Photodynamic treatment combining light and a photosensitizer molecule can be an effective method to inactivate pathogenic bacteria. This study identified vitamin K5 as an efficient photosensitizer for ultraviolet light A (UVA)-induced bacterial inactivation. Six bacterial species, Bacillus cereus (vegetative form), Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Klebsiella pneumoniae, and two species of antibiotic-resistant bacteria, Pseudomonas aeruginosa* and Staphylococcus aureus*, were suspended in aqueous solutions with or without vitamin K5 and exposed to UVA irradiation. UVA irradiation (5.8 J cm−2) with vitamin K5 (1600 μmol l−1) reduced the colony forming units (CFU) of these bacteria by three to seven logs. Antibiotic resistant bacteria were also susceptible to the bactericidal effects of UVA and vitamin K5 combination treatment. Inactivation of bacteria in human plasma required higher doses of UVA light and vitamin K5. UVA irradiation (30 J cm−2) with vitamin K5 (2000 μmol l−1) reduced E. coli and S. aureus spiked into human plasma by seven logs CFU/ml. Reactive oxygen species, such as superoxide anion radicals and hydroxyl radicals, were found to be generated in vitamin K5 aqueous solution after UVA irradiation, suggesting these oxygen species may mediate the inactivation of the bacteria.
紫外光可见光照射内源性光敏剂( 如核黄素即维生素B2)产生单线态氧和光动力疗法
Singlet Oxygen Generation by UVA Light Exposure of Endogenous Photosensitizers
---内源性光敏剂包括:血红素、胆绿素、胆红素、核黄素即维生素B2、FMN即黄素单核苷酸、叶酸
摘要
已经显示UVA光(320-400nm)由于由诸如黄素 (Flavin)或尿刊酸(urocanic acid)的物质产生单线态氧而在组织中产生有害的生物效应。核黄素(riboflavin, 维生素B2),黄素单核苷酸(FMN),黄素腺嘌呤二核苷酸(FAD),β-烟酰胺腺嘌呤二核苷酸(NAD)和溶液中的β-烟酰胺腺嘌呤二核苷酸磷酸(NADP),尿酸或胆固醇在355nm激发。
通过在1270nm处发光的时间分辨测量直接检测单线态氧。 NAD,NADP和胆固醇显示没有发光信号可能是由于在355nm处的非常低的吸收系数。
可以清楚地检测到尿刊酸的单线态氧发光,但信号太弱而无法量化量子产率。核黄素(ΦΔ= 0.54±0.07),FMN(ΦΔ= 0.51±0.07)和FAD(ΦΔ= 0.07±0.02)精确测定单线态氧的量子产率。
在通气溶液中,核黄素和FMN比外源光敏剂如Photofrin产生更多的单线态氧,后者用于光动力疗法以杀死癌细胞。
随着氧浓度的降低,单线态氧产生的量子产率降低(而其他氧自由基如过氧化氢H2O2,羟自由基增加),这在评估单线态氧在低氧浓度(组织内部)中的作用时必须考虑。
图1:图A是PN,PNS的吸收截面光谱,核黄素,FMN和FAD溶解在H2O中的吸收截面光谱如图1B所示。核黄素(维生素B2)对于波长短于300nm具有高吸收值,但350到550nm的波长也具有高吸收值。激发激光(355 nm)的光在这些分子中被很好地吸收。
To compare with our excitation wavelength, the absorption cross-section spectra of riboflavin, FMN, and FAD dissolved in H2O are shown in Fig. 1 B. The molecules have high absorption values for wavelengths shorter than 300 nm, but also from 350 to 550 nm. The light of our excitation laser (355 nm) is well absorbed in these molecules.
图4
在1270nm处单线态氧的发光的时间积分信号对于H 2 O中PNS,核黄素,FMN和FAD的空气饱和溶液的吸收能量。通过在355nm处吸收敏化剂来校正每个斜率。使用简单的线性拟合(y(x)= ax + b)将实线拟合到实验数据点。
Time-integrated signal of luminescence of singlet oxygen at 1270 nm versus absorbed energy for air-saturated solutions of PNS, riboflavin, FMN, and FAD in H2O. Each slope is corrected by the absorption of the sensitizers at 355 nm. The solid lines have been fitted to the experimental data points using a simple linear fit (y(x) = ax + b).
另外,在激发诸如核黄素之类的敏化剂之后,在氧自由基(I型)和单线态氧(II型反应)的产生之间总是存在竞争。该竞争取决于相应实验装置中的氧浓度。在完全充气的条件下([O2]≈280μM),UVA光有效地转换为单线态氧(ΦΔ= 0.54)。在低氧浓度([O2] <2μM)下,单线态生成降低至ΦΔ<0.20。这很重要,因为大多数内源性光敏剂位于细胞内,细胞内的氧分压可以是4托([O2] =7.5μM)甚至更低(38)。同时,其他活性氧物质(例如氧自由基)的产生可能增加。这与核黄素溶液在低氧条件下照射时由于H2O2产生增加比在空气中显示出更强的细胞毒性的结果很好地相关(39)。我们的研究结果也支持了最近的研究结果,即6-磷酸脱氢酶(G6PD)的失活是由于在I型光敏反应中核黄素的激发三重态直接氧化,其效率在低氧浓度下增加(40)。
结论
在过去十年中,许多文章都指出,UVA光暴露主要通过单线态氧引起皮肤老化甚至皮肤癌(1,3-7,24,41,42)。然而,缺少内源性光敏剂产生单线态氧的精确测量,特别是在不同的氧浓度下。
将UVA光应用于尿刊酸,可以清楚地检测到单线态氧发光,但信号太弱而无法量化各自的量子产率。激发核黄素,FMN和FAD,检测到单线态氧的强发光信号。对于这些物质,使用PNS作为参考,在空气饱和的溶剂中成功测定了量子产率(核黄素ΦΔ= 0.54±0.07,FMNΦΔ= 0.51±0.07,并且FADΦΔ= 0.07±0.02)。取决于它们在皮肤中的浓度,黄素是单线态氧的潜在产生剂,甚至比用于光动力疗法中的杀死癌细胞的外源性卟啉更有效。鉴于这些高值,包括尿刊酸在内的这些物质在UVA暴露期间可提供足够的单线态氧,从而导致基因调节,光老化甚至致癌作用似乎是合理的。
当测量在不同氧浓度下单线态氧产生的功效时,对于低氧浓度,单线态氧产生(PT)的功效显着降低。当用UVA光照射例如核黄素时,与通气环境(例如体外)中的条件相比,在皮肤中产生至少少2倍的单线态氧。
Abstract
UVA light (320–400 nm) has been shown to produce deleterious biological effects in tissue due to the generation of singlet oxygen by substances like flavins or urocanic acid. Riboflavin, flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), β-nicotinamide adenine dinucleotide (NAD), and β-nicotinamide adenine dinucleotide phosphate (NADP), urocanic acid, or cholesterol in solution were excited at 355 nm.
Singlet oxygen was directly detected by time-resolved measurement of its luminescence at 1270 nm. NAD, NADP, and cholesterol showed no luminescence signal possibly due to the very low absorption coefficient at 355 nm.
Singlet oxygen luminescence of urocanic acid was clearly detected but the signal was too weak to quantify a quantum yield. The quantum yield of singlet oxygen was precisely determined for riboflavin (ΦΔ = 0.54 ± 0.07), FMN (ΦΔ = 0.51 ± 0.07), and FAD (ΦΔ = 0.07 ± 0.02).
In aerated solution, riboflavin and FMN generate more singlet oxygen than exogenous photosensitizers such as Photofrin, which are applied in photodynamic therapy to kill cancer cells.
With decreasing oxygen concentration, the quantum yield of singlet oxygen generation decreased, which must be considered when assessing the role of singlet oxygen at low oxygen concentrations (inside tissue).
