¡¡
https://www.sinobiological.com/Th17-cytokines.html
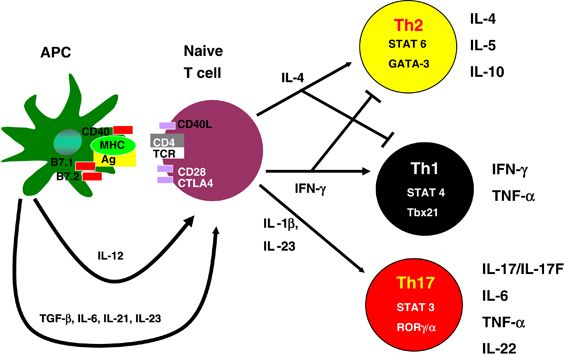
H pylori alters the DC-polarized Th17/Treg balance toward a Treg-biased response, which suppresses the effective induction of H pylori-specific Th17 immunity.
Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/19931266
https://www.immunology.org/public-information/bitesized-immunology/cells/th17-cells
¡¡
¡¡
Th17 Cells Require You to Chew before You Swallow
Marc VeldhoenInstituto de Medicina Molecular, Faculdade de Medicina da Universidade de Lisboa, Av. Professor Egas Moniz, Lisbon 1649-028, Portugal
Detailed knowledge of CD4+ T helper (Th) cell biology has been gained over the last decades. Initially split in two subsets, Th1 cells and Th2 cells, the discovery of Th17 cells did not contribute just a third effector arm of the CD4+ lineage. Th17 cells are rare in secondary lymphoid organs but enriched at epithelial barriers. There, they can be generated under the influence of microorganisms. In this issue of Immunity, Dutzan et al. (2017) report an important role for mechanical-damage-induced factors in specifically maintaining a Th17 cell population at the oral mucosa.
Epithelia are prime locations at risk to microbial invasion, occupied by a variety of microorganisms that make contributions to the health of the tissue as well as the host. Immune networks at epithelia balance tolerance, keep potential pathogens at bay, enable moderate immunity when minor breaches occur, and initiate comprehensive responses when encountering danger. Due to the large area it constitutes and the varied composition of its microorganisms, the intestine has attracted the most scrutiny and has been used to study and define these immune networks. Subsequently, attention was focused on the skin and lungs, with the realization that, similar to the intestine, these tissues are under the influence of micro-organisms as well. It became apparent that not just the microbial players differ between barrier organs, but that immune networks and cellular compositions vary as well. For example, the mouse skin and small intestine contain a specialized population of lymphocytes, the intraepithelial lymphocytes, that is not present in the lung or the colon and are not able to secrete IL-17 (Li et al., 2011).
Immune networks are tuned to tissue-specific needs. IL-17-producing lymphocytes present at all barrier sites are critical for protection against extracellular bacteria and fungi. They consist of those expressing a T cell receptor (TCR) formed with ¦Á and ¦Â chains (Th17 cells), such as in the lamia propria of the intestine, or ¦Ã¦Ä chains such as are prevalent in lungs and dermis. The role of IL-17-producing lymphocytes encompasses regulating microbial occupancy via co-production of IL-17 and IL-22, synergistically increasing the production of antimicrobial factors (Kolls et al., 2008). The activity of Th17 cells is, however, not restricted to making IL-17 and IL-22, and they show a large degree of plasticity (Hirota et al., 2011, Hirota et al., 2013). Th17 cells are hence an important component of the immune network at epithelia, maintaining epithelial integrity, managing microbial occupation, and responding to microbial threats robustly and with flexibility.
Th17 cell differentiation and maintenance has been closely associated with components of the microbiota such as, but not exclusively, segmented filamentous bacteria (SFB) (Berer et al., 2011, Ivanov et al., 2008). Under the influence of micro-organisms, the cytokines IL-6, IL-1¦Â, tumor necrosis factor (TNF), transforming growth factor (TGF)-¦Â, and IL-23 are produced with direct effects on the generation, function, and maintenance of Th17 cells. It is clear that SFB are not present outside the small intestine, raising the question of how IL-17-mediated immunity is regulated at other tissues.
The oral cavity is known to contain a substantial population of IL-17 cells, especially offering protecting against fungi (Conti et al., 2014). Loss of the ability to make or respond to IL-17 results in mucocutaneous candidiasis on the skin, vagina, and mouth. The cellular source of IL-17 in the oral cavity was shown to consist of at least tongue-resident populations of ¦Ã¦Ä T cells as well as Th17 cells, with redundancy between these populations. The response of Th17 cells upon candida infection is swift and can be mobilized within a few hours to days (Conti et al., 2014, Hirota et al., 2011), suggesting that Th17 cell responses may be derived from a preexisting ¡°natural¡± Th17 cell population derived from the thymus (Marks et al., 2009). The Th17 and ¦Ã¦Ä T cells found in the tongue did, however, adhere to similar cues as those in the small intestine and completely depended on the presence of a microbiota for their presence.
