¡¡
1.inhibit urease
2.increase collagen synthesis
3.increase mucus production via increasing PGE2 synthesis
4.In normal humans, vitamin C is vigorously transported into and concentrated in gastric juice; high concentration of ascorbate in gastric juice can inactivate and denature urease secreted by H. pylori at low pH mediated by H2O2 in the presence of Fe(3+) ions, preventing H. pylori survival and colonization into acidic stomach (Krajewska and Brindell, 2011; Pal et al., 2011).
5. Vitamin C levels both in gastric acid and serum have constantly been affirmed to be low in subjects with H. pylori infected gastritis and peptic ulcers. Ascorbic acid supplementation likely relates to reduced incidences of bleeding from peptic ulcers and gastric cancer.
6. H. pylori eradication is shown to increase vitamin C levels.
7. ascorbic acid deficiency has been related to high occurrence of gastritis and bleeding from gastric and duodenal ulcers as well
8. Even with supplementation approaching maximally tolerated oral doses at 3¨C4 g, plasma ascorbate concentrations will just reach a plateau of about 200 ~ 300 ¦Ìmol/L. In contrast, with intravenous ascorbate intake, pharmacologic ascorbate concentration of 25 ~ 30 mmol/L has been safely attained to treat various cancers, severe burns, sepsis, and other diseases
9. Low plasma ascorbate level, or hypovitaminosis C (plasma vitamin C concentration: 11.4 ~ 27 ¦Ìmol/L vs normal range 27 ~ 100 ¦Ìmol/L ) associated with a variety of disease complexes including cancer, sepsis, gastric ulcer, etc, may affect ~ 10% of the general population
10.Pharmacologic ascorbate can be used as a pro-drug for the formation of H2O2; the H2O2 concentration in extracellular fluid can reach as high as 200 ¦Ìmol/L, which leads to production of large amounts of ROS inside or outside of cancer cells via iron mediated Fenton reactions and thus cause damage on macromolecules in cancer cells
11. Intravenous vitamin C, also termed pharmacological ascorbate could achieve 25 ~ 30 mmol/L and form high concentration of H2O2 as a pro-oxidant drug, which was been extensively used to treat and prevent many disorders like various cancers and other diseases.
¡¡
¡¡
¡¡
¡¡
Vitamin C: A Preventative, Therapeutic Agent Against Helicobacter pylori
Azhar Hussain, Elsa Tabrez, Jagannadha Peela, Prasanna Honnavar Dr, Shams S.M. Tabrez
Azhar Hussain Corresponding Author
Medicine, Xavier University School of Medicine, Oranjestad, ABW
Business Administration, Davenport University, College of Health Professionals, Grand Rapids, USA
Elsa Tabrez
Internal Medicine, American University of Integrative Sciences, Bridgetown, BRB
Jagannadha Peela
Faculty of Medicine/Professor of Medical Genetics and Biochemistry, St. Matthew's University School of Medicine, Grand Cayman, CYM
Prasanna Honnavar Dr
Microbiology and Immunology/Faculty of Medicine, Xavier University School of Medicine, Oranjestad, ABW
Shams S.M. Tabrez
Board Certified Gastroenterologist and Hepatologist, University of Central Florida College of Medicine, Orlando, USA
Abstract
The treatment of Helicobacter pylori (H. pylori) induced infections using antibiotic therapies is clinically well accepted; however, using a noninvasive approach with the implementation of therapeutic agents such as vitamin C is not well investigated. Vitamin C has certain characteristics, which allow for it to be considered as a potential treatment option for patients with H. pylori infections. Vitamin C¡¯s hostility and mechanism of action towards H. pylori infection in peptic ulcer disease can be classified into two categories: as a preventative agent and alternatively as a therapeutic agent. Preventatively vitamin C acts as a biological antioxidant as well as an immune boosting agent, while therapeutically it acts as an inhibitor of urease, a potential collagen synthesizing agent, and a stimulant in prostaglandin synthesis. As a result, the dosage of vitamin C should be highly regulated. Furthermore, numerous studies have shown that vitamin C supplementation if taken with antibiotics can increase the efficiency of the treatment leading to an increased possibility of eradication of H. pylori in infected individuals. This paper will investigate the recent studies that show different mechanisms through which vitamin C can be used as a preventative or a therapeutic agent for the treatment of H. pylori related infections.
Introduction & Background
Helicobacter pylori (H. pylori) is a Gram-negative, microaerophilic, spiral-shaped bacterium that colonizes on the mucosal lining of the stomach [1]. H. pylori is one of the primary causes of upper gastrointestinal diseases, including dyspepsia, peptic ulcer diseases, heartburn, and gastroesophageal reflux disease. Chronic disease due to H. pylori has been associated with the advancement of gastric adenocarcinoma and lymphoma involving mucosa-associated lymphoid tissue (MALT) [2]. Some 95% of the patients with H. pylori infection develop duodenal ulcers, 80% of the patients develop gastric ulcers, and 10%-15% of the patients develop peptic ulcers. The bacteria transmit via oro-oral, oro-fecal, or oro-gastric route. Recent studies showed that over 50% of the global population is infected by H. pylori infection in which 1%-3% develop gastric cancer. As a result, the World Health Organization classified H. pylori as a group 1 carcinogen [1].
The proton pump inhibitor (PPI) (e.g., omeprazole 20 mg BID, lansoprazole 30 mg BID, or pantoprazole 40 mg QID) with two antibiotics treatment (such as amoxicillin 1000 mg BID and clarithromycin 500 mg BID) is considered as a standard triple therapy and as a first-line treatment option for H. pylori infection [3]. The optimal duration of a standard triple therapy is 14 days achieving a H. pylori eradication rate of 81.9%, as compared to 7 days triple therapy which attains an eradication rate of only 72.9% [4]. The standard triple therapy is followed by a second-line treatment, which is a quadruple therapy that consists of a PPI or H2 receptor antagonist (e.g., lansoprazole 30 mg BID or ranitidine 150 mg BID) plus bismuth subsalicylate 525 mg QID, metronidazole 250 mg QID, and tetracycline 500 mg QID for additional 10-14 days for 90.4% eradication [3], but the eradication rate of infection is minimal due to antibiotic resistance and compliance. However, several nonantibiotic treatments have been investigated as potential adjuvants for the treatment of H. pylori; these include phytomedicines, probiotics, and antioxidants [5]. The vitamin C content in gastric juice has recently pulled in numerous researchers, suggesting that vitamin C might be a protective agent against the H. pylori infection especially against the development of gastric cancer [6]. N-nitroso compounds (NOCs) are strong carcinogens and are closely related to food and nutrition [7]. It has been demonstrated that vitamin C is anticarcinogenic because it inhibits the development of N-nitroso mixes (NOCs) in gastric juice [8].
There have been several clinical studies which demonstrated that high H. pylori infection rate is related to low vitamin C (ascorbic acid) level in the gastric juice as well as in the serum [9-10]. Nevertheless, many studies demonstrated that a high dose of vitamin C would inhibit the growth and colonization of H. pylori and even eradicate them [11-12]. Understanding the mechanism would help to design more clinical studies in more reasonable ways to formulate appropriate anti-H. pylori agents.
Survival of H. pylori at low gastric pH
Helicobacter pylori is not an acidophile, but the main reason for its ability to overcome the acidic gastric environment is due to its ability to synthesize a large amount of urease enzyme that catalyzes the hydrolysis of urea to yield ammonia and carbonic acid [13]. The activation of the urease is a key factor in the successful colonization of bacteria into the gastric mucosa because it can allow the bacteria to survive at a very low acidic pH of 2.5 [1]. However, in the absence of the enzyme urease, the bacteria can only survive at a pH of 4.0-8.0 [13]. Autolysis of the H. pylori colony results in the release of cystolic urease into the gastric mucosa, which attaches to the surface of the H. pylori bacteria [5, 14]. In the gastric mucosa, the deprotonated carbonic acid and protonated ammonia are in equilibrium [15]. The effect of this reaction is an increase in pH and formation of a basic ammonium cloud around the bacteria allowing H. pylori to survive and to colonize on the gastric epithelium [15]. On successful colonization, H. pylori resides below the gastric mucus which has a higher pH than the gastric lumen [16].
The motility of the H. pylori plays an important role in the pathogenesis and successful colonization into gastric mucosa. For this purpose, H. pylori has two to six polar sheathed flagellae, which allow the movement of the bacterium into the highly viscous mucus layer of the gastric epithelium. These flagellae are composed of three main structures: the basal body, which serves as a cell anchor and contains the proteins required for rotation and chemotaxis, a curved hook, and the helically shaped flagellar filament [17]. The lining of the stomach is a spongy gel-like state because of the acid content which the bacterium is unable to penetrate. However, by using its flagella, H. pylori releases an adhesion molecule, which allows the bacterium to bind to the host cell [18]. The bacterium then releases a high amount of urease enzyme, which can neutralize the acid by converting urea into carbon dioxide, and ammonia, and drills in the mucoid lining of the gastric epithelium [16].
Discussion: mechanisms of action of vitamin C against H. pylori
Vitamin C can be used as a preventative agent as summarized in Table 1 and as a therapeutic agent as summarized in Table 2. This review focuses on the mechanism through which vitamin C can be preventative: as beneficially used to prevent H. pylori infection as well as therapeutic: as to control the infection and eradicate the bacteria.
