西安交通大学:人参靶向低氧诱导因子 阻断卵巢癌转移
Ginsenoside 20(S)-Rg3 Targets HIF-1α to Block Hypoxia-Induced Epithelial-Mesenchymal Transition in Ovarian Cancer Cells
卵巢癌是以上皮性卵巢癌(epi卵巢癌,EOC)的形式存在,是妇科最致命的恶性肿瘤。卵巢癌患者的低生存率与其恶性侵袭转移有关。导致卵巢癌细胞侵袭和转移的关键是缺氧的肿瘤微环境, 导致稳定的低氧诱导因子- 1α(HIF-1α)介导肿瘤细胞浸润和转移。
卵巢癌是以上皮性卵巢癌的形式存在,是妇科最致命的恶性肿瘤
卵巢癌患者的预后仍然很差,主要是由于恶性肿瘤进展。由于上皮-间充质转化(epi- mesenchymal transition, EMT)是介导癌细胞侵袭转移的重要机制,靶向EMT过程中更有效、毒性更小的化合物抑制转移对卵巢癌的治疗有很大的治疗价值。
EMT最初是在胚胎发育过程中发现的,后来发现它与肿瘤的侵袭转移过程密切相关。EMT过程中细胞经历一个从极化上皮表型间质表型,一个生物事件的特点是细胞形态学变化从一个鹅卵石形状分散成形状,失去上皮特异性蛋白质收购间叶细胞特异性标记和属性,和增强细胞的能动性和入侵的过渡。
我们首次发现,中药人参的药理活性成分人参皂苷20(S)-Rg3,在体外和体内,能有效阻断低氧诱导的卵巢癌细胞转化(EMT)和转移。
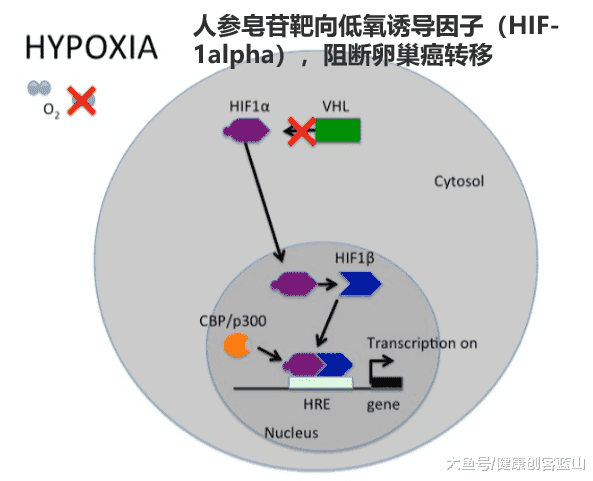
人参皂苷20(S)-Rg3靶向低氧诱导因子,阻断卵巢癌转移
机械的研究证实20(S)-Rg3的作用方式,通过激活ubiquitin-proteasome途径促进HIF-1α退化从而降低低氧诱导因子1α的表达(HIF-1α)。
HIF-1α的减少导致通过上皮细胞特异性标记物E-cadherin Snail的转录抑制的上调,
和在低氧条件下间质细胞特异性标记物vimentin的下调。
重要的是,20(S)-Rg3在卵巢癌裸鼠异种移植模型中有效抑制了上皮-间充质转化(EMT),有望成为一种新的抗癌治疗药物。
Ginsenoside 20(S)-Rg3 Targets HIF-1α to Block Hypoxia-Induced Epithelial-Mesenchymal Transition in Ovarian Cancer Cells
Ting Liu,# 1 Le Zhao,# 1 Yan Zhang, 1 Wei Chen, 2 Dan Liu, 3 Huilian Hou, 4 Lu Ding, 1 and Xu Li 1 , *Joseph Najbauer, Editor1 Center for Translational Medicine, the First Affiliated Hospital School of Medicine, Xi'an Jiaotong University, Xi'an, China,2 Center for Laboratory Medicine, the First Affiliated Hospital School of Medicine, Xi'an Jiaotong University, Xi'an, China,3 Department of Gynaecology and Obstetrics, the First Affiliated Hospital School of Medicine, Xi'an Jiaotong University, Xi'an, China,4 Department of Pathology, the First Affiliated Hospital School of Medicine, Xi'an Jiaotong University, Xi'an, China,
Ovarian cancer, existing predominantly in the form of epithelial ovarian cancer (EOC), is the most lethal gynecologic malignancy [1], [2]. The low survival rate of patients with ovarian cancer is associated with its vicious nature of invasion and metastasis. Among the critical contributors to the invasive and metastatic capability of ovarian cancer cells is hypoxia of the tumor microenvironment [3], [4], which mediates tumor cell invasion and metastasis through stabilization of hypoxia-inducible factor-1 alpha (HIF-1α) [5], [6].
