Theranostics. 2016; 6(11): 1887¨C1898.
Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes
Bingdi Chen,1,* Wenjun Le,1,* Yilong Wang,1 Zhuoquan Li,1 Dong Wang,2 Lei Ren,2 Ling Lin,3 Shaobin Cui,1 Jennifer J. Hu,4 Yihui Hu,1 Pengyuan Yang,3 Rodney C. Ewing,5 Donglu Shi,1,6,✉ and Zheng Cui1,7,✉
Author information Article notes Copyright and License information Disclaimer
1The Institute for Translational Nanomedicine, Shanghai East Hospital, The Institute for Biomedical Engineering & Nano Science, Tongji University School of Medicine, Shanghai, 200120, China;
2Department of Biomaterials, College of Materials, Xiamen University, Xiamen 361005, China;
3Institutes of Biomedical Sciences, Fudan University, Shanghai, 200032, China;
4Public Health Sciences, University of Miami School of Medicine, Miami, FL 33136, USA;
5Department of Geological Sciences, School of Earth, Energy & Environmental Sciences, Stanford University, Stanford, CA 94305-2115, USA;
6Materials Science and Engineering Program, Department of Mechanical and Materials Engineering, College of Engineering and Applied Science, University of Cincinnati, Cincinnati, Ohio, USA;
7Department of Pathology, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Abstract
A set of electrostatically charged, fluorescent, and superparamagnetic nanoprobes was developed for targeting cancer cells without using any molecular biomarkers. The surface electrostatic properties of the established cancer cell lines and primary normal cells were characterized by using these nanoprobes with various electrostatic signs and amplitudes. All twenty two randomly selected cancer cell lines of different organs, but not normal control cells, bound specifically to the positively charged nanoprobes. The relative surface charges of cancer cells could be quantified by the percentage of cells captured magnetically. The activities of glucose metabolism had a profound impact on the surface charge level of cancer cells. The data indicate that an elevated glycolysis in the cancer cells led to a higher level secretion of lactate. The secreted lactate anions are known to remove the positive ions, leaving behind the negative changes on the cell surfaces. This unique metabolic behavior is responsible for generating negative cancer surface charges in a perpetuating fashion. The metabolically active cancer cells are shown to a unique surface electrostatic pattern that can be used for recovering cancer cells from the circulating blood and other solutions.
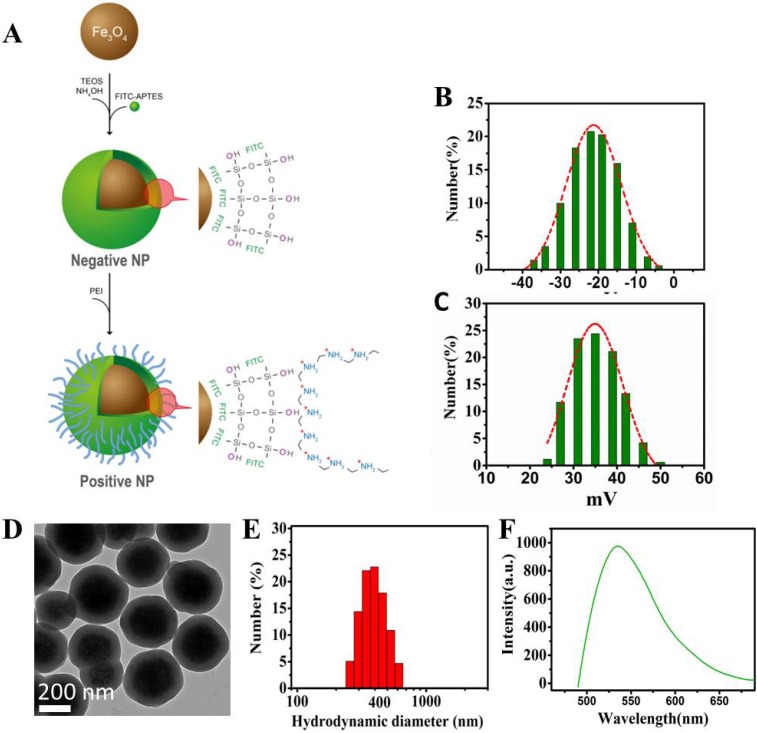
Design and characterization of the nanoprobes. (A) Schematic diagram showing the design of the Fe3O4 multi-functional nanoprobe which is rendered fluorescent with FITC. (B) Zeta potential distributions of negative NPs. (C) Zeta potential distributions of positive NPs. (D) Transmission electron microscopy showing the image of NPs with a thin layer of silica coating. (E) Light scattering histogram showing the hydrodynamic diameter distributions of NPs. (F) Fluorescence spectroscopy showing the emission of NPs having a peak around 525 nm.
