俄勒冈大学 叶绿素和叶绿酸有效地限制了黄曲霉毒素在人体中的吸收
Chlorophyll and chlorophyllin effective in limiting aflatoxin absorption in humans
一项新的研究发现,叶绿素及其衍生物叶绿素在限制人体对黄曲霉毒素的吸收方面是有效的。黄曲霉毒素是由一种真菌产生的,这种真菌是玉米、花生和大豆等谷物的污染物;众所周知,它会导致肝癌,并与其他健康问题(如肝炎)协同工作。
叶绿素及其衍生物叶绿素在限制人体对黄曲霉毒素的吸收方面是有效的
在美国,黄曲霉毒素的水平得到了严格的控制,但在发展中国家的食品供应中经常发现黄曲霉毒素的含量,尤其是那些储存设施较差的国家。
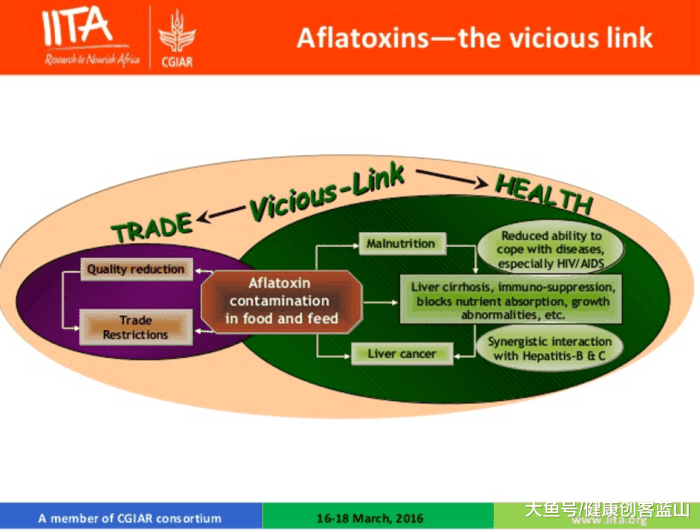
黄曲霉素(Aflatoxins)的恶性循环
俄勒冈州立大学(OSU)的科学家乔治·贝利(George Bailey)是著名的环境和分子毒理学教授,他是中国黄曲霉毒素研究的先驱。他在中国发现,在一个地区,每10名成年人中就有1人死于肝癌。
黄曲霉素是已知的最强致癌物
但这种后续研究的科学世界特别感兴趣是研究人员使用的方法——一个新的“0”阶段的方法,在人类志愿者中安全测试低水平的致癌物质的总暴露量以及膳食叶绿素对黄曲霉素吸收的阻断作用。
这项研究的结果发表在《癌症预防研究》杂志上。
贝利和其他几位研究人员,包括主要作者卡罗尔·朱伯特,都参与了最近的研究。该杂志还收录了约翰霍普金斯大学的两位研究人员——托马斯肯斯勒和约翰格鲁普曼——撰写的一篇观点,他们称赞了这一方法,并建议应该扩大这些0阶段“微剂量”研究。
他们写道:“致癌物的微剂量研究有可能为化学预防干预提供重要的见解,并提高预防性药物的整体临床开发和安全性评估。”
OSU莱纳斯鲍林研究所贝利实验室的前研究员朱贝尔说,第0阶段的研究“可能会为各种新研究打开大门”。Jubert现在为生命微系统公司工作,这是一个OSU的分支公司,希望继续与俄勒冈州的天然产品合作,包括纯叶绿素。
“这项技术并不特别困难,”她补充说。“这只是评估人类接触毒素的一种新方法。”
在他们的研究中,朱贝特和她的同事给四名志愿者注射了低剂量的黄曲霉毒素,这些毒素被贴上了碳14同位素的标签作为示踪剂。然后,他们给志愿者同样剂量的黄曲霉毒素和叶绿素或叶绿素,这在之前已经被证明可以降低鳟鱼和大鼠的致癌物生物利用度。利用加速器质谱仪,他们测量了毒素生物利用度。研究人员说,这项技术非常敏感,可以测量任何标记化合物的微量含量。
他们的研究表明黄曲霉毒素的快速吸收在叶绿素和叶绿素处理后明显受限。
俄勒冈州立大学药理学家、该项研究的第二作者约翰•马塔(John Mata)表示:“这种‘第0阶段’研究的好处在于,它使用了超敏感技术和环境致癌物的‘微剂量’来研究人体内的毒物动力学。”“这些测量方法很重要,因为它们让我们能够更好地设计未来的研究,以了解膳食成分对癌症风险的影响。”
“在这种情况下,很明显,研究结果值得进一步研究,”Mata补充道。“我们发现黄曲霉毒素吸收非常迅速,叶绿素和叶绿素有改善作用,防止毒素进入血液。进一步的研究可以更精确地探索相互作用,以及剂量水平。
朱伯特和玛塔还测试了在人体接触其他毒素时使用类似技术的可行性,这些毒素包括通过吸烟摄入致癌物的吸烟者。
马塔是俄勒冈州立大学兽医学院的教授,是一位药理学家,曾在制药行业工作过。他说,第一阶段研究的目的是观察一种化合物是否安全;第二阶段扩大了项目的范围,第三阶段着眼于化合物的功效。第0阶段代表了一个新概念——一种通过使用对志愿者造成的风险很小的极小剂量来测量药物动力学的方法。
在这种情况下,志愿者受到的辐射量等于你在一小时的飞行中所遇到的辐射量;黄曲霉毒素的含量是美国食品和药物管理局规定的花生酱三明治含量的1/30。
来源:
俄勒冈州立大学
https://s.click.taobao.com/dWJDNNw
Chlorophyll and chlorophyllin effective in limiting aflatoxin absorption in humans
Download PDF Copy
December 30, 2009
A new study has found that chlorophyll and its derivative chlorophyllin are effective in limiting the absorption of aflatoxin in humans. Aflatoxin is produced by a fungus that is a contaminant of grains including corn, peanuts and soybeans; it is known to cause liver cancer - and can work in concert with other health concerns, such as hepatitis.
