining health, wellbeing, and freedom from cardiovascular disease [CVD] (1-51).
So just what does the fragile endothelial glycocalyx specifically do that is so important to our overall health and wellbeing? Early on, it became apparent that the endothelial glycocalyx serves as a barrier structure to prevent circulating red blood cells from contacting the inner-most endothelial cells lining blood vessels (1-4, 9, 12). Further functions of the glycocalyx have been identified including the prevention of circulating white blood cells (leukocytes) and platelets (blood clotting components) from adhering to the endothelial cells that make up the lining of blood vessels (4-6, 12, 15, 17, 18, 26, 33). The glycocalyx also regulates permeability (6, 12) of blood vessels to blood borne elements including LDL cholesterol – the “bad” cholesterol (20, 22, 28) and transmission of blood flow shear stress information (turbulent or non-turbulent flow) to endothelial cells (1, 7, 21, 23).
Finally, the glycocalyx can modulate the inflammatory process (8, 14, 19, 77). The glycocalyx is a dynamic structure, which is shed in response to inflammation (8, 14, 19, 77) and other factors, but is also continually re-synthesized. The process of glycocalyx shedding is a normal response to injury or infection because it allows leukocytes to infiltrate the endothelium and enter the arterial intima where these immune cells can begin healing or dispose of damaged or diseased blood vessel tissues.
THE GLYCOCALYX AND ATHEROSCLEROSIS
Figure 4 below represents a diagrammatic view of the atherosclerotic process that clogs arteries and causes heart attacks and strokes. At the top of the figure, the inside (lumen) of an artery is pictured with a glycocalyx that has been shed. Notice the layer of flat endothelial cells
which represent the inner most barrier to blood borne elements. Below the endothelial cell layer is the sub-endothelial space, called the intima, which is where atherosclerotic plaques form and accumulate. Also, notice the LDL cholesterol molecules entering the intima. Under normal conditions, they are restricted to the circulating bloodstream by an intact endothelial glycocalyx, which impedes their entry into the intima (20, 22, 28).
Figure 4. Schematic diagram of the atherosclerotic (artery clogging process).
Figure 4. Schematic diagram of the atherosclerotic
When an atherosclerotic plaque forms in the intima of arteries, a number of key steps occur which can ultimately lead to cardiovascular disease: 1) Inflammation (8, 14, 19, 77) causes the endothelial glycocalyx to be shed (14-29), promoting further inflammation. 2) The glycocalyx becomes thinner in areas of turbulent blood flow (1, 7, 21, 23) where atherosclerotic plaques form. 3) The shedding of the glycocalyx promotes movement of LDL cholesterol from the bloodstream into the intima (20, 22, 28). 4) The LDL cholesterol becomes oxidized and continues to promote glycocalyx shedding. 5) Leukocytes, monocytes, T cells, other leukocytes and platelets, attach to adhesion molecules on the surface of endothelial cells (4-6, 12, 15, 17, 18, 26, 33) and then enter the intima. 6) After entry into the arterial intima, a specific type of leukocyte, called monocytes, are transformed into macrophages. 7) Macrophages consume oxidized LDL cholesterol and other elements to become foam cells. 8) Over time and continued inflammation, these foam cells become the atherosclerotic plaques that clog arteries. 9) Eventually a fibrous cap forms over the plaque which, if ruptured via the continued inflammatory processes, can precipitate heart attacks and strokes.
The atherosclerotic process is more complex than these simple nine steps, but these steps will provide you with sufficient information to understand how a high salt diet promotes atherosclerosis at nearly every step along the way.
However, before I show you how a high salt diet damages the endothelial glycocalyx and promotes CVD, heart attacks and strokes, one more important detail must be known. An additional revealing clue to the atherosclerotic process is the anatomical location of atherosclerotic plaques within arteries. As far back as 1971, C.G. Caro and colleagues (52, 53) showed that early atherosclerotic lesions developed almost exclusively in areas of turbulent blood flow near bifurcations (forks) of arteries, and rarely in areas of non-turbulent (laminar) blood flow. This fact has been verified in all modern studies of atherosclerosis (1, 7, 21, 21, 23, 53). Figure 5 below shows this phenomenon of plaque formation in areas of turbulent flow where the thickness and density of the endothelial glycocalyx is reduced. Current evidence indicates that atherosclerosis arises in these areas of turbulent flow because they are less able (than areas of non-turbulent flow) to respond to inflammatory conditions in the bloodstream, which promote glycocalyx shedding (8, 14, 19, 77).
