加州大学圣地亚哥分校 亚麻籽中的亚麻酸可以杀死幽门螺旋杆菌
Mechanism of Antibacterial Activity of Liposomal Linolenic Acid against Helicobacter pylori
https://www.researchgate.net/publication/259589624_Liposome-like_Nanostructures_for_Drug_Delivery
https://en.wikibooks.org/wiki/Structural_Biochemistry/Lipids/Lipid_Bilayer
图示:Ulcer-causing Bacterium (H.Pylori) Crossing Mucus Layer of Stomach, using flagella 利用鞭毛穿过胃粘膜,形成溃疡的细菌(H.Pylori)
幽门螺杆菌感染约占世界人口的一半,是引起胃炎、消化性溃疡和胃癌的主要原因。此外,这种细菌对所有主要的抗生素都有抗药性。
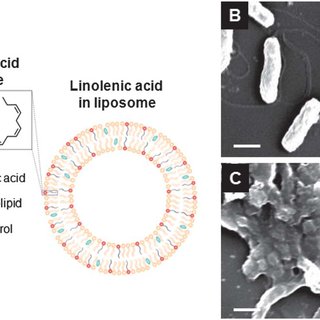
最近,我们开发了一种新型脂质体亚麻酸(LipoLLA)制剂,对几种临床分离的幽门螺杆菌耐药菌株(包括螺旋型和球菌型)具有很强的杀菌活性。
此外,LipoLLA在体内治疗效果优于标准的三联疗法。我们的数据显示LipoLLA与幽门螺杆菌细胞膜有关。
因此,在本研究中,我们研究了LipoLLA对幽门螺旋杆菌可能的抗菌机制。
与脂质体硬脂酸(LipoSA, C18:0)和油酸(LipoOA, C18:1)相比,LipoLLA (C18:3)的抗菌活性。LipoLLA杀菌效果最强,在5分钟内完全杀死了幽门螺杆菌。
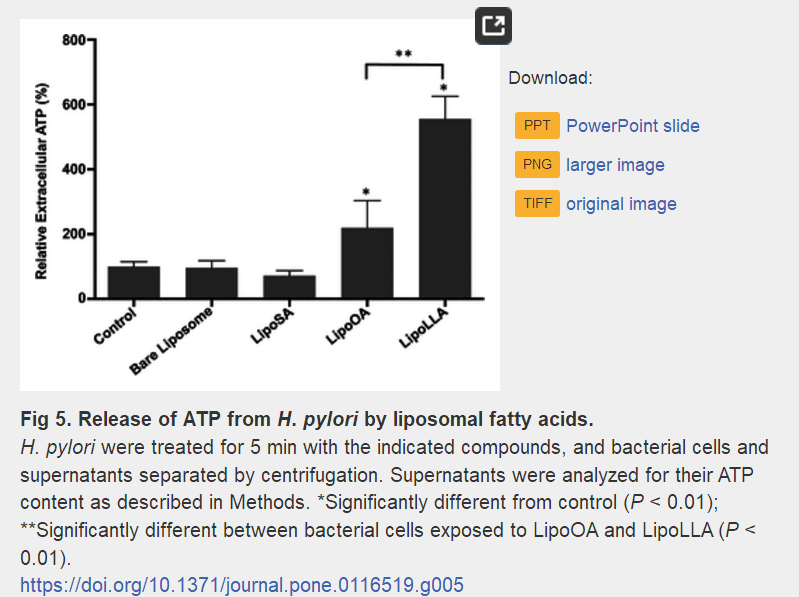
LipoOA和LipoLLA处理后,幽门螺杆菌外膜的通透性增加。此外,通过检测细菌释放的三磷酸腺苷(ATP),我们发现LipoLLA处理的幽门螺杆菌的菌质膜比LipoOA处理的菌质膜具有明显更高的通透性,导致细菌细胞死亡。
通过透射电镜(TEM)和扫描电镜(SEM)观察,LipoLLA在5min内造成了细菌膜的结构变化,影响了膜的完整性,导致细胞质内容物渗漏。
我们的发现表明LipoLLA具有快速杀菌作用,这表明它是一种很有前途的新型有效的抗幽门螺旋杆菌药物。
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0116519
Mechanism of Antibacterial Activity of Liposomal Linolenic Acid against Helicobacter pylori
Helicobacter pylori infects approximately half of the world population and is a major cause of gastritis, peptic ulcer, and gastric cancer. Moreover, this bacterium has quickly developed resistance to all major antibiotics.
Recently, we developed a novel liposomal linolenic acid (LipoLLA) formulation, which showed potent bactericidal activity against several clinical isolated antibiotic-resistant strains of H. pylori including both the spiral and coccoid form.
In addition, LipoLLA had superior in vivo efficacy compared to the standard triple therapy.
Our data showed that LipoLLA associated with H. pylori cell membrane.
Therefore, in this study, we investigated the possible antibacterial mechanism of LipoLLA against H. pylori. The antibacterial activity of LipoLLA (C18:3) was compared to that of liposomal stearic acid (LipoSA, C18:0) and oleic acid (LipoOA, C18:1).
LipoLLA showed the most potent bactericidal effect and completely killed H. pylori within 5 min. The permeability of the outer membrane of H. pylori increased when treated with LipoOA and LipoLLA.
Moreover, by detecting released adenosine triphosphate (ATP) from bacteria, we found that bacterial plasma membrane of H. pylori treated with LipoLLA exhibited significantly higher permeability than those treated with LipoOA, resulting in bacteria cell death.
Furthermore, LipoLLA caused structural changes in the bacterial membrane within 5 min affecting membrane integrity and leading to leakage of cytoplasmic contents, observed by both transmission electron microscopy (TEM) and scanning electron microscopy (SEM).
Our findings showing rapid bactericidal effect of LipoLLA suggest it is a very promising new, effective anti-H. pylori agent.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0116519
Omega-3 Phospholipids - ScienceDirect
https://www.sciencedirect.com/science/article/pii/B9781630670443500182
The majority of the n-3 fatty acids, in particular EPA and DHA, found in krill oil are bound to phospholipids, whereas other marine oils confine these to TAGs or ethyl ester forms; the majority of these two fatty acids, EPA and DHA, are contained in the PC form (Phleger et al., 2002).
Author: Kangyi Zhang
Publish Year: 2015
Standard liposomal preparations-drug delivery system
chemistry of lipids phospholiplids
nanoparticles and liposome
b) Sonication of the phospholipid described in part a) would most likely lead to the formation of (micelles, liposomes, two insoluble liquid phases).
