﹛
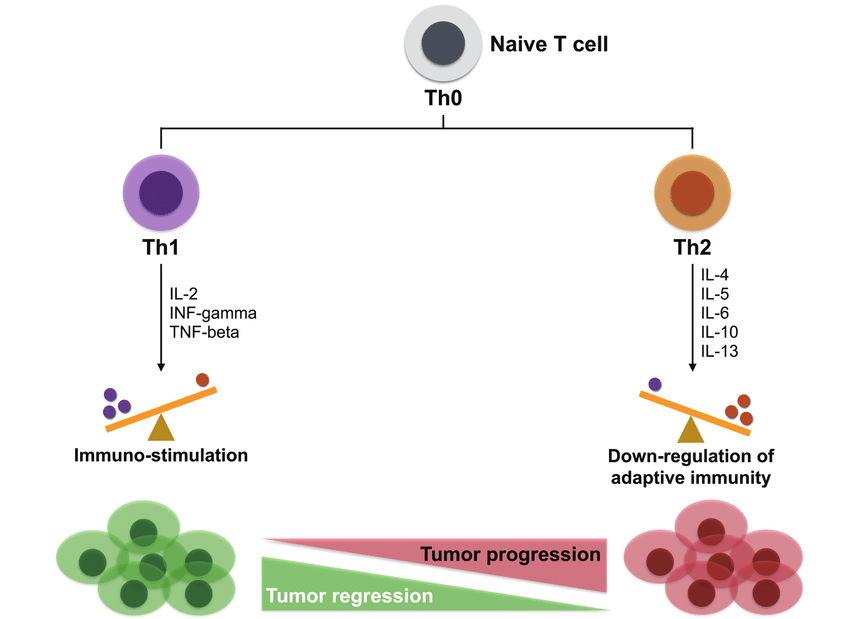
Th1-Th2-Th17 Responses
﹛
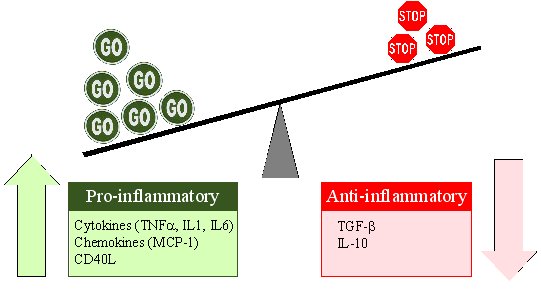
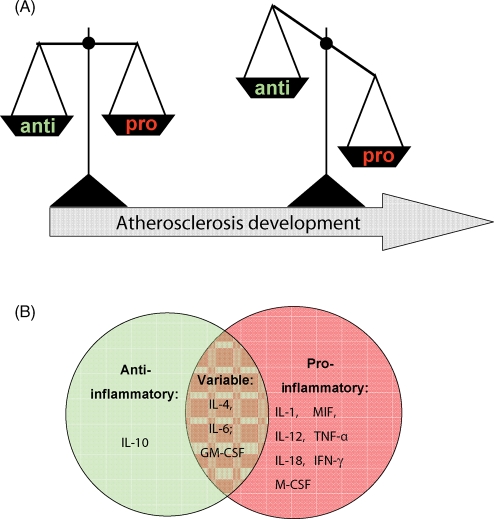
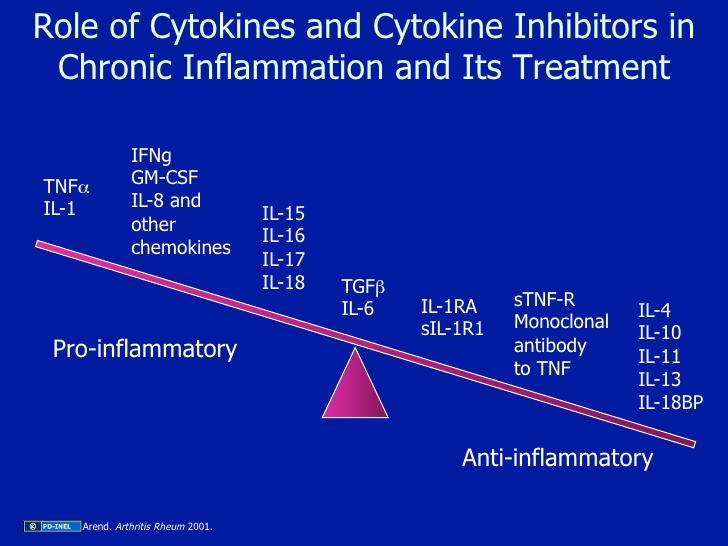
The yin-yang of cytokines. The balance between pro-inflammatory cytokines and anti-inflammatory cytokines is critical in determining outcome. Chemokines have preferred partners that link cell trafficking to function, as indicated. Angiogenesis, tissue replacement (fibrosis) and regeneration predominantly fall within the influence of the more anti-inflammatory axis.
