°°
https://www.spandidos-publications.com/ijmm/38/4/995?text=fulltext
°°
http://www.mdpi.com/2072-6643/5/12/5161/htm
°°
FEBS J. 2007 Jan;274(1):1-22.
Vitamin C. Biosynthesis, recycling and degradation in mammals.
Linster CL1, Van Schaftingen E.
Author information
1
Universit®¶ Catholique de Louvain, Christian de Duve Institute of Cellular Pathology, Brussels, Belgium.
Abstract
Vitamin C, a reducing agent and antioxidant, is a cofactor in reactions catalyzed by Cu(+)-dependent monooxygenases and Fe(2+)-dependent dioxygenases. It is synthesized, in vertebrates having this capacity, from d-glucuronate. The latter is formed through direct hydrolysis of uridine diphosphate (UDP)-glucuronate by enzyme(s) bound to the endoplasmic reticulum membrane, sharing many properties with, and most likely identical to, UDP-glucuronosyltransferases. Non-glucuronidable xenobiotics (aminopyrine, metyrapone, chloretone and others) stimulate the enzymatic hydrolysis of UDP-glucuronate, accounting for their effect to increase vitamin C formation in vivo. Glucuronate is converted to l-gulonate by aldehyde reductase, an enzyme of the aldo-keto reductase superfamily. l-Gulonate is converted to l-gulonolactone by a lactonase identified as SMP30 or regucalcin, whose absence in mice leads to vitamin C deficiency.The last step in the pathway of vitamin C synthesis is the oxidation of l-gulonolactone to l-ascorbic acid by l-gulonolactone oxidase, an enzyme associated with the endoplasmic reticulum membrane and deficient in man, guinea pig and other species due to mutations in its gene.
Another fate of glucuronate is its conversion to d-xylulose in a five-step pathway, the pentose pathway, involving identified oxidoreductases and an unknown decarboxylase. Semidehydroascorbate, a major oxidation product of vitamin C, is reconverted to ascorbate in the cytosol by cytochrome b(5) reductase and thioredoxin reductase in reactions involving NADH and NADPH, respectively. Transmembrane electron transfer systems using ascorbate or NADH as electron donors serve to reduce semidehydroascorbate present in neuroendocrine secretory vesicles and in the extracellular medium.
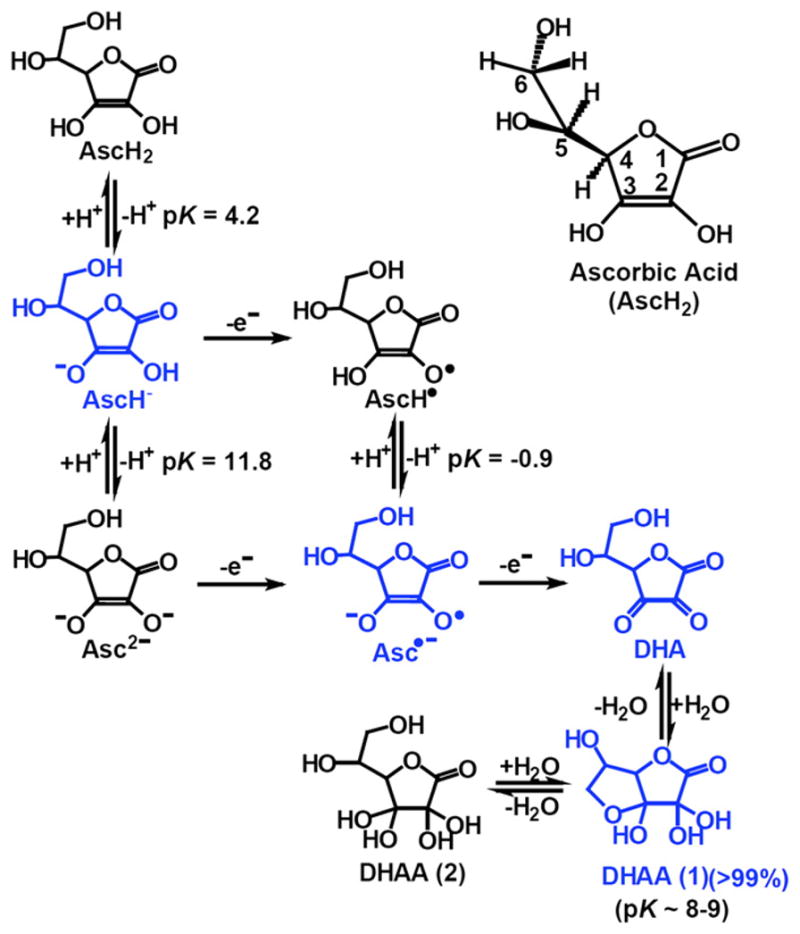
Dehydroascorbate, the fully oxidized form of vitamin C, is reduced spontaneously by glutathione, as well as enzymatically in reactions using glutathione or NADPH.