Quantum yield of singlet oxygen ΦΔ
Comparable to exogenous photosensitizers, endogenous molecules absorb UVA light in the skin and can generate singlet oxygen. The efficacy of a molecule to generate singlet oxygen is expressed by the quantum yield of singlet oxygen (ΦΔ). The molecules such as the flavins or urocanic acid are assumed to play a major role regarding the photooxidative damage of the skin (1,3,4,6,7,24,25) and the eye lens (8,26).
Riboflavin and FMN exhibit quantum yields higher than for exogenous photosensitizers such as hematoporphyrin derivative (Photofrin, ΦΔ = 0.35) (20), which are used in photodynamic therapy to kill cancer cells. Our results confirm that riboflavin and FMN are potential type II sensitizers under fully aerated conditions. Even the complex molecule FAD retains the ability of the flavin group to generate singlet oxygen. Interestingly, the quantum yield decreases with complexity of molecules going from riboflavin, to FMN and to FAD.
FIGURE 4
Time-integrated signal of luminescence of singlet oxygen at 1270 nm versus absorbed energy for air-saturated solutions of PNS, riboflavin, FMN, and FAD in H2O. Each slope is corrected by the absorption of the sensitizers at 355 nm. The solid lines have been fitted to the experimental data points using a simple linear fit (y(x) = ax + b).
The role of oxygen concentration
The detection of singlet oxygen by its luminescence is a powerful tool even in living cells in vitro (15,34). As already stated above, the efficacy of singlet oxygen generation decreases with decreasing oxygen concentration, i.e., decreasing oxygen partial pressure. That is shown in Fig. 3 C (ΦΔ,max) for riboflavin, which is similar to other sensitizers (14,16) and the other flavins. To elucidate the role of flavins, experiments are carried out frequently in vitro under aerated conditions, which is equivalent to an oxygen partial pressure of ∼150 Torr (150 mmHg or [O2] = 280 μM). Under in vivo conditions, e.g., in living skin, the oxygen partial pressure is only 20 Torr (20 mmHg or [O2] = 37 μM) at the dermal-epidermal junction or even less inside the cells (21). In view of this difference in oxygen partial pressure, the singlet oxygen generation by riboflavin decreases approximately twofold at most. These results are important when comparing experiments that are performed at different oxygen partial pressure.
Recently, it was shown that irradiated riboflavin can damage nicotine by antibody-catalyzed oxidative degradation (35). However, that experiment was performed in aerated solution and therefore at a high efficacy of singlet oxygen generation, which might not reflect the degradation under low oxygen conditions in vivo. Riboflavin-sensitized photodynamic modifications of high-molecular-weight Kininogen were also investigated only in vitro and singlet oxygen was found to be an important mediator (36). According to experiments under aerobic conditions it was stated that photoexcitation of riboflavin may also potentially occur in vivo in the organs and tissues that are permeable to light, such as the eye or skin, and damage hyaluronic acid and other cell-matrix components, to cause inflammation and accelerate aging (37). In view of our results, one must be careful when judging the relevance of singlet oxygen in vivo based on experiments in vitro.
Additionally, after excitation of sensitizers such as riboflavin, there is always a competition between the generation of oxygen radicals (type I) and singlet oxygen (type II reaction). That competition depends on the oxygen concentration in the respective experimental setup. At fully aerated conditions ([O2] ≈ 280 μM), the UVA light is effectively converted to singlet oxygen (ΦΔ = 0.54). At low oxygen concentrations ([O2] < 2 μM), the singlet generation decreases to ΦΔ < 0.20. This is important since most of the endogenous photosensitizers are located inside cells and the oxygen partial pressure inside a cell can be 4 Torr ([O2] = 7.5 μM) and even less (38). At the same time, the generation of other reactive oxygen species (e.g., oxygen radicals) may increase. This correlates well to findings that riboflavin solution showed stronger cytotoxicity during irradiation under hypoxia than under air due to the heightened generation of H2O2 (39). Our results also support the very recent findings that the inactivation of 6-phosphate dehydrogenase (G6PD) results from its direct oxidation by the excited triplet state of riboflavin in a Type-I-photosensitized reaction whose efficiency increases at low oxygen concentration (40).
CONCLUSIONS
In the last decade, numerous articles have stated that UVA light exposure cause skin aging or even skin cancer mainly by singlet oxygen (1,3–7,24,41,42). However, precise measurements of singlet oxygen generation by endogenous photosensitizers were missing, in particular at different oxygen concentrations.
Applying UVA light to urocanic acid, singlet oxygen luminescence was clearly detected, but the signal was too weak to quantify the respective quantum yield. Exciting riboflavin, FMN, and FAD, strong luminescence signal of singlet oxygen was detected. For these substances the quantum yield were successfully determined in air-saturated solvents using PNS as reference (riboflavin ΦΔ = 0.54 ± 0.07, FMN ΦΔ = 0.51 ± 0.07, and FAD ΦΔ = 0.07 ± 0.02). Depending on their concentration in the skin, the flavins are potential generators of singlet oxygen, even more effective than exogenous porphyrins used for cell killing in photodynamic therapy. In view of these high values, it seems to be reasonable that these substances including urocanic acid can provide sufficient singlet oxygen during UVA exposure leading to gene regulation, photoaging, and even carcinogenesis.
When measuring the efficacy of singlet oxygen generation at different oxygen concentrations, the efficacy of singlet oxygen generation (PT) decreased significantly for low oxygen concentrations. When irradiating, e.g., riboflavin with UVA light, at least a factor-2-less singlet oxygen is generated in the skin as compared to the condition in an aerated environment (e.g., in vitro).
SOURCE:
Jürgen Baier,* Tim Maisch,* Max Maier,† Eva Engel,‡ Michael Landthaler,* and Wolfgang Bäumler*
Department of Dermatology, †Department of Physics, and ‡Department of Organic Chemistry, University of Regensburg, Regensburg, Germany
Singlet Oxygen Generation by UVA Light Exposure of Endogenous Photosensitizers https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1518628/
Toxicity Testing of a Novel Riboflavin-Based Technology for Pathogen Reduction and White Blood Cell Inactivation
The Mirasol PRT System (Gambro BCT, Lakewood, CO) for platelets and plasma uses riboflavin and UV light to reduce pathogens and inactivate white blood cells in donated blood products. An extensive toxicology program, developed in accordance with International Organisation for Standardisation (ISO) 10993 guidelines, was performed for the Mirasol PRT system. Test and control articles for most of the reported studies were treated (test) or untreated (control) blood products. For some studies, pure lumichrome (the major photoproduct of riboflavin) or photolyzed riboflavin solution was used. Systemic toxicity was evaluated with in vivo animal studies in the acute and subchronic settings. Developmental toxicity was evaluated with an in vivo animal study. Genotoxicity and neoantigenicity were evaluated with in vitro and in vivo tests. Hemocompatibility and cytotoxicity were assessed with standard, in vitro assays. The pharmacokinteics, excretion, and tissue distribution of 14C-riboflavin and its photoproducts was evaluated with an in vivo animal study. The possible presence of leachable or extractable compounds (from the disposable set) was evaluated with novel assays for measuring these compounds in blood. No treatment-related toxicity was observed in any of the studies.
Transfusion Medicine Reviews
Volume 22, Issue 2, April 2008, Pages 133-153
Author links open overlay panelHeather L.ReddyAnthony D.Dayan1JoyCavagnaroShayneGadJunzhiLiRaymond P.Goodrich
Show more
https://doi.org/10.1016/j.tmrv.2007.12.003Get rights and content
https://www.sciencedirect.com/science/article/pii/S0887796307001125
Pathogen Reduction technology
The Mirasol Pathogen Reduction Technology (PRT) System renders a broad range of disease-causing viruses, bacteria and parasites less pathogenic, and inactivates residual white blood cells found in blood components.