Figure 2
Schematic diagram showing the procedure of magnet capturing of the cells. The cells in suspension are mixed with NPs of different electrical charges. Cell/NP bindings take place due to the opposite charges. The captured cells are magnetically attracted to the side of the tube by a permanent magnet.Design and characterization of the nanoprobes. (A) Schematic diagram showing the design of the Fe3O4 multi-functional nanoprobe which is rendered fluorescent with FITC. (B) Zeta potential distributions of negative NPs. (C) Zeta potential distributions of positive NPs. (D) Transmission electron microscopy showing the image of NPs with a thin layer of silica coating. (E) Light scattering histogram showing the hydrodynamic diameter distributions of NPs. (F) Fluorescence spectroscopy showing the emission of NPs having a peak around 525 nm.
Mechanism of surface charge generation via lactate secretion
Nearly all metabolically active cancer cells, whether in vivo or in vitro, are known to secrete a large amount of lactate as mobile anions 22. This is a result of increased glycolysis in which the levels of glucose uptake and lactate secretion can be up to 30 times greater than that of normal cells. Therefore, we propose that the negative charges on the surface of cancer cells are largely generated by the increased glycolysis and the associated lactate secretion across the plasma membrane.
Figure 5
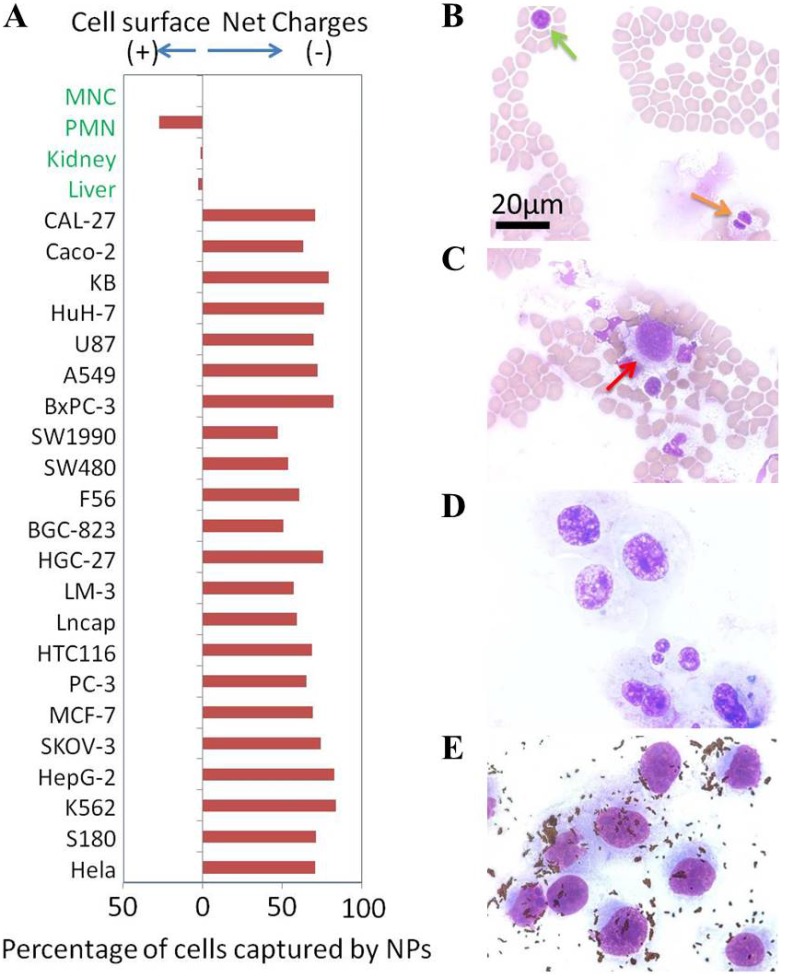
(A) Net surface charges of cells. The calculated net surface charges expressed in this figure is the net captured percentage of cells by one type charge sign NPs subtracted by the cells captured by the NPs with opposite charge sign as controls. Microscope photographs of blood smear (Green arrow to MNC cell & orange arrow to PMN cell) (B), blood smear with breast cancer MDA-MB-231 cells (Red arrow to MDA-MB-231 cell) (C), MDA-MB-231 cells smear (D) and positive NPs captured MDA-MB-231 cells from blood (E).