In normoxic cells, HIF-1α is hydroxylated by a family of proline hydroxylases (PHD1-3) at Pro402 and Pro564, leading to a conformational change that promotes HIF-1α binding to the von Hippel Lindau protein (VHL) - a component of the large E3 ubiquitin ligase complex that mediates proteasomal degradation of HIF-1α [7], [8]. Under hypoxic conditions, low oxygen levels preclude proline hydroxylation, which leads to HIF-1α stabilization and subsequent formation of a heterodimer with constitutively expressed HIF-1β. The bioactive HIF-1 dimer regulates, as a transcription factor, the expression of a broad range of genes involved not only in cell cycle, apoptosis [9], angiogenesis [10], [11] and metabolism [12] in response to the hypoxic environment, but also in a cellular process called epithelial-mesenchymal transition (EMT) [13]–[15].
Identified first in embryonic development, EMT has been found later to be closely involved in the process of cancer invasion and metastasis [16], [17]. During the EMT process cells undergo a transition from a polarized epithelial phenotype to a mesenchymal phenotype [18], a biological event characterized by cell morphology change from a cobble-stone shape to a dispersed fibroblastoid shape, loss of epithelial cell-specific protein markers and acquisition of mesenchymal cell-specific properties, and enhanced cell motility and invasion.
The prognosis of patients with ovarian cancer has remained poor mainly because of aggressive cancer progression. Since epithelial-mesenchymal transition (EMT) is an important mechanism mediating invasion and metastasis of cancer cells, targeting the EMT process with more efficacious and less toxic compounds to inhibit metastasis is of great therapeutic value for the treatment of ovarian cancer. We have found for the first time that the ginsenoside 20(S)-Rg3, a pharmacologically active component of the traditional Chinese herb Panax ginseng, potently blocks hypoxia-induced EMT of ovarian cancer cells in vitro and in vivo. Mechanistic studies confirm the mode of action of 20(S)-Rg3, which reduces the expression of hypoxia-inducible factor 1α (HIF-1α) by activating the ubiquitin-proteasome pathway to promote HIF-1α degradation. A decrease in HIF-1α in turn leads to up-regulation, via transcriptional suppression of Snail, of the epithelial cell-specific marker E-cadherin and down-regulation of the mesenchymal cell-specific marker vimentin under hypoxic conditions. Importantly, 20(S)-Rg3 effectively inhibits EMT in nude mouse xenograft models of ovarian cancer, promising a novel therapeutic agent for anticancer therapy.
Ginsenoside 20(S)-Rg3 Targets HIF-1α to Block Hypoxia-Induced Epithelial-Mesenchymal Transition... - Europe PMC Article - Europe PMC
References
1. Cho KR, Shih Ie M (2009) Ovarian cancer. Annual Review of Pathology 4: 287–313. [PMC free article] [PubMed]
2. Swisher EM, Taniguchi T, Karlan BY (2012) Molecular scores to predict ovarian cancer outcomes: a worthy goal, but not ready for prime time. J Natl Cancer Inst 104: 642–645. [PMC free article] [PubMed]
3. Vaupel P (2004) Tumor microenvironmental physiology and its implications for radiation oncology. Seminars in radiation oncology 14: 198–206. [PubMed]
4. Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93: 266–276. [PubMed]
5. Weidemann A, Johnson RS (2008) Biology of HIF-1alpha. Cell Death and Differentiation 15: 621–627. [PubMed]
6. Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, et al. (2008) Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nature cell biology 10: 295–305. [PubMed]
7. Semenza GL (2001) HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107: 1–3. [PubMed]
8. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, et al. (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472. [PubMed]
9. Goda N, Dozier SJ, Johnson RS (2003) HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxidants & redox signaling 5: 467–473. [PubMed]
10. Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, et al. (1998) Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490. [PubMed]
11. Saponaro C, Malfettone A, Ranieri G, Danza K, Simone G, et al. (2013) VEGF, HIF-1alpha expression and MVD as an angiogenic network in familial breast cancer. PLoS One 8: e53070. [PMC free article] [PubMed]
12. Gordan JD, Thompson CB, Simon MC (2007) HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 12: 108–113. [PMC free article] [PubMed]
13. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, et al. (2007) Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. The Journal of clinical investigation 117: 3810–3820. [PubMed]
14. Micalizzi DS, Farabaugh SM, Ford HL (2010) Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 15: 117–134. [PMC free article] [PubMed]
15. Haase VH (2009) Oxygen regulates epithelial-to-mesenchymal transition: insights into molecular mechanisms and relevance to disease. Kidney Int 76: 492–499. [PMC free article] [PubMed]
16. Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Research 66: 8319–8326. [PubMed]