I. Vitamin C as a Preventative Agent
Preventative agent
Role Function
Biological antioxidant Ascorbic acid scavenges and eliminates free radicals
Immune booster 100-fold increase of vitamin C inside immune cells and decrease of plasma vitamin C
Table 1: Role of vitamin C as a preventative agent.
Biological Antioxidant
Vitamin C is a nonessential, potent, water-soluble micronutrient that can neutralize a wide range of pro-oxidants, due to its low redox potential [19-20]. Vitamin C functions as a biological antioxidant, an oxidative stress reducer, a factor in immune function and in enzyme activation as shown in Table 1 [19]. Vitamin C also acts as a cofactor in the biosynthesis of collagen, catecholamines, and peptide hormones [21]. Vitamin C exists in two major forms: reduced form as ascorbic acid, as well as its oxidized form as dehydroascorbic acid, which may be interconvertible [20], by a dehydroascorbic acid reductase, glutaredoxins or other thiols acting as an electron donor [5]. The reduced form as ascorbic acid has scavenger properties and may be beneficial to eliminate free radicals under the formation of semidehydroascorbic acid, which is a nonreactive radical [22]. The dehydroascorbic acid may spontaneously hydrolyze and dehydrate; however, the ascorbic acid is more stable and does not show the same tendency to irreversibly hydrolyze particularly at pH > 4 [5]. This mechanism is essential for the inhibition of the growth of H. pylori [16, 18, 23].
Immune Boosting Agent
The immune system within the human body acts as a protective agent against pathogens that cause infections and diseases. These immune responses are divided into two categories; an innate system is the first nonspecific immune response, while the adaptive immune response is pathogen-specific and develops over time subsequent to the introduction of that particular pathogen [23-24]. The innate immune response is particularly very important in children because it exists at birth and offers initial protection against foreign pathogens. Adaptive immune responses are different in that they are dependent upon prior exposure to the antigens. The adaptive immune response, therefore, has mature plasma B cells and antibodies, which are capable of recognizing specific previously encountered antigens. Each successful immune response concludes with phagocytic engulfing of pathogens by macrophages [24].
One of the most important functions of vitamin C is that it helps in the activation of the immune system of the body. Within the plasma membrane of the immune cells, there are active transporters of vitamin C that bind to it and actively transport the vitamin C into the cell [25]. For example, during inflammation due to infection, these transporters increase the influx of vitamin C up to 100-fold compared to the amount of vitamin C present in the plasma [26]. As a result, plasma vitamin C concentration can be depleted during infection. Studies show that the aging of the immune system can be reversed by the supplementation of vitamin C [27]. This study shows significant results in geriatric patients whose overall immune function is in a process of degradation.
II. Vitamin C as a Therapeutic Agent
Therapeutic agent
Function Mechanism of action
Urease maturation (potent virulence factor required for survival of H. pylori in acidic environments) Increase in vitamin C leads to the reduction of nickel of urease enzyme
Collagen synthesis Vitamin C acts as a cofactor for synthesizing collagen type IV required for synthesis of lamina propria in the stomach lining. The absence of vitamin C allows easy penetration of H. pylori
Prostaglandin synthesis Phospholipid molecule converts to arachidonic acid via the enzyme phospholipase A2. Arachidonic acid is then converted to prostaglandin via the enzymes cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX2)
Table 2: Role of vitamin C as a therapeutic agent.
Inactivation of Urease
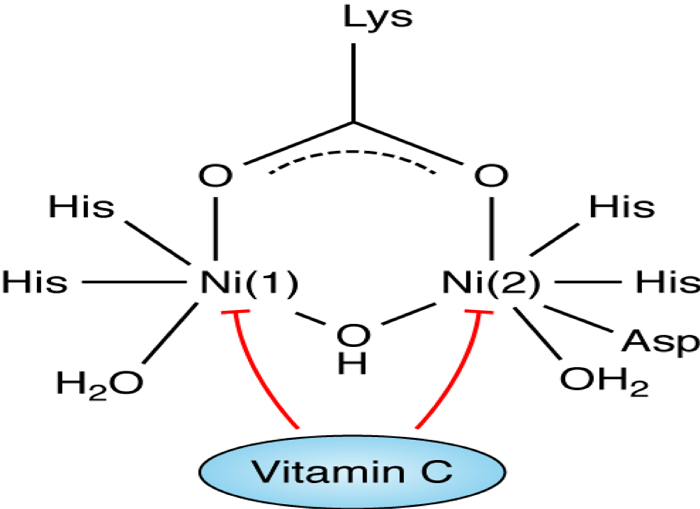
Urease is an important enzyme that constitutes approximately 5%-6% of the total protein of H. pylori and it is linked to its pathogenicity as shown in Table 2 [28], due to its ability to colonize on the gastric mucosa at a low pH [23]. The structure of urease enzyme is composed of two half subunits held together by a noncovalent bond [5], each subunit containing a specific active site. These active sites contain two nickel ions that are bound by a carbamylated lysine and an oxygen donor [29]. In addition to the binding, the first nickel ion is held by two histidine amino acids and a water molecule and the second nickel ion which is similar in composition to the first nickel ion, also contains the amino acid aspartate as shown in Figure 1 [5, 14, 28-29]. The ability of vitamin C to inhibit urease action plays an important role in understanding the mechanism of H. pylori infection and bacterial eradication [30]. Studies show that the high concentration of vitamin C favors reduction of the nickel center in the urease enzyme [5], which in turn inhibits the activity of the enzyme and may reduce the H. pylori manifestations.
¡¡
The-structure-of-urease-and-the-role-of-vitamin-C-as-an-inhibitor.
Figure 1: The structure of urease and the role of vitamin C as an inhibitor.
Vitamin C, which is a relatively strong acid (pKa = 4.1) further lowers the pH of the gastric lumen [28]. Vitamin C, a reducing agent of urease, when added to the gastric lumen results in urease becoming structurally unstable, therefore, irreversibly losing its enzyme activity [30]. Therefore, vitamin C can be beneficial in inhibiting the growth, colonization, and endurance of H. pylori at an earlier period of the infection and may be helpful in the eradication of the bacteria [23].
Collagen Synthesizing Agent
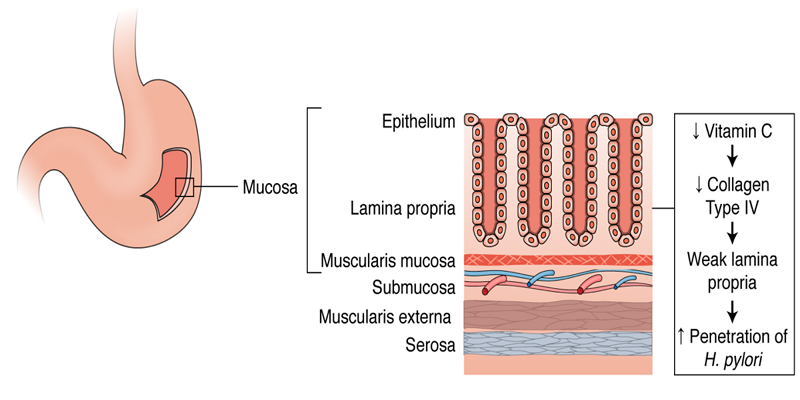
The stomach inner lining itself is a layered structure containing four sublayers which are the mucosa, the submucosa, the muscular, and the serosa. The mucosa layer of the inner stomach lining is composed of epithelial cells specific to the stomach including parietal cells, chief cells, and gastric enteroendocrine cells. Below this layer of epithelial cells in the mucosa is the lamina propria. Under the mucosa layer is the submucosa which entirely contains blood vessels. H. pylori can bind to the extracellular matrix (ECM) proteins on the surface of the epithelial cells and infiltrate the cells by releasing toxins as shown in Figure 2 [31]. Once H. pylori infiltrates the cells, it can further penetrate the deeper layers of the stomach lining and travel through the bloodstream. Presuming that H. pylori can only penetrate farther into tissues if it passes through the lamina propria, a durable lamina propria has the potential to prevent penetration. The lamina propria is composed predominantly of collagen fibers, specifically collagen type IV which provides structure and support for the epithelial cells located within the gastric mucosa [32]. Vitamin C is a known cofactor in the synthesis and strengthening of collagen [33]. Vitamin C's collagen strengthening abilities could potentially be a significant reason as to why patients with an increased serum and plasma vitamin C experience very little to no infestation of H. pylori [34]. Stronger collagen in the lamina propria under the epithelium could attribute to a more difficult infiltration mechanism of H. pylori into the tissues and bloodstream, therefore, resulting in decreased penetration and decreased overall prevalence of H. pylori linked diseases [33].
¡¡
Role-of-vitamin-C-in-collagen-synthesis.
Figure 2: Role of vitamin C in collagen synthesis.