Built for efficiency and ease of use, the novel Mirasol PRT system helps improve the safety of the blood supply by reducing the infectious levels of disease-causing agents in platelets and plasma while still maintaining quality blood components
The Mirasol Pathogen Reduction Technology (PRT) System uses a combination of riboflavin (vitamin B2), a non-toxic, naturally occurring compound, and a specific spectrum of ultraviolet (UV) light to inactivate viruses, bacteria, parasites and white blood cells that may be present in collected blood products.
The Mirasol PRT system consists of three main components:
• A disposable kit — includes an illumination/storage bag and sterile riboflavin solution
• The Mirasol Illuminator — provides UV light and agitation for the Mirasol PRT process
• Mirasol® Managersoftware — integrates and manages data reporting and storage
During treatment, a blood product is mixed with the riboflavin solution and placed into the Illuminator where it is exposed to UV light for about five to ten minutes. There is no need to remove riboflavin or its photoproducts; after illumination, the treated products are ready for transfusion or placement into storage.
How the Mirasol PRT system inactivates pathogens and white blood cells:
UV light + riboflavin = irreversible inactivation
Riboflavin molecules associate with nucleic acids of pathogens. Exposure to UV light activates riboflavin and when it is associated with pathogen nucleic acid, riboflavin causes a chemical alteration to functional groups of the nucleic acids (primarily guanine bases), making pathogens unable to replicate.
Treating platelets and plasma in three simple steps:
1. The product is transferred to the Mirasol PRT illumination/storage bag
2. Riboflavin solution is added and mixed with the product
3. The mixture is then exposed to UV light for about five to ten minutes
The Mirasol PRT System
You know better than anyone—these are challenging times. The Mirasol PRT system allows you to meet the needs of your business by optimizing the balance of cost, efficacy and safety of your blood products.2,3
SAFE
The Mirasol PRT system is the only PRT system:
Shown to reduce incidence of a transfusion-transmitted disease in humans1
That uses riboflavin (vitamin B2), a non-toxic, non-mutagenic compound, to inactivate pathogens and white blood cells1,5,6,7
SIMPLE
The Mirasol process has fewer steps than other PRT methods and the device can be used to treat platelets, plasma and whole blood. This simplicity helps minimize the impact to operation.8,9,10
EFFECTIVE
The Mirasol PRT system is effective in protecting against a broad spectrum of emerging tested and untested pathogens, including bacteria, parasites, and enveloped and non-enveloped viruses; it also inactivates white blood cells, adding an extra layer of safety for patients. Blood products treated with the Mirasol PRT system maintain efficacy and help save patients’ lives.11,12,13,14,15,16,17,18,19
AFFORDABLE
The Mirasol PRT system treatment can be used as an alternative to some safety procedures without introducing new risks for operators or patients.20,21,22 Treatment with the Mirasol PRT system can help reduce product discard rates for blood centers and reduce the overall cost of transfusion for hospitals.2,3,8,9
TRUSTED PARTNER
The Mirasol PRT system comes from Terumo BCT, a global leader in blood component technologies.
Mirasol – Rontis Medical http://rontismedical.com/mirasol/
https://www.terumobct.com/mirasol
哈佛大学:维生素B2是氰化物中毒的最有效解药
~化学和代谢组学筛选鉴定新的生物标记物和氰化物中毒的解毒剂。
抽象
暴露于氰化物会导致一系列可能致命的心脏,神经和代谢功能障碍。需要改进的氰化物解毒剂,但目标的理想生物途径尚不清楚。为了更好地了解氰化物的代谢作用并发现新的氰化物解毒剂,我们开发了一种斑马鱼氰化物暴露模型,并对其进行了高通量化学筛选。
在3120个小分子的筛选中,我们发现了4种阻止氰化物毒性的新型解毒剂。最有效的解毒剂是核黄素。
氰化物处理的斑马鱼的代谢组学分析揭示了胆汁酸和嘌呤代谢的变化,最显着的是肌苷水平的增加。核黄素使斑马鱼中许多氰化物引起的神经和代谢紊乱正常化。在斑马鱼中观察到的氰化物的代谢作用在氰化物毒性的兔模型中是保守的。此外,用硝普钠(一种释放一氧化氮和氰化物离子的药物)治疗的人显示出增加的循环胆汁酸和肌苷。
总之,核黄素可能是一种新的氰化物毒性治疗和硝普钠治疗期间的预防措施,肌苷可以作为氰化物暴露的生物标志物,胆汁酸和嘌呤代谢途径中的代谢物可以揭示对逆转氰化物毒性至关重要的途径。。
Chemical and metabolomic screens identify novel biomarkers and antidotes for cyanide exposure.
Abstract
Exposure to cyanide causes a spectrum of cardiac, neurological, and metabolic dysfunctions that can be fatal. Improved cyanide antidotes are needed, but the ideal biological pathways to target are not known. To understand better the metabolic effects of cyanide and to discover novel cyanide antidotes, we developed a zebrafish model of cyanide exposure and scaled it for high-throughput chemical screening. In a screen of 3120 small molecules, we discovered 4 novel antidotes that block cyanide toxicity. The most potent antidote was riboflavin. Metabolomic profiling of cyanide-treated zebrafish revealed changes in bile acid and purine metabolism, most notably by an increase in inosine levels. Riboflavin normalizes many of the cyanide-induced neurological and metabolic perturbations in zebrafish. The metabolic effects of cyanide observed in zebrafish were conserved in a rabbit model of cyanide toxicity. Further, humans treated with nitroprusside, a drug that releases nitric oxide and cyanide ions, display increased circulating bile acids and inosine. In summary, riboflavin may be a novel treatment for cyanide toxicity and prophylactic measure during nitroprusside treatment, inosine may serve as a biomarker of cyanide exposure, and metabolites in the bile acid and purine metabolism pathways may shed light on the pathways critical to reversing cyanide toxicity.
Nath AK1, Roberts LD, Liu Y, Mahon SB, Kim S, Ryu JH, Werdich A, Januzzi JL, Boss GR, Rockwood GA, MacRae CA, Brenner M, Gerszten RE, Peterson RT.
Author information
Cardiovascular Research Center, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA 02129, USA.
PMID: 23345455 PMCID: PMC3633825 DOI: 10.1096/fj.12-225037
[Indexed for MEDLINE] Free PMC Article
FASEB J. 2013 May;27(5):1928-38. doi: 10.1096/fj.12-225037. Epub 2013 Jan 23.
Chemical and metabolomic screens identify novel biomarkers and antidotes for cyanide exposure. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/23345455
核黄素通过激活新的信号转导途径诱导植物的抗病性
Riboflavin induces disease resistance in plants by activating a novel signal transduction pathway
Dong H,Beer SV。
抽象
证明了核黄素作为系统抗性的激发子和植物中新信号传导过程的激活剂的作用。在用核黄素处理后,拟南芥(Arabidopsis thaliana)对寄生霜霉(Peronospora parasitica)和丁香假单胞菌(Pseudomonas syringae pv。)产生了系统性抗性。番茄和烟草对烟草花叶病毒(TMV)和链格孢菌(Alternaria alternata)产生了系统性抗性。在抗性诱导所需的浓度下,核黄素不会在植物中引起细胞死亡或直接影响可培养病原体的生长。核黄素诱导植物中发病相关(PR)基因的表达,表明其能够触发导致全身抗性的信号转导途径。蛋白激酶抑制剂K252a和控制防御基因转录的NIM1 / NPR1基因突变均损害对核黄素的反应性。相反,核黄素在NahG植物中诱导抗性和PR基因表达,其不能积累水杨酸(SA)。因此,核黄素诱导的抗性需要蛋白激酶信号传导机制和功能性NIM1 / NPR1基因,但不需要SA的积累。核黄素是系统抗性的激发子,它以不同的方式触发抗性信号转导。
Phytopathology. 2000 Aug;90(8):801-11. doi: 10.1094/PHYTO.2000.90.8.801.