Solutions for Sample Midterm 2 - Biology LibreTexts
https://bio.libretexts.org/Courses/University_of_California_Davis/BIS_102:_Structure_and_Function_of_Biomolecules_(Gasser)/Reader/Chapter_4/Solutions_for_Sample_Midterm_2
Lipids in Transdermal and Topical Drug Delivery | American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
https://www.americanpharmaceuticalreview.com/Featured-Articles/170872-Lipids-in-Transdermal-and-Topical-Drug-Delivery/
Micelle To Liposome Formation Diagram - Glucose Phosphate
https://www.barnardhealth.us/glucose-phosphate/info-vzl.html
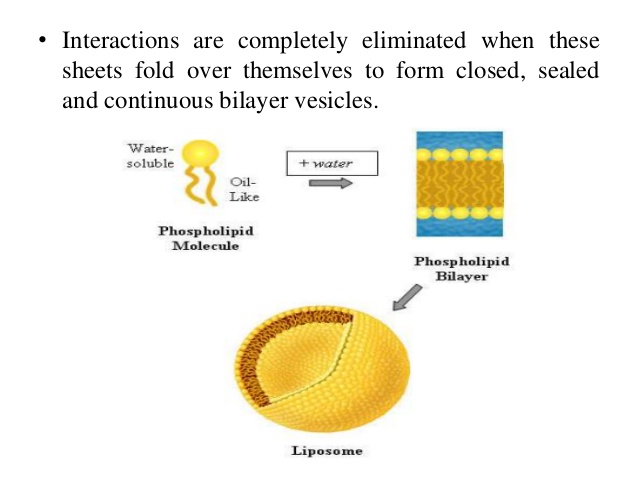
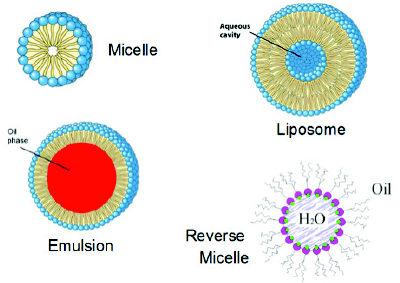
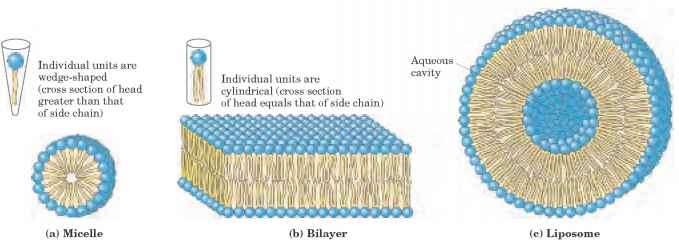
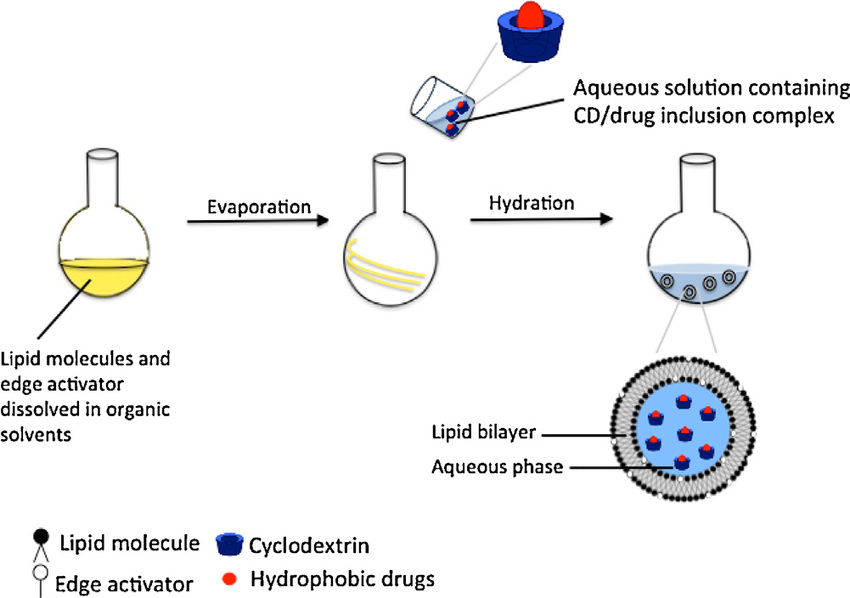
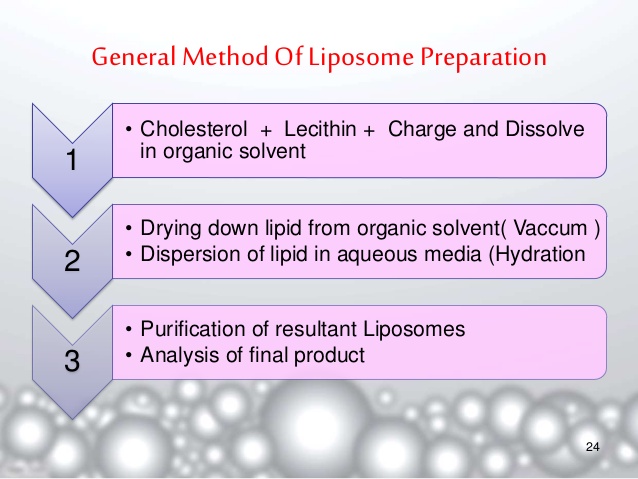
4.Liposomes
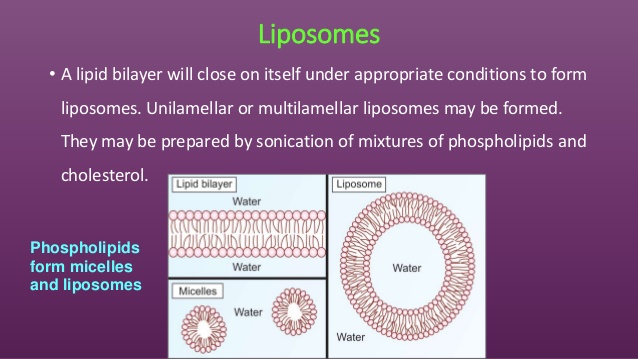
Liposomes are nanosized artificial vesicles of spherical shape that can be produced from natural or synthetic phospholipids. The polar head groups are located at the surfaceof the membranes, in contact with the medium, whereas the fatty acid chains form the hydrophobic core of the membranes, shieldedfromthewater.Polarmoleculescanbeencapsulated in the aqueous core, while hydrophobic molecules dissolve in the bilayers of liposomes [19]. Lipid film hydration is the simplest method for the encapsulation of water-soluble drugs; a lipid film is hydrated with an aqueous solution containing the drug (Figure 2). Entrapment efficiency may vary depending on: (i) the compound itself (charge, size, solubility, etc.), (ii) the lipid variations (a single type or a mixture of lipids, composition, charge, etc.), and (iii) the preparation methods. The manipulation and design of liposomes endowed with the ability for targeting specific cell sites (or alternately, the temporary avoidance of these sites), results inlongcirculation,increasedbiodistribution,andfavourable pharmacodynamics [98–100]. Currently, a number of liposome formulations are in clinical use to combat cancer and infectiousdiseases,whileothersawaitclinicaltrialoutcomes. For example, while DaunoXome, Doxil, and Ambisome are currently clinically approved, CPX-1 and LE-SN38 are examples of liposomal-based drugs that encapsulate a topoisomerase I inhibitor and are currently in Phase-II clinical trials for the treatment of colon or colorectal cancer [100]. Someofthesetbacksanddisadvantagesofliposomesinclude time-consuming preparation techniques, low entrapment volumes, and toxicity concerns due to the presence of residual toxic organic compounds during preparation [98]. The lipids used for the preparation of liposomes are predominantly phospholipids or surfactants which form bilayers similar to those found in biological membranes. The surfactants dimyristoyl-phosphatidyl-glycerol (DMPG), dimyristoyl-phosphatidylcholine (DMPC), dipalmitoyl-phosphatidylcholine (DPPC), and desaturated-phosphatidylcholine (DSPC) are naturally occurring but can be produced synthetically as well. Extensive testing of these phospholipids has revealed them to be remarkably safe for pharmaceutical use. For example, phosphatidylcholine (i.e., DPPC) (a phospholipid that carries no net charges, is the major constituent of cell membranes, and it provides a structural framework for the membrane and maintains the permeability barrier) is well tolerated in animal and human studies. Inhaled nondrug containing liposomes (15 and 150mg of lipid DPPC/mL) for 1 hour on pulmonary function and on oximetry in healthy nonsmoking volunteers showed that liposome inhalation is well tolerated, and no oxygen desaturation, decrements in pulmonary function, or side effects were noted [101]. Other investigators have utilizedfluorescein-labelledliposomestoexaminetheclearance ofaerosolizedliposomesfromthelungsofhumanvolunteers and reported no untoward side effects [102]. Intravenous administration of DPPC (5 to 50mg of lipid) did not induce immediate or delayed toxicity in mice and did not produce any changes in body weight and weight of major organs 2 weeks after administration. It has been reported that the toxicity of intravenously administered liposomes composed of DPPC is so low that accurate assessment of an LD50 value is difficult and has been estimated to be of the order of 10g/kg in mice [103]. However, it is important to note that addition of other constituents to liposomes in order to alter stabilityorkineticscanresultinanincreaseintoxicpotential, particularlyonparenteraladministrationofliposomes[104].