Vitamin C is specifically required for hydroxylation in collagen synthesis, a post-translational modification [35]. Once pre-procollagen is translated from mRNA, it enters the rough endoplasmic reticulum for elongation, hydroxylation, and glycosylation prior to its maturation. Hydroxylation of pre-procollagen allows for further glycosylation of the molecule and subsequent triple helix formation. Both enzymes, lysyl hydroxylase and prolyl hydroxylase are necessary for hydroxylation and require vitamin C as a cofactor amongst other elements such as O2, ferrous (Fe2+), and alpha-ketoglutarate [34]. Without vitamin C, the enzymes are not able to function and additional modification of pre-procollagen does not take place, in which case the molecule does not mature to collagen [35]. An increase of vitamin C in the plasma of an individual could account for an increase in collagen synthesis, therefore, resulting in a tougher collagen-saturated lamina propria [32]. This relationship has the potential for being a possible correlating factor between the high vitamin C serum and plasma concentrations and low occurrence of H. pylori penetrance in certain individuals.
Prostaglandin Synthesis
Prostaglandins (PG) are lipid-derived compounds, subdivided into PGA, PGB, PGE, and PGF [36], which have inflammatory responses and hormone-like effects on various reactions within the body [37]. The effect of specific prostaglandins is dependent on its location of the action. Prostaglandin E2 (PGE2) is found in high concentrations within the gastric juice and mucosa, and thus has forceful protective effects on the gastric mucosa layer of the inner stomach lining [38]. PGE2s are protective in the sense that they stimulate mucosal blood flow as well as mucus and bicarbonate secretion within the lumen of the stomach. The release and activation of PGE2s are contingent upon injury to the mucosal layer [39]. Subsequent to H. pylori penetration of the epithelial cells within the mucosa [37], PGE2s are released to secrete mucus and act as defensive agents [39]. A high degree of prostaglandin synthesis is most likely related to a high defense against bacterial agents like H. pylori [38].
http://clincancerres.aacrjournals.org/content/11/16/6087
Certain studies have identified a correlation between vitamin C and the synthesis of prostaglandin E2 specifically [40]. A study was conducted using inbred mice to assess the influence of vitamin C on prostaglandin E2 synthesis. A 90%-100% increase in PGE2 output was noted upon the introduction of vitamin C [41]. Prostaglandin synthesis begins with the conversion of a phospholipid molecule to arachidonic acid in the presence of the enzyme phospholipase A2. Arachidonic acid undergoes a cyclooxygenase reaction and a peroxidase reaction in the presence of enzymes COX-1 and COX-2, to produce prostaglandin which becomes tissue-specific depending upon location [42]. El Attar et al. showed that vitamin C has dose-dependent effects on the release of arachidonic acid which leads to further PGE2 synthesis. In addition to that, vitamin C acts as a stimulant of PGE2 synthesis by inducing the release of exogenous arachidonic acid in fibroblast cells [40]. Similar findings observed by Siegel et al. show an abundance of mucous production upon abrasion of the mucosal layer from H. pylori [41].
Dose regulation of vitamin C
Vitamin C is a water-soluble vitamin and an essential nutrient that must be taken from the diet. The optimum dietary vitamin C intake of 200 mg per day is essential to increase vitamin C¡¯s health benefits with the least risk of adverse effects in the majority of the adult population [43]. Normally an excess dose of a water-soluble vitamin simply passes through the body without causing any toxic effects; however, if vitamin C consumption is more than 2000 mg per day, it can cause kidney stones as well as osmotic diarrhea due to limited absorption [44].
Recently there have been numerous studies that discuss the dosage of vitamin C in the treatment of H. pylori. Zojaji et al. conducted a study in which two groups were given amoxicillin 1 g with metronidazole 500 mg BID and bismuth 240 mg BID with omeprazole 40 mg QID in two divided doses. The second group was given an additional 500 mg of vitamin C. Experimental results showed that 78% of individuals of group two with the additional vitamin C were able to eradicate H. pylori as compared to 48.8% of individuals from group one [45].
Similar results were shown byJarosz et al. in which two groups of patients with H. pylori infection were treated without the administration of any antibiotics. The control group was treated with antacids for four weeks whereas the second group was treated with the same antacids for four weeks with an additional dose of 5 g of vitamin C daily for a span of four weeks. Plasma and gastric juice total vitamin C levels were measured at baseline, at the end of four weeks¡¯ treatment, and again four weeks after treatment cessation. In the control group, H. pylori infection remained unchanged in all the patients; however, the patients with vitamin C treatment were able to eradicate the H. pylori (p = 0.01) [46]. These studies do show promising results; however, more research must be conducted to determine the best treatment options accompanied by vitamin C dosages for the treatment of H. pylori.
Additional clinical trials
Table 3 shows the clinical trials that have been previously conducted to assess the effectiveness of vitamin C with or without the use of antibiotics. These clinical trials further suggest that vitamin C may be beneficial for decreasing the incidence rate for H. pylori related infections.
Author Publication date Patients Treatment Results
Sezikli et al. [47] April 2012 30 patients with severe gastritis (H. pylori related infection) Vitamin C 500 mg BID and vitamin E 200 IU BID for four weeks orally Increase of eradication of H. pylori
Zojaji et al. [45] September 2009 312 patients with H. pylori infection Group A:162 patients received amoxicillin 1 g, metronidazole 500 mg BID, bismuth 240 mg BID, and omeprazole 40 QID in two doses Group B: 150 patients received the same regimen plus 500 mg vitamin C 48.8% of the patients in Group A and 78% in Group B responded to eradication therapy
Sasazuki et al. [48] April 2003 635 patients diagnosed with chronic gastritis (H. pylori related infection), but only 244 finished the treatment 120 patients given low-dose vitamin C (50 mg) and 124 patients given high-dose vitamin C (500 mg) completing five-year supplementation H. pylori titer was significantly reduced by both low-dose and high-dose vitamin C
Jarosz et al. [46] December 1998 60 patients with dyspeptic symptoms and proven chronic gastritis, and H. pylori infection Group 1: 28 patients Group 2: 32 patients Group 1 was treated with antacid for four weeks whereas Group B was treated with antacids for four weeks with an addition of 5 g of vitamin C for four weeks, but no antibiotic treatment in both groups In the Group A, H. pylori infection remained unchanged in all patients. In Group B, the eradication of H. pylori was 30%.
Chuang et al. [49] October 2002 104 patients with H. pylori infection Group 1 was treated with lansoprazole, amoxicillin, and metronidazole BID for a week. Group 2 was treated with lansoprazole, amoxicillin, and metronidazole plus vitamin C (250 mg) and vitamin E (200 mg) BID for a week, followed by vitamin C and vitamin E QD for six consecutive weeks Vitamin C and vitamin E in combination with triple therapy is not effective and showed no H. pylori eradication
Chuang et al. [50] January¨CFebruary 2007 171 H. pylorii nfected patients Group 1: 55 patients received 20 mg omeprazole, 1 g amoxicillin, and 250 mg clarithromycin BID Group 2: 61 patients received 20 mg omeprazole, 1 g amoxicillin, and 250 mg clarithromycin with additional 500 mg vitamin C BID Group 3: 55 patients 20 mg omeprazole, 1 g amoxicillin, and 500 mg clarithromycin Group 2 had a higher eradication rate than Group 1 but had an equivalent rate to Group 3. Results indicate that an addition of vitamin C to one week triple therapy can allow for a reduction of the dosage of clarithromycin
Table 3: Clinical trial data analysis.
However, there are certain studies, as shown in Table 3, that show that vitamin C, if added with the triple therapy regimen, may not improve the H. pylori eradication rate. The studies¡¯ inability to elicit a positive correlation between vitamin C and H. pylori eradication could be attributed to a particularly low dose [49], a short duration time of the therapy, or the patients¡¯ noncompliance to vitamin C treatment protocols. Further research investigation is needed for the proper protocol through which vitamin C can be added to the triple therapy regimen to increase the eradication of the H. pylori infection. These studies must focus on determining the optimum vitamin C dosage and treatment duration required for H. pylori eradication.
Conclusions
When assessing treatment mechanisms with regard to H. pylori infections, vitamin C¡¯s role as a potential preventative and therapeutic agent is distinctive. High serum vitamin C levels are associated with a low incidence rate of H. pylori infection upon exposure. Vitamin C properties such as biological antioxidant and immune regulator act as a preventative agent against H. pylori related infections. Vitamin C also acts as a therapeutic agent by functioning as an inhibitor of urease, a synthesizing agent for collagen, and a stimulant in prostaglandin synthesis. Therefore, while vitamin C¡¯s preventative and therapeutic capacity is significantly under-investigated, current studies have established a distinct positive correlation between vitamin C levels and the body¡¯s ability to combat H. pylori infections.Cureus | Vitamin C: A Preventative, Therapeutic Agent Against Helicobacter pylori
https://www.cureus.com/articles/13566-vitamin-c-a-preventative-therapeutic-agent-against-helicobacter-pylori#¡¡
Indian Journal of Pharmacology 2011
Vitamin-C as anti-Helicobacter pylori agent: More prophylactic than curative- Critical review
Jagannath Pal1, Madhusudana Girija Sanal2, Gopal Jee Gopal3
1 Department of Medical Oncology, Dana Farber Cancer Institute, USA
2 Department of Research, Institute of Liver and Biliary Sciences, D1, Vasant Kunj, New Delhi - 110070, India
3 Special Center for Molecular Medicine, Jawaharlal Nehru University, New Delhi - 110067, India
Potential of nonantibiotic therapies for treatment of Helicobacter pylori-related acid peptic disease remains underexplored. Several clinical studies have shown that higher prevalence of H. pylori infection is associated with low Vitamin C (Vit C) level in serum and gastric juice. However, there is no consensus regarding the usefulness of Vit C supplementation in the management of H. pylori infection.Surveying the existing literature we conclude that high concentration of Vit C in gastric juice might inactivate H. pylori urease, the key enzyme for the pathogen's survival and colonization into acidic stomach.