Riboflavin induces disease resistance in plants by activating a novel signal transduction pathway.
Dong H, Beer SV.
Abstract
The role of riboflavin as an elicitor of systemic resistance and an activator of a novel signaling process in plants was demonstrated. Following treatment with riboflavin, Arabidopsis thaliana developed systemic resistance to Peronospora parasitica and Pseudomonas syringae pv. Tomato, and tobacco developed systemic resistance to Tobacco mosaic virus (TMV) and Alternaria alternata. Riboflavin, at concentrations necessary for resistance induction, did not cause cell death in plants or directly affect growth of the culturable pathogens. Riboflavin induced expression of pathogenesis-related (PR) genes in the plants, suggesting its ability to trigger a signal transduction pathway that leads to systemic resistance. Both the protein kinase inhibitor K252a and mutation in the NIM1/NPR1 gene which controls transcription of defense genes, impaired responsiveness to riboflavin. In contrast, riboflavin induced resistance and PR gene expression in NahG plants, which fail to accumulate salicylic acid (SA). Thus, riboflavin-induced resistance requires protein kinase signaling mechanisms and a functional NIM1/NPR1 gene, but not accumulation of SA. Riboflavin is an elicitor of systemic resistance, and it triggers resistance signal transduction in a distinct manner.
PMID: 18944500 DOI: 10.1094/PHYTO.2000.90.8.801
Free full text
Riboflavin induces disease resistance in plants by activating a novel signal transduction pathway. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/18944500
The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production
Riboflavin (vitamin B2) is required for normal plant growth and development. Previous studies have shown that riboflavin application can enhance pathogen resistance in plants. Here, we investigated the role of riboflavin in increasing drought tolerance (10 % PEG6000 treatment) in plants. We treated 4 week-old tobacco plants with five different levels of riboflavin (0, 4, 20, 100 and 500 μM) for 5 days and examined their antioxidant responses and levels of drought tolerance. Compared with the controls, low and moderate levels of riboflavin treatment enhanced drought tolerance in the tobacco plants, whereas higher concentrations of riboflavin (500 μM) impaired drought tolerance. Further analysis revealed that plants treated with 500 μM riboflavin accumulated higher levels of ROS (O2 − and H2O2) and lipid peroxide than the control plants or plants treated with low levels of riboflavin. Consistent with this observation, the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) were higher in plants treated with low or moderate (4, 20 and 100 μM) levels of riboflavin compared with the control. We also found that chlorophyll degraded rapidly in control and 500 μM riboflavin-treated plants under drought stress conditions. In addition, the survival times of the riboflavin-treated plants were significantly modified by treatment with reduced glutathione, a well-known ROS scavenger, under drought stress conditions. Thus, riboflavin-mediated ROS production may determine the effects of riboflavin on drought tolerance in tobacco plants.
The regulatory role of riboflavin in the drought tolerance of tobacco plants depends on ROS production | SpringerLink https://link.springer.com/article/10.1007%2Fs10725-013-9858-8
Photodynamic therapy
From Wikipedia, the free encyclopedia
Synonyms photochemotherapy
Photodynamic therapy (PDT), is a form of phototherapy involving light and a photosensitizing chemical substance, used in conjunction with molecular oxygen to elicit cell death (phototoxicity). PDT has proven ability to kill microbial cells, including bacteria, fungi and viruses.[1] PDT is popularly used in treating acne. It is used clinically to treat a wide range of medical conditions, including wet age-related macular degeneration, psoriasis, atherosclerosis and has shown some efficacy in anti-viral treatments, including herpes. It also treats malignant cancers[2] including head and neck, lung, bladder and particular skin. The technology has also been tested for treatment of prostate cancer, both in a dog model[3] and in human prostate cancer patients.[4]
It is recognised as a treatment strategy that is both minimally invasive and minimally toxic. Other light-based and laser therapies such as laser wound healing and rejuvenation, or intense pulsed light hair removal do not require a photosensitizer.[5] Photosensitisers have been employed to sterilise blood plasma and water in order to remove blood-borne viruses and microbes and have been considered for agricultural uses, including herbicides and insecticides.
Photodynamic therapy's advantages lessen the need for delicate surgery and lengthy recuperation and minimal formation of scar tissue and disfigurement. A side effect is the associated photosensitisation of skin tissue.[5]
Basics
PDT applications involve three components:[2] a photosensitizer, a light source and tissue oxygen. The wavelength of the light source needs to be appropriate for exciting the photosensitizer to produce radicals and/or reactive oxygen species. These are free radicals (Type I) generated through electron abstraction or transfer from a substrate molecule and highly reactive state of oxygen known as singlet oxygen (Type II).
PDT is a multi-stage process. First a photosensitiser with negligible dark toxicity is administered, either systemically or topically, in the absence of light. When a sufficient amount of photosensitiser appears in diseased tissue, the photosensitiser is activated by exposure to light for a specified period. The light dose supplies sufficient energy to stimulate the photosensitiser, but not enough to damage neighbouring healthy tissue. The reactive oxygen kills the target cells.[5]
Reactive oxygen species
In air and tissue, molecular oxygen (O2) occurs in a triplet state, whereas almost all other molecules are in a singlet state. Reactions between triplet and singlet molecules are forbidden by quantum mechanics, making oxygen relatively non-reactive at physiological conditions. A photosensitizer is a chemical compound that can be promoted to an excited state upon absorption of light and undergo intersystem crossing (ISC) with oxygen to produce singlet oxygen. This species is highly cytotoxic, rapidly attacking any organic compounds it encounters. It is rapidly eliminated from cells, in an average of 3 µs.[6]
Photochemical processes
When a photosensitiser is in its excited state (3Psen*) it can interact with molecular triplet oxygen (3O2) and produce radicals and reactive oxygen species (ROS), crucial to the Type II mechanism. These species include singlet oxygen (1O2), hydroxyl radicals (•OH) and superoxide (O2−) ions. They can interact with cellular components including unsaturated lipids, amino acid residues and nucleic acids. If sufficient oxidative damage ensues, this will result in target-cell death (only within the illuminated area).[5]
Photosensitisers
----Some photosensitizers;hemin, riboflavin,rose bengal, methylene, chorophyll
Many photosensitizers for PDT exist. They divide into porphyrins, chlorins and dyes.[7] Examples include aminolevulinic acid (ALA), Silicon Phthalocyanine Pc 4, m-tetrahydroxyphenylchlorin (mTHPC) and mono-L-aspartyl chlorin e6 (NPe6).
Photosensitizers commercially available for clinical use include Allumera, Photofrin, Visudyne, Levulan, Foscan, Metvix, Hexvix, Cysview and Laserphyrin, with others in development, e.g. Antrin, Photochlor, Photosens, Photrex, Lumacan, Cevira, Visonac, BF-200 ALA,[7][8] Amphinex[9] and Azadipyrromethenes.
The major difference between photosensitizers is the parts of the cell that they target. Unlike in radiation therapy, where damage is done by targeting cell DNA, most photosensitizers target other cell structures. For example, mTHPC localizes in the nuclear envelope.[10] In contrast, ALA localizes in the mitochondria[11] and methylene blue in the lysosomes.[12]
Cyclic tetrapyrrolic chromophores
Cyclic tetrapyrrolic molecules are fluorophores and photosensitisers. Cyclic tetrapyrrolic derivatives have an inherent similarity to the naturally occurring porphyrins present in living matter.