A potential problem with conventional liposomes, particularly when delivered intravenously, is their rapid removal from circulation by cells of the reticuloendothelial system (RES) particularly in the liver and spleen [14, 105]. To circumvent the phagocytic cells of the immune system and hence enhance their half-life in the circulation, “stealth liposomes” have been designed [14, 105]. Stealth liposomes are created by coating the liposomes with a layer of polyethylene glycol-phosphatidylethanolamine (PEG liposomes). PEGylation is the process of covalent attachment of polyethylene glycol polymer to another molecule masking the agent from the host’s immune and metabolic systems and creating a shield around the pegylated agent due to its large hydrodynamic volume, thus protecting it from renal clearance and consequently prolonging its circulatory time [14, 105, 106].
Preparation of liposomal-N-acetylcysteine (L-NAC). L-NAC is prepared from a mixture of DPPC and NAC in a 1 : 1 molar ratio by a dehydration-rehydration method. The lipids are dissolved in chloroform in a round-bottomed flask and dried at 45°C with a rotary evaporator. The lipid film is dried with nitrogen to eliminate traces of chloroform and hydrated with a solution of NAC and subsequently sonicated. Sonication is a simple method for reducing the size of liposomes. Upon rehydration, free NAC is separated by high-speed centrifugation (24400 g at 4°C, for 30 min), a step performed twice. At the end of this procedure, the liposomal vesicle size is usually below 200 nm mean diameter with an encapsulation efficiency of 35% NAC.
Biodegradable liposome-encapsulated hydrogels for biomedical applications: a marriage of convenience
Santiago Grijalvo ab, Judith Mayr c, Ramon Eritja ab and David Díaz Díaz *ac
aInstitute of Advanced Chemistry of Catalonia (IQAC-CSIC), Spain
bBiomedical Research Networking Center in Bioengineering, Biomaterials and Nanomedicine (CIBER BBN), Spain
cInstitute of Organic Chemistry, University of Regensburg, Universitätstrasse. 31, D-93040 Regensburg, Germany. E-mail: David.Diaz@chemie.uni-regensburg.de; Fax: +49 941 943-4121; Tel: +49 941 943-4373
Received 26th October 2015 , Accepted 12th January 2016
First published on 28th January 2016
Hydrogels are hydrophilic three-dimensional networks with demonstrated potential for medical and pharmaceutical applications. Specifically, biopolymer-based hydrogels offer certain advantages over synthetic polymers in terms of biocompatibility and biodegradability. Because of their inherent properties, hydrogels are able to efficiently encapsulate and liberate in a controlled release manner, different hydrophobic and hydrophilic therapeutic molecules, including nucleic acids, proteins and antibodies. Several strategies have been reported in the literature to minimize the potential burst release of encapsulated drugs, thus preventing their local accumulation and consequent toxic responses. Within this context, liposomes embedded in hydrogels have emerged as an attractive strategy to reduce this undesirable effect. This tutorial review covers a selection of the most promising cationic, neutral and anionic biopolymer-based hydrogels containing liposomes, niosomes or vesicles for drug delivery or tissue engineering applications.
Injectable Lipid Emulsions—Advancements, Opportunities and Challenges
University of Mississippi
Injectable Lipid Emulsions—Advancements, Opportunities and Challenges
University of Mississippi
Key unit operations for preparing lipid emulsions
Injectable lipid emulsions, for decades, have been clinically used as an energy source for hospitalized patients by providing essential fatty acids and vitamins. Recent interest in utilizing lipid emulsions for delivering lipid soluble therapeutic agents, intravenously, has been continuously growing due to the biocompatible nature of the lipid-based delivery systems.
Advancements in the area of novel lipids (olive oil and fish oil) have opened a new area for future clinical application of lipid-based injectable delivery systems that may provide a better safety profile over traditionally used long- and medium-chain triglycerides to critically ill patients. Formulation components and process parameters play critical role in the success of lipid injectable emulsions as drug delivery vehicles and hence need to be well integrated in the formulation development strategies. Physico-chemical properties of active therapeutic agents significantly impact pharmacokinetics and tissue disposition following intravenous administration of drug-containing lipid emulsion and hence need special attention while selecting such delivery vehicles. In summary, this review provides a broad overview of recent advancements in the field of novel lipids, opportunities for intravenous drug delivery, and challenges associated with injectable lipid emulsions.
Emulsifiers
Emulsions are thermodynamically unstable systems and will eventually undergo physical changes (e.g., aggregation, creaming, and droplet growth) over time. Emulsifiers stabilize emulsions by reducing the interfacial tension of the system and by providing enough surface charge for droplet–droplet repulsion. The choice of emulsifier is driven by its toxicity profile, intended site of delivery, and stabilizing potential. Natural lecithin, obtained from egg yolk, has been used extensively to stabilize injectable emulsions (29). These emulsifiers are biocompatible, nontoxic, and are metabolized like natural fat (30). However, hydrolysis of natural lecithin during emulsification, sterilization and storage leads to the formation of lysophospholipids, with detergent like properties, and causes hemolysis. Although, such effects have been rarely reported in clinics lysophospholipidlevelsmustbecontrolled(50).Combination of synthetic surfactants with lecithin, use of purified lecithin, and addition of free fatty acids has been suggested to reduce the formation of lysophospholipids (46,50). Polyethylene glycol (PEG) lipids such as polyethylene glycol-modified phosphatidylethanolamine (PEG-PE) have been used as emulsifiers/coemulsifiers to sterically stabilize emulsion formulations through the presence of PEG head groups at the emulsion surface (51–53). Additionally, the steric stabilization and/or increased hydrophilicity imparted by these emulsifiers have been demonstrated to reduce the affinity of the emulsion droplet for the mononuclear phagocyte systems (53). Non-ionic surfactants, especially Pluronic® F68, also hold great potential. Injectable emulsions stabilized with Pluronic® F68, either alone or in combination with phospholipids, have been shown to improve the stability of emulsions. However, long-term administration of emulsionscontainingPluronic®F68hasbeenassociatedwithsocalled “overloading” syndrome characterized by hyperlipidemia, fever, anorexia, and pain in the upper stomach, hemolysis, and anemia (46,54–57). Systemic toxicity, mainly hemolysis, and problems during autoclaving have limited the use of a number of, otherwise excellent, emulsifying agents (58).