Levels of aflatoxin are carefully regulated in the United States, but are often found in the food supplies of developing nations, especially those with poor storage facilities.
OSU scientist George Bailey, a distinguished professor of environmental and molecular toxicology, pioneered studies of aflatoxin in China, where he found that in one region, one out of every 10 adults died from liver cancer.
But what has the science world particularly intrigued with this follow-up study is the methodology used by the researchers - a new "Phase 0" approach that safely tests low levels of carcinogens in human volunteers to measure the total aflatoxin exposure and to determine the effect of dietary chlorophlls on reducing this exposure.
Results of the study were just published in the journal Cancer Prevention Research.
Bailey and several other researchers, including lead author Carole Jubert, were part of the recent study. The journal also included a perspective written by a pair of Johns Hopkins researchers - Thomas Kensler and John Groopman - who praise the methodology and suggest that these Phase 0 "microdosing" studies should be expanded.
They wrote: "-microdosing studies with carcinogens have the potential to provide important insights into chemopreventive interventions and to enhance the overall clinical development and safety evaluation of preventive agents."
The Phase 0 study "-may open the door for all kinds of new research," said Jubert, a former researcher in Bailey's lab at OSU's Linus Pauling Institute. Jubert now works for Life Microsystems, an OSU spinoff company that hopes to continue work with natural products grown in Oregon, including pure chlorophylls.
"The technology is not particularly difficult," she added. "It's just a novel approach to evaluate toxin exposure in humans."
In their study, Jubert and her colleagues gave very low doses of aflatoxin labeled with carbon-14 isotopes as a tracer to four human volunteers. They then gave the volunteers the same doses of aflatoxin along with doses of either chlorophyll or chlorophyllin, which previously had been shown to reduce carcinogen bioavailability in trout and rats. Using an accelerator mass spectrometer, they measured the rate of aflaxtoxin bioavailability. This technique is extremely sensitive, the researchers say, allowing measurement of minute amounts of any labeled compound.
Related Stories
Biomarker predicts kidney cancer risk years before diagnosis
Researchers identify way to grow immune cells at large scale for preventing cancer reoccurrence
Scientists reveal role of 'junk DNA' in cancer dissemination
Their research revealed rapid absorption of aflatoxin, which was significantly limited after the chlorophyll and chlorophyllin treatments.
"The beauty of this kind of 'Phase 0' study is the use of ultra-sensitive technology and 'microdoses' of environmental carcinogens to study toxicokinetics within the human body," said John Mata, an OSU pharmacologist and second author on the study. "These measurements can be important because they allow us to better design future studies to understand the effects of dietary constituents on cancer risk.
"In this case, clearly the results merit further study," Mata added. "We showed that aflatoxin is absorbed quite rapidly and that chlorophyll and chlorophyllin have an ameliorating effect, preventing the toxin from getting into the bloodstream. Further studies can more precisely explore the interactions, as well as dosage levels."
Jubert and Mata also have tested the feasibility of using similar technology on human exposure to other toxins, including smokers who ingest carcinogens through cigarette smoke.
Mata, a professor in OSU's College of Veterinary Medicine, is a pharmacologist who previously worked in the drug industry. He said Phase 1 studies are designed to see if a compound is safe; Phase 2 expands the scope of the project, and Phase 3 looks at the compounds' efficacy. Phase 0 represents a new concept - a way to measure the kinetics of a drug by using extremely small doses that pose little risk to the volunteers.
In this case, the amount of radiation given the human volunteers was equal to that you would encounter from a one-hour airplane ride; the level of aflatoxin administered was 1/30th the amount the Food and Drug Administration allows in a peanut butter sandwich.
Source:
Oregon State University



.png)