Porphyrins
Porphyrins are a group of naturally occurring and intensely coloured compounds, whose name is drawn from the Greek word porphura, or purple. These molecules perform biologically important roles, including oxygen transport and photosynthesis and have applications in fields ranging from fluorescent imaging to medicine. Porphyrins are tetrapyrrolic molecules, with the heart of the skeleton a heterocyclic macrocycle, known as a porphine. The fundamental porphine frame consists of four pyrrolic sub-units linked on opposing sides (α-positions, numbered 1, 4, 6, 9, 11, 14, 16 and 19) through four methine (CH) bridges (5, 10, 15 and 20), known as the meso-carbon atoms/positions. The resulting conjugated planar macrocycle may be substituted at the meso- and/or β-positions (2, 3, 7, 8, 12, 13, 17 and 18): if the meso- and β-hydrogens are substituted with non-hydrogen atoms or groups, the resulting compounds are known as porphyrins.[5]
The inner two protons of a free-base porphyrin can be removed by strong bases such as alkoxides, forming a dianionic molecule; conversely, the inner two pyrrolenine nitrogens can be protonated with acids such as trifluoroacetic acid affording a dicationic intermediate. The tetradentate anionic species can readily form complexes with most metals.[5]
Absorption spectroscopy
Porphyrin's highly conjugated skeleton produces a characteristic ultra-violet visible (UV-VIS) spectrum. The spectrum typically consists of an intense, narrow absorption band (ε > 200000 l mol−1 cm−1) at around 400 nm, known as the Soret band or B band, followed by four longer wavelength (450–700 nm), weaker absorptions (ε > 20000 L⋅mol−1⋅cm−1 (free-base porphyrins)) referred to as the Q bands.
The Soret band arises from a strong electronic transition from the ground state to the second excited singlet state (S0 → S2); whereas the Q band is a result of a weak transition to the first excited singlet state (S0 → S1). The dissipation of energy via internal conversion (IC) is so rapid that fluorescence is only observed from depopulation of the first excited singlet state to the lower-energy ground state (S1 → S0).[5]
Ideal photosensitisers
The key characteristic of a photosensitiser is the ability to preferentially accumulate in diseased tissue and induce a desired biological effect via the generation of cytotoxic species. Specific criteria:[13]
Strong absorption with a high extinction coefficient in the red/near infrared region of the electromagnetic spectrum (600–850 nm)—allows deeper tissue penetration. (Tissue is much more transparent at longer wavelengths (~700–850 nm). Longer wavelengths allow the light to penetrate deeper[9] and treat larger structures.)[9]
Suitable photophysical characteristics: a high-quantum yield of triplet formation (ΦT ≥ 0.5); a high singlet oxygen quantum yield (ΦΔ ≥ 0.5); a relatively long triplet state lifetime (τT, μs range); and a high triplet-state energy (≥ 94 kJ mol−1). Values of ΦT= 0.83 and ΦΔ = 0.65 (haematoporphyrin); ΦT = 0.83 and ΦΔ = 0.72 (etiopurpurin); and ΦT = 0.96 and ΦΔ = 0.82 (tin etiopurpurin) have been achieved
Low dark toxicity and negligible cytotoxicity in the absence of light. (The photosensitizer should not be harmful to the target tissue until the treatment beam is applied.)
Preferential accumulation in diseased/target tissue over healthy tissue
Rapid clearance from the body post-procedure
High chemical stability: single, well-characterised compounds, with a known and constant composition
Short and high-yielding synthetic route (with easy translation into multi-gram scales/reactions)
Simple and stable formulation
Soluble in biological media, allowing intravenous administration. Otherwise, a hydrophilic delivery system must enable efficient and effective transportation of the photosensitiser to the target site via the bloodstream.
Low photobleaching to prevent degradation of the photosensitizer so it can continue producing singlet oxygen
Natural fluorescence (Many optical dosimetry techniques, such as fluorescence spectroscopy, depend on fluorescence.)[14]
Applications
Photoimmunotherapy
Photoimmunotherapy is an oncological treatment for various cancers that combines photodynamic therapy of tumor with immunotherapy treatment. Combining photodynamic therapy with immunotherapy enhances the immunostimulating response and has synergistic effects for metastatic cancer treatment.[19][20][21]
Vascular targeting
Some photosensitisers naturally accumulate in the endothelial cells of vascular tissue allowing 'vascular targeted' PDT.
Verteporfin was shown to target the neovasculature resulting from macular degeneration in the macula within the first thirty minutes after intravenous administration of the drug.
Compared to normal tissues, most types of cancers are especially active in both the uptake and accumulation of photosensitizers agents, which makes cancers especially vulnerable to PDT.[22] Since photosensitizers can also have a high affinity for vascular endothelial cells.[23]
Acne
PDT is currently in clinical trials as a treatment for severe acne. Initial results have shown for it to be effective as a treatment only for severe acne.[24] The treatment causes severe redness and moderate to severe pain and burning sensation. (see also: Levulan) One phase II trial, while it showed improvement, was not superior to blue/violet light alone.[25]
Ophthalmology
As cited above, verteporfin was widely approved for the treatment of wet AMD beginning in 1999. The drug targets the neovasculature that is caused by the condition.
Photodynamic therapy - Wikipedia https://en.wikipedia.org/wiki/Photodynamic_therapy
光敏化胶原交联与光敏剂核黄素和370 nm UVA光对人角膜巩膜组织的结构和生物力学影响
Structural and Biomechanical Effects of Photooxidative Collagen Cross-Linking with Photosensitizer Riboflavin and 370 nm UVA Light on Human Corneoscleral Tissues
摘要
该研究使用组织学,厚度,扫描电子显微镜和原子力显微镜分析,定量研究光致抗原胶原交联处理与光敏剂核黄素(RF)和370nm UVA光在体外人角膜巩膜胶原纤维中的直接影响。
使用解剖刀从供体组织切开地切开20个8×2mm的角膜巩膜条带。研究了四个参数,包括胶原交联处理之前和之后的角膜巩膜组织的密度,厚度,粘附力和硬度。 RFUVA催化的胶原交联处理导致角膜(8%)和巩膜(23%)基质胶原的密度增加。然而,角膜巩膜厚度没有差异。此外,RFUVA催化的胶原交联治疗导致角膜巩膜的生物力学反应增加:角膜巩膜硬度增加25%和8%,角巩膜粘连力增加24%和22%。通过RF敏化光反应的胶原交联处理可以引起角膜和巩膜的胶原纤维网络的结构和生物力学变化。这是由于纤维间距和基质水肿变窄。
Structural and Biomechanical Effects of Photooxidative Collagen Cross-Linking with Photosensitizer Riboflavin and 370 nm UVA Light on Human Corneoscleral Tissues
Abstract
This study quantitatively investigated the immediate effects of a photooxidative collagen cross-linking treatment with photosensitizer riboflavin (RF) and 370 nm UVA light in in vitro human corneoscleral collagen fibrils using histology, thickness, scanning electron microscopy, and atomic force microscopy analyses. Twenty 8 × 2 mm corneoscleral strips were dissected sagittally from donor tissue using a scalpel. Four parameters were investigated, including the density, thickness, adhesion force, and stiffness of corneoscleral tissues before and after the collagen cross-linking treatment. The RFUVA-catalyzed collagen cross-linking treatment led to an increase in the density of both corneal (8%) and scleral (23%) stromal collagens. However, there was no difference in corneoscleral thickness. Furthermore, RFUVA-catalyzed collagen cross-linking treatment led to an increased biomechanical response of corneosclera: 25 and 8% increases in corneoscleral stiffness, and 24 and 22% increases in corneoscleral adhesion force. The collagen cross-linking treatment through RF-sensitized photoreaction may cause structural and biomechanical changes in the collagen fibril network of the cornea and the sclera. This is due to narrowing of the interfibrillar spacing and the stromal edema.