MANUFACTURING
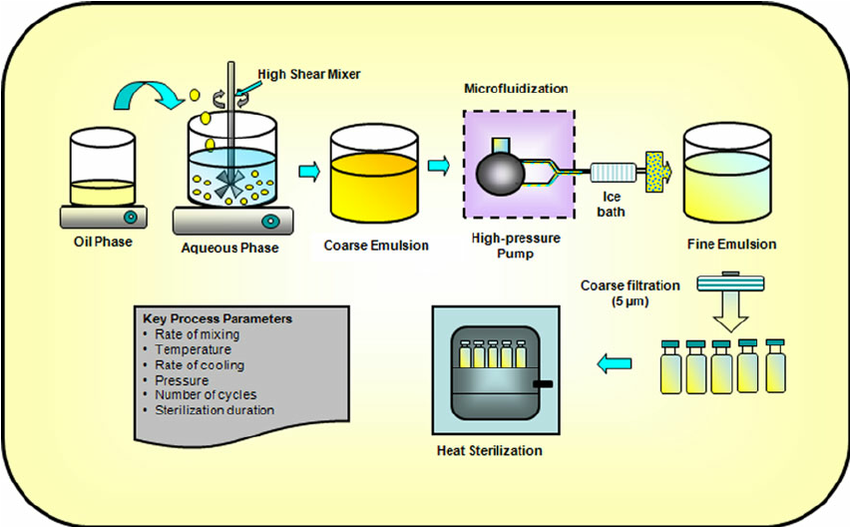
Formulation Process
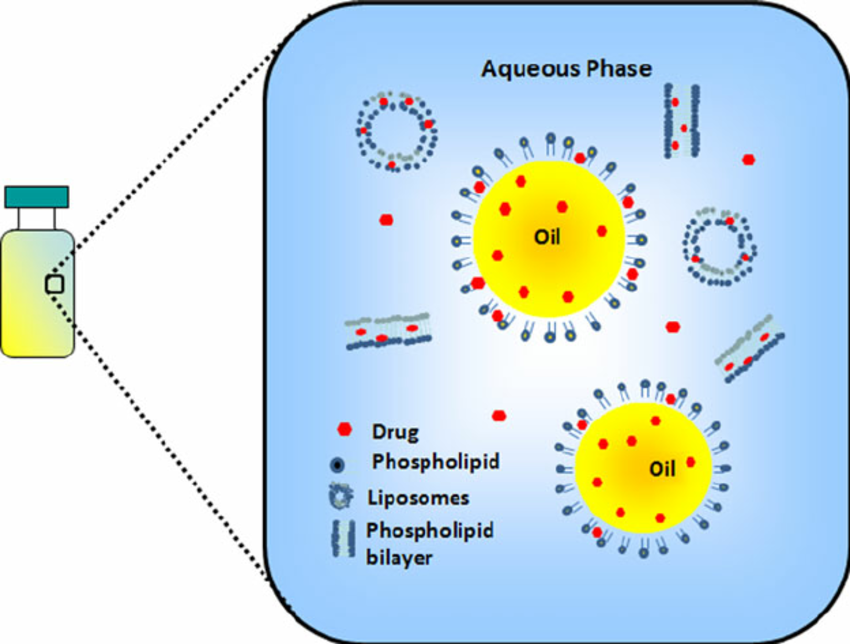
Figure 1 depicts the key processes involved in the production of injectable lipid emulsions. Water soluble and oil-soluble ingredients are generally dissolved in the aqueous phase and oil phase, respectively. Emulsifiers, such as phosphatides, can be dispersed in either oil or aqueous phase. Both phases are adequately heated and stirred to disperse or dissolve the ingredients. The lipid phase is then generally added to the aqueous phase under controlled temperature and agitation (using high-shear mixers) to form a homogenously dispersed coarse emulsion (46,58). Coarse emulsions with a droplet size smaller than 20 μm generally produces unimodal and physically stable fine emulsions (64). The coarse emulsion is then homogenized (using a microfluidizer or a high-pressure homogenizer) at optimized pressure, temperature, and number of cycles to further reduce the droplet size and form fine emulsion (65,66). Factors such as type and concentration of oil phase and surfactants, operating temperature, pressure, number of cycles, etc. can influence the mean droplet size during high-pressure homogenization and microfluidization. The USP <729> specifies that throughout the shelf-life mean droplet size and PFAT5 (volumeweighted percentage of fat globules ≥5 μm) of an injectable fine emulsion should be ≤500 nm and ≤0.05%, respectively (67,68). For example, the mean droplet size of Intralipid 10% and 20% has been reported to be 276 and 324 nm, respectively (65). The pH of the resulting fine emulsion is then adjusted to the desired value and the emulsion is filtered through 1–5 μm filters (64). The fine emulsions are usually packed in USP type I glass containers. Siliconized containers are sometimes used to prevent droplet size growth (58). Plastic containers are permeable to oxygen and contain oilsoluble plasticizers and are thus usually avoided (46,58). Additionally, teflon-coated vial plugs/stoppers are usually used to prevent oxygen permeation and softening on contact with the oil phase (46,58). The entire process (filtration/ coarse and fine emulsion preparation) should be carried out under nitrogen atmosphere whenever possible and especially in cases where the excipients and drugs are sensitive to oxidation (46,58,60).
Drug Incorporation Methods
Water-insoluble drugs, with or without the aid of cosolvents, can be incorporated into the emulsions by dissolving the drug in the oil phase prior to emulsification (de novo method) or added to pre-prepared emulsions (extemporaneous addition). For drugs that are highly oil soluble, the de novo method, which involves dissolving the therapeutic agent into the oil phase prior to emulsification, is usually adopted (42,69). In some cases, elevation of temperature and use of fatty acids as lipophilic counter-ions can help in the solubilization process (33,70). Alternately, oil-soluble drugs that are liquid at room temperature, such as halothane and propofol, can be extemporaneously added to pre-formed emulsions (e.g., Intralipid®) whereon the drug preferentially partitions into the oil phase (42). Recently, a solvent-free novel SolEmuls® Technology has been developed that localizes the drug at the interface of the emulsion. In this approach, the drug, as ultra-fine powders/nanocrystals, is added to preformed emulsions (e.g., Lipofundin® and Intralipid®) or to coarse emulsions, and the mixture is then homogenized until thedrugcrystalsaredissolved,resultinginlocalizationofdrugat the interface (69,71,72). Amphotericin B formulated using this technology has been shown to be more effective and less toxic than the commercially available formulation (73). However, it has been suggested that in order to take advantage of emulsion dosage forms it is desirable to incorporate the drug into the innermost phase of the emulsion (70).
under reduced pressure in round bottom flasks to form a thin film. Upon sonication with the aqueous phase, a liposome-like dispersion is formed. Addition of the oil phase to this drugliposome dispersion followed by emulsification results in an emulsion formulation (60). However, the use of co-solvents warrants careful assessment of drug precipitation, physical and chemical stability of emulsions and drug partitioning in the formulation (42). Figure 2 depicts the emulsion structure and possible drug molecule distribution within the emulsion system. Drug may possibly get incorporated within the oil phase, aqueous phase, phospholipid rich phase (PLR) or the mesophase. Centrifuging the emulsions will separate these phases. The PLR has been suggested to be composed of phospholipids that formed a layer at theinterfacebetween theoilphaseandthe aqueousphase as well as excess phospholipids dispersed in the emulsion system. The mesophase is thought to essentially consist of liposomes, also formed from excess phospholipids (74,75). Recently, Sila-on et al. investigated the effect of drug incorporation method (de novo versus extemporaneous addition) on partitioning behavior of four lipophilic drugs, diazepam (logP 2.23), clonazepam (logP 1.46), lorazepam (logP 0.99), and alprazolam (logP 0.54) in parental lipid emulsions (soybean oil (10% w/w) and Epikuron® 200) (74). Partitioning of diazepam was unaffectedbydrugincorporationmethod;bothmethodsyielded highdrugconcentrationsintheinneroilphaseandPLR.Onthe otherhand,partitioning of thelesslipophilicdrugsclonazepam, lorazepam, and alprazolam was dependent on the method of incorporation. De novo emulsification and extemporaneous addition resulted in higher drug localization in PLR, and aqueous and mesophase, respectively (74).
Drugs that are slightly soluble in oil can be incorporated into the emulsions with the aid of co-solvents (42,64). The solvents are evaporated during the manufacturing process. Another approach involves dissolving drug and phospholipids inorganicsolventsfollowedbyevaporationoftheorganicphase
droplet size and morphology of the droplets (29). Detailed review of application of these techniques in submicron emulsions has been published (77,78).
Zeta Potential. Zeta potential is defined as the electrical potential at the shear plane of the emulsion droplet and is a useful parameter for stability assessment. A number of factors such as pH, ionic strength, type and concentration of emulsifiers and presence of electrolytes can affect the zeta potential of the system (78). A zeta potential value of ±25 mV has been suggested to produce a stable emulsion (79).
Viscosity. The rheological properties of emulsions have been reviewed by Sherman et al.(80). These properties can be complex and depends on a number of factors such as surfactants and oils used, ratio of dispersed and continuous phase, droplet size distribution and other factors. Flocculation of emulsions will generally increase the viscosity during storage and is important for assessing stability and shelf-life of the emulsion system.
pH. The pH of these lipid emulsions decrease during sterilization and storage as a result of increase in FFA content due to the hydrolysis of phosphatidylcholine (PC) and phosphatidylethanolamine (PE), the lysoderivatives of PC and PE, and the emulsified triglycerides (81). A decrease in pH can lead to a decrease in the zeta potential of the emulsion droplets and ultimately lead to emulsion instability. Thus, pH of the system should be maintained throughout the shelf-life of the emulsion (82).