Once infection established, urease is not very important for its survival. The role of Vit-C as anti-H. pylori agent in peptic ulcer diseases appears to be preventive rather than curative.
Rather than supplementing high dose of Vit C along with conventional triple therapy, it is preferable to complete the conventional therapy and thereafter start Vit C supplementation for extended period which would prevent reinfection in susceptible individuals, provided the patients are not achlorhydric. Further studies are required to prove the role of Vit C in susceptible population.
Keywords: Helicobacter pylori, prophylactic, supplementation, urease, vitamin C
Vitamin-C as anti-Helicobacter pylori agent: More prophylactic than curative- Critical review Pal J, Sanal MG, Gopal GJ - Indian J Pharmacol
http://www.ijp-online.com/article.asp?issn=0253-7613;year=2011;volume=43;issue=6;spage=624;epage=627;aulast=Pal¡¡
INTRODUCTION
In spite of initial success of eradication treatment for Helicobacter pylori in peptic ulcer diseases using combination of a proton pump inhibitor and antibiotics, emergence of antibiotics resistance in recent years, leads to frequent treatment failure in at least 10-20% of the patients. [1]Nonantibiotic therapies, including phytomedicines, probiotics and antioxidants have been increasingly investigated as potential adjuvants for the treatment of H. pylori. [2] Several clinical studies have shown that high H. pylori infection rate is related to low ascorbic acid (AA)/Vitamin C (Vit C) level in serum as well as in gastric juice. [3],[4],[5] On the other hand high dose of Vit C have been shown to inhibit H. pylori growth, colonization or even eradication of H. pylori infection in few studies while others gave nonconclusive results. [2],[6],[7],[8],[9],[10],[11] But still it is not clear how Vit C level can affect the course of H. pylori infection in stomach and how the infection affects the level of Vit C in serum and gastric juice. Moreover doses of Vit C used in those experiments were extremely higher compared to the physiological concentration of Vit C in the stomach. These high concentrations cannot be achieved in physiological condition due to its pro-oxidant activity at high concentration (500 mg/day or more) particularly in presence of high body iron stores leading to several adverse effects including DNA damage and an increase risk of cancer. [12],[13] So the actual role of Vit C at physiological or therapeutic level, in the course of H. pylori infection in the stomach is still inconclusive. One study shows that AA does not inhibit growth of H. pylori at a concentration (400 Mm) within the physiological range. [14] This experiment was carried out in buffered condition at pH 7.4, but in vivo H. pylori have to counter unbuffered acidic pH (pH 3) to colonize successfully into the stomach. At low pH the effect of AA on survival of H. pylori might be different. So these ex vivo studies also do not rule out the role of AA as anti-H. pylori agent.
At this juncture a new query emerges; whether antioxidant effect of Vit C has any effect on the survival of H. pylori at low pH? Till date very limited studies have been carried out in this direction. A systematic analysis of the available information would lead to some interesting logical derivations which might explain the existing confusion associated with the role of Vit C in H. pylori infection. This would help us to design more clinical studies in a logical way and to formulate appropriate antipylori regimen.
» Mechanism of H. pylori Survival At Low Gastric pH
H. pylori synthesizes large amount of urease, which is found in its cytosol. The cytosolic urease is released into the gastric juice upon spontaneous autolysis of a subpopulation of H. pylori and subsequently it is adsorbed onto the surface of intact bacteria. The urease catalyses the hydrolysis of urea present in the gastric juice, to yield carbonic acid and ammonia. Thus H. pylori makes a cloud of ammonia on its surface to neutralize the gastric acid which enables it to colonize the gastric epithelium. [15],[16] Once successfully colonized, H. pylori resides below the gastric mucus layer which has a higher pH than gastric lumen. [17],[18] So in chronic infection, the role played by urease, in survival of the bacteria seems less important. However, besides protecting from acid, urease also aids in colonization by providing ammonia for bacterial protein synthesis.
» Structural Features of H. pylori Urease
In the active site of urease there are two Ni (Ni ++ ) centers held by co-ordination bonding. Ni(1) is coordinated by two imidazole ligands from two histidine residues and a water molecule. Ni(2) is coordinated by two histidine residues as well as with an aspartate and a water molecule. [19] It has been found that purified urease is inactivated at pH<5 (in buffered solution). So it had long been unanswered that how extracellular urease on H. pylori cell surface remains active in low gastric pH. Moreover cytosolic pH (pH 6) of H. pylori is also suboptimal for maximal urease activity. Ha et al; 2001 have convincingly shown that supramolecular assembly of urease create a pore within the complex which serves as a pathway for diffusion of urea toward the 12 clustered active sites which protect each other from acid inactivation by producing localized cloud of ammonia from breakdown of urea. [20]
» Vit C as Biological Antioxidant
Vit C is an acidic molecule with strong reducing activity and is an essential component of most living tissues. It has two major redox forms: AA and DHA, the reduced and oxidized form, respectively, and they are all interconvertible. Within the cell DHA is rapidly converted to AA by the specific enzyme systems like DHA reductase, glutaredoxins and protein disulfide isomerase in presence of glutathione or other thiols as electron donors [21],[22] Unlike AA, DHA is relatively unstable and undergo rapid spontaneous irreversible hydrolysis particularly at a pH > 4. [23]
» Vit C as a Reducing Agent for Transition Metals
Few transition metals like Fe(III), Cu(II), Hg(II), Cr(VI) are able to accept electron from AA, resulting in their reduction and simultaneous oxidation of AA to DHA. In presence of strong biological oxidants like oxygen, hydrogen peroxide the oxidation of AA is accelerated, where the metal ions act predominantly as catalyst. In aqueous solutions at neutral pH, metal ions, having relatively low redox potential like Zn(II), Ni(II), Co(II), Pb(II) unable to oxidize AA by themselves nor serve as a catalyst for oxidation by molecular oxygen. However, in biological solutions (e.g.: tissue fluid, blood, serum) redox chemistry of these metal ions is different due to presence of different natural metal complexing agents like amino acids, peptides, proteins, nucleotides, etc. These natural ligands are also able to form ternary complexes with the metal ions and Vit C. Among all biological ligands, histidine or histidine containing peptides are shown to be more efficient in binding transition metals like Ni(II) and form stable ternary complexes with AA. The assembly favors oxidation of AA by the metal ion and the process is relatively faster in presence of oxygen. [24],[25],[26]
» Vit-C as an Anti-H. pylori Agent
Normally AA is actively secreted from plasma to the gastric juice. [27],[28] High concentration of AA in gastric juice favor reduction of Ni ++ centers, coordinated to the histidine residues of the urease, secreted from H. pylori, leading to inactivation of the enzyme followed by acid denaturation [Figure 1]. This reduction of the transition metal ion by AA, also accelerated at low pH of gastric juice as in case of reduction of ferric iron by AA. [10],[11] Recently Krajewska et al. showed that in a buffered system neither AA nor DHA themselves are inhibitors of urease. The inhibitory effect of AA and DHA was seen in the presence of Fe(3+) ions and, unlike reported in the literature, they found that it was mediated by H 2 O 2 . Interestingly the resulting inhibition by DHA-Fe(3+) consisted of enzyme thiol oxidation and its effectiveness grew with increasing pH. [29] This could be another interesting mechanism by which AA can act against H. pylori especially in the initial stages of infection.
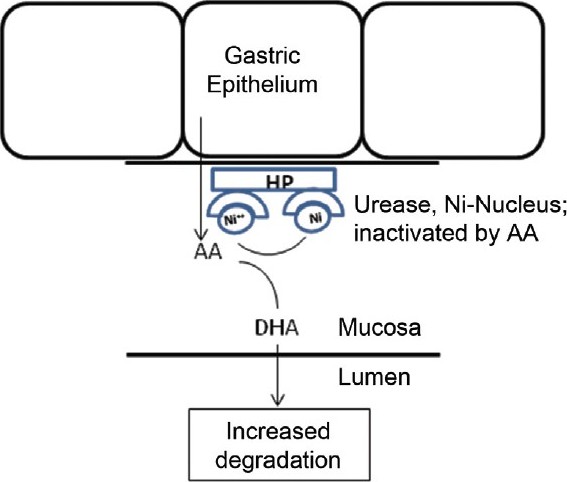
Figure 1: Inactivation of H. pylori urease by Vit.C in H. pylori-infected gastric mucosa. Abbreviation: Ni ¨C nickel (reduced form), AA- ascorbic acid/Vit. C, DHA- dehydroascorbic acid, HP- H. pylori
In low gastric pH, once urease is inactive it becomes difficult for H. pylori to survive and colonize in stomach. But once it successfully colonizes into stomach wall, H. pylori stays within the gastric mucosa, where the pH is suitable for the survival of the bacteria, due to the bicarbonate buffer from gastric epithelium secreted into the luminal surface. [17],[18] Moreover after chronic infection, a proportion of patients develop relative achlorhydria leading to higher pH of gastric juice. [30] So at this stage of infection, role of urease for its survival is relatively less. This might explain the fact that even a high dose AA supplementation is not able to eradicate H. pylori infection in a significant percentage of cases [11] though AA have been shown to inhibit urease in vitro. [29],[31] In chronically infected stomach, the bacteria continue to produce urease, which capture the free Ni ++ from the gastric juice. As discussed above, this bound Ni ++ is more preferred substrate for reduction by AA than free Ni ++ . As a consequence of reduction of the Ni ++ centers of urease, AA itself oxidized to DHA and rapidly exhausted by spontaneous hydrolysis particularly when passed through alkaline medium of intestine. This might be one of the explanations of low serum and gastric juice AA level in chronically H. pylori-infected patients. Though local ROS, generated by chronic gastritis may also be responsible partially for conversion of AA to DHA. At the site of inflammation extra cellular DHA is taken up by leucocytes and rapidly converted to AA and thus recycled. [32],[33] So the local gastric inflammation may not have significant effect on Vit C deficiency in H. pylori induced gastritis. There is also evidence that, in Ni ++ toxicity, there is depletion of serum AA and the toxic effect is reversed by addition of AA. [26] This seems to be due to reduction of Ni ++ by AA in the biological solution in vivo. On the other hand, patients with already low serum AA likely to be more prone to get infection by H. pylori, because low AA in gastric juice might favor colonization by the bacteria as explained above. Moreover low antioxidant level in chronically infected gastric mucosa, causes elevation of ROS contributing perpetuation of inflammation and infection cycle. [34],[35] Thus it is possible that AA can have an anti-H. pylori effect during initiation, spreading and perpetuation of the infection although major effect could be during initiation of colonization.