﹛
﹛
Dendritic cell interaction with T-lymphocytes | Download Scientific Diagram
https://www.researchgate.net/figure/Dendritic-cell-interaction-with-T-lymphocytes_fig4_257837657﹛
﹛
﹛
﹛
https://www.researchgate.net/figure/Pro-and-Anti-inflammatory-cytokines-balance-IgA_fig1_305405169
https://www.selfhacked.com/blog/il-10/
Higher IL-10 was associated with more white matter volume in visual areas and tracts [12].
https://www.selfhacked.com/blog/il-10/
﹛
﹛
https://www.sinobiological.com/Proinflammatory-cytokines-list.html
﹛
裘翌棒呫 18汕-Glycyrrhetinic Acid Triggers Curative Th1 Response and Nitric Oxide Up-Regulation
File:Th1-Th2-Th17-Treg origin.png - Wikipedia
https://en.wikipedia.org/wiki/File:Th1-Th2-Th17-Treg_origin.pngA growing body of evidence supports the host protective role of IL-12-induced differentiation of Th1 cells that produce IFN-污, which activates macrophages to produce NO (38).
Transcript levels of IL-10 and IL-4 were reduced in mice given GRA therapy, whereas those for IL-12 p40 and IFN-污 were significantly elevated. GRA also increased the levels of TNF-汐, another inflammatory cytokine with well-defined antileishmanial effects that is known to act either alone or with IFN-污 to induce the production of reactive nitrogen and oxygen intermediates (39, 40)
transcriptional effects of GRA on genes regulating the expression of iNOS and Th1 cytokines might result in part from increased translocation of NF-百B into the nucleus of activated cells.kinase activity of IKK was stimulated in cells that were activated in the presence of GRA through a mechanism that most likely involves upstream signaling pathwaysㄛlikely NF-百B-inducing kinase, PI3K, or MAPK (48).
裘翌棒呫 18汕-Glycyrrhetinic Acid Triggers Curative Th1 Response and Nitric Oxide Up-Regulation in Experimental Visceral Leishmaniasis Associated with the Activation of NF-百B | The Journal of Immunology
https://www.jimmunol.org/content/175/2/1161﹛
﹛
﹛
Inflammation and Cancer
https://oncohemakey.com/inflammation-and-cancer/
﹛
Cell-Mediated Immunity
Cell-mediated immunity is the result of cooperation between innate and adaptive immunity to
destroy virus infected cells before they can produce more virus
damage pathogenic bacteria, fungi, and parasites
eliminiate cancerous cells that lack normal cell surface proteins
Cells involved in immunity can be divided into
primary effector cells such as
granulocytes
macrophages
CD8+ T-cells
natural killer (NK) cells
secondary support cells such as
CD4+ helper T-cells
dendritic cells
Cell-mediated immunity can result from
innate immune response
activation of T-cells
coordination of cells via cytokines
Important Cytokines in Cellular Immunity
Cytokines are key coordinators of the cellular immune response by promoting
vascular changes near the site of inflammation
recruiting of other target cells
differentiation of target cells
signaling to distant organs
Cytokines can be secreted by a variety of cells including
activated macrophages
helper T-cells
killer T-cells
Effector Mechanisms
Macrophage and neutrophil killing depends upon
oxygen-dependent mechanisms such as respiratory burst
oxygen-independent mechanisms including
hydrolytic enzymes that destroy peptides
defensins that form holes in bacterial membranes
lactoferrin that binds iron and denies it to bacteria
lysozyme that cleaves bacterial peptidoglycan walls
NK and cytotoxic CD8+ T-cell killing depends upon three mechanisms including
exocytosis of cytotoxic granules containing
granzymes that are apoptosis activating serine proteases
perforin that makes a hole in membranes
Fas ligand that directly signals target cells to undergo apoptosis
cytokine signaling mainly through TNF pathways that also induce apoptosis
Notably NK cells are inhibited by MHC complexes on the surface of cellsCell-Mediated Immunity - Immunology - Medbullets Step 1
https://step1.medbullets.com/immunology/105050/cell-mediated-immunity﹛
Potential mechanism behind the hygiene hypothesis
Potential mechanism behind the hygiene hypothesis - Thryve - Medium
https://medium.com/@thryve/potential-mechanism-behind-the-hygiene-hypothesis-b26ab5d212c5﹛
Radiation Research 178(6) ﹞ October 2012
Cytokinnes in Radiobiological Response: An Review
University of California, Los Angeles
Dörthe Schauem Evelyn L Kachikwu, William H Mcbride
Cytokines function in many roles that are highly relevant to radiation research.