In Vitro Release. Characterizing in vitro drug release from emulsionsisa challengingtask because ofthe submicron sizeof the droplets and difficulty in separating the continuous and dispersed phase (58). A number of experimental techniques such as dialysis bag method, diffusion cell method, centrifugal ultrafiltration technique, ultrafiltration at low pressure, and continuous and in situ flow methods have been investigated to measurethereleaseofdrugfromcolloidaldispersions.Detailed descriptions of these methods have been given elsewhere (58,83). However each of the above methods is associated with certain drawbacks. Ultrafiltration techniques use filtration and centrifugation steps to separate the drug released into the continuousphasefromtheoildroplets.However,applicationof external energy can result in emulsion destabilization and increase in the drug release rates (58,83).
the residual carrier bound drug. However, this method is not suitable for all compounds as it requires an analytical method which detects the drug without interference from the emulsion system (86). The continuous flow method involves addition of the drug carrier to a filtration cell containing the sink medium. The sink medium is continuously replaced with fresh medium and analyzed simultaneously. Clogging of the filter, and emulsion destabilization, limits the use of these systems for measuring true release rates. Reverse bulk equilibrium dialysis avoids the above drawbacks. In this method, the drug incorporated emulsion is added to a sink media containing a number of small dialysis bags, previously filled and equilibrated with the sink solution. At appropriate time points these small dialysis bags are removed and the content analyzed. This method avoids violation of sink condition, destabilization of emulsion and the need for filtration and centrifugation. Additionally, this technique has the capability to mimic the in vivo situation where the drug is administered intravenously (86).
...
Effect of Droplet Size
All the factors listed above, in particular type and concentration of lipid and emulsifier used, can significantly affect the droplet size (57,88,90,112,141,142). An increase in the total interfacial area with a decrease in droplet size facilitates LPL and HL activity (143,144). However, larger sized droplets (>than 250 nm compared to <100 nm) were cleared faster, indicating a greater role of MPS, compared to LPL, in the clearance of these emulsion (144,145). Takino et al. and Kurihara et al. also demonstrated that compared to small sized emulsion, large size emulsions were rapidly eliminated from the blood circulation and were taken up by the MPS (107,113). Moreover, droplet size has been shown to determine distribution within tumor and other peripheral tissues (146). Emulsions with droplet size larger than 200 nm effectively inhibited drug penetration into the bone marrow, small intestine and other non-MPS organs, indicating size controlled disposition in the body (Table III)(107).
An emulsion with water, linoleic acid and emulsifier ...
https://www.researchgate.net/post/An_emulsion_with_water_linoleic_acid_and_emulsifier...
Emulsions of linoleic acid and other unsaturated fatty acids, when incubated at 37 °C in air with cysteine, glutathione and sulphydryl proteins such as papain, cause a rapid destruction of —SH groups. The rate of —SH group destruction is slowed down in a nitrogen atmosphere but considerably increased in oxygen.
Rationale for using new lipid emulsions in parenteral ...
https://www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/...
May 11, 2009 · 3.Sprecher, H (2002) The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 67, 79–83. 4.Edgren, B & Wrtelind, A (1963) The theoretical background of the intravenous nutrition with fat emulsions.
Author: Philip C. Calder
Publish Year: 2009
Review of Intravenous Lipid Emulsion Therapy
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5102272
Nov 17, 2016 · The emulsion is a mixture of 3 fatty acids: omega-6, -3, and -9. In addition, alpha linolenic acid (ALA), which is used as a stabilizing agent for the emulsion, makes up a small percentage of the emulsion.1 Vitamin E is also added to help reduce the oxidative stress of the emulsion components, as well as the oxidative stress on patients.1–3
Cited by: 2
Publish Year: 2016
Author: Jeffery W. Spray
5 Dangers of Eating Saponins
Saponins are Antinutrients and Disrupt Fat Metabolism
Saponins Increase Intestinal Permeability
Saponins Cleave Cholesterol
Saponins Disrupt Endocrine Function
Saponins are Toxic to Cells
What Are Saponins?
Saponins…Not just a shorthand way to say hello to someone. “What’s saponin?”
Saponins are a class of bitter-tasting compounds that produce soap-like foam when added to water. Most saponins occur naturally in plants, but some are manmade for scientific or industrial purposes.
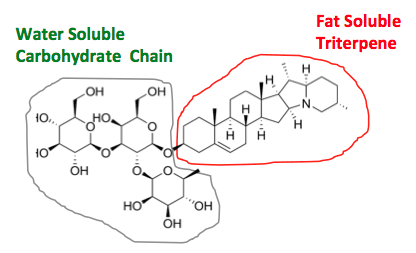
All saponins have a hydrophilic (water soluble) carbohydrate bonded to a lipophilic (fat soluble) triterpene or steroid structure. The hydrophilic and hydrophobic properties interact with the surface tension of water to create bubbles in aqueous solutions.
Hydrophilic and Lipophilic
Like many plant compounds, saponins evolved as a defense system against herbivores and insects[*]. Unlike human beings, they don’t like being submerged in a bubble bath.
Are Saponins Healthy?
Overall, the effects of dietary saponins aren’t very well-studied. Some researchers believe they have medicinal effects or health benefits, but any such effects appear to be hormetic in nature.
Hormesis is a favorable biological response that sometimes occurs when cells or organisms are exposed to manageable amounts of toxins or stress.
According to the authors of a 2018 paper,
“Emerging evidence suggests that hormetic phytochemicals produced by environmentally stressed plants can activate the moderate cellular stress response mechanisms at a subtoxic level in humans, which may enhance tolerance against severe dysfunction or disease.”[*]
In other words, by taking in low levels of toxic compounds, you might make your cells stronger. As Nietzsche said “What doesn’t kill you makes you stronger…but quinoa still tastes like cardboard.”
The dose makes the poison. And unfortunately, no one really knows what the correct dose of saponins is for these effects.
Someone who eats saponin-rich foods every day could be well above the threshold for any theoretical beneficial hormetic effects. And if you are already unwell, they could easily make you sicker.
That’s why I recommend exercise, fasting, cold exposure, and other forms of hormesis instead.
They’re safer because you can control the dose and gauge the effects much more easily
Some studies also show that Ketones and BHB upregulate the same antioxidant pathways, without the side effects [*].
Even if saponins are beneficial from an antioxidant perspective, they have side effects. It’s like smoking a cigarette combined with quinoa, because it has antioxidants.
Keep reading to learn 5 reasons why eating saponin-rich plant foods is unwise.
Foods Highest In Saponins
Watch out for these foods saponin content
Licorice root (22.2-32.3 grams per 100g)[*]
Legumes, especially peanuts, soybeans (3.9-5.6 grams per 100g), and chickpeas (3.6-5 grams per 100g)[*][*]
Quinoa (up to 0.73g per 100g)[*]
Spinach (0.5g per 100g)[*][*]
Oats (0.1-0.3g per 100g)[*][*]
5 Reasons to Avoid Saponins
#1: Saponins are Antinutrients and Disrupt Fat Metabolism
Similar to oxalates, phytins, lectins, and tannins, saponins are antinutrients[*]. These jerks bully your cells and steal their nutrients.
Research shows that saponins in plant foods may interfere with the absorption and digestion of glucose, protein, and vitamins A, B12, D, and E[*][*][*].
They can also form protein complexes that have unknown effects when combined with proteins like casein from milk[*].
Not only that, saponins can disrupt the digestion of cholesterol and saturated fat.
Saponins form complexes with cholesterol that cause up to 44% of dietary cholesterol to be excreted rather than digested[*][*]. And in the case of saturated fats, they can decrease absorption by up to 87% by forming calcium-containing complexes[*].
Saponins and its effect on the human body
They also inhibit pancreatic lipase which can cause oily diarrhea and liver failure[*][*]. The only people saponins benefit: the executives of Charmin toilet paper.
Vegetables are not this horcrux that solves all your problems. Turns out they can even make you fat.
Some saponins may even inhibit lipolysis (the release of free fatty acids) and hepatic gluconeogenesis, which could prevent your body from burning stored fat[*].