Few clinical studies have been carried out where different conventional anti-H. pylori regimens supplemented with Vit C were used. The results of these studies [36],[37],[38],[39],[40],[41] range from no benefit on Vit C supplementation with conventional anti-H. pylori regimens to studies which reported that Vit C decreased effectiveness of conventional anti-H. pylori particularly where a triple therapy containing metronidazol was used. [36],[37] In all those anti-H. pylori regimens proton pump inhibitor was one of the critical components which increased the gastric pH. Ex vivo studies using physiological concentration of AA and pH (7.4) in H. pylori culture media also support these views. Moreover it have been reported that PPI reduce bioavailability and stability of Vit C in gastric juice. [42],[43] Secondly in the anerobic micro-organisms, metmronidazol enter into the bacterial cell through passive diffusion and is reduced to generate active free radicals. Thus a flow of metronidazol is maintained into the cells along a concentration gradient. [9],[44],[45] In presence of high dose of AA extracelluar reduction of metronidazole might take place, hampering effective accumulation of the drug into the bacterial cells. More over active free radical forms of metronidazol might be stabilized by high concentration of Vit C which leads to reduced H. pylori eradication rates when this combined regimen is used. Vit C supplementation at a dose of 500 mg b.d. following triple therapy seems to be quite effective as shown from a preliminary study by Sezikli et al. [38] More clinical trials are required in this direction to determine the optimum dosage and regimen keeping in mind the increasing pro-oxidative role of Vit C at higher dose (500 mg/day) as observed by Podmore et al. [13] It is important to particularly consider long-term Vit C supplementation therapy. However, a few recent studies report an increased eradication of H. pylori when triple therapy was supplemented with Vit C. [38],[39],[40],[41] Yet there is no convincing evidence if supplementation of Vit C along with triple therapy could be beneficial in eradicating resistant H. pylori infection. [41] Interestingly, in one study Vit C was supplemented two more weeks following completion of triple therapy showing better eradication rate than achievable by triple therapy alone. [38]
» Conclusions Top
The role of AA as anti-H. pylori agent in peptic ulcer diseases is most likely to be preventive rather than curative. Rather than supplementing high doses of AA along with conventional antipylori regimen it is preferable to complete the standard course of antipylori regimen, which might then be followed by Vit C supplementation therapy for extended period which would prevent from reinfection in susceptible subjects and also might eradicate the residual H. pylori infection, provided the patients are not achlorhydric or under prolonged acid suppressive therapy. It is also essential to maintain a constant protective level of Vit C in gastric juice or in serum to inhibit colonization or reinfection by H. pylori in gastric epithelium. Considering the short biological half life of this water soluble vitamin and the chances of toxicity from single daily bolus dose, split multiple doses/sustained release formulations would be more effective to maintain constant protective level of Vit C in plasma and gastric juice. More clinical trials are required in this direction to determine the actual dosage and regimen. It would be interesting to study the incidence of H. pylori infection among the large cohorts of population having varied concentrations of plasma Vit C. Future studies should be directed toward comparison of the reinfection rates in cohorts undergone the standard course of anti-H. ylori pylori regimen versus those who are maintained on Vit C supplementation after the completion of the standard therapy. It is clear from this review that well-controlled, well-designed studies are required in this direction as H. pylori infection and consequent gastric cancer is a major public health problem.Vitamin-C as anti-Helicobacter pylori agent: More prophylactic than curative- Critical review Pal J, Sanal MG, Gopal GJ - Indian J Pharmacol
http://www.ijp-online.com/article.asp?issn=0253-7613;year=2011;volume=43;issue=6;spage=624;epage=627;aulast=Pal¡¡
Urease activity and L-ascorbic acid
Article in Journal of Enzyme Inhibition and Medicinal Chemistry, June 2011
Barbara Krajewska
Malgorzata Brindell
agiellonian University
In this work, we studied the behaviours of urease in the presence of l-ascorbic acid (AA) and dehydroascorbic acid (DHA) in different conditions. The inactivations of urease were carried out in an unbuffered and buffered system.We show that in the unbuffered system AA inactivated urease in a biphasic manner by denaturation brought about by AA-lowered pH. Further, we show that in the buffered system neither AA nor DHA themselves are inhibitors of urease. The inhibitory action of AA and DHA was revealed in the presence of Fe(3+) ions and most importantly, unlike reported in the literature, it was found to be primarily mediated by H(2)O(2). The resulting inhibition by DHA-Fe(3+) consisted of enzyme thiol oxidation and its effectiveness grew with increasing pH.
The results may shed light on the roles of AA in therapies applied in ureolytic bacteria infections, notably those with Helicobacter pylori.
... In addition to the binding, the first nickel ion is held by two histidine amino acids and a water molecule and the second nickel ion which is similar in composition to the first nickel ion, also contains the amino acid aspartate as shown in Figure 1 [5,14,[28][29]. The ability of vitamin C to inhibit urease action plays an important role in understanding the mechanism of H. pylori infection and bacterial eradication [30]. Studies show that the high concentration of vitamin C favors reduction of the nickel center in the urease enzyme [5], which in turn inhibits the activity of the enzyme and may reduce the H. pylori manifestations. ...
... Vitamin C, which is a relatively strong acid (pKa = 4.1) further lowers the pH of the gastric lumen [28]. Vitamin C, a reducing agent of urease, when added to the gastric lumen results in urease becoming structurally unstable, therefore, irreversibly losing its enzyme activity [30]. Therefore, vitamin C can be beneficial in inhibiting the growth, colonization, and endurance of H. pylori at an earlier period of the infection and may be helpful in the eradication of the bacteria [23]. ...¡¡
... Organics may also stabilise amorphous ferric oxide (AFO) in colloidal form with this material presumably able to penetrate but ultimately clog membrane pores. While citrate is recognised as a strong Fe(III)-binding ligand and may promote removal of Fe(III)-containing gels and oxides from the membrane surface and pores via ligand-promoted dissolution , reductive dissolution using agents such as ascorbic acid (Asc) or dithionite might be expected to be more effective given the relative rates of ligand-promoted versus reduction-promoted dissolution [20,232425262728 . In terms of the standard reduction potential , both Asc (0.06 V) [29] and dithionite ( À0.66 V) [30] have much lower reduction potential than that of Fe(III) (0.77 V) [31] though the actual reduction potentials in the wastewater environment will depend very much on speciation and concentration with pH likely to play an important role. ...
Urease activity and L-ascorbic acid | Request PDF
https://www.researchgate.net/publication/45582213_Urease_activity_and_L-ascorbic_acid¡¡
Published: 21 August 1943
Urease Activity and Ascorbic Acid
J. H. QUASTEL
Nature volume 152, page215(1943)Cite this article
Agricultural Research Council Unit of Soil Enzyme Chemistry, Bothamsted Experimental Station, Harpenden
Abstract
WITH reference to Mr. Elson's observation1 that ascorbic acid at low concentrations inhibits urease activity, and that this inhibition disappears in the presence of cysteine, it is worth noting that certain polyhydric phenols, for example, catechol and quinol, also exert at low concentrations (one part in two millions) highly inhibitory effects on urease activity, this inhibition disappearing in presence of thiol compounds such as cysteine2. It has been shown that the inhibition in this case is due, not to the phenol, but to the corresponding oxidized product, that is, the quinone present in solution with the phenol. The alleviating action of cysteine is due to the reduction of the quinone to the inert phenol. It may be suggested that, in an analogous manner, the toxicity of ascorbic acid in dilute solution is due to the oxidized form of ascorbic acid, namely, the diketone, present in solution, and not to the ascorbic acid itself. The effect of cysteine will be to reduce the dehydro-ascorbic acid to the inert ascorbic acid, a phenomenon which is known to take place, as shown by Crook3.