Figure 7
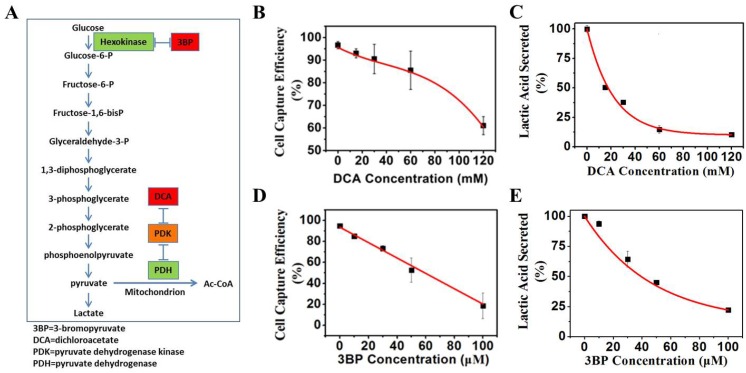
(A) Mechanisms of glycolysis inhibition via dichloroacetate (DCA) and bromopyruvate (3BP) for varying the glucose levels of culture media. (B) K562 cell capture efficiency vs. the concentration of glycolysis inhibitor DCA when reacting with the positive NPs. (C) Lactic acid secreted by K562 cancer cells vs. the glycolysis inhibitor DCA. (D) K562 cancer cell capture capacity vs. the glycolysis inhibitor 3BP when reacted with the positive NPs. (E) Lactic acid secreted by K562 cancer cells vs. direct glycolysis inhibitor 3BP.¡¡
Figure 8
(A) K562 cell capture capacity vs. extra lactic concentration when reacted with the positive NPs. (B) Lactic acid secreted by the K562 cells vs. extra lacate concentration.Discussion
We have shown the negative surface charges as a unique pattern of cancer cells. In contrast, the normal cells, including all blood cells, do not possess this property. Using the uniquely designed NPs, we have recovered cancer cells from human blood with high efficiency and purity. The negative charges were found to be generated from the large quantity of lactate secretion, a known property of all metabolically active cancer cells. These findings are summarized in Figure Figure99.
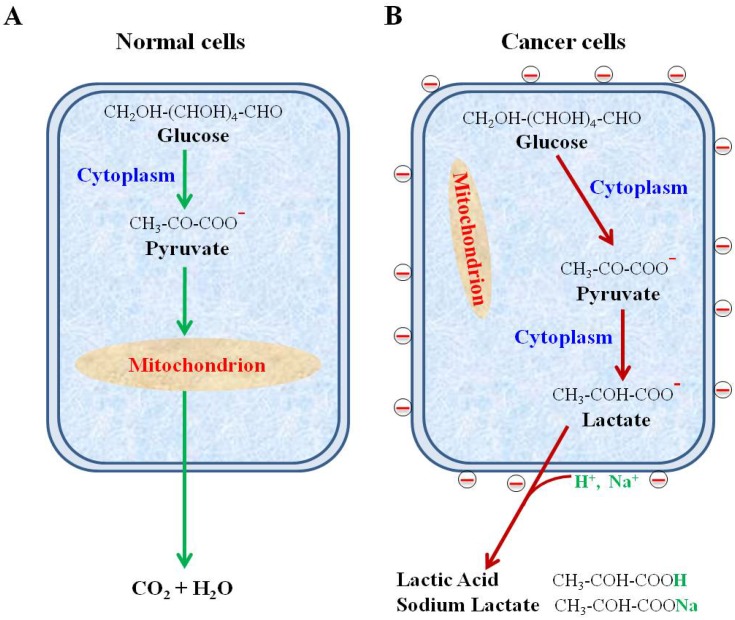
Schematic diagram showing secretion of lactate anions leading to a loss of cations from cancer cell surface and leaving behind the negative charges. (A) Glucose metabolism via glycolysis and oxidative phosphorylation in normal cells. (B) Glucose metabolism via highly elevated glycolysis and suppressed oxidative phosphorylation in cancer cells.