This review focuses on how cytokines are structurally organized, how they are induced by radiation, and how they orchestrate mesenchymal, epithelial and immune cell interactions in irradiated tissues.
Pro-inflammatory cytokines are the major components of immediate early gene programs and as such can be rapidly activated after tissue irradiation. They converge with the effects of ionizing radiation in that both generate free radicals including reactive oxygen and nitrogen species (ROS/RNS). "Self" molecules secreted or released from cells after irradiation feed the same paradigm by signaling for ROS and cytokine production.
As a result, multilayered feedback control circuits can be generated that perpetuate the radiation tissue damage response. The pro-inflammatory phase persists until such times as perceived challenges to host integrity are eliminated.
Antioxidant, anti-inflammatory cytokines then act to restore homeostasis. The balance between pro-inflammatory and anti-inflammatory forces may shift to and fro for a long time after radiation exposure, creating waves as the host tries to deal with persisting pathogenesis.
Individual cytokines function within socially interconnected groups to direct these integrated cellular responses. They hunt in packs and form complex cytokine networks that are nested within each other so as to form mutually reinforcing or antagonistic forces. This yin-yang balance appears to have redox as a fulcrum.
Because of their social organization, cytokines appear to have a considerable degree of redundancy and it follows that an elevated level of a specific cytokine in a disease situation or after irradiation does not necessarily implicate it causally in pathogenesis.
In spite of this, "driver" cytokines are emerging in pathogenic situations that can clearly be targeted for therapeutic benefit, including in radiation settings. Cytokines can greatly affect intrinsic cellular radiosensitivity, the incidence and type of radiation tissue complications, bystander effects, genomic instability and cancer. Minor and not so minor, polymorphisms in cytokine genes give considerable diversity within populations and are relevant to causation of disease. Therapeutic intervention is made difficult by such complexity; but the potential prize is great.
﹛
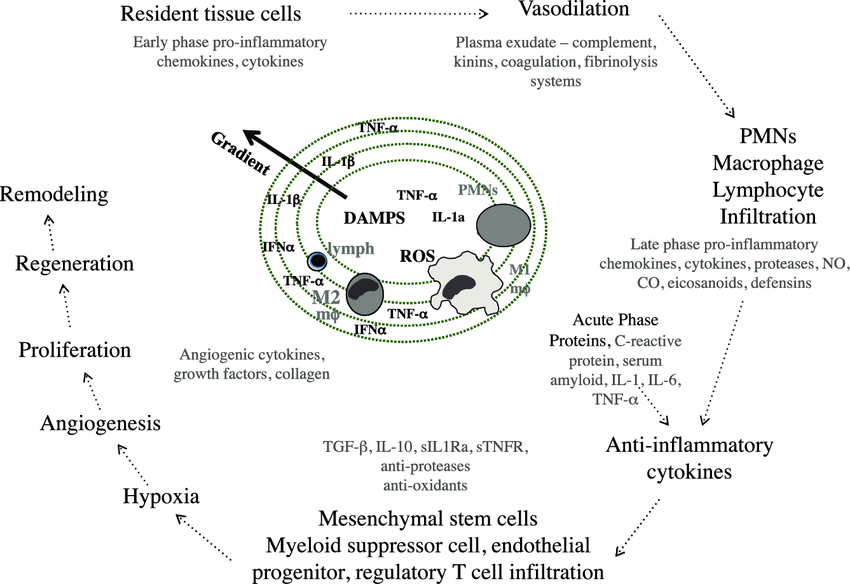
The yin-yang of cytokines. The balance between pro-inflammatory cytokines and anti-inflammatory cytokines is critical in determining outcome. Chemokines have preferred partners that link cell trafficking to function, as indicated. Angiogenesis, tissue replacement (fibrosis) and regeneration predominantly fall within the influence of the more anti-inflammatory axis.