Samjin Choi (a1) (a2), Jae-Ho Shin (a3), Youjin Cheong (a1), Kyung-Hyun Jin (a3) ...
https://doi.org/10.1017/S1431927613001669Published online: 06 June 2013
Structural and Biomechanical Effects of Photooxidative Collagen Cross-Linking with Photosensitizer Riboflavin and 370 nm UVA Light on Human Corneoscleral Tissues | Microscopy and Microanalysis | Cambridge Core https://www.cambridge.org/core/journals/microscopy-and-microanalysis/article/structural-and-biomechanical-effects-of-photooxidative-collagen-crosslinking-with-photosensitizer-riboflavin-and-370-nm-uva-light-on-human-corneoscleral-tissues/FE95CA7DA7B59A29BACB396B8400480B
单线态氧在细胞的寿命和扩散
Lifetime and Diffusion of Singlet Oxygen in a Cell
在时间和空间分辨的实验中,使用聚焦激光束照射掺入细胞核中的敏化剂,在单个神经细胞中产生单线态分子氧O2(a1Δg)。由此产生的单线态氧通过其红外磷光检测。获得的数据表明,与普通感知相反,这种活性物质在细胞中可以是相当长寿的,并且因此可以在可感知的距离上扩散,包括穿过细胞膜进入细胞外环境。这些结果为光诱导细胞死亡和细胞内信号传导的机理研究提供了新的视角。
Lifetime and Diffusion of Singlet Oxygen in a Cell
In time- and spatially resolved experiments, singlet molecular oxygen, O2(a1Δg), was created in a single nerve cell upon irradiation of a sensitizer incorporated in the cell nucleus using a focused laser beam. The singlet oxygen thus produced was detected by its infrared phosphorescence. Data obtained indicate that, contrary to common perception, this reactive species can be quite long-lived in a cell and, as such, can diffuse over appreciable distances including across the cell membrane into the extra-cellular environment. These results provide a new perspective for mechanistic studies of photoinduced cell death and intracellular signaling.
Esben Skovsen†, John W. Snyder†, John D. C. Lambert‡, and Peter R. Ogilby*†
Department of Chemistry, and Department of Physiology, University of Aarhus, DK-8000 Århus, Denmark
J. Phys. Chem. B, 2005, 109 (18), pp 8570–8573
DOI: 10.1021/jp051163i
Publication Date (Web): April 8, 2005
Copyright © 2005 American Chemical Society
Lifetime and Diffusion of Singlet Oxygen in a Cell - The Journal of Physical Chemistry B (ACS Publications) https://pubs.acs.org/doi/abs/10.1021/jp051163i
抗真菌光动力疗法Antifungal photodynamic therapy
在光动力抗微生物化学疗法(PACT)中,致敏药物和可见光的组合导致微生物细胞的选择性破坏。
光药物组合杀死微生物的能力已有100多年的历史。然而,直到最近才开始寻找抗生素抗性病原体的替代疗法,这一现象已被详细研究过。大量研究表明,PACT在体外破坏病毒和原生动物以及革兰氏阳性和革兰氏阴性细菌和真菌方面非常有效。
实验研究的结果已经最终证明,使用吩噻嗪鎓,卟啉和酞菁光敏剂,通过光动力作用可以有效地杀死皮肤菌和酵母。重要的是,已经证明对真菌比对人细胞具有相当大的选择性,没有关于真菌抗性的报道,并且该处理与对真菌或人细胞的遗传毒性或诱变作用无关。
尽管细胞培养研究取得了成功,但只有极少数的体内动物和人体试验已经发表。本文回顾了迄今为止发表的关于PACT抗真菌应用的研究,旨在提高对这一研究领域的认识,该领域有可能对未来真菌感染的治疗产生重大影响。
Antifungal photodynamic therapy
In photodynamic antimicrobial chemotherapy (PACT), a combination of a sensitising drug and visible light causes selective destruction of microbial cells. The ability of light–drug combinations to kill microorganisms has been known for over 100 years. However, it is only recently with the beginning of the search for alternative treatments for antibiotic-resistant pathogens that the phenomenon has been investigated in detail. Numerous studies have shown PACT to be highly effective in the in vitro destruction of viruses and protozoa, as well as Gram-positive and Gram-negative bacteria and fungi. Results of experimental investigations have demonstrated conclusively that both dermatomycetes and yeasts can be effectively killed by photodynamic action employing phenothiazinium, porphyrin and phthalocyanine photosensitisers. Importantly, considerable selectivity for fungi over human cells has been demonstrated, no reports of fungal resistance exist and the treatment is not associated with genotoxic or mutagenic effects to fungi or human cells. In spite of the success of cell culture investigations, only a very small number of in vivo animal and human trials have been published. The present paper reviews the studies published to date on antifungal applications of PACT and aims to raise awareness of this area of research, which has the potential to make a significant impact in future treatment of fungal infections.
Microbiological Research
Volume 163, Issue 1, 15 January 2008, Pages 1-12
Microbiological Research
Author links open overlay panelRyan F.DonnellyPaul A.McCarronMichael M.Tunney
Show more
https://doi.org/10.1016/j.micres.2007.08.001
Antifungal photodynamic therapy - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0944501307000912
霍普金斯大学:细胞内(维生素B2)自发荧光:上皮癌干细胞的生物标志物
Intracellular autofluorescence: A biomarker for epithelial cancer stem cells
抽象
癌症干细胞(CSCs)被认为可以促进肿瘤生长,转移和化疗耐药。尽管表面标志物如CD133和CD44已成功用于分离CSC,但它们的表达并不仅仅与CSC表型相关并且易于发生环境改变。
我们鉴定了具有自发荧光亚细胞区室的细胞,其专门显示跨越不同人类肿瘤类型的CSC特征。
原发性肿瘤来源的自体荧光细胞与侧群(SP)细胞不重叠,在球体培养中富集,在化学疗法期间,强烈表达多能性相关基因,高度转移并显示长期体内致瘤性,即使在单一 - 细胞水平。
自发荧光是由于在具有ATP依赖性ABCG2转运蛋白的膜结合的细胞质结构中核黄素积累。
总之,我们在不同上皮癌的CSC中鉴定并表征了内在的自发荧光表型,并使用该标记分离和表征这些细胞。
Intracellular autofluorescence: A biomarker for epithelial cancer stem cells
Abstract
Cancer stem cells (CSCs) are thought to drive tumor growth, metastasis and chemoresistance. Although surface markers such as CD133 and CD44 have been successfully used to isolate CSCs, their expression is not exclusively linked to the CSC phenotype and is prone to environmental alteration.
We identified cells with an autofluorescent subcellular compartment that exclusively showed CSC features across different human tumor types.
Primary tumor-derived autofluorescent cells did not overlap with side-population (SP) cells, were enriched in sphere culture and during chemotherapy, strongly expressed pluripotency-associated genes, were highly metastatic and showed long-term in vivo tumorigenicity, even at the single-cell level.
Autofluorescence was due to riboflavin accumulation in membrane-bounded cytoplasmic structures bearing ATP-dependent ABCG2 transporters.
In summary, we identified and characterized an intrinsic autofluorescent phenotype in CSCs of diverse epithelial cancers and used this marker to isolate and characterize these cells.
SOURCE:
JOHN HOPKINS UNIVERSITY
Irene Miranda-Lorenzo, Jorge Dorado, Enza Lonardo, Sonia Alcala, Alicia G. Serrano, Jenifer Clausell-Tormos, Michele Cioffi, Diego Megias, Sladjana Zagorac, Anamaria Balic, Manuel Hidalgo, Mert Erkan, Joerg Kleeff, Aldo Scarpa, Bruno Sainz, Christopher Heeschen
Intracellular autofluorescence: A biomarker for epithelial cancer stem cells — Johns Hopkins University https://jhu.pure.elsevier.com/en/publications/intracellular-autofluorescence-a-biomarker-for-epithelial-cancer--3
https://pubs.acs.org/doi/abs/10.1021/mp800046m
Folic acid conjugated ferritins as photosensitizer carriers for photodynamic therapy†
Zipeng Zhen,a,b Wei Tang,a,b Weizhong Zhang,a,b and Jin Xiecorresponding authora,b
Author information Copyright and License information Disclaimer
aDepartment of Chemistry, University of Georgia, Athens, Georgia 30602, United States
bBio-Imaging Research Center, University of Georgia, Athens, Georgia 30602, United States
corresponding authorCorresponding author.