Figure 5. Effect of turbulent blood flow and laminar (non-turbulent) blood flow upon the distribution of atherosclerotic plaques in arteries.
Figure 5 Turbulent Blood Flow Effects
HOW A HIGH SALT DIET PROMOTES ATHEROSCLEROSIS AND CARDIOVASCULAR DISEASE (CVD)
Normally, plasma concentrations of sodium are maintained within a narrow range that varies in the general population from 134 to 148 mmol/L (54-56). Increasing dietary salt in subjects without hypertension elevates plasma sodium concentrations within or beyond the normal range (57, 58).
In the Paleo diet community, a common perception is that adding salt, or particularly sea salt, to a Paleo diet has little or no adverse effect upon health and wellbeing (59-68). Nevertheless, the data reveals that salt, even added to the diet of normotensives (normal blood pressure), elevates plasma sodium concentrations and has important and far-reaching health considerations; particularly if the salt habit is continued throughout life.
Experiments conducted in 2007 on living human endothelial cells incubated outside the body (ex vivo) demonstrated that stiffness of the cells was unaffected by sodium concentrations of < 135 mmol/L, but stiffness rose steeply at concentrations between 135 and 145 mmol/L (70). Further, higher plasma sodium concentrations (~143-150 mmol/L), which are still mostly within normal ranges and can easily be achieved in living humans by adding salt to their diet (54-58), caused the endothelial cells to stiffen and reduce their production of nitric oxide (70, 72): a compound found throughout the body. In blood vessels, nitric oxide suppresses cell inflammation and adhesion of leukocytes and platelets to the endothelium (71). In this way, normal nitric oxide production by arterial endothelial cells inhibits blood clotting and promotes normal blood flow. Nitric oxide metabolism and production is diminished with age and CVD (71).
One of the problems with the ex vivo experiment conducted in 2007 (70) was that the endothelial cells were not only incubated with sodium, but also with a hormone called aldosterone. Accordingly, the interpretation of the experiment was muddled. Was it the sodium, the aldosterone, or both that caused a stiffening of the endothelial cells and decreased production of nitric oxide? In the ensuing years, others researchers replicated these results (46, 72), but the question still remained: what caused the adverse effects upon endothelial function and nitric oxide production?
This question was finally answered in 2016 in a living (in vitro) mouse model that had a genetic mutation preventing it from synthesizing aldosterone [aldosterone synthase knockout mouse] (69). A high salt diet, without aldosterone, increased plasma sodium to 150 mmol/L, increased endothelial cell stiffness by 44 percent, and suppressed nitric oxide production. These results are consistent with two recent randomized controlled human trials showing that a high salt meal (65 mmol Na) increased plasma sodium concentrations and impaired endothelial function while increasing arterial stiffness (74, 75).
In a living (in vivo) animal model, high salt diets fed to mice increased the responsiveness of arterial endothelial cells to a pro-inflammatory cytokine (TNFα), at areas of turbulent blood flow within the arteries. High salt diets also increased adhesion of leukocytes to these same areas of turbulent flow (76). Both of these physiological changes are characteristic of, and precede the development of CVD in humans. As I previously mentioned, high salt diets promote inflammation by impairing the release of nitric oxide (69, 70, 72), while TNFα directly causes endothelial cells to shed the glycocalyx (8, 14, 19, 77). Accordingly, glycocalyx shedding initiated by the inflammation produced from a high salt diet facilitates the formation of atherosclerotic plaques and the development of CVD. Figure 6 below summarizes the effects of high salt diets upon the development of CVD.
