The Mystery of Allergy 过敏之谜
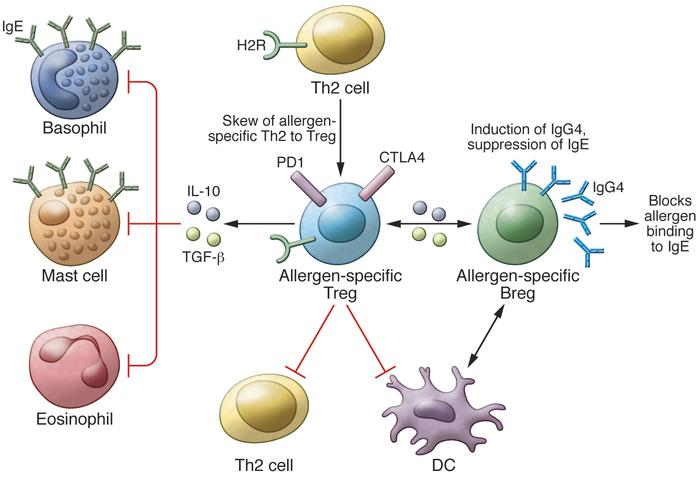
Mechanisms of allergen tolerance. The induction of allergen-specific Tregs, which switch from allergen-specific Th2 cells is one of the initial events in the development of allergen tolerance. The effector cells of allergic inflammation — mast cells, basophils, and eosinophils — are regulated by suppressive and regulatory functions of Tregs in several ways. Treg-secreted IL-10 and TGF-β suppress these cells. Tregs also suppress Th2 cells and their cytokines, preventing the provision of survival factors for these allergy effector cells. IL-10 and TGF-β suppress IgE production by B cells, and meanwhile, IL-10 induces IgG4. IL-10–producing regulatory B cells (Bregs) play a role in suppression of allergen-specific T cells and mainly switch to IgG4-producing plasma cells. H2R plays a role in the suppression of Th2 cells, inflammatory dendritic cells, and basophils.
过敏原耐受机制。 从过敏原特异的Th2细胞转换而来的过敏原特异Tregs的诱导是过敏原耐受性发展的最初事件之一。 过敏性炎症的效应细胞-肥大细胞,嗜碱性粒细胞和嗜酸性粒细胞-通过多种方式通过Treg的抑制和调节功能进行调节。 Treg分泌的IL-10和TGF-β抑制这些细胞。 Tregs还抑制Th2细胞及其细胞因子,从而阻止为这些过敏效应细胞提供生存因子。 IL-10和TGF-β抑制B细胞产生IgE,同时,IL-10诱导IgG4。 产生IL-10的调节性B细胞(Bregs)在抑制变应原特异性T细胞中发挥作用,主要转换为产生IgG4的浆细胞。 H2R在抑制Th2细胞,炎性树突状细胞和嗜碱性粒细胞中起作用。
Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs
Cezmi A. Akdis1,2 and Mübeccel Akdis1
1Swiss Institute of Allergy and Asthma Research (SIAF), University of Zurich, Davos, Switzerland. 2Christine Kühne – Center for Allergy Research and Education (CK-CARE), Davos, Switzerland.
During the past 20 years, major advances have been made in understanding the molecular and cellular mechanisms of allergen tolerance in humans. The demonstration of T cell tolerance, particularly that mediated by the immune-suppressive functions of IL-10, led to a major conceptual change in this area. Currently, the known essential components of allergen tolerance include the induction of allergen-specific regulatory subsets of T and B cells, the immune-suppressive function of secreted factors, such as IL-10 and TGF-β, the production of IgG4 isotype allergen–specific blocking antibodies, and decreased allergic inflammatory responses by mast cells, basophils, and eosinophils in inflamed tissues.
T cells and the allergic response
Immune tolerance can develop against any immune-activating substance, and multiple mechanisms mediate this process. Deregulation of immune tolerance may lead to the development of allergies, asthma, tumors, chronic infections, rejection of transplanted organs, graft-versus-host disease, and many autoimmune diseases. Allergic diseases are characterized by the induction of a type 2 immune response that includes Th2 cells and type 2 innate lymphoid cells (ILC2s), together with the production of allergen-specific IgE antibodies and increased eosinophil numbers in the affected tissues and sometimes in peripheral blood (1). Although there are many different ways to treat allergic disease–associated symptoms, currently, the only long-term curative treatment is allergen-specific immunotherapy (AIT), which involves the administration of increasing doses of the causative allergen. Over time, AIT induces a state of allergen-specific immune tolerance. AIT has been performed for more than 100 years, though it has only been within the past two decades that the mechanisms that mediate the action of AIT have been slowly uncovered (2). After the discovery of Th1 and Th2 cells in 1986 (3), it was suggested that a Th2 response underlies the development of allergic diseases and that Th1 responses are predominant in infections and autoimmunity. Following these initial findings, the general dogma was that a switch toward a Th1 response would be required for the successful treatment of allergies by AIT, and a switch toward a Th2 response would be beneficial for the treatment of autoimmunity.