Jin Xie: ude.agu@eixnij
The target, folic acid receptor, is found to be overexpressed in about 40% human cancers, and is able to mediate endocytosis of folic acid conjugated cargos.20–22
We coupled folic acid as a tumour targeting ligand to the surface of ferritins and loaded them with ZnF16Pc. The resulting nanoconjugates can efficiently home to 4T1 tumours in vivo, and, with photoirradiation, leading to suppressed tumour growth and tumour metastasis.
Conclusions
Overall, we have shown that folic acid can be coupled to ferritins that are loaded with photosensitizers like ZnF16Pc. The resulting nanoconjugates after systematic injection can efficiently home to tumours. With photoirradiation, the treatment caused efficient tumour growth suppression while minimally affecting normal tissues. More interestingly, it was observed that PDT treatment helped suppress tumour metastasis to the lung, which is likely attributed to a PDT-stimulated anti-tumour response. These observations confirm ferritin as a safe and powerful nanoplatform for efficient delivery of photosensitizers.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4885642/
Sensitive detection of intracellular environment of normal and cancer cells by autofluorescence lifetime imaging
Author links open overlay panelKamleshAwasthiaDaikiMoriyabTakakazuNakabayashicLimingLibNobuhiroOhtaa
a
Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung University, 1001, Ta-Hsueh Road, Hsinchu 30010, Taiwan
b
Department of Bio- and Material Photonics, Chitose Institute of Science and Technology, Chitose 066-8655, Japan
c
Graduate School of Pharmaceutical Sciences, Tohoku University, Aoba-ku, Sendai 980-8578, Japan
Received 17 June 2016, Revised 18 October 2016, Accepted 19 October 2016, Available online 24 October 2016.
crossmark-logo
Show less
https://doi.org/10.1016/j.jphotobiol.2016.10.023Get rights and content
Highlights
• Autofluorescence lifetime images of normal and cancer cells have been observed.
• NADH fluorescence lifetime is shorter in cancer cells than that in normal cells.
• FAD fluorescence lifetime is also shorter in cancer cells than that in normal cell.
• NADH or FAD fluorescence lifetime measurements are applicable for diagnosis of cancer cells.
• Time-resolved autofluorescence spectra of NADH were measured for normal and cancer cells.
Abstract
Intracellular fluorescence lifetime images of the endogenous fluorophores of nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD), which are well known as autofluorescence chromophores, were obtained from rat normal fibroblast cells (WFB) and H-ras oncogene-transfected cancer cells among WFB (W31). The average lifetime of the NADH and FAD autofluorescence was shorter in cancer cells than in normal cells, indicating that the difference in metabolism between healthy and cancer cells alters the conditions for coenzymes such as NADH and FAD and that the autofluorescence lifetime measurement of NADH and FAD is applicable for the noninvasive diagnosis of cancer cells. The pico- and nano-second time-resolved fluorescence spectra of NADH obtained with different time windows were similar in normal and cancer cells, indicating that every fluorescence decay component gives the same spectrum in both cell types. These results as well as the fluorescence lifetime images of exogenous fluorophores stained with sodium pheophorbide a in normal and cancer cells suggest that the difference in the fluorescence lifetime between normal and cancer cells cannot be attributed to a difference in the intracellular pH or refractive index but to the difference in the bound condition between proteins and NADH or FAD under the different intracellular environments of normal and cancer cells.
Sensitive detection of intracellular environment of normal and cancer cells by autofluorescence lifetime imaging - ScienceDirect https://www.sciencedirect.com/science/article/pii/S1011134416304699
Light (phototherapy)-induced riboflavin deficiency in the neonate
The Journal of Pediatrics
Volume 90, Issue 1, January 1977, Pages 118-122
The Journal of Pediatrics
Author links open overlay panelM.D.Donald S.GromischM.D.RafaelLopezM.D.Harold S.ColePh.D.Jack M.Cooperman
Show more
https://doi.org/10.1016/S0022-3476(77)80784-1Get rights and content
Phototherapy with blue light decomposes riboflavin, which has a maximum absorption at 450 nm. A study was designed to determine whether riboflavin deficiency developed in neonates who received phototherapy for moderate hyperbilirubinemia. Twenty-one infants with normal erythrocyte glucose-6-phosphate dehydrogenase activity were investigated. Five infants with moderate hyperbilirubinemia who did not require phototherapy served as the controls. Riboflavin deficiency was determined from the degree of saturation of erythrocyte glutathione reductase, a method shown to reflect riboflavin nutritional status in the neonate. Sixteen of 21 infants who were exposed to phototherapy developed riboflavin deficiency; all who had phototherapy for 49 hours or more developed the deficiency. That the concentration of serum bilirubin or the duration of hyperbilirubinemia was not a factor is supported by the fact that none of the controls became deficient. This observation may have important metabolic and clinical consequences for the neonate.
Light (phototherapy)-induced riboflavin deficiency in the neonate - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0022347677807841
血液成分
使用基于维生素B2(核黄素)和紫外线的光化学处理灭活血小板和血浆产品中的病毒
背景
多层次血液安全计划可降低输血传播疾病的风险;然而,仍然存在窗口期传播筛选病毒以及从无症状捐赠者传播未筛查和新出现的病毒的风险。为了降低这种风险,针对八种病毒剂评估了基于核黄素和紫外线的病原体减少过程。
研究设计和方法
核黄素和紫外线照射评估以下八种病毒剂:脑心肌炎病毒(EMC),甲型肝炎病毒(HAV),丙型肝炎病毒(HCV),甲型流感(FLUAV),拉克罗斯病毒(LACV),伪狂犬病病毒(PRV) ),辛德毕斯病毒(SINV)和水疱性口炎病毒(VSV)。在处理之前,取出样品以确定产品的初始病毒载量。处理后,重新评估产品的病毒载量并计算对数减少。
结果
用核黄素和UV光处理后病毒减少在血小板(PLT)和血浆单位中是等效的,如通过血浆,PLT和含有35%血浆的PLT添加剂溶液中的EMC降低3.2-log所证明的。另外,观察到以下病毒减少值:HAV 1.8log,HCV至少4.1log,FLUAV至少5.0log,LACV至少3.5log,PRV 2.5log,SINV 3.2log和VSV至少6.3log。
结论
在该研究中观察到的结果表明,用基于核黄素和UV光的病原体减少过程处理PLT和血浆产品可能潜在地消除筛选的病毒的窗口期传播并且大大降低未经筛选的病毒的输血传播的风险。
BLOOD COMPONENTS
Inactivation of viruses in platelet and plasma products using a riboflavin‐and‐UV–based photochemical treatment
BACKGROUND
Multilayered blood safety programs reduce the risk of transfusion‐transmitted diseases; however, there remains a risk of window period transmission of screened viruses and transmission of unscreened and emerging viruses from asymptomatic donors. To reduce this risk, a riboflavin‐and‐UV‐light–based pathogen reduction process was evaluated against eight viral agents.
STUDY DESIGN AND METHODS
Riboflavin and UV light was evaluated against the following eight viral agents: encephalomyocarditis virus (EMC), hepatitis A virus (HAV), hepatitis C virus (HCV), influenza A (FLUAV), La Crosse virus (LACV), pseudorabies virus (PRV), sindbis virus (SINV), and vesicular stomatitis virus (VSV). Before treatment, a sample was removed to determine the product's initial viral load. After treatment the product's viral load was reevaluated and the log reduction was calculated.