The mainstream medical paradigm considers antinutrients that disrupt cholesterol and fat metabolism “beneficial” because of the narrative that cholesterol and fat are responsible for heart disease and the obesity epidemic.
However, nothing could be further from the truth. You need cholesterol and saturated fat to make hormones and stay alive, and higher intake levels correlate to a healthier, longer life.
#2: Saponins Increase Intestinal Permeability
Along with preventing absorption of some nutrients, eating too many saponins can cause leaky gut.
According to the authors of a study published in the Journal of Nutrition,
“The results indicate that some saponins readily increase the permeability of the small intestinal mucosal cells, thereby inhibiting active nutrient transport, and facilitating the uptake of materials to which the gut would normally be impermeable.”[*]
Basically, increased permeability of your intestines can allow bacteria, metabolites, and small food molecules to “leak” into your bloodstream and cause autoimmune issues and inflammation[*]. Your gut, stops holding the door.
Hodor
Think of your gut like an exclusive club. The bouncers are the single layer of epithelial cells. In line, there are both toxins — underrage and uncool kids — and nutrients — celebrities and fashionistas you want inside. When your gut bouncers reject the toxins, saponins beat them over the head and find their way in anyways.
The club turns from something cool, fun and exclusive to a drunk, under-age mosh pit. It’s a disaster for everyone.
Mouse studies of high doses of saponins demonstrate intestinal hemorrhage, erosion of mucosa, and damage to the small intestine, liver, and kidney[*].
Although it’s unlikely anyone eats enough saponins to cause hemorrhaging, some researchers think that elevated saponins in modern diets might be responsible for gut and immune issues[*].
“Leaky gut syndrome” may be responsible for rising rates of celiac disease, too[*].
#3: Saponins Cleave Cholesterol
Remember how saponins form complexes in your gut that prevent cholesterol from absorbing?
Saponins can also interfere with cholesterol in your cells and cell membranes[*]. All your cells contain cholesterol, and they require it for normal functioning[*].
In fact, scientists often use saponins to cleave cholesterol from cell membranes intentionally, to better examine them[*]. Instead of eating leftover quinoa, scientists put it in a test tube to conduct their tests. Okay, not actually. These scientists use chemically isolated saponins — but it’s the same exact chemical.
Plant saponins also have the ability to strip away phospholipids, another vital part of cells[*].
According to a 2013 peer-reviewed paper, “the general cytotoxicity of saponins is mainly dependent on their membrane toxicity and that the membrane toxicity might be caused by the loss of cholesterol from the cell membrane”[*].
Keep that in mind next time you hear someone talking about the benefits of “lowering your cholesterol” with plant compounds or drugs.
Cholesterol is needed to survive
#4: Saponins Can Disrupt Endocrine Function
Saponins can disrupt male and female hormones in two different ways.
First of all, even though your cells can make cholesterol, you need adequate dietary cholesterol to sustain production of sex hormones[*][*]. And as we’ve already learned, saponins prevent your body from absorbing cholesterol.
Second, many saponins have a phytoestrogenic effect and may act as an endocrine disruptor[*].
In men, endocrine disruptors can reduce testosterone levels, lower sperm count, and cause feminization[*]. We all knew eating quinoa was lame, but this takes it to a whole new level.
Saponins and Testosterone
And research links endocrine disruption in women to higher rates of breast cancer, infertility, and children with birth defects[*].
Finally, children may be more susceptible to endocrine disruption because their brains and organs are still in development[*][*].
That’s why I recommend that everyone, but especially kids, avoid saponins. Why take the risk?
#5: Saponins are Toxic to Cells
We’ve already covered the fact that saponins can cleave cholesterol from cells and increase gut permeability.
However, the cytotoxicity (cellular toxicity) of saponins extends beyond those effects.
While some scientists think it could make saponins useful for treating cancer, cytotoxicity is a double-edged sword (at best)[*].
For example, saponins remove the cell membrane from erythrocytes and destroy red blood cells[*].
Saponins are a one-two punch. They first damage your gut junctions and allow molecules into your bloodstream. Then once inside, they destroy your cellular integrity.
In vitro studies also show that saponins can damage and dissolve the endothelium (delicate, single-cell lining) of blood vessels[*].
Do people typically eat enough saponins to cause these effects?
No one knows for sure, but if you eat a standard vegan cocktail of soy, chickpeas, quinoa, or take saponin supplements like ginseng or licorice root, you could be getting several grams of saponins each day.
Studies show that saponins cleave cell membranes in concentrations of micrograms per milliliter [*]. Do you really want to unleash these toxic termites into your bloodstream?
Last but not least, plant saponins are also genotoxic, meaning they cause DNA damage and interfere with cell replication[*].
According to a 2015 study, a triterpenoid saponin caused DNA damage in concentrations as low as 5 micrograms per milliliter[*]. That translates to human serum levels as low as a few milligrams of saponins.
Essentially, the evidence shows that saponins are likely to cause problems no matter where they end up in your body.
Final Thoughts
Are saponins helpful or harmful?
For the most part, they’re harmful.
Most of the purported health benefits involve lowering blood glucose, insulin levels, cholesterol levels, or triglycerides[*]. But if you eat a healthy diet to begin with, you don’t have to worry about any of those things.
Perhaps in the future, someone will discover that saponins can help cancer patients. But you wouldn’t chow down on chemotherapy drugs at every meal to prevent cancer, would you?
Bottom line: instead of taking “plant medicine” to heal the effects of a toxic diet, try eating a diet that isn’t toxic.
Want to learn how to use the carnivore diet to heal? The best way is to click the button below to obtain the in-depth, free guide I’ve made for you.
And if you want to ask questions and learn together with a like-minded group of carnivores, check out my Facebook group Carnivore Nation. I’m also active on Twitter and Instagram daily.The Science of Saponins: 5 Dangers of Eating Them
https://carnivoreaurelius.com/saponins/
Ultrasonic Homogenizers for Liquid Processing - Hielscher ...
https://www.hielscher.com
When mixing immiscible liquids into an emulsion, droplet size and distribution are a key factor for the stability of the emulsion. Ultrasonics can create very fine size droplets and narrow size distributions. In most cases, our ultrasonic mixers can achieve submicron droplets when preparing emulsions in …
Liposome: classification, preparation, and applications
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3599573
Feb 22, 2013 · Briefly, first, the water-in-oil emulsion is shaped by brief sonication of a two-phase system, containing phospholipids in organic solvent such as isopropyl ether or diethyl ether or a mixture of isopropyl ether and chloroform with aqueous buffer. The organic solvents are detached under reduced pressure, resulting in the creation of a viscous gel.
Cited by: 1346
Publish Year: 2013
Author: Abolfazl Akbarzadeh, Rogaie Rezaei-Sadabady, Ro
BMC Complement Altern Med. 2019; 19: 46.
Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria
Xiaoyan Sun,#1,2 Xiushi Yang,#2 Peng Xue,2 Zhiguo Zhang,corresponding author1 and Guixing Rencorresponding author2
1College of Food Science and Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan, 250353 China
2Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing, 100081 China
Abstract
Background
Quinoa is a food crop native to the Andes. The process of dehulling quinoa can produce approximately 8–12% husk, which is often discarded because it contains bitter saponin. Saponin derived from quinoa has been reported to exhibit anti-inflammatory and antifungal activity. However, the antibacterial effects of quinoa saponin against halitosis-related bacteria are still unclear.
Methods
In this study, quinoa saponin (QS) and alkali-transformed saponin (ATS) were separated by AB-2 resin to obtain QS-30, QS-80, ATS-30 and ATS-80. Halitosis-related bacteria included Porphyromonas gingivalis (P. gingivalis), Clostridium perfringens (C. perfringens) and Fusobacterium nucleatum (F. nucleatum). The MIC and MBC were determined using gradient dilutions in 96-well plates, and the saponins were identified by HPLC and mass spectrometry. The changes in membrane integrity were tested using a microplate reader, the membrane potential was tested by spectrofluorometry, and the morphological characteristics were examined using a transmission electron microscope to explore the antibacterial mechanisms.