In the mid-1990s, our group was working on the mechanisms of action of AIT in conventional and T cell epitope peptide immunotherapies (4, 5). We were using honey bee venom allergy and its major allergen phospholipase A2 as a model to investigate antigen/allergen-specific immune response development in humans (4). In a 1996 article published in the JCI, we demonstrated that allergen-specific T cell tolerance was induced during the course of AIT (4). Two months after the beginning of venom immunotherapy, the proliferation of T cells specifically targeting phospholipase A2 and its T cell epitope peptides as well as the secretion of both Th2 (IL-4, IL-5, and IL-13) and Th1 (IL-2 and IFN-γ) cytokines were abolished. These data directly challenged the dogma that switching the balance between Th1 and Th2 responses is playing a role in treatment and suggested that the development of full T cell tolerance and suppression of both Th1 and Th2 subsets are taking place. As a control antigen, we demonstrated that tetanus toxoid–induced T cell proliferation and cytokine production remained unchanged following venom-specific AIT, demonstrating that the immune tolerance was specific to venom antigens and did not extend to other antigens. In addition, the state of T cell tolerance induced by AIT was altered in vitro by treatment with IL-2, IL-4, and IL-15. This was one of the first demonstrations that antigen-specific effector T cells have plasticity, suggesting that microenvironmental cytokines are important for determining success or failure in AIT (4). During these experiments, we were fortunate to be working with highly pure synthetic peptides and purified bee venom antigens collected in a sterile way. This enabled us to demonstrate immune tolerance, because any lipopolysaccharide contamination during collection and extraction of environmental allergens, such as house dust mites and pollens, would have led to results showing a skew from Th2 toward Th1.
IL-10–producing Tregs in immune tolerance
In hindsight, our research continued to elucidate the molecular and cellular mechanisms of T cell tolerance to allergens, and in our 1998 study published in the JCI, we demonstrated that human IL-10–producing Tregs (Tr1 cells) are linked to an antigen-specific suppressor function (Figure 1 and ref. 6). Intracytoplasmic cytokine staining of circulating lymphocytes revealed that IL-10 was initially produced by CD4+CD25+ allergen–specific Tr1 cells and then by B cells and monocytes. These IL-10–secreting Tr1 cells were substantially increased as early as seven days after the start of bee venom AIT in allergic individuals. Following these initial findings, one major question was whether these Tr1 cells also develop during other types of AIT against different allergens. It took many years to demonstrate that Tr1 cells are generated in response to other immunotherapies such as sublingual immunotherapy, other allergens such as grass pollen and house dust mites, and peptide immunotherapies in allergy and autoimmune diseases (7–12). In addition, Tr1 cells were demonstrated to be present in high-dose exposure to allergen models, such as nonallergic beekeepers and cat owners (6, 13, 14).
Tr1 cells and recently discovered Br1 cells support a healthy immune response to allergens
Studies during the past two decades have demonstrated that there are two broad subsets of CD3+CD4+ Tregs. One subset is the naturally occurring thymus-derived CD4+CD25+ FOXP3+ Tregs, also called natural Tregs (nTregs), and the other subset is the inducible Tregs (iTregs). Three main subsets of iTregs have been characterized: FOXP3-expressing iTregs, CD4+FOXP3- IL-10–producing Tr1 cells, and TGF-β–expressing Th3 cells. It has been repeatedly shown that all three subsets of iTregs coexist and overlap in many immune tolerance–related situations in humans. For example, antigen-specific CD4+ T cells that express IL-10, TGF-β, and FOXP3, or some combination of these markers, increase in nasal biopsies (12, 15–17) and peripheral blood (7–9) in response to AIT at the healing phase.
Tr1 cells have been shown to be important for the maintenance of a healthy immune response in different diseases, such as allergy, many autoimmune conditions, transplantation tolerance, and graft-versus-host disease in both humans and mice (13, 18–26). Although IL-10 is the main cytokine produced by Tr1 cells, these cells also produce TGF-β and low-to-medium levels of IFN-γ and IL-5, but not IL-4 or IL-2 (27, 28). It took almost a decade to demonstrate that human Tr1 cells suppress effector T cell responses by multiple mechanisms that depend on IL-10, TGF-β (7), PD-1, CTLA-4 (29), and histamine receptor 2 (H2R) (13).
IL-10 has a potent immunosuppressive capacity that is crucial not only for the establishment of peripheral tolerance to allergens, but also in protecting the host from exaggerated inflammatory responses to pathogens as well as to autoimmune diseases (30). IL-10 directly inhibits T cells through suppression of CD28- and ICOS-dependent T cell costimulation (31). In addition, IL-10 inhibits the production of proinflammatory cytokines, chemokines, and chemokine receptors as well as the expression of MHC class II and costimulatory molecules CD80/CD86 on monocytes/macrophages and dendritic cells (30).