Figure 6. How high salt diets promote the development of cardiovascular disease (CVD)
Figure 6 How Salt Promotes CVD
Clinical data from the Atherosclerosis Risk in Communities Study involving 12,779 subjects demonstrated that plasma sodium concentrations represent a significant predictor of 10-years risk of coronary heart disease (77). These findings show that elevation of plasma sodium even within the normal physiological range (134 to 148 mmol/L) is accompanied by cardiovascular changes that facilitate the development of CVD (77).
PRACTICAL IMPLICATIONS AND FINAL THOUGHTS
Perhaps the most obvious implication of this recent, enormous and persuasive scientific literature, is simply not to add salt or sea salt to your already healthful, natural and fresh Paleo foods and recipes. Unadulterated, natural, fresh Paleo foods have very little normally occurring sodium. In a previous blog table, I have demonstrated how it is virtually impossible to ingest more than 2300 mg of sodium from natural, un-salted Paleo foods. From this table, also notice how the potassium content of natural, un-salted Paleo diets typically contains about five times more potassium than sodium.
High dietary levels of potassium are known to overcome many of the adverse cardiovascular effects of sodium by softening vascular endothelial cells (78), increasing nitric oxide production (78) and preventing sodium induced impairment of endothelial function (75). So, practically speaking, it may not always be possible to avoid foods with added salt. If this is the case, try to counter high salt foods with plenty of potassium rich fruits and vegetables. Some of my favorite high potassium foods are peaches, cherries, bananas, dates, raisins, dried figs, pecans, filberts, tomatoes, steamed broccoli and summer squash.
Another practical way to counter the effects of temporary overindulgence in salty foods is to drink more water. For an average person with an elevated plasma sodium concentration of 143 mmol/L, drinking one liter of water over a six to eight-hour period can reduce this value to the lower range of 137 mmol/L, providing no additional high salt foods are consumed (77).
So, the take home message to prevent salt induced CVD is this: 1) don’t put added salt or sea salt into fresh, healthy Paleo foods; 2) avoid salted, processed foods whenever possible. If you happen to overindulge in salted foods: 3) eat plenty of potassium rich fruits and vegetables and 4) drink more water.
http://thepaleodiet.com/dietary-salt-impairs-the-endothelial-glycocalyx-the-most-important-cardiovascular-disease-risk-factor-you-may-never-have-heard-about/
REFERENCES
1. Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000 Sep;440(5):653-66
2. Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996 Sep;79(3):581-9.
3. van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003 Apr 4;92(6):592-4.
4. Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014 Jul;69(7):777-84.
5. Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010 Jul 15;87(2):300-10.
6. Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010 Nov;105(6):687-701
7. Gouverneur M, Van Den Berg B, Nieuwdorp M, Stroes E & Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. Journal of Internal Medicine 2006; 259: 393–40
8. Kolářová H, Ambrůzová B, Svihálková Šindlerová L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm. 2014;2014:694312. doi: 10.1155/2014/694312. Epub 2014 Apr 3
9. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch Eur J Physiol (2007) 454:345–359
10. Reitsma S, oude Egbrink MG, Vink H, van den Berg BM, Passos VL, Engels W, Slaaf DW, van Zandvoort MA. Endothelial glycocalyx structure in the intact carotid artery: a two-photon laser scanning microscopy study. J Vasc Res. 2011;48(4):297-306.
11. Rorije N, Engberink RO, van den Born B, Verberne H, Vogt L. 7d.06: Effects of an acute and chronic salt load on microvascular permeability in healthy subjects. J Hypertens. 2015 Jun;33 Suppl 1:e101. doi: 10.1097/01.hjh.0000467623.14967.fc.
12. van den Berg BM, Nieuwdorp M, Stroes ES, Vink H. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep. 2006;58 Suppl:75-80
13. Vincent PE, Weinberg PD. Flow-dependent concentration polarization and the endothelial glycocalyx layer: multi-scale aspects of arterial mass transport and their implications for atherosclerosis. Biomech Model Mechanobiol. 2014 Apr;13(2):313-26
14. Cancel LM, Ebong EE, Mensah S, Hirschberg C, Tarbell JM. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis. 2016 Sep;252:136-46.
15. Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, Conzen P, Jacob B, Becker BF. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur J Anaesthesiol. 2014 Sep;31(9):474-81.
16. Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, Conzen P, Becker BF, Rehm M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014 Oct 13;18(5):538. [Epub ahead of print] 17. Chappell D, Dörfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010 Aug;34(2):133-9
18. Constantinescu AA, Vink H and Spaan JA. Endothelial surface endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 2003;23;1541-1547.
19. Henry, CBS, Duling, BR. TNF-a increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000; 279: H2815–H2823.
20. Kang H, Fan Y, Sun A, Deng X. Compositional or charge density modification of the endothelial glycocalyx accelerates flow-dependent concentration polarization of low-density lipoproteins. Exp Biol Med (Maywood). 2011 Jul;236(7):800-7
21. Koo A, Dewey CF Jr, García-Cardeña G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol. 2013 Jan 15;304(2):C137-46
22. Liu X, Fan Y, Deng X. Effect of the endothelial glycocalyx layer on arterial LDL transport under normal and high pressure. J Theor Biol. 2011 Aug 21;283(1):71-81
23. Lopez-Quintero SV, Cancel LM, Pierides A, Antonetti D, Spray DC, Tarbell JM. High glucose attenuates shear-induced changes in endothelial hydraulic conductivity by degrading the glycocalyx. PLoS One. 2013 Nov 18;8(11):e78954. doi: 10.1371/journal.pone.0078954
24. Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WH, Mullens W. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015 Feb 3;65(4):378-88.
25. Noble MI, Drake-Holland AJ, Vink H. Hypothesis: arterial glycocalyx dysfunction is the first step in the atherothrombotic process. QJM. 2008 Jul;101(7):513-8
26. Reitsma S, Oude Egbrink MG, Heijnen VV, Megens RT, Engels W, Vink H, Slaaf DW, van Zandvoort MA. Endothelial glycocalyx thickness and platelet-vessel wall interactions during atherogenesis. Thromb Haemost. 2011 Nov;106(5):939-46
27. van den Berg BM, Spaan JA, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H915-20.
28. van den Berg BM, Spaan JA, Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 2009 Apr;457(6):1199-206
29. Voyvodic PL, Min D, Liu R, Williams E, Chitalia V, Dunn AK, Baker AB.
Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem. 2014 Apr 4;289(14):9547-59.
30. Bruegger D, Schwartz L, Chappell D, Jacob M, Rehm M, Vogeser M, Christ F, Reichart B, Becker BF. Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on- and off-pump coronary artery bypass surgery. Basic Res Cardiol. 2011 Nov;106(6):1111-21
31. Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol. 2005 Nov;289(5):H1993-9.
32. Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M, Conzen P, Becker BF, Rehm M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014 Oct 13;18(5):538. doi: 10.1186/s13054-014-0538-5.
33. Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, Conzen P, Jacob B, Becker BF. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur J Anaesthesiol. 2014 Sep;31(9):474-81
34. Dane M, van den Berg B, Rabelink T. The endothelial glycocalyx: scratching the surface for cardiovascular disease in kidney failure. Atherosclerosis. 2014 Jul;235(1):56-7. doi: 10.1016/j.atherosclerosis.2014.04.005. Epub 2014 Apr 25.
35. Kliche K, Gerth U, Pavenstädt H, Oberleithner H. Recharging red blood cell surface by hemodialysis. Cell Physiol Biochem. 2015;35(3):1107-15.
36. Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Arch. 2012 Feb;463(2):269-78.
37. Lee DH, Dane MJ, van den Berg BM, Boels MG, van Teeffelen JW, de Mutsert R, den Heijer M, Rosendaal FR, van der Vlag J, van Zonneveld AJ, Vink H, Rabelink TJ; NEO study group. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS One. 2014 May 9;9(5):e96477. doi: 0.1371/journal .pone.0096477
38. Oberleithner H, Wilhelmi M. Vascular glycocalyx sodium store – determinant of salt sensitivity? Blood Purif. 2015;39(1-3):7-10.
39. Oberleithner H. Sodium selective erythrocyte glycocalyx and salt sensitivity in man. Pflugers Arch. 2015 Jun;467(6):1319-25.