Results
Antibacterial assays indicated that QS-80 and ATS-80 showed inhibitory activity. In addition, ATS-80 exerted a stronger inhibitory effect than QS-80, especially against Fusobacterium nucleatum, with a lower minimum inhibitory concentration (31.3 μg/mL) and a lower minimum bactericidal concentration (125 μg/mL). ATS-80 destroyed the bacterial membrane structure, leading to bacterial death.
Conclusions
Based on the excellent antibacterial activity and economic prospects of quinoa husk, ATS-80 could be used as an antibacterial agent to treat halitosis.
Keywords: Quinoa, Halitosis, Alkali-transformation, Antibacterial activity, Antibacterial mechanism
Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6373059/
皂苷(saponin)别称:碱皂体;皂素;皂甙(zao dai);皂角苷;或皂草苷。“皂苷”一词由英文名 Saponin 意译而来,英文名则源于拉丁语的 Sapo,意为肥皂。皂苷(Saponin)是苷元为三萜或螺旋甾烷类化合物的一类糖苷,主要分布于陆地高等植物中,也少量存在于海星和海参等海洋生物中。 许多中草药如人参、远志、桔梗、甘草、知母和柴胡等的主要有效成分都含有皂苷。有些皂苷还具有抗菌的活性或解热、镇静、抗癌等有价值的生物活性。
结构
“皂苷”一词由英文名 Saponin 意译而来,英文名则源于拉丁语的 Sapo,意为肥皂。 皂苷是苷类中结构比较复杂的化合物。它们广泛存在于植物体内,种类繁多,组成复杂。国际上对皂苷的研究十分活跃,80年代已进入一个新的高潮。不仅一些结构复杂的皂苷的结构得到证实,而且纠正了以往结构鉴定中的某些错误,他们系统研究了不少重要中草药中的皂苷。
1.其外观呈白色至浅黄棕色粉末,或茶棕色至棕黑色糊状体(固形物60%)。
2.粉末体部分易溶于水,未溶部分溶于乙醇水溶液。pH4.5~5.5。
3.灰分10%~12%。与水一起振摇则成胶体状溶液,显示优良的起泡作用和乳化作用。
4.质量最优的是从产于智利、秘鲁、玻利维亚的蔷薇科的常绿乔木Quillaja saponaria取得的三萜系化合物的皂树皮皂角苷,比从其他原料取得者佳。是FDA承认的惟一食品用皂角苷。
皂苷由皂苷元与糖构成。组成皂苷的糖常见的有葡萄糖、半乳糖、鼠李糖、阿拉伯糖、木糖、葡萄糖醛酸和半乳糖醛酸等。
苷元为螺旋甾烷类(C-27甾体化合物)的皂苷称为甾体皂苷,主要存在于薯蓣科、百合科和玄参科等。分子中不含羧基,呈中性。燕麦皂苷D和薯蓣皂苷为常见的甾体皂苷。
苷元为三萜类的皂苷称为三萜皂苷,主要存在于五加科、豆科、远志科及葫芦科等,其种类比甾体皂苷多,分布也更为广泛。大部分三萜皂苷呈酸性,少数呈中性。
柴胡皂苷
柴胡皂苷
皂苷根据苷元连接糖链数目的不同,可分为单糖链皂苷、双糖链皂苷及三糖链皂苷。在一些皂苷的糖链上,还通过酯键连有其他基团。
皂苷的化学结构中,由于苷元具有不同程度的亲脂性,糖链具有较强的亲水性,使皂苷成为一种表面活性剂,水溶液振摇后能产生持久性的肥皂样泡沫。一些富含皂苷的植物提取物被用于制造乳化剂、洗洁剂和发泡剂等。
功能
皂苷的溶血性
1.溶血原因:
皂苷与胆甾醇结合生成不溶性分子复合物,破坏血红细胞的渗透性而发生崩解。
2.溶血指数(衡量溶血强度大小):
在一定条件(等渗、缓冲、恒温)下能使同一动物来源的血液中红细胞完全溶解的最低浓度。
3.溶血作用
(1)溶血作用的有无与分子结构中皂苷元有关。
例如人参中皂苷没有溶血现象,但是经过分离后的以人参三醇及齐墩果酸为苷元的人参皂苷却有显著的溶血作用,而以人参二醇为苷元的人参皂苷则有抗溶血作用。
(2)皂苷的溶血作用的强弱和糖部分有关,单糖链皂苷作用显著,某些双糖链皂苷则无溶血作用,但经酶转化成单糖链皂苷后便有了溶血作用。
4.溶血强度规律:
单皂苷>双皂苷、中性皂苷
5.某些植物的树脂、脂肪酸、挥发油也溶血。
多数皂苷能降低液体(水)的表面张力,具有起泡沫性质和乳化剂作用,能用作清洁剂, 还有溶血和毒鱼的作用。有许多含皂苷类成分的中药如远志、桔梗等有祛痰止咳的功效;有些皂苷还具有抗菌的活性或解热、镇静、抗癌等有价值的生物活性。个别皂苷有特殊的生理活性,如人参皂苷能增进DNA和蛋白质的生物合成,提高机体的免疫能力。甘草酸具有促进肾上腺皮质激素的作用,并有止咳和治疗胃溃疡病的功效。
皂苷是一组结构多样的自然产生的化合物,主要发现在植物中,这些皂苷透出一股苦味,在水溶液中容易起泡沫。皂苷被认为对冷血动物是有毒的,对哺乳类动物的口服毒性却是很低的。在食品中天然存在的皂苷是无毒的,甚至可能对人类饮食有益。
理化性质
物理性质
苷类的一种。能形成水溶液或胶体溶液并能形成肥皂状泡沫的植物糖苷统称。是由皂苷元和糖、糖醛酸或其他有机酸组成的。根据已知皂苷元的分子结构,可以将皂苷分为两大类,一类为甾体皂苷,另一类为三萜皂苷。皂苷多为白色或乳白色无定形粉末,少数为晶体,味苦而辛辣,对黏膜有刺激性。皂苷一般可溶于水、甲醇和稀乙醇,易溶于热水、热甲醇及热乙醇,不溶于乙醚、氯仿及苯。皂苷是很强的表面活性剂,即使高度稀释也能形成皂液。皂苷对心脏有刺激作用;又是很强的溶血剂。
化学性质
一类较复杂的苷类化合物,与水混合振摇时可生成持久性的似肥皂泡沫状物。在植物界分布很广,许多中药例如人参、三七、知母、远志、甘草、桔梗、柴胡等都含有皂苷;中国从前用皂荚洗衣服,就是由于其中含有皂苷类化合物。皂苷由皂苷配基与糖、糖醛酸或其他有机酸组成。组成皂苷的糖常见的有D-葡萄糖、L-鼠李糖、D-半乳糖、L-阿拉伯糖、L-木糖。常见的糖醛酸有葡萄糖醛酸、半乳糖醛酸,这些糖或糖醛酸往往先结合成低聚糖糖链,然后与皂苷配基分子中C3─OH 相缩合,或由两个糖链分别与皂苷配基分子中两个不同位置上的OH相缩合,皂苷配基分子中的─COOH也可能与糖连接,形成酯苷键。
皂苷来源
含有皂苷的植物油豆科、蔷薇科、葫芦科、苋科等,动物有海参和海星。人参皂苷是人参成分中最有效的药用成分,人参皂苷种类有近30种,每一种人参皂苷都有其特定的药理功能。 [2]
大豆皂苷Soyasaponin Aa Acetylsoyasaponin A4对人体不仅无毒害作用,而且具有许多有益的生理功能。近20年来研究结果表明,大豆皂苷具有多种生理活性和良好药理作用,具有抗癌、调节免疫功能、降低血清中胆固醇含量、防治心血管疾病、抗菌、抗病毒,护肝、减肥等多重生理功效,除用作药物外,大豆皂苷还可以作为高级化妆品、食品添加剂和表面活性剂应用于化学工业。 [3]
Methods Mol Biol. 2010;588:63-6. doi: 10.1007/978-1-59745-324-0_9.
Permeabilization of cell membranes.
Jamur MC1, Oliver C.