The degradation of vitamin C in mammals is initiated by the hydrolysis of dehydroascorbate to 2,3-diketo-l-gulonate, which is spontaneously degraded to oxalate, CO(2) and l-erythrulose.
This is at variance with bacteria such as Escherichia coli, which have enzymatic degradation pathways for ascorbate and probably also dehydroascorbate.
PMID: 17222174 DOI: 10.1111/j.1742-4658.2006.05607.x
Vitamin C. Biosynthesis, recycling and degradation in mammals. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/17222174°°
Evol Med Public Health. 2019 Aug 28;2019(1):221-231. doi: 10.1093/emph/eoz024. eCollection 2019.
Glut-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis: The electron transfer hypothesis.
Hornung TC1, Biesalski HK1.
Author information
Abstract
INTRODUCTION:
During evolution, some species including humans, monkeys and fruit bats lost the ability for ascorbic acid (AA) biosynthesis due to inactivation of the enzyme l-gulono-lactone oxidase (GLO) and subsequently became dependent on dietary vitamin C. There are four current hypotheses in relation to the benefit of vitamin C dependence in the context of adaptation and reproduction. Here we advance and test a new 'electron transfer hypothesis', which focusses on the role of the expression of glucose transporter 1 (Glut-1) in red blood cells (RBCs) in recycling vitamin C, thereby increasing the efficiency of micronutrient uptake.
METHODS:
To evaluate the benefit of Glut-1 expression, we determined vitamin C uptake into RBCs and potential release from two different species, humans with l-Gulono-lactone-oxidase (GLO-loss) and pigs with functional GLO.
RESULTS:
The oxidized form of vitamin C (dehydroascorbate, DHA) was transported into human RBCs via Glut-1. There was no transport of either the reduced (AA) or the oxidized vitamin in pig erythrocytes.
CONCLUSION:
We propose that the transport of vitamin C increases an intracellular electron pool, which transfers electrons from intracellular ascorbate to extracellular substances like ascorbyl free radical or DHA, resulting in 100-fold smaller daily requirement of this essential redox sensitive micronutrient. This would be an advantage during seasonal changes of the availability from food and may be the key for the survival of individuals without vitamin C biosynthesis.
LAY SUMMARY:
40 million years ago some individuals lost the ability to synthesize vitamin C. Why did they survive such as humans until now? Individuals with a specific glucose transporter Glut-1 on their erythrocytes which transports vitamin C need less and are protected from scarcity due to seasons and food competitors.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Foundation for Evolution, Medicine, and Public Health.Glut-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis: The electron transfer hypothesis. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/31857900°°
EBioMedicine. 2015 Oct 3;2(11):1735-50. doi: 10.1016/j.ebiom.2015.09.049. eCollection 2015 Nov.
Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascorbic Acid.
Tu H1, Li H1, Wang Y1, Niyyati M1, Wang Y1, Leshin J1, Levine M1.
Author information
Abstract
Strategies to prevent diabetic microvascular angiopathy focus on the vascular endothelium. Because red blood cells (RBCs) are less deformable in diabetes, we explored an original concept linking decreased RBC deformability to RBC ascorbate and hyperglycemia.We characterized ascorbate concentrations from human and mouse RBCs and plasma, and showed an inverse relationship between RBC ascorbate concentrations and deformability, measured by osmotic fragility. RBCs from ascorbate deficient mice were osmotically sensitive, appeared as spherocytes, and had decreased ¶¬-spectrin.These aberrancies reversed with ascorbate repletion in vivo.
Under physiologic conditions, only ascorbate's oxidation product dehydroascorbic acid (DHA), a substrate for facilitated glucose transporters, was transported into mouse and human RBCs, with immediate intracellular reduction to ascorbate.
In vitro, glucose inhibited entry of physiologic concentrations of dehydroascorbic acid into mouse and human RBCs. In vivo, plasma glucose concentrations in normal and diabetic mice and humans were inversely related to respective RBC ascorbate concentrations, as was osmotic fragility.
Human RBC ¶¬-spectrin declined as diabetes worsened. Taken together, hyperglycemia in diabetes produced lower RBC ascorbate with increased RBC rigidity, a candidate to drive microvascular angiopathy. Because glucose transporter expression, DHA transport, and its inhibition by glucose differed for mouse versus human RBCs, human experimentation is indicated.