﹛
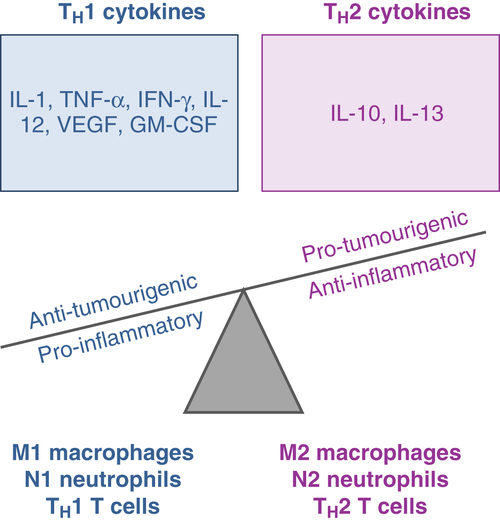
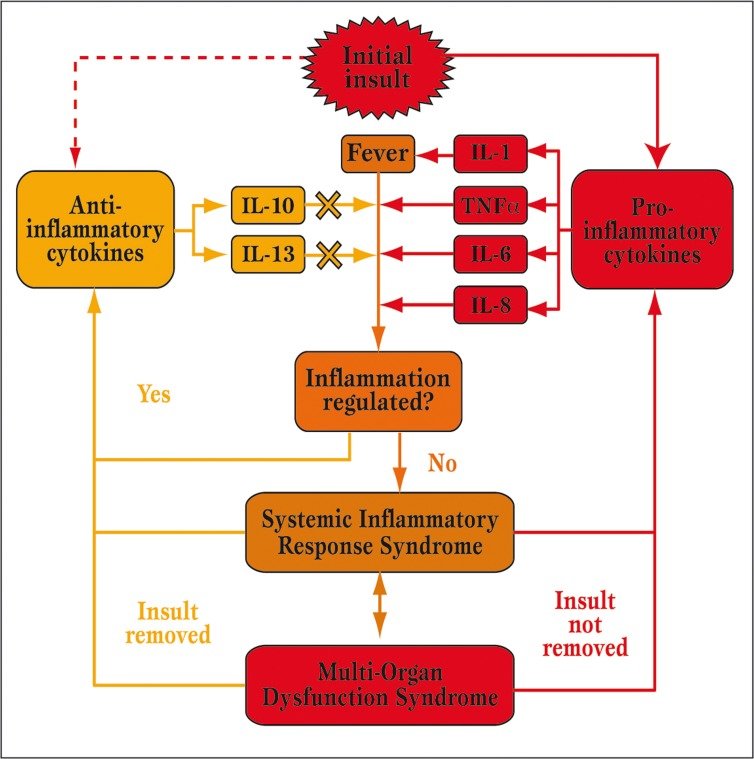
Cytokines drive the formation of inflammatory lesions working together with DAMPS to generate a pro-inflammatory, pro-oxidant microenvironment. The vasculature becomes leaky, allowing infiltration by neutrophils, and then macrophages and lymphocytes that migrate along chemokine gradients. Acute phase proteins, including cytokines, are generated along with a fair measure of cell death. In the periphery, cells may become more resistant to death and infection. Hypoxia may occur and in time the lesion resolves under the influence of anti-inflammatory cytokines and cells. Macrophages develop an M2 rather than an M1 phenotype. Angiogenesis and/or vasculogenesis assists either tissue regeneration or replacement with extracellular materials (fibrosis).