17. Gardberg M, Kaipio K, Lehtinen L, Mikkonen P, Heuser VD, et al. (2013) FHOD1, a Formin Upregulated in Epithelial-Mesenchymal Transition, Participates in Cancer Cell Migration and Invasion. PLoS One 8: e74923. [PMC free article] [PubMed]
18. Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7: 131–142. [PubMed]
19. Lim S, Becker A, Zimmer A, Lu J, Buettner R, et al. (2013) SNAI1-mediated epithelial-mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance. PLoS One 8: e66558. [PMC free article] [PubMed]
20. Klymkowsky MW, Savagner P (2009) Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. The American journal of pathology 174: 1588–1593. [PMC free article] [PubMed]
21. Singh A, Settleman J (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29: 4741–4751. [PMC free article] [PubMed]
22. Gillis CN (1997) Panax ginseng pharmacology: a nitric oxide link? Biochemical pharmacology 54: 1–8. [PubMed]
23. Liao B, Newmark H, Zhou R (2002) Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Experimental neurology 173: 224–234. [PubMed]
24. Nag SA, Qin JJ, Wang W, Wang MH, Wang H, et al. (2012) Ginsenosides as Anticancer Agents: In vitro and in vivo Activities, Structure-Activity Relationships, and Molecular Mechanisms of Action. Front Pharmacol 3: 25. [PMC free article] [PubMed]
25. Lu JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7: 293–302. [PMC free article] [PubMed]
26. Jeong KJ, Kim GW, Chung SH (2014) AMP-activated protein kinase: An emerging target for ginseng. J Ginseng Res 38: 83–88. [PMC free article] [PubMed]
27. Choi JS, Chun KS, Kundu J, Kundu JK (2013) Biochemical basis of cancer chemoprevention and/or chemotherapy with ginsenosides (Review). Int J Mol Med 32: 1227–1238. [PubMed]
28. Li J, Wei Q, Zuo GW, Xia J, You ZM, et al. (2014) Ginsenoside Rg1 induces apoptosis through inhibition of the EpoR-mediated JAK2/STAT5 signalling pathway in the TF-1/Epo human leukemia cell line. Asian Pac J Cancer Prev 15: 2453–2459. [PubMed]
29. Lee SY, Kim GT, Roh SH, Song JS, Kim HJ, et al. (2009) Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Bioscience, biotechnology, and biochemistry 73: 811–816. [PubMed]
30. Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, et al. (1995) Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biological & Pharmaceutical Bulletin 18: 1197–1202. [PubMed]
31. Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, et al. (2008) Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl) 121: 1394–1397. [PubMed]
32. Xu TM, Xin Y, Cui MH, Jiang X, Gu LP (2007) Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chin Med J (Engl) 120: 584–588. [PubMed]
33. Bae EA, Kim EJ, Park JS, Kim HS, Ryu JH, et al. (2006) Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial cells. Planta medica 72: 627–633. [PubMed]
34. Keum YS, Han SS, Chun KS, Park KK, Park JH, et al. (2003) Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutation research 523–524: 75–85. [PubMed]
35. Cui W, Cheng L, Hu C, Li H, Zhang Y, et al. (2013) Electrospun poly(L-lactide) fiber with ginsenoside rg3 for inhibiting scar hyperplasia of skin. PLoS One 8: e68771. [PMC free article] [PubMed]
36. Yue PY, Wong DY, Wu PK, Leung PY, Mak NK, et al. (2006) The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochemical pharmacology 72: 437–445. [PubMed]
37. Ahmed N, Abubaker K, Findlay J, Quinn M (2010) Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer. Curr Cancer Drug Targets 10: 268–278. [PubMed]
38. Huang RY, Chung VY, Thiery JP (2012) Targeting pathways contributing to epithelial-mesenchymal transition (EMT) in epithelial ovarian cancer. Curr Drug Targets 13: 1649–1653. [PubMed]
39. Kizaka-Kondoh S, Tanaka S, Harada H, Hiraoka M (2009) The HIF-1-active microenvironment: an environmental target for cancer therapy. Adv Drug Deliv Rev 61: 623–632. [PubMed]
40. Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732. [PubMed]
41. Lee JY, Jung KH, Morgan MJ, Kang YR, Lee HS, et al. (2013) Sensitization of TRAIL-Induced Cell Death by 20(S)-Ginsenoside Rg3 via CHOP-Mediated DR5 Upregulation in Human Hepatocellular Carcinoma Cells. Molecular Cancer Therapeutics 12: 274–285. [PubMed]
42. Kim SM, Lee SY, Cho JS, Son SM, Choi SS, et al. (2010) Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-kappaB. European journal of pharmacology 631: 1–9. [PubMed]
43. Wang W, Zhang X, Qin JJ, Voruganti S, Nag SA, et al. (2012) Natural product ginsenoside 25-OCH3-PPD inhibits breast cancer growth and metastasis through down-regulating MDM2. Plos One 7: e41586. [PMC free article] [PubMed]
44. Xie XS, Yang M, Liu HC, Zuo C, Li HJ, et al. (2009) Ginsenoside Rg1, a major active component isolated from Panax notoginseng, restrains tubular epithelial to myofibroblast transition in vitro. Journal of ethnopharmacology 122: 35–41. [PubMed]
45. Bae JS, Park HS, Park JW, Li SH, Chun YS (2012) Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J Nat Med 66: 476–485. [PubMed]
http://europepmc.org/articles/PMC4157750/
.png)