40. Oberleithner H, Wälte M, Kusche-Vihrog K. Sodium renders endothelial cells sticky for red blood cells. Front Physiol. 2015 Jun 30;6:188. doi: 10.3389/fphys.2015.00188.
41. Oberleithner H. Sodium selective erythrocyte glycocalyx and salt sensitivity in man. Pflugers Arch. 2014 Jul 17. [Epub ahead of print] PMID: 25027385
42. Oberleithner H. Vascular endothelium: a vulnerable transit zone for merciless sodium. Nephrol Dial Transplant. 2014 Feb;29(2):240-6.
43. Oberleithner H, Wilhelmi M. Determination of erythrocyte sodium sensitivity in man. Pflugers Arch. 2013 Oct;465(10):1459-66
44. Oberleithner H. Vascular endothelium leaves fingerprints on the surface of erythrocytes. Pflugers Arch. 2013 Oct;465(10):1451-8.
45. Oberleithner H. Two barriers for sodium in vascular endothelium? Ann Med. 2012 Jun;44 Suppl 1:S143-8
46. Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, Oberleithner K. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. 2011 Oct;462(4):519-28.
47. Paar M, Pavenstädt H, Kusche-Vihrog K, Drüppel V, Oberleithner H, Kliche K. Endothelial sodium channels trigger endothelial salt sensitivity with aging. Hypertension. 2014 Aug;64(2):391-6.
48. Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, Lukasz A, Oberleithner H, Pavenstädt H, Brand M, Kümpers P. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014 Jun;234(2):335-43.
49. Krähling H, Mally S, Eble JA, Noël J, Schwab A, Stock C. The glycocalyx maintains a cell surface pH nanoenvironment crucial for integrin-mediated migration of human melanoma cells. Pflugers Arch. 2009 Oct;458(6):1069-83.
50. Cai B, Fan J, Zeng M, Zhang L, Fu BM. Adhesion of malignant mammary tumor cells MDA-MB-231 to microvessel wall increases microvascular permeability via degradation of endothelial surface glycocalyx. J Appl Physiol (1985). 2012 Oct;113(7):1141-53.
51. Reitsma S, oude Egbrink MG, Vink H, van den Berg BM, Passos VL, Engels W, Slaaf DW, van Zandvoort MA. Endothelial glycocalyx structure in the intact carotid artery: a two-photon laser scanning microscopy study. J Vasc Res. 2011;48(4):297-306
52. Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc Royal Soc. 1971;B.177:109–159.
53. Caro CG. Discovery of the role of wall shear in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009 Feb;29(2):158-61.
54. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride and Sulfate / Appendix J. Serum Electrolyte Concentrations NHANESIII. 1988-94. In: Standing Committee on the Scientific Evaluation of Dietary Reference Intakes FaNB, editor. Panel on Dietary Reference Intakes for Electrolytes and Water. Washington, DC 20001: THE NATIONAL ACADEMIES PRESS;2005. P. 558-63.
55. Oh SW, Baek SH, An JN, Goo HS, Kim S, Na KY, Chae DW, Kim S, Chin HJ. Small increases in plasma sodium are associated with higher risk of mortality in a healthy population.
J Korean Med Sci. 2013 Jul;28(7):1034-40.
56. Komiya I, Yamada T, Takasu N, Asawa T, Akamine H, Yagi N, Nagasawa Y, Ohtsuka H, Miyahara Y, Sakai H, Sato A, Aizawa T. An abnormal sodium metabolism in Japanese patients with essential hypertension, judged by serum sodium distribution, renal function and the renin-aldosterone system. J Hypertens. 1997 Jan;15(1):65-72.
57. He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension. 2005 Jan;45(1):98-102.
58. Suckling RJ, He FJ, Markandu ND, MacGregor GA. Dietary salt influences postprandial plasma sodium concentration and systolic blood pressure. Kidney Int. 2012 Feb;81(4):407-11.