﹛
﹛
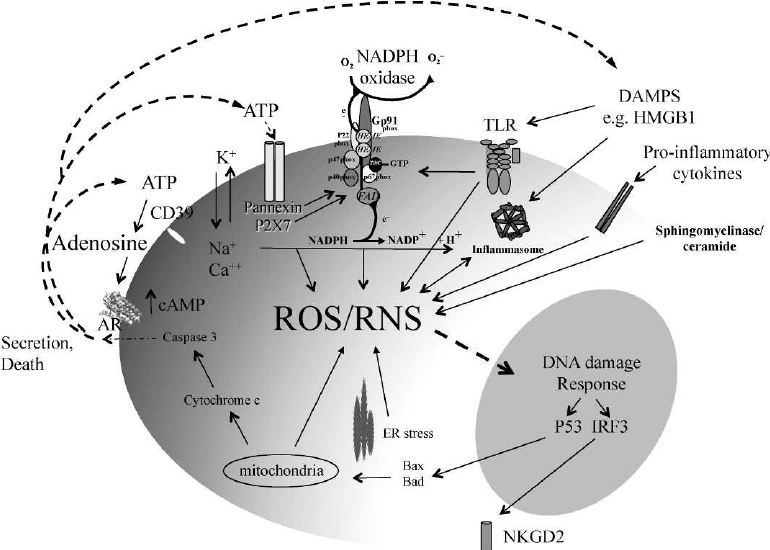
ROS can be generated from many sources following irradiation. Released nucleotides including ATP can activate P2X purinergic receptors to open the cation pore and trigger calcium-dependent intracellular processes. This is required for activation of NADPH oxidases that can also be activated by TLR signaling to generate superoxide. Radiation damage to mitochondria is another potential source of ROS. Further DAMP and pro-inflammatory cytokines signaling, the DNA damage response through Bax, and the formation of inflammasomes can all perpetuate ROS generation by forming positive feedback circuits. Adenosine can be generated from nucleotides by ectonucleotidases such as CD39 to signal through the adenosine receptors (AR) to negatively regulate inflammation, as does the production of anti-inflammatory cytokines.
﹛
(PDF) Cytokines in Radiobiological Responses: A Review
https://www.researchgate.net/publication/232736146_Cytokines_in_Radiobiological_Responses_A_Review﹛
﹛
﹛
How to Increase IL-10 Anti-Inflammatory Cytokine
Written by Joe Cohen, BS | Last updated: March 3, 2020
The Good
We usually think of cytokines as ※bad§ and inflammatory. IL-10 is an important exception.
IL-10 is anti-inflammatory cytokine because it decreases various immune cells such as Th1 AND Th2 cells [2, 1], neutrophils [5], macrophages and natural killer cells [6]. The last three are the guns of the immune system.
It also decreases a host of cytokines (IFNy, IL-2, TNF, and GM-CSF) and other alarm bells of the immune system (MHCII) [7].
It inhibits Nf-kB [6], the master control of inflammation (in two ways: by suppressing IKK activity and NF-百B DNA binding [8]).
It inhibits COX-2 [9], which is involved in migraines, pain, and inflammation. COX-2 is classically blocked by NSAIDs such as aspirin and ibuprofen.
By inhibiting mast cells, it counteracts the inflammatory effect that these cells have at the site of an allergic reaction [10].
IL-10 decreases obesity by reducing overeating and decreasing insulin and leptin resistance in the hypothalamus, the gland that controls appetite (by inhibiting cytokines, Nf-kB, and ER stress) [11].How to Increase IL-10 Anti-Inflammatory Cytokine - SelfHack
https://selfhack.com/blog/il-10/﹛
﹛
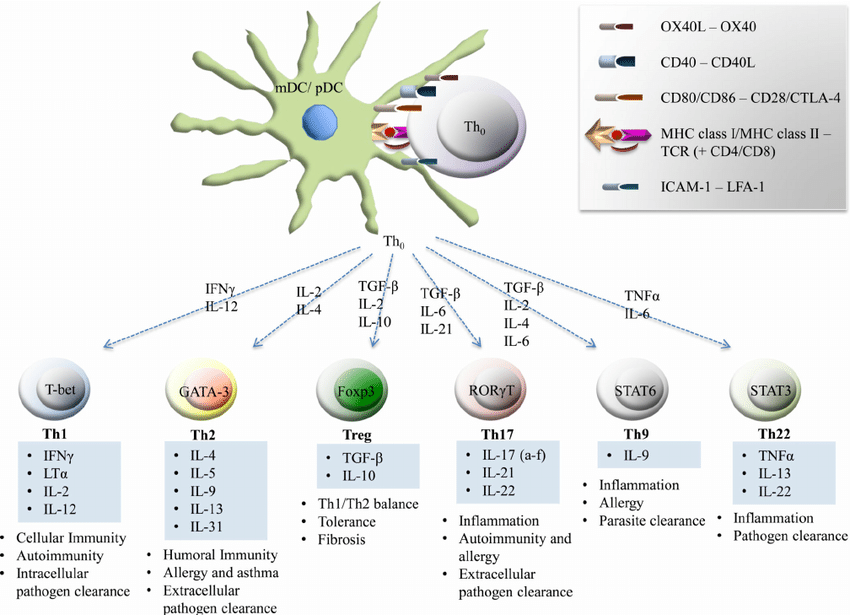
Cooperation between Innate and Adaptive Immune Response
Schematic representation of immune response against pathogens based on the cooperation between innate and adaptive immunity components.