References
1 Elson, L. H., Nature, 152, 49 (1943).
2 Quastel, J. H., Biochem. J., 27, 1116 (1933).
3 Crook, E. M., Biochem. J., 35, 226 (1941).Urease Activity and Ascorbic Acid | Nature
https://www.nature.com/articles/152215b0¡¡
NATURAL UREASE INHIBITOR INCLUDING L-ASCORBIC ACID AND HEALTHY FOODS THEREOF, THE INHIBITING METHOD OF AMMONIA FORMATION THEREBY
LEE JEONG SANG, UM HAN CHEON, SURH YOUNG JOON
1. LEE JEONG SANG
Patent: Examined Patent Application - Republic of Korea
Patent
Share this article Share with emailShare with twitterShare with linkedinShare with facebook
Abstract
An anti-Helicobacter pylori composition containing vitamin C is provided to prevent the side effects of antibiotics, the appearance of antibiotics-resistant bacteria, and reinfection in the inhibition of Helicobacter pylori. A growth inhibitor of Helicobacter pylori comprises an urease inhibitor containing vitamin C. A healthful food containing vitamin C has an urease inhibiting effect. Vitamin C is effective in inhibiting the generation of ammonia caused by the activity of urease in livestock excretions, therefore an ammonia inhibitor containing vitamin C is used as an environment improving agent.NATURAL UREASE INHIBITOR INCLUDING L-ASCORBIC ACID AND HEALTHY FOODS THEREOF, THE INHIBITING METHOD OF AMMONIA FORMATION THEREBY - Abstract - Europe PMC
http://europepmc.org/article/PAT/KR20080056505¡¡
Front Physiol. 2018; 9: 1103.
Vitamin C and Helicobacter pylori Infection: Current Knowledge and Future Prospects
Haixin Mei1,* and Hongbin Tu2,*
1Department of Gastroenterology, Xinyang Central Hospital, Xinyang, China
2National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, MD, United States
Edited by: Gareth Davison, Ulster University, United Kingdom
Reviewed by: Ana Cipak Gasparovic, Rudjer Boskovic Institute, Croatia; Marcos Lopez, University of Chicago, United States
Abstract
The gram-negative bacterium, Helicobacter pylori (H. pylori), infection is predominantly known for its strong association with development of gastric diseases, including gastritis, peptic ulcers, and stomach cancer. Numerous clinical reports show that ascorbic acid deficiency has been connect with gastritis. Vitamin C levels both in gastric acid and serum have constantly been affirmed to be low in subjects with H. pylori infected gastritis and peptic ulcers. Ascorbic acid supplementation likely relates to reduced incidences of bleeding from peptic ulcers and gastric cancer. H. pylori eradication is shown to increase vitamin C levels, while the benefits of ascorbic acid oral intake to increase the effectiveness of H. pylori-eradication therapy are controversial. Recent studies suggest that ascorbate intake intravenously, but not orally; pharmacologic ascorbate concentrations up to 30 mmol/L in blood, several millimolar in tissues as well as in interstitial fluid, are easily and safely achieved. Pharmacologic ascorbate can exert pro-oxidant effects locally as a drug by mediating hydrogen peroxide (H2O2) formation, which was applied to animal and clinical trials of cancer, sepsis, and severe burns etc. In this review, we summarize current understanding of the associations of vitamin C and H. pylori infection, and outline some potential strategies for H. pylori intervention from emerging advances on ascorbic acid physiology and pharmacology.
Keywords: Helicobacter pylori, gastric diseases, vitamin C, concentration-function relationship, pharmacologic ascorbate, oral ingestion, I.V. administration, hydrogen peroxide (H2O2)Vitamin C and Helicobacter pylori Infection: Current Knowledge and Future Prospects
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6102328/¡¡
Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori†
Hui©\Min Zhang M.D., Ph.D. Noriko Wakisaka M.S. Osamu Maeda M.S. Tatsuo Yamamoto Ph.D.
First published: 20 November 2000 https://doi.org/10.1002/(SICI)1097-0142(19971115)80:10<1897::AID-CNCR4>3.0.CO;2-LCitations: 67
† Presented in part at the 70th Annual Meeting of the Japanese Society for Bacteriology, Utsunomiya©\shi, Tochigi, Japan, March 29©\31, 1997.
Abstract
BACKGROUND
Helicobacter pylori infection is a risk factor for gastric carcinogenesis. High dietary vitamin C intake appears to protect against gastric carcinoma. It has been suggested that vitamin C exerts the protective effect by scavenging free radicals that may be enhanced by H. pylori. However, vitamin C has not been investigated in relation to the direct action on H. pylori. In this study, the authors attempted to clarify this possibility both in vitro and in vivo.
METHODS
Susceptibility testing of H. pylori (64 strains) was performed by the agar dilution method. Bactericidal actions were determined by a broth cultivation technique. The effect of vitamin C on in vivo H. pylori colonization was evaluated by using the Mongolian gerbil model.
RESULTS
At concentrations of 2048, 512, and 128 ¦Ìg/mL (minimum inhibitory concentrations [MICs]), vitamin C could inhibit the growth of 90% of the bacterial stains incubated at pH values of 7.4, 6.0, and 5.5, respectively. The broth cultures exposed to the MICs of vitamin C displayed a 1.57 ∼ 2.5©\log decrease in the number of viable bacteria, and the loss of viability was observed in 24 hours at concentrations 8©\fold higher than the MICs. In an in vivo experiment, H. pylori colonies decreased significantly in animals treated with vitamin C after oral administration of vitamin C (10 mg/head/day) for 7 days.
CONCLUSIONS
High doses of vitamin C inhibit the growth of H. pylori in vitro as well as in vivo. Cancer 1997; 80:1897©\903. © 1997 American Cancer Society.
Helicobacter pylori is a microaerophilic, gram negative, urease positive, spiral bacterium that causes chronic infection of the human stomach.1 The chronic H. pylori infection may lead to peptic ulceration2 and may be a potential risk factor for gastric carcinoma.3 Epidemiologic evidence suggests that high dietary vitamin C (ascorbic acid) reduces gastric carcinoma risk.4 It may do this by either reducing N©\nitroso compound formation in gastric juice5 or by scavenging reactive oxygen species in gastric mucosa.6 Significantly lower concentrations of vitamin C in gastric juice have been reported in H. pylori infected patients and it has been found that the concentrations of vitamin C rise after eradication of H. pylori.7, 8 There is a possibility that vitamin C has anti©\H. pylori activity and therefore H. pylori has to interfere with vitamin C secretion or degrade vitamin C to maintain chronic infection. Based on this assumption, the current study examined the inhibitory effects of vitamin C on H. pylori growth in vitro and on H. pylori colonization in the Mongolian gerbil stomach.RESULTS
In Vitro Effects of Vitamin C, Vitamin E, and Other Chemicals on the Growth of H. pylori
The MICs of vitamin C for 64 strains of H. pylori were determined by the agar dilution method. There was satisfactory growth of most of the bacteria tested on the control plates with different pH values. Only the colonies that grew well in control plates were subjected to MIC determination. Table 1 shows the ranges of MICs and the concentrations required to inhibit 50% and 90% of the strains (MIC50, MIC90), which were determined after 3 days of incubation. At a pH of 7.4, 90% of H. pylori were inhibited at concentrations of 2048 ¦Ìg/mL. However, the activity increased markedly by 4©\ and 16©\fold at pH values of 6.0 and 5.5, respectively. MIC90 were 512 and 128 ¦Ìg/mL when media pH was adjusted to 6.0 and 5.5, respectively. The results of MICs show that vitamin C has an inhibitory activity against H. pylori and this activity is pH©\dependent.
Table 1. MIC Values of Vitamin C and Other Chemicals Against Helicobacter pylori¡¡
Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori - Zhang - 1997 - Cancer - Wiley Online Library
https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/%28SICI%291097-0142%2819971115%2980%3A10%3C1897%3A%3AAID-CNCR4%3E3.0.CO%3B2-L¡¡
¡¡
Digestive and Liver Disease Volume 41, Issue 9, September 2009, Pages 644-647
The efficacy of Helicobacter pylori eradication regimen with and without vitamin C supplementation
panelH.ZojajiaR.TalaieaD.MirsattariaM.HaghazaliaM.MolaeibN.MohsenianaF.DerakhshanaM.R.Zalia
a Research Center for Gastroenterology and Liver Disease, Shahid Beheshti University of Medical Sciences, Tehran, Iran
b Dept. of Pathology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Received 13 April 2008, Accepted 4 September 2008, Available online 2 June 2009.
Abstract
Background
Vitamin C in gastric juice and in vitro has been shown to inhibit the growth of Helicobacter pylori (H. pylori).
Aims
The purpose of this study was to investigate the effect of addition of vitamin C to eradication regimen on H. pylori eradication rate.
Patients
This randomised controlled clinical trial was conducted on 312 patients with H. pylori infection who had referred to the Taleghani Research Center of Gastroenterology and Liver Disease.
Methods
Patients were randomly divided into two groups. Group A patients (162 patients) received amoxicillin 1 g and metronidazole 500 mg b.i.d., bismuth 240 mg b.i.d. and omeprazole 40 mg q.i.d. in two divided doses. Patients in group B (150 patients) received the same regimen plus 500 mg vitamin C per day. All patients received therapy for 2 weeks. Four weeks later all patients underwent urea breath test and results were compared.