Author information
1 Department of Cell and Molecular Biology, Faculdade de Medicina de Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil.
Abstract
In order to detect intracellular antigens, cells must first be permeabilized especially after fixation with cross-linking agents such as formaldehyde and glutaraldehyde. Permeabilization provides access to intracellular or intraorganellar antigens. Two general types of reagents are commonly used: organic solvents, such as methanol and acetone, and detergents such as saponin, Triton X-100 and Tween-20. The organic solvents dissolve lipids from cell membranes making them permeable to antibodies. Because the organic solvents also coagulate proteins, they can be used to fix and permeabilize cells at the same time.
Saponin interacts with membrane cholesterol, selectively removing it and leaving holes in the membrane. The disadvantage of detergents such as Triton X-100 and Tween-20 is that they are non-selective in nature and may extract proteins along with the lipids. This chapter provides methods for the use of organic solvents and detergents to permeabilize cell membranes.
为了检测细胞内抗原,必须首先对细胞进行渗透,特别是用甲醛、戊二醛等交联剂固定后。渗透作用提供了获得细胞内或胞内抗原的途径。常用的试剂有两种:有机溶剂,如甲醇和丙酮;洗涤剂,如皂苷、Triton X-100和Tween-20。有机溶剂溶解细胞膜上的脂质,使其可被抗体渗透。因为有机溶剂也会使蛋白质凝固,所以它们可以同时用于固定和渗透细胞。皂苷与膜胆固醇相互作用,有选择地去除胆固醇并在膜上留下孔洞。Triton X-100和Tween-20等洗涤剂的缺点是,它们在性质上是无选择性的,可能会从脂质中提取蛋白质。本章提供了使用有机溶剂和洗涤剂对细胞膜进行渗透的方法。
Permeabilization of cell membranes. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/20012820
Saponin vs Triton X 100
instead of solubilizing the plasma membranes of the epithelial cells,Saponin pokes holes in it leaving the underlying structures and proteins in the cell intact. I used to use it all the time to detect Toxoplasma (parasite) inside epithelial cells since the holes will allow antibodies through into the interior of the cell and the parasitophorous membrane, but leave the parasites in tact. TritonX-100, though a mild detergent actually solubilizes the membranes resulting everything spilling out.
My guess is people use Saponin to keep the bacteria intact to measure the gentamicin in some way, sory I've never done that assay per se.
here's some info from wikipedia that explains a bit why this occurs
One research use of the saponin class of natural products involves their complexation with cholesterol to form pores in cell membrane bilayers, e.g., in red cell (erythrocyte) membranes, where complexation leads to red cell lysis (hemolysis) on intravenous injection.[8] In addition, the amphipathic nature of the class gives them activity as surfactants that can be used to enhance penetration of macromolecules such as proteins through cell membranes.[7] Saponins have also has been used as adjuvants in vaccines.
(http://en.wikipedia.org/wiki/Saponin)
Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study In Vitro and In Vivo
Muhammad Imran Khan ,1 Abdulatef Ahhmed,2 Jin Hyuk Shin,1 Jun Soo Baek,1 Min Yong Kim,3,4 and Jong Deog Kim
Antibiotics are substances that inhibit or kill the microorganisms. Antibiotics are quite important and very necessary in order to provide a feasible solution to control or inhibit the pathogenic bacteria. Bacteria resistivity and adverse side effects of synthetic antibiotics chemicals and search of novel antibiotics from natural sources are highly recommended. Natural products are comparatively safe, effective, and crucial materials for maltipurpose use and applications. Saponins are one of the diverse groups of plant sources compounds with valuable medial values and bioactivates. Saponins possess detergent-like properties and might increase the permeability of bacterial cell membranes; this activity might facilitate antibiotic influx through the bacterial cell wall membrane [38]. Saponins extracts from Quillaja saponaria are in use as foaming agents in beverages or emulsifiers in foods [39].
3.2 Antibacterial Mechanisms of Saponin/Bacterial Cell Lysis Potential of Saponins
For determination the lysis of bacterial cell by saponins we measured the AKP (Alkaline phosphatase) contents of each strain after exposing to saponins.
Saponins caused lysis of bacterial wall as AKP contents were increased rapidly after the addition of saponins to the bacteria culture. The APK contents were found to be increased with increasing concentration of the saponins and it was higher in all cases as compared to control (Figure 8). AKP contents were highest at MIC after 3 hrs treatment in case of S. aureus. The results suggested lysis of bacterial cell by saponins and leakage of the AKP contents.
3.3 3.3. Effects on the Contents of Soluble Proteins of Bacteria
The contents of soluble proteins were found to be increased with the increasing concentration of the saponins (Figure 9). The concentrations of soluble proteins were maximum for strain S. aureus and minimum for strain E. coli at 2 h. For all groups proteins contents were higher as compared to the control group. The results demonstrated that saponins may cause bacterial lysis or damage to the cell wall and member and lead to liberation of internal proteins contents to the external media. The higher amount of proteins contents represents higher lysis and damage of bacteria cells by saponins. Proteins contents were found to be decreased with time as used by bacteria.
Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study In Vitro and In Vivo
https://www.hindawi.com/journals/ecam/2018/3486106/
Hemolysis of human erythrocytes with saponin affects the membrane structure
Article in Acta Histochemica 102(1):21-35 · March
Friedrich Schiller University Jena
Incubation of cells and tissues with saponin makes the lipid bilayer permeable to macromolecules. Ghosts (membrane preparations) of saponin-lysed erythrocytes do not reseal, thus indicating an irreversible damage of the lipid bilayer. We investigated the influence of disturbance of the lipid bilayer on membrane proteins by comparing ghosts of saponin-lysed erythrocytes with ghosts of cells lysed in hypotonic buffer. Transmission electron microscopy revealed destruction of the lipid bilayer and emergence of multilamellar buds in saponin-lysed ghosts. Freeze-fracture electron microscopy showed regions with crystalline lipids and an increase in particle-free areas on fracture faces. The number of protein sulfhydryl groups and the binding of hemoglobin were diminished in saponin-lysed ghosts. A Scatchard plot of hemoglobin binding revealed the decrease of high affinity binding sites. All these results indicate an aggregation of band 3 protein also demonstrated by laser scanning microscopy after incubation of cells labelled with eosin-5-maleimide with sublytic concentration of saponin. Hemolysis with saponin also affected the interaction between transmembrane proteins and the cytoskeleton. Dissociation of peripheral membrane proteins by incubation of ghosts in low salt buffer or by blocking sulfhydryl groups was increased and the association of spectrin with spectrin-depleted vesicles was decreased. The increased incorporation of the fluorescent probe Merocyanine 540 into saponin-lysed ghosts and the increased relative fluorescence quantum yield confirmed the perturbation of the lipid bilayer and the changed interaction between membrane lipids and intrinsic membrane proteins. Our results suggest that permeabilization of the lipid bilayer with saponin to admit the access of antibodies to the cytoplasmic surface of cells can aggregate transmembrane proteins and affect the immunocytochemical localization of associated proteins of the cytoskeleton.
Hemolysis of human erythrocytes with saponin affects the membrane structure | Request PDF
https://www.researchgate.net/publication/12590095_Hemolysis_of_human_erythrocytes_with_saponin_affects_the_membrane_structure
As nouns the difference between phospholipid and lysophospholipid is that phospholipid is (chemistry) any lipid, such as lecithin or cephalin, consisting of a diglyceride combined with a phosphate group and a simple organic molecule such as choline or ethanolamine; they are important constituents of biological membranes while lysophospholipid is (organic chemistry) any derivative of a phospholipid in which one or both acyl derivatives have been removed by hydrolysis.
作为名词,磷脂和溶血磷脂的区别在于磷脂是(化学上)任何一种脂质,如卵磷脂或脑磷脂,由二甘油酯与磷酸基和简单的有机分子(如胆碱或乙醇胺)组成;它们是生物膜的重要组成部分,而溶血磷脂是(有机化学)磷脂的任何衍生物,其中一个或两个酰基衍生物已被水解除去。
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)