Immunity is a state of sufficient biological defenses to avoid infection, disease or other unwanted biological invasion. It is divided in a non-specific (innate) and specific (adaptive) components. The term innate immunity is due to its constant presency in healthy individuals and generally refers to responses that do not require previous exposures to the pathogen, whereas adaptive immunity develops more slowly and is specific to a particular pathogen and involves immunological memory. The adaptive immunity consists of humoral immunity in which antibodies neutralize and eradicate extracellular microbes and toxins and cell-mediated immunity in which T-lymphocytes play a key role. The most important is the cooperation between innate and adaptive immunity.
Until recently innate immunity was largely ignored as the adaptive immunity was thought to play a key role in the immune response. Now it became clear that innate immunity has an important role as the first line of defense against pathogens as well as in development of an adaptive immune response. The polarization of immune response largely depends on the initial signals that are brought by innate immunity. There are some essential signals, required to induce T- and B-cell responses. Innate immune signals modulate the quantity and the quality of adaptive immune response and are required to initiate an effective immune response, that mainly depends on the nature of the pathogen. There are two separated ways of antigen processing, which enable the immune system to appropriately respond against extracellular and intracellular pathogens, respectively.
Briefly, we will focus on the immune response against extracellular pathogens. As the pathogen invades the host, its pathogen associated molecular pattern (PAMP) is being recognised by the pathogen recognition receptor (PRR) on APC, for instance dendritic cell (signal 1 in immune activation). Pathogen recognition and APC activation is the first signal needed for activation of immune response. After internalisation (phagocytosis) antigens are processed into peptides. These antigen-derived peptides are presented by major histocompatibility complex MHC class 2 (MHC II) molecules on APC surface for recognition by CD4+ T lymphocytes that have T-cell receptor specific for the same peptides. TCR recognition of specific peptide represents signal 2 in immune activation. The last step - signal 3 in immune activation is co-stimulation, which depends on the interactions between costimulatory molecules, such as B7 and CD40 on APC and CD-28 and CD40L on CD4+ T. All this is happening at the first stages of immune response and leads to the expression of appropriate immune mediators, such as cytokines, that enable the polarization of immune response in a direction, that is potentially optimal for pathogen eradication.