In addition to T cell regulation, IL-10 secreted from Tr1 cells plays a major role in the induction of IgG4 and suppression of IgE (6, 32). Moreover, it was recently demonstrated that IL-10–secreting B regulatory (Br1) cells essentially contribute to allergen tolerance (ref. 33 and Figure 1). Phospholipase A2–specific Br1 cells from nonallergic beekeepers and those that are induced after AIT showed increased expression of IL-10 with an antigen-specific suppressor capacity. A major finding of this study was that IgG4 production is specifically confined to Br1 cells. IgG4 represents a noninflammatory Ig isotype that does not activate complement and plays an IgE-blocking antibody role for the degranulation of mast cells and basophils (33).
Conclusions
Specific Tr1 cells and immune tolerance development are essential for the induction and maintenance of healthy immune responses to allergens. The relationship between clinical allergen tolerance and immune tolerance has been observed by direct analysis of the affected tissues and skin during late-phase responses in humans who have undergone AIT with whole allergen and T cell epitope peptides as well as in individuals who have been exposed to naturally high doses of allergens. As of today, the concept that Tr1 cells mediate antigen-specific T cell tolerance has been demonstrated and generally accepted after the publication of several thousand studies on immune regulation.
对过敏原的免疫耐受机制:IL-10和Treg的作用
Cezmi A.Akdis1,2和MübeccelAkdis1 1苏黎世大学瑞士过敏和哮喘研究所(SIAF),瑞士达沃斯。 2克里斯汀·库恩(ChristineKühne)–瑞士达沃斯过敏研究与教育中心(CK-CARE)。
在过去的20年中,在理解人类过敏原耐受性的分子和细胞机制方面取得了重大进展。 T细胞耐受性的证明,特别是由IL-10的免疫抑制功能介导的耐受性,导致了这一领域的重大观念变化。目前,已知的变应原耐受性的必要组成部分包括诱导T细胞和B细胞的变应原特异性调节子集,分泌因子(例如IL-10和TGF-β)的免疫抑制功能,IgG4同种型变应原–特异性阻断抗体,并减少发炎组织中肥大细胞,嗜碱性粒细胞和嗜酸性粒细胞的过敏性炎症反应。
T细胞和过敏反应
对任何免疫激活物质都可能产生免疫耐受,并且多种机制介导了这一过程。免疫耐受的失调可能导致过敏,哮喘,肿瘤,慢性感染,移植器官排斥,移植物抗宿主病和许多自身免疫病的发展。过敏性疾病的特征在于诱导包括Th2细胞和2型先天性淋巴样细胞(ILC2s)的2型免疫反应,以及在受影响的组织中以及有时在外周血中产生过敏原特异性IgE抗体和增加嗜酸性粒细胞数量(1)。尽管治疗过敏性疾病相关症状的方法有很多,但是,目前唯一的长期治疗方法是过敏原特异性免疫疗法(AIT),其中涉及增加剂量的致病性过敏原。随着时间的流逝,AIT诱导了过敏原特异性免疫耐受的状态。 AIT已经执行了100多年,尽管只是在过去的二十年中才慢慢发现了调解AIT行为的机制(2)。在1986年发现Th1和Th2细胞后(3),有人认为Th2反应是过敏性疾病发展的基础,而Th1反应在感染和自身免疫中占主导地位。根据这些最初的发现,一般的教条是,要通过AIT成功治疗过敏,就需要转向Th1反应,而转向Th2反应对自身免疫治疗是有益的。
JCI - Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs
https://www.jci.org/articles/view/78891
Recent Pat Inflamm Allergy Drug Discov
. 2012 May
Phytochemicals to prevent inflammation and allergy
Yuva Bellik 1, Si M Hammoudi, Fatiha Abdellah, Mokrane Iguer-Ouada, Laïd Boukraâ
Recently, much interest has been generated for a wide range of phyto-constituents with reports demonstrating their role in the modulation of inflammatory responses, including phenolics, alkaloids, and terpenoids. Natural products have long been, over the years, contributed to the development of modern therapeutic drugs. At present, steroids, antihistaminic drugs, suppressants or inhibitors of the release of mediators and the like have been used as anti-allergic agents. However, some of them lack immediate effectiveness or have central side effects. Drug discovery from plants involves a multidisciplinary approach combining botanical, ethno-botanical, phytochemical and biological techniques. Several natural product drugs of plant origin are in clinical use and some are undergoing Phase II and Phase III clinical trials. A major effort was directed toward discovery of novel anti-inflammatory and anti-allergic agents, which resulted in the invention of several patented formulations. These formulations concern a variety of pharmaceutical preparations which can be used as solid or liquid dosage forms or encapsulated as a soft or hard gelatin capsule. The present article is a short review of recent patents on the role of phytochemicals in preventing inflammation and allergy.Phytochemicals to prevent inflammation and allergy - PubMed
https://pubmed.ncbi.nlm.nih.gov/22352960/