Results
A total of 140 patients in group A and 141 in group B completed the study. On intention-to-treat analysis 48.8% of patients in group A in comparison to 78% in group B responded to eradication therapy and had negative urea breath test (p < 0.0001).
Conclusion
Addition of vitamin C to H. pylori treatment regimen of amoxicillin, metronidazole and bismuth can significantly increases H. pylori eradication rate.The efficacy of Helicobacter pylori eradication regimen with and without vitamin C supplementation - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S1590865808006361¡¡
Vitamin C: A Preventative, Therapeutic Agent Against Helicobacter pylori
Azhar Hussain, Elsa Tabrez, Jagannadha Peela, Prasanna Honnavar Dr, Shams S.M. Tabrez
Published: July 30, 2018
Abstract
The treatment of Helicobacter pylori (H. pylori) induced infections using antibiotic therapies is clinically well accepted; however, using a noninvasive approach with the implementation of therapeutic agents such as vitamin C is not well investigated. Vitamin C has certain characteristics, which allow for it to be considered as a potential treatment option for patients with H. pylori infections. Vitamin C¡¯s hostility and mechanism of action towards H. pylori infection in peptic ulcer disease can be classified into two categories: as a preventative agent and alternatively as a therapeutic agent. Preventatively vitamin C acts as a biological antioxidant as well as an immune boosting agent, while therapeutically it acts as an inhibitor of urease, a potential collagen synthesizing agent, and a stimulant in prostaglandin synthesis. As a result, the dosage of vitamin C should be highly regulated. Furthermore, numerous studies have shown that vitamin C supplementation if taken with antibiotics can increase the efficiency of the treatment leading to an increased possibility of eradication of H. pylori in infected individuals. This paper will investigate the recent studies that show different mechanisms through which vitamin C can be used as a preventative or a therapeutic agent for the treatment of H. pylori related infections.Inactivation of Urease
Urease is an important enzyme that constitutes approximately 5%-6% of the total protein of H. pylori and it is linked to its pathogenicity as shown in Table 2 [28], due to its ability to colonize on the gastric mucosa at a low pH [23]. The structure of urease enzyme is composed of two half subunits held together by a noncovalent bond [5], each subunit containing a specific active site. These active sites contain two nickel ions that are bound by a carbamylated lysine and an oxygen donor [29]. In addition to the binding, the first nickel ion is held by two histidine amino acids and a water molecule and the second nickel ion which is similar in composition to the first nickel ion, also contains the amino acid aspartate as shown in Figure 1 [5, 14, 28-29]. The ability of vitamin C to inhibit urease action plays an important role in understanding the mechanism of H. pylori infection and bacterial eradication [30]. Studies show that the high concentration of vitamin C favors reduction of the nickel center in the urease enzyme [5], which in turn inhibits the activity of the enzyme and may reduce the H. pylori manifestations.
Figure 1: The structure of urease and the role of vitamin C as an inhibitor.
Urease maturation (potent virulence factor required for survival of H. pyloriin acidic environments) Increase in vitamin C leads to the reduction of nickel of urease enzyme Vitamin C, which is a relatively strong acid (pKa = 4.1) further lowers the pH of the gastric lumen [28]. Vitamin C, a reducing agent of urease, when added to the gastric lumen results in urease becoming structurally unstable, therefore, irreversibly losing its enzyme activity [30]. Therefore, vitamin C can be beneficial in inhibiting the growth, colonization, and endurance of H. pylori at an earlier period of the infection and may be helpful in the eradication of the bacteria [23].
Cureus | Vitamin C: A Preventative, Therapeutic Agent Against Helicobacter pylori
https://www.cureus.com/articles/13566-vitamin-c-a-preventative-therapeutic-agent-against-helicobacter-pylori#¡¡
Antimicrob Agents Chemother. 2020 Feb 18. pii: AAC.02192-19. doi: 10.1128/AAC.02192-19. [Epub ahead of print]
Hydrogen peroxide-mediated oxygen enrichment eradicates Helicobacter pylori in vitro and in vivo.
Di J1,2, Zhang J2, Cao L1, Huang TT1, Zhang JX1, Mi YN1, Xiao X1, Yan PP1, Wu ML1, Yao T1, Liu DZ1, Liu J1, Cao YX3.
Author information
1 Department of Pharmacology, School of Basic Medical Science, Xi'an Jiaotong University Health Science Center, Xi'an, 710061, China.
2 Department of Gastroenterology, Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an, 710004, China.
3 Department of Pharmacology, School of Basic Medical Science, Xi'an Jiaotong University Health Science Center, Xi'an, 710061, China yxy@xjtu.edu.cn.
Abstract
Helicobacter pylori (H. pylori) is an important risk factor for gastric ulcers. However, antibacterial therapies increase the resistance rate and decrease the eradication rate of H. pylori Inspired by microaerophilic characteristics of H. pylori, we aim at effectively establishing an oxygen-enriched environment to eradicate and prevent the recurrence of H. pylori The effect and the mechanism of an oxygen-enriched environment in eradicating H. pylori and preventing the recurrence were explored in vitro and in vivo During oral administration and after drugs withdrawal, H. pylori counts were both evaluated by Giemsa stain in animal cohorts.An oxygen-enriched environment was successfully established by adding hydrogen peroxide into solutions and rabbit gastric juice, in which H. pylori could not survive. Hydrogen peroxide effectively killed H. pylori in Columbia blood agar and special peptone broth. Minimum inhibition concentrations and minimum bactericidal concentrations of hydrogen peroxide were both relatively stable after promotion of resistance for 30 generations, and these results indicated that hydrogen peroxide did not easily induce resistance to H. pylori
Models of Mongolian gerbils and Kunming mice showed that hydrogen peroxide significantly eradicated and effectively prevented the recurrence of H. pylori without toxicity and damage to the gastric mucosa.
The mechanism of hydrogen peroxide on H. pylori death was related to the disruption of bacterial cell membranes. Oxygen-enriched environment achieved by hydrogen peroxide eradicates and prevents the recurrence of H. pylori by damaging bacterial cell membranes. Hydrogen peroxide provides an attractive candidate for anti-H. pylori treatment.
Copyright © 2020 American Society for Microbiology.
PMID: 32071054 DOI: 10.1128/AAC.02192-19Hydrogen peroxide-mediated oxygen enrichment eradicates Helicobacter pylori in vitro and in vivo. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/32071054¡¡
Hydrogen peroxide-induced inactivation of urease: Mechanism, kinetics and inhibitory potency
Article in Journal of Molecular Catalysis B Enzymatic 68(3):262-269 ¡¤ March 2011
Barbara Krajewska
Evidence has been collected that H2O2 resulting from redox reactions taking place in some inhibitor systems, is responsible for the inactivation of urease. Accordingly, in this study the inactivation by H2O2 exogenously added to urease (jack bean) was investigated. The reaction accountable for the inactivation was the oxidation of the enzyme thiol groups. The reaction was studied at pHs over 6.2¨C8.2. At each pH, the first-order kinetics was obeyed, the resulting dependences of kobs on H2O2 concentration being hyperbolic. Analyzed with Kitz-Wilson method, the kinact appeared to be invariant with pH, while the inhibitor binding constant KI decreased with increasing pH. Consequently, the process grew faster with an increase in pH, the second-order rate constants increasing from 0.0032M−1s−1 at pH 6.2 to 0.0615M−1s−1 at pH 8.2, consistent with higher susceptibility of thiolate anions to oxidation by H2O2 compared to reduced thiols. The reactions always resulted in irreversible inactivation of urease, indicative of the oxidation of thiol groups to either sulfinic or sulfonic acid, independent of the extent of inactivation. Analysis of the numbers of urease thiol groups modified with H2O2 revealed that for the complete inactivation, out of 36 thiols/molecule available under non-denaturating conditions, the rapid oxidation of 30 highly reactive ¨CSH groups was responsible for a 50% loss in enzyme activity, and the slower oxidation of the remaining six ¨CSH groups, for the other 50%. The enzyme was protected by active-site binding inhibitors, boric acid and fluoride, from the inactivation, suggesting that the six thiols are the active-site flap Cys-592s. For comparison with other urease inhibitors, the IC50 was determined for 20min incubation with H2O2, its value changing from 242mM at pH 6.2 to 11mM at pH 8.2.... Thiol-containing compounds exert protective effects by interacting with the sulfhydryl group (-SH) of urease to restrict the ability of inhibitors to access the active site [40,41]. We therefore performed an assay to investigate the potential effects of thiol-containing compounds Control 0 ¡À 01.1 ¡À 0.3Urea 472 ¡À 571.3 ¡À 0.2Acetohydroxamic acid 0 ¡À 0 100 1.7 ¡À 0.4 0 Coptisine (50) 129 ¡À 31 72.7 1.0 ¡À 0. (dithiothreitol (DTT), L-Cysteine (L-Cys), and glutathione (GSH)) on coptisine-treated urease. ...