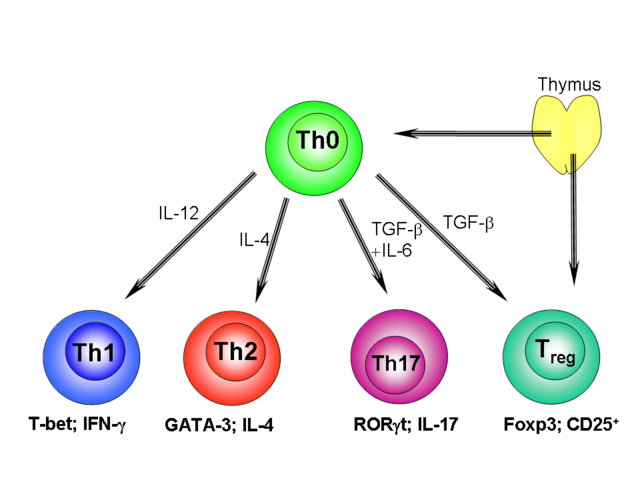
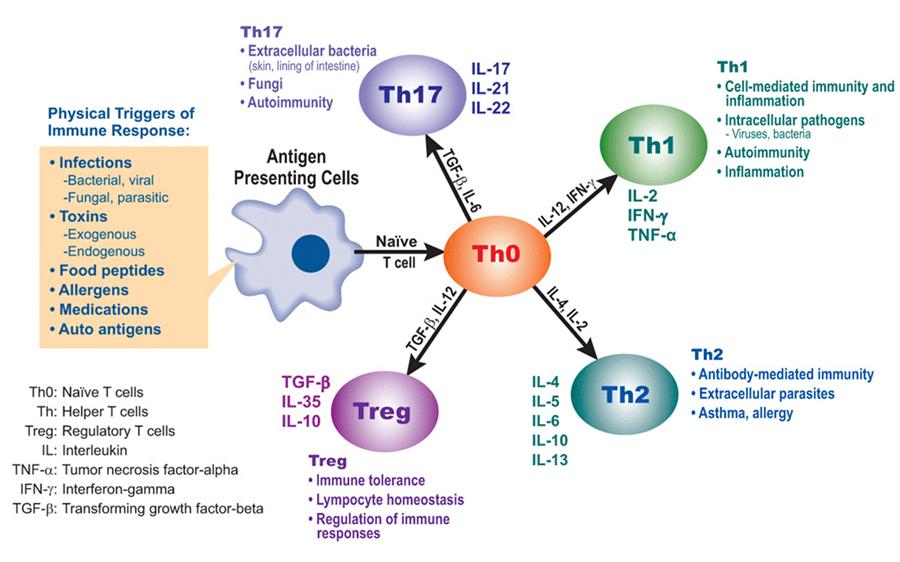
For example, when IL-12 is absent or set of interleukins like IL-4 and IL-10 are present, T-helper 2 (Th2) cell polarization occurs. Th2 cells produce interleukin IL-4, IL-5, IL-6, IL-10, IL-13 and participate in phagocytosis-independent responses. They provide B-cell help and the induction of IgG1, IgE and IgA, which all provide the defense against extracellular pathogens. For instance, IL-4, that is produced by Th2 cells, promotes IgE heavy chain class switching. These antibodies are effective against helminths, since IgE has Fc, which is recognized by Fc-缶RI on eosinophills, that finally eradicate the parasitic infection.
So it is obvious, that interactions between Th and B-cells is of a great importance. B-cells are a special type of APC's that are a part of adaptive immune response. In contrast to macrophages and dendritic cells, which have extracellulary exposed PAMP's, B-cells retain specific B cell receptors (BCR) that are basically membrane-bound immunoglobulins, which bind a particular antigen. After B cell encounters and recognizes a fitting (cognate) native antigen with its B-cell receptor, internalization, antigen processing and presentation of antigen derived peptides in MHC II molecules occurs (for B cell is APC). This leads to the interaction with previously activated Th cell that carries T-cell receptor, specific for the same antigenic component, that is presented in MHC II molecules of B cell. Only after this specific interaction B-cell receives an additional signals from a T-helper cell which leads to a clonal expansion and differentiation of a B-cell that carries an appropriate BCR. The net result is a production of antibodies of the same specifity and later on, also affinity maturation and heavy chain class switching, which all is necessary for effective humoral response. The final stage is an establishment of a memory B-cell repertoire, that quickly responds to a subsequent exposition to the cognate antigen.
In contrary, defense against intracellular pathogens is carried out by different strategy since humoral response is not effective against intracellular patogens, such as viruses and intracellular bacteria. In that case, cytosolic antigens are presented within MHC class 1 (MHC I) molecules, which can be found on all nucleated cells, for all these cells could be infected by viruses. Antigenic peptides, presented in MHC I molecules, are recognised by CD8+ T cells, which then, with help of Th cells differentiate into CTL. These kill infected cells and eradicate infection.