Despite of the decades-long efforts, there has not been any theoretical consensus or clinical application based on the electrical properties of cancer cells. This study has, for the first time, provided unequivocal evidence for a unique electrical pattern of cancer cell surfaces from as many as 22 different types of cancer cells in comparison to primary normal cells. The experimental results of this study significantly differ from the previous reports on how to measure the cell surface charges. For example, the human granulocytes were reportedly to have the negatively charged surfaces whereas the data of this study clearly indicate their positive charged surfaces 28. We attribute this contrast to the different methodologies employed by these studies. The multifunctional NPs developed in this study enabled direct assessment of the cell surface charge via electrostatic targeting, binding, and magnetic capturing of cancer cells from various solutions including blood. The findings of this study reveal a fundamental property of cancer cells and have profound potentials in clinical applications.
The phenomenon of the elevated glycolysis is unique for cancer cells and has been used widely for cancer diagnosis for decades. The level of glucose uptake and lactate secretion in cancer cells can be up to 30 folds higher than the normal cells, giving a basis for the cancer detection technologies, such as PET (Positron Emission Tomography) scan 29. Radioactively labeled glucose can be preferentially taken up by cancer cells for imaging. Thus, the elevated glucose metabolism is a significant hallmark of cancer cells. In comparison to the glucose uptake, the secretion of lactate is also an inevitable outcome of glycolysis 30. Our results show that lactate secretion is a direct cause for the negative charges on the cancer cell surfaces. It is not unreasonable to assume that the surface charges are consequentially linked to another well-established hallmark of cancer cells. Due to instability of genomes, the protein profiles can be vastly different even in the adjacent cancer cells of the same lesion. The search for consistent biomarker molecules on the surfaces of cancer cells has proven to be very difficult. In comparison to any molecular ligand, the biophysical property of cancer cells associated with the unique metabolism in cancer cells may prove to be a much more reliable property for detection.
The phenomenon of the elevated glycolysis is unique for cancer cells and has been used widely for cancer diagnosis for decades. The level of glucose uptake and lactate secretion in cancer cells can be up to 30 folds higher than the normal cells, giving a basis for the cancer detection technologies, such as PET (Positron Emission Tomography) scan 29. Radioactively labeled glucose can be preferentially taken up by cancer cells for imaging. Thus, the elevated glucose metabolism is a significant hallmark of cancer cells. In comparison to the glucose uptake, the secretion of lactate is also an inevitable outcome of glycolysis 30. Our results show that lactate secretion is a direct cause for the negative charges on the cancer cell surfaces.
Figure 9
Schematic diagram showing secretion of lactate anions leading to a loss of cations from cancer cell surface and leaving behind the negative charges. (A) Glucose metabolism via glycolysis and oxidative phosphorylation in normal cells. (B) Glucose metabolism via highly elevated glycolysis and suppressed oxidative phosphorylation in cancer cells.¡¡
Conclusions
All movements of ions across plasma membranes cause significant changes of surface charge. Cancer cells are well-known to secrete a large amount of lactate anions across plasma membranes as a hallmark. Our studies have now revealed that this hallmark may have led to a generation of unique negative charges on the surfaces of cancer cells. Without an elevated glycolysis, the surfaces of normal cells remain charge-neutral or slightly positive. This unique biophysical property can allow cancer cells to be identified and captured using a nanotechnology-based method without having to use any specific molecular marker. This technology could also be used to study the electrical property of all cells, such as neurons and other excitable cells. In clinical settings, our findings and technologies may have significant implications in developing novel diagnostic assay and probably therapies for cancer.
Keywords: Targeting, Biomarker, Nanoprobe, Surface Charge, Cell Metabolism, Lactate Secretion, Glycolysis.Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4997244/¡¡