... Thiol-containing compounds exert protective effects by interacting with the sulfhydryl group (-SH) of urease to restrict the ability of inhibitors to access the active site [40,41]. We therefore performed an assay to investigate the potential effects of thiol-containing compounds (dithiothreitol (DTT), L-Cysteine (L-Cys), and glutathione (GSH)) on coptisine-treated urease. ...¡¡
Hydrogen peroxide-induced inactivation of urease: Mechanism, kinetics and inhibitory potency
https://www.researchgate.net/publication/251671757_Hydrogen_peroxide-induced_inactivation_of_urease_Mechanism_kinetics_and_inhibitory_potency¡¡
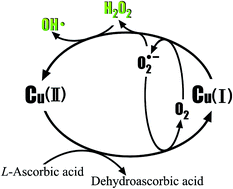
Generation of hydrogen peroxide and hydroxyl radical resulting from oxygen-dependent oxidation of l-ascorbic acid via copper redox-catalyzed reactions
Peng Zhou,a Jing Zhang,*a Yongli Zhang,a Ya Liu,a Juan Liang,a Bei Liua and Wei Zhanga
College of Architecture and Environment, Sichuan University, Chengdu 610065, P. R. China
Abstract
The generation of hydrogen peroxide (H2O2) and hydroxyl radical (HO¨B) during the oxidation of L-ascorbic acid (L-AA) by oxygen with copper as a catalyst was investigated to set up the O2/Cu/L-AA process with benzoic acid (BA) as a probe reagent. The high concentration of H2O2 that is generated undergoes an intramolecular two-electron transfer and is further activated by the intermediate cuprous copper [Cu(I)] to yield HO¨B as a product, resulting in significant degradation of BA.Dehydroascorbic acid, 2,3-diketogulonic acid, and L-xylosone were the predominant detected products of the oxidation of L-AA. However, the generation of H2O2 and degradation of BA were regulated by variations in pH, which results from the contradiction between protonated L-AA that is difficult to chelate with Cu(II) via electron transfer and hydrogen ions (H+), which are indispensable for the generation of H2O2.
Furthermore, the concentration of H2O2 and degradation of BA increased with an increase in the dosage of L-AA. Trace amounts of Cu(II) are effective for catalyzing the oxidation of L-AA, whereas the generation of H2O2 and degradation of BA increased with an increase in the dosage of Cu(II). Owing to the formation of Cu(I) chloride complexes or Cu(II) chloride complexes, the addition of chloride (Cl−) could inhibit the generation of H2O2 and degradation of BA.
Generation of hydrogen peroxide and hydroxyl radical resulting from oxygen-dependent oxidation of l-ascorbic acid via copper redox-catalyzed reactions - RSC Advances (RSC Publishing)
https://pubs.rsc.org/en/content/articlelanding/2016/ra/c6ra02843h#!divAbstract¡¡
¡¡
Anti-Helicobacter Effects of Vitamin C
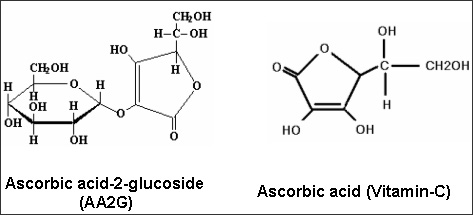
In an in vitro study, 10 to 20 mg vitamin C per ml could effectively inhibit Helicobacter pylori growth under microaerobic conditions, whereas in an aerobic milieu, vitamin C even promoted H. pylori survival in concentrations ranging from 2 to 20 mg/mL [105]. These observations might be explained by the antioxidant properties of vitamin C, protecting microaerophilic bacteria against toxic effects of ROS. Following one-week treatment of H. pylori-infected Mongolian gerbils with 10 mg vitamin C daily, gastric pathogen loads could be significantly lowered [106]. In support, several clinical studies reported more effective H. pylori eradication upon vitamin C application to infected humans [107¨C109]. In addition, oxidative stress, apoptotic responses, and decreased cellular viability that had been induced in an H. pylori-infected human gastric adenocarcinoma cell line could be counteracted by vitamin C application in its L-ascorbic acid-2-glucoside form [110].
Immunomodulatory and Antimicrobial Effects of Vitamin C
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6798581/¡¡
Biological Chemistry
Formation of a Stable Ascorbic Acid 2-Glucoside by Specific Transglucosylation with Rice Seed ¦Á-Glucosidase
Norio Muto, Sadaharu Suga, Kazuko Fujii, Kazuko Goto & Itaru Yamamoto
Pages 1697-1703 | Received 19 Jan 1990, Published online: 08 Sep 2014
Abstract
The enzymatic transglucosylation to synthesize a chemically stable form of l-ascorbic acid (AA) was further investigated by using commercially available enzymes. Among various glycosidases examined, only rice seed ¦Á-glucosidase could produce a nonreducing and stable glucoside of AA, which was identified as 2-O-¦Á-d-glucopyranosyl-l-ascorbic acid (AA-2G). The enzyme showed the same regioselective transglucosylase activity as rat intestinal ¦Á-glucosidase that had been demonstrated by us to be effective in this reaction, although these two enzymes had different pH optima for maltose hydrolysis. The substrate specificity of rice ¦Á-glucosidase for AA-2G formation was considerably different from that of rat ¦Á-glucosidase. However, both ¦Á-glucosidases had high specificity for the ¦Á-1,4-glucosidic linkage but not ¦Á-1,6, suggesting that they can catalyze a preferential transglucosylation to the 2-position but not to the 6-position of AA. Thus, these results allows us to produce a sufficient amount of AA-2G with rice seed ¦Á-glucosidase for its application to medicinal and other uses.Formation of a Stable Ascorbic Acid 2-Glucoside by Specific Transglucosylation with Rice Seed ¦Á-Glucosidase: Agricultural and Biological Chemistry: Vol 54, No 7
https://www.tandfonline.com/doi/abs/10.1080/00021369.1990.10870201¡¡
Including results for vitamin c enhance activity of ho-1.
Do you want results only for vitaminc enhance sactivity of HO-1?
Vitamin C, a Multi-Tasking Molecule, Finds a Molecular ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5959041
While the mechanism involved in the induction of HO-1 remains to be determined, it is likely that vitamin C provokes a pro-oxidant state in cells that subsequently activates HO-1 gene expression. Indeed, HO-1 is known as an oxidative stress-responsive gene, and as described next, vitamin C at pharmacological doses behaves as a pro-oxidant. 4.3.
Cited by: 4
Publish Year: 2016
Author: Y. Robert Li
Gastroprotection by vitamin C¡ªa heme oxygenase-1-dependent mechanism?
Author links open overlay panelJan CBeckeraNinaGrosserbPeterBoknikcHenningSchröderbWolframDomschkeaThorstenPohlea
a
Department of Medicine B, University of M¨¹nster, M¨¹nster, Germany
b
Department of Pharmacology and Toxicology, School of Pharmacy, University of Halle, Halle, Germany
c
Institute of Pharmacology und Toxicology, University of M¨¹nster, M¨¹nster, Germany
Received 21 October 2003, Available online 13 November 2003.
Abstract
Free oxygen radicals contribute to gastric mucosal damage induced by acetylic¨Csalicylic acid (ASA). Vitamin C has been shown to reduce gastric toxicity of ASA in humans.We intended to assess the role of heme oxygenase-1 (HO-1) in this process by application of these substances to AGS and KATO III cells. HO-1 expression was monitored by real-time RT-PCR, Western blot, and HO activity measurement. HO-1 mRNA was significantly elevated by either ASA or vitamin C in gastric epithelial cells, combination of both substances further increased expression.
HO-1 protein and enzyme activity rose in cells exposed to vitamin C alone or combined with ASA, but not after stimulation with ASA alone.
In contrast to endothelia, in which ASA simultaneously induces HO-1 mRNA and protein expression, gastric epithelial cells require vitamin C to translate HO-1 mRNA into active protein, which then may exert gastroprotection by its antioxidant and vasodilative properties.
Keywords
Acetylic¨Csalicylic acidGastric epithelial cellsGastroprotectionHeme oxygenaseProtein expressionReal-time RT-PCRVitamin CGastroprotection by vitamin C¡ªa heme oxygenase-1-dependent mechanism? - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0006291X03022460¡¡
¡¡
¡¡
Crit Rev Immunol. 2001;21(5):399-425.
Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue.
Mills CD1.
Author information
1
Department of Surgery and Diabetes Institute for Immunology and Transplantation, University of Minnesota Hospitals and Clinics, Minneapolis 55455, USA. mills002@tc.umn.edu
Abstract
Macrophages can metabolize arginine to nitric oxide in quantities that inhibit pathogens or nearby host cells. They can instead metabolize arginine to ornithine (a precursor of polyamines and collagen) in quantities that stimulate pathogens or nearby host cells.Macrophages are essentially the only circulating cells that can make these life or death decisions with arginine. Macrophages expressing these destructive or constructive phenotypes have been termed M-1 or M-2 because they also stimulate TH1 or TH2 responses, respectively.
Factors that influence whether a macrophage expresses the M-1 or M-2 phenotype and the real or potential impact on immune responses and other host processes are discussed.
PMID: 11942557
[Indexed for MEDLINE]https://www.ncbi.nlm.nih.gov/pubmed/11942557
¡¡
6. Rudi J, et al. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J Clin Invest. 1998;102:1506¨C1514. [PMC free article] [PubMed] [Google Scholar]
¡¡
¡¡