Very simplified, we can say, that the result (polarization) of immune response depends on which player of innate branch of immune response (e.g., dendritic cell) recognized invading pathogen, how the peptides derived from antigenic components were presented (MHC I or MHC II molecules) and which immunomodulatory molecules were produced (e.g. IL-12). Therefore, cooperation between initial innate and subsequent adaptive response is needed for effective functioning of the immune system and immune response.Team:Slovenia/Background/Immune response - 2008.igem.org
http://2008.igem.org/Team:Slovenia/Background/Immune_response﹛
About Hydroxytyrosol | Olive Wellness Institute
https://olivewellnessinstitute.org/article/about-hydroxytyrosol
Hydroxytyrosol has a potent antioxidant activity 每 it has one of the highest know ORAC (oxygen radical absorbance capacity) results known for a natural antioxidant. (ORAC is a method used to determine the antioxidant capacity of a food or chemical substance). 2
Other articles from olivewellnessinstitute.org
Hydroxytyrosol: The Phytochemical Responsibl#
Hydroxytyrosol, Tyrosol and Derivatives and Th#
Hydroxytyrosol Modulates Adipocyte Gene an#
Hydroxytyrosol - an overview | ScienceDirect Topics
https://www.sciencedirect.com/topics/neuroscience/hydroxytyrosol
Hydroxytyrosol is a metabolite of oleuropein with antioxidant effects. Often olive extracts are standardized for hydroxytyrosol, a compound devoid of antihypertensive effect. Benolea (EFLA943), standardized to oleuropein (16% to 24%) and polyphenols, given at 1000 mg/day (500 mg b.i.d.), reduced systolic BP by 11.5 mm Hg and diastolic by 4.8 mm ...﹛
Th2 and Th1 Responses: Clear and Hidden Sides of Immunity Against Intestinal Helminths
Alba Cort谷s
Carla Muñoz-Antoli
J. Guillermo Esteban
Rafael Toledo
Trends
Th2 responses are associated with protection against intestinal helminths through the activation of several effector mechanisms at the host每parasite interface.
Novel functions for epithelial cells and mucosal innate immune cells have been shown to be crucial for initiating and regulating type 2 immunity.
The generation of Th1 responses associated with susceptibility to infection is underestimated, though mechanisms leading to Th1 activation are key for a better understanding of the immune regulation of these infections.
Cross-talk between innate and adaptive components of the immune system is critical in the polarization of the response and differ among helminth每mouse models.
An understanding of the early mechanisms determining the development of protective type 2 immunity or Th1 responses, associated with chronicity, is key for the development of control tools against intestinal helminths.
Intestinal helminthiases affect millions of people worldwide, mainly in developing regions, where they cause a significant negative impact on human health and socioeconomic growth of affected populations. However, intestinal helminthiases are still among the most neglected tropical diseases. Protective immunity against intestinal helminths is associated with development of type 2 responses. Nevertheless, in some host每intestinal helminth combinations, local Th1 responses are initiated, inducing chronicity. The usage of helminth每mouse models is useful for elucidating the mechanisms behind the initiation of each type of response. Herein, the current knowledge on these topics is reviewed, paying particular attention to the earliest events of the immune cascade which eventually lead to either susceptibility or resistance to infection.
Th2 and Th1 Responses: Clear and Hidden Sides of Immunity Against Intestinal Helminths: Trends in Parasitology
https://www.cell.com/trends/parasitology/fulltext/S1471-4922(17)30127-7﹛
Article
Published: 17 March 2010
Human primary gastric dendritic cells induce a Th1 response to H. pylori
D Bimczok, R H Clements, K B Waites, L Novak, D E Eckhoff, P J Mannon, P D Smith & L E Smythies
Mucosal Immunology volume 3, pages260每269(2010)Cite this articleDepartment of Medicine (Gastroenterology), University of Alabama at Birmingham, Birmingham, Alabama, USA
Abstract
Adaptive CD4 T-cell responses are important in the pathogenesis of chronic Helicobacter pylori gastritis. However, the gastric antigen-presenting cells that induce these responses have not yet been identified. Here we show that dendritic cells (DCs) are present in the gastric mucosa of healthy subjects and are more prevalent and more activated in the gastric mucosa of H. pylori-infected subjects. H. pylori induced gastric DCs isolated from noninfected subjects to express increased levels of CD11c, CD86 and CD83, and to secrete proinflammatory cytokines, particularly interleukin (IL)-6 and IL-8. Importantly, gastric DCs pulsed with live H. pylori, but not control DCs, mediated T-cell secretion of interferon-污. The ability of H. pylori to induce gastric DC maturation and stimulate gastric DC activation of Th1 cells implicates gastric DCs as initiators of the immune response to H. pylori.Human primary gastric dendritic cells induce a Th1 response to H. pylori | Mucosal Immunology
https://www.nature.com/articles/mi201010﹛
﹛

.png)