Dutzan et al. (2017) investigated Th17 cells present in the gingiva, the soft tissue surrounding the teeth. The gingiva supports dentition and harbors a complex microbiome. Periodontitis is a common disease in humans that can result in loss of teeth and is linked with cardiovascular disease and rheumatoid arthritis (RA), yet there is limited understanding of the immune network present in the gingiva. Besides a protective role, uncontrolled Th17 cell responses at the gingiva can be detrimental and, like in RA, promotes bone loss. Hence a better understanding of Th17 cell accumulation and maintenance at this site is important. In this study by Dutzan et al. (2017), Th17 cells, in contrast to Th1 and regulatory T cells, were found to accumulate in the gingiva with age, from 8 to 24 weeks in mice and 18¨C25 to 40¨C50 years in humans. This accumulation was specific to the gingiva and was not observed at the skin, intestine, spleen, or even oral-draining lymph node. This enrichment of Th17 cells was linked with increased proliferation (Dutzan et al., 2017).
Dutzan et al. (2017) next investigated the reason for the accumulation of Th17 cells in the gingiva. They did not observe significant differences in load, diversity, or composition of the microbiota in mice at 8 versus 24 weeks of age. SFB were not found in the oral cavity. Furthermore, presence or absence of intestinal SFB had no effect on Th17 cell accumulation in the gingiva. Gingiva Th17 cells accumulated in mice in the absence of dectin-1 and mannose receptor signaling, thereby excluding a role for fungi. TCR transgenic mice did not show an increase in Th17 cells in the gingiva, suggesting that Th17 cell accumulation was antigen specific. Interestingly, in marked contrast to the intestine, skin, or tongue, the maintenance of gingival Th17 cells was independent of the microbiota, as germ-free mice showed a similar accumulation of Th17 cells with age.
The authors went on to look for the signals driving Th17 cell accumulation in the gingiva. They ruled out a role for IL-1¦Á, IL-1¦Â, IL-12, and IL-23 by using genetically modified animals. However, they found that IL-6 was essential for the accumulation of Th17 cells in the gingiva and it acted intrinsically on Th17 cells. This result was surprising since IL-6 production was linked to microbial stimulation. Since the authors had found that the microbiota were dispensable for the accumulation, they looked for the source of IL-6 in the gingiva. Surprisingly, they found that IL-6, but not IL-1¦Â, was derived from the epithelium due to tissue damage or mechanical pressure alone, occurring physiologically in the mouth through abrasion and by chewing, thereby enabling the accumulation of Th17 cells. In line with this hypothesis, mice harbored increasing numbers of Th17 cells in the gingiva based on the level of abrasion experienced. Mice placed on soft diet had fewer Th17 cells than those on standard chow, which had fewer Th17 cells than those receiving additional rubbing of the gingiva (Figure 1). Although mechanical pressure may result in expression of chemoattractants, Th17 cells were not selectively recruited to the gingiva. Instead, the presence of IL-6 correlated with increased local proliferation of Th17 cells. This highlights that the cues required to accumulate Th17 cells at barrier sites involve IL-6 but that the induction of this cytokine can be achieved in more ways than via microbial stimulation.
Figure thumbnail gr1
Figure 1Chewing and Tissue Damage Results in the Accumulation of Th17 Cells in the Gingiva
Show full caption
View Large Image Figure ViewerDownload Hi-res image Download (PPT)
It remains to be investigated whether damage or mechanical sensing in the gingiva constitute a tissue-specific cue. The involvement of IL-6 would suggest this could be a general mechanism of relevance at other sites, albeit that the mastication provides continues signal in the gingiva. In line with this, Dutzan et al. (2017) showed that damage induced in the skin also resulted in the accumulation of Th17 cells, suggesting that microbial induction as well as the tissue-damage response can enhance Th17 cell numbers.
IL-17 stimulation of many cell types, such as fibroblasts, macrophages, chondrocytes, and osteocytes, often in synergy with other cytokines, results in the production of IL-6, IL-1¦Â, and TNF, as well as chemokines such as CCL20. This positive-feedback loop would strengthen the accumulation of Th17 cells and recruitment of granulocytes. The results by Dutzan et al. (2017) emphasize the importance of IL-6 in maintaining Th17 cells. The mechanism may find additional relevance in autoimmune disorders such as RA, where continuous bone destruction by Th17 cells is a major symptom of disease, but which may also be directly involved, via the damage-induced secretion of IL-6, in sustaining disease.
The work by Dutzan et al. (2017) shows that tissues have the ability to maintain numbers of Th17 cells via different mechanisms. In the case of the gingiva, the maintenance of a resident population of Th17 cells depends on the how well you chew your food.¡¡
Th17 Cells Require You to Chew before You Swallow: Immunity
https://www.cell.com/immunity/fulltext/S1074-7613(16)30522-2¡¡

.png)