KEYWORDS:
3-O-MG, 3-O-methylglucose; AA, ascorbic acid; Ascorbic Acid; DHA, dehydroascorbic acid; Dehydroascorbic Acid; Diabetes; GLUT, facilitated glucose transporter; Glucose Transport; Gulo-/-, gulonolactone oxidase knockout mouse unable to synthesize ascorbate; PBS, phosphate buffered saline; RBCs, red blood cells; RIPA, Western blot cell lysis buffer; Red Blood Cells; SVCT, sodium-dependent vitamin C transporter; TCEP, Tris(2-carboxyethyl)phosphine; WT, wildtype mouse; ¶¬-Spectrin
Comment in
Famine From Feast: Low Red Cell Vitamin C Levels in Diabetes. [EBioMedicine. 2015]Low Red Blood Cell Vitamin C Concentrations Induce Red Blood Cell Fragility: A Link to Diabetes Via Glucose, Glucose Transporters, and Dehydroascor... - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/26870799Bench-to-bedside review: Glucose and stress conditions in the intensive care unit | Critical Care | Full Text
https://ccforum.biomedcentral.com/articles/10.1186/cc9100°°
Ascorbic Acid and Dehydroascorbic Acid Concentrations in Plasma and Peripheral Blood Mononuclear Cells after Oral Liposomal-Encapsulated or Intravenous Ascorbic Acid Delivery
Author(s): Nina A. Mikirova, PhD1
1Bio-communication Research Institute, Riordan Clinic 3100 North Hillside, Wichita, KS, USA email: nmikirova@riordanclinic.orgCurrently there are about 12 liposome-based drugs approved for clinical use and more are in various stages of clinical trials (Chang & Yeh, 2012; Torchilin, 2005). Most liposomal drug formulations are approved for intravenous application. Other administration routes such as intramuscular delivery have also been approved. Oral delivery of drugs has been examined; however, this route of administration is more troublesome due to the potential for liposomal breakdown following exposure to bile salts (Shaji & Patole, 2008). The studies of the oral liposomal formulations of ascorbic acid include a study published by Devis and coworkers (Davis, Paris, Beals, et al., 2016), in which the authors compared oral absorbance of liposomal ascorbic acid to oral absorbance of a non-encapsulated form, and intravenous delivery. On four separate randomly ordered occasions, 11 men and women were administered an oral placebo, or 4 g of vitamin C via oral, oral liposomal, or intravenous delivery. The data indicate that oral delivery of 4 g of vitamin C encapsulated in liposomes produces circulating concentrations of vitamin C that are greater than non-encapsulated oral, but less than achieved by intravenous administration. In addition, liposomal vitamin C provides protection from ischemia reperfusion-mediated oxidative stress that is similar to the protection provided by un-encapsulated oral and intravenous administrations. The levels of ascorbate that were achieved in plasma after 4 grams vitamin C were 1419 uM for intravenous ascorbate, 124 uM for un-encupsulated and 170 uM for encapsulated ascorbate.
in the paper of Hickey (Hickey, Roberts, & Miller, 2008), the highest achievable plasma ascorbic acid concentrations by oral intake of liposomal formulations of ascorbic acid were determined for dosages of 5 grams, 20 grams and 36 grams. Two subjects took part in this study, and blood plasma measurements were obtained over the first 6 hours of absorption. Circulating concentrations of vitamin C following oral delivery of 5 g of ascorbic acid encapsulated in liposomes were compared with concentrations following 5 g of un-encapsulated ascorbic acid and showed that both produced similar response curves. Plasma levels following a 36 g oral dose of liposomal ascorbic acid resulted in peak plasma levels in the region of 400 uM/L with delayed the peak plasma level and broader response.
According to our data, concentration of ascorbate in plasma, after administration of 25 grams intravenous vitamin C, increased from 112 to 140 times. The oral liposomal intake of 25 grams of ascorbate resulted in increasing concentrations in plasma seven times and reached in an average value 230 uM. According to another study (Hickey, Roberts, & Miller, 2008), this level in plasma can be achieved by less doses of standard ascorbic acid supplementation. The concentration of dehydroascorbic acid in plasma was raised 14 -20 times after intravenous ascorbate and 2 times after liposomal ascorbate.
Research: Realistic Observations On High Dose Intravenous ...
Pharmacokinetic studies later revealed substantial differences in the maximum achieved blood concentrations of vitamin C based on the route of administration. When vitamin C is taken orally, plasma concentrations of the vitamin are tightly controlled, with a peak achievable concentration less than 300 µM.
°°
Pharmacokinetic studies have found that the peak concentration of vitamin C achievable in blood plasma following oral ingestion is about 220 ¶Őmol/L, whereas at least 15 mmol/L is achievable following IVC administration [ 7 ].www.ncbi.nlm.nih.gov/pmc/articles/PMC5981254/°°
°°