59. Kresser C. Shaking Up The Salt Myth: Healthy Salt Recommendations. May 4, 2012. //chriskresser.com/shaking-up-the-salt-myth-healthy-salt-recommendations/
60. Staley B, Mason H. Make it Paleo: Over 200 Grain-Free Recipes for Any Occasion. Foreword by Mark Sisson. Victory Belt Publishing, Las Vegas NV, 2011.
61. //www.marksdailyapple.com/salt-what-is-it-good-for/
62. Fragoso S. Everyday Paleo. Foreword by Robb Wolf. Victory Belt Publishing, Las Vegas NV, 2011.
63. Gower C. Paleo Slow Cooking: Gluten Free Recipes Made Simple. Foreword by Robb Wolf. Victory Belt Publishing, Las Vegas NV, 2012.
64. Mayfield J, Mayfield C. Paleo Comfort Foods: Homestyle Cooking for a Gluten-Free Kitchen. Foreword by Robb Wolf. Victory Belt Publishing, Las Vegas NV, 2011.
65. https://www.thepaleomom.com/is-salt-paleo/
66. //paleoleap.com/salt-and-a-paleo-diet/
67. //www.paleoplan.com/ingredients/salt/
68. //synergyhw.blogspot.com/2013/05/salt-one-of-your-best-friends-on-paleo.html
69. Jeggle P, Hofschröer V, Maase M, Bertog M, Kusche-Vihrog K. Aldosterone synthase knockout mouse as a model for sodium-induced endothelial sodium channel up-regulation in vascular endothelium. FASEB J. 2016 Jan;30(1):45-53.
70. Oberleithner H, Riethmüller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007 Oct 9;104(41):16281-16286.
71. Ghimire K, Altmann HM, Straub A, Isenberg JS. Nitric oxide: What’s new to NO? Am J Physiol Cell Physiol. 2016 Dec 14:ajpcell.00315.2016. doi: 10.1152/ajpcell.00315.2016. [Epub ahead of print] 72. Kusche-Vihrog K, Schmitz B, Brand E. Salt controls endothelial and vascular phenotype. Pflugers Arch. 2015 Mar;467(3):499-512
73. Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens. 2015 Jan;24(1):8-13
74. Dickinson KM, Clifton PM, Burrell LM, Barrett PH, Keogh JB. Postprandial effects of a high salt meal on serum sodium, arterial stiffness, markers of nitric oxide production and markers of endothelial function. Atherosclerosis. 2014 Jan;232(1):211-6.
75. Blanch N, Clifton PM, Petersen KS, Keogh JB.Effect of sodium and potassium supplementation on vascular and endothelial function: a randomized controlled trial. Am J Clin Nutr. 2015 May;101(5):939-46
76. Wild J, Soehnlein O, Dietel B, Urschel K, Garlichs CD, Cicha I. Rubbing salt into wounded endothelium: sodium potentiates proatherogenic effects of TNF-α under non-uniform shear stress. Thromb Haemost. 2014 Jul 3;112(1):183-95.
77. Dmitrieva NI, Burg MB. Elevated sodium and dehydration stimulate inflammatory signaling in endothelial cells and promote atherosclerosis. PLoS One. 2015 Jun 4;10(6):e0128870. doi: 10.1371/journal.pone.0128870.
78. Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmüller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2829-34
About Loren Cordain, PhD, Professor Emeritus
Loren Cordain, PhD, Professor EmeritusDr. Loren Cordain is Professor Emeritus of the Department of Health and Exercise Science at Colorado State University in Fort Collins, Colorado. His research emphasis over the past 20 years has focused upon the evolutionary and anthropological basis for diet, health and well being in modern humans. Dr. Cordain’s scientific publications have examined the nutritional characteristics of worldwide hunter-gatherer diets as well as the nutrient composition of wild plant and animal foods consumed by foraging humans. He is the world’s leading expert on Paleolithic diets and has lectured extensively on the Paleolithic nutrition worldwide. Dr. Cordain is the author of six popular bestselling books including The Real Paleo Diet Cookbook, The Paleo Diet, The Paleo Answer, and The Paleo Diet Cookbook, summarizing his research findings.
.png)
.png)
.png)
.png)
.png)
.png)
.png)