RESULTS
Virus reduction after treatment with riboflavin and UV light is equivalent in platelet (PLT) and plasma units, as demonstrated by a 3.2‐log reduction of EMC in plasma, PLTs, and PLT additive solution containing 35% plasma. Additionally, the following viral reductions values were observed: HAV 1.8 log, HCV at least 4.1 log, FLUAV at least 5.0 log, LACV at least 3.5 log, PRV 2.5 log, SINV 3.2 log, and VSV at least 6.3 log.
CONCLUSIONS
The results observed in this study suggest that treating PLT and plasma products with a riboflavin‐and‐UV‐light–based pathogen reduction process could potentially eliminate window period transmission of screened viruses and greatly reduce the risk of transfusion transmission of unscreened viruses.
Shawn D. Keil Abderrahmane Bengrine Richard Bowen Susanne Marschner Nick Hovenga Lindsay Rouse Denise Gilmour Gilles Duverlie Raymond P. Goodrich
First published: 03 March 2015 https://doi.org/10.1111/trf.13030 Cited by: 16
Inactivation of viruses in platelet and plasma products using a riboflavin‐and‐UV–based photochemical treatment - Keil - 2015 - Transfusion - Wiley Online Library https://onlinelibrary.wiley.com/doi/abs/10.1111/trf.13030
使用核黄素光化学法破坏血源性病毒核酸的基于流动的装置的有效性
强调
• 我们开发了一种基于流动的处理装置,用于使用核黄素光化学方法对血浆中的病毒灭活。
• 我们已经通过使用指示病毒的细胞病变效应方法证明了灭活效果。
• 我们通过核酸试验证明了核酸在血源性病毒中的损伤效力。
• 装置处理具有剂量效应,并且有效至低于NAT检测限的水平。
• 所有数据显示该装置可用于输血医学领域。
摘要
背景
使用指示病毒的细胞病变效应方法证明了使用核黄素光化学的基于流动的治疗装置的有效性。然而,需要评估针对真实血源性病毒的灭活效力,尤其是在核酸水平。
材料与方法
使用严格的血液选择程序选择具有不同浓度的血源性病毒的特殊血浆样品,并用装置处理(DT)处理。使用聚合酶链反应荧光法的核酸测试(NAT)用于检测病毒拷贝。
结果
使用DT,高乙型肝炎病毒(HBV)浓度的血浆中的NAT值降低至1330。经过100倍稀释后,NAT值低于DT的NAT检测限,而没有DT则为23.0。中等HBV浓度的血浆中NAT值为61.9,DT降低至37.8,稀释10倍后,NAT值低于NAT检测限,而未检测DT低于20。低浓度血源性病毒的血浆Ct值低于DT的NAT检测限。
结论
DT具有剂量效应,其在血液传播的病毒中有效地将核酸破坏至低于NAT检测限的水平。
Effectiveness of a flow-based device using riboflavin photochemistry in damaging blood-borne viral nucleic acids
Highlights
• We had developed a flow-based treatment device for virus inactivation in plasma using riboflavin photochemical method.
• We had demonstrated the inactivation effectiveness by cytopathic effect method using indicator viruses.
• We demonstrated the damage effectiveness of nucleic acid in blood-borne viruses by nucleic acid test.
• Device treatment had a dose effect and was effective to a level below the NAT detection limits.
• All data showed the device was useful to application in transfusion medicine field.
Abstract
Background
Effectiveness of a flow-based treatment device using riboflavin photochemistry was demonstrated by cytopathic effect method using indicator viruses. However, inactivation efficacy against real blood-borne viruses needs to be evaluated, especially at nucleic acid level.
Material and Methods
Special plasma samples with varying concentrations of blood-borne virus were selected using a strict blood selection procedure and were treated with device treatment (DT). Nucleic acid test (NAT) using polymerase chain reaction fluorescence method was used to detect virus copies.
Results
The NAT value of 4325 in plasma with high Hepatitis B Virus (HBV) concentrations decreased to 1330 with DT. After 100-fold dilution, the NAT value was below the NAT detection limits with DT compared with 23.0 that without DT. The NAT value of 61.9 in plasma with medium HBV concentrations decreased to 37.8 with DT, and after 10-fold dilution, the NAT value was below the NAT detection limits with DT compared with below 20 that without DT. The Ct values of plasma with low concentrations of blood-borne viruses were below the NAT detection limits with DT.
Conclusion
There was a dose effect with DT which was effective in blood-borne viruses damaging nucleic acids to a level below the NAT detection limits.
Previous article in issueNext article in issue
Abbreviations
CPEcytopathic effectCtcycle thresholdDTDevice treatmentELISAenzyme-linked immunosorbent assayHBVHepatitis B virusHCVHepatitis C virusHIVHuman immunodeficiency virusNATnucleic acid testPCRPolymerase chain reactionRPMround per minutesUVultraviolet
Journal of Photochemistry and Photobiology B: Biology
Volume 183, June 2018, Pages 391-396
Author links open overlay panelLiguoZhuaHongliTongcShufangWangaYangYuaZhongLiubChangqingLibDeqingWanga
a Department of Blood Transfusion, Chinese PLA General Hospital, Beijing, China
b Institute of Blood Transfusion, Chinese Academic of Medical Science and Peking Union Medical College, Chengdu, China
c Department of Biochemistry, Chinese PLA General Hospital, Beijing, China
Effectiveness of a flow-based device using riboflavin photochemistry in damaging blood-borne viral nucleic acids - ScienceDirect https://www.sciencedirect.com/science/article/pii/S1011134417315415#!
Riboflavin photosensitized hemolysis of rat erythrocytes in the presence of serum.
Abstract
Rat erythrocytes incubated in photoactivated riboflavin system in the presence of serum caused a rapid hemolysis. During the course of illumination, a marked efflux of intracellular K+, increase in osmotic fragility and promotion of lipid peroxidation of the cell occurred prior to hemolysis indicating that riboflavin-induced photohemolysis is colloidosmotic process. In the absence of serum, however, no hemolysis was observed at any time of incubation, suggesting that membrane damages are enhanced by the illumination in the presence of serum. Furthermore, the illumination of cells in the photoactivated riboflavin system in the presence of serum under continued anaerobic conditions did not exhibit any significant hemolysis. These results indicate that the photohemolysis of cells is due to oxidative damages of cell membrane that initiated the hemolysis. Singlet oxygen (1O2) scavengers employed in this study strongly inhibited the photohemolysis, suggesting that cells are damaged oxidatively by 1O2 generated in photoactivated riboflavin system in the presence of serum. Furthermore, triplet quenchers and alpha-tocopherol suppressed this photohemolysis. A reference was made concerning a possible adverse effect of riboflavin on infant erythrocytes in phototherapy.
J Pharmacobiodyn. 1982 Aug;5(8):568-75.
Suzuki Y, Miura T, Ogiso T.
Riboflavin photosensitized hemolysis of rat erythrocytes in the presence of serum. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/7153830
Hemolysis of Human Red Blood Cells by Riboflavin-Cu(II) System: Enhancement by Azide
Abstract
Photoactivated riboflavin in the presence of Cu(II) generates reactive oxygen species (ROS) which can hemolyze human red blood cells (RBC). In the present work we examined the effect of sodium azide (NaN3) on RBC in the presence of riboflavin and Cu(II). The addition of NaN3 to the riboflavin-Cu(II) system enhanced K+ loss and hemolysis. The extent of K+ loss and hemolysis were time and concentration dependent. Bathocuproine, a Cu(I)-sequestering agent, inhibited the hemolysis completely. Among various free radical scavengers used to identify the major ROS involved in the reaction, thiourea was found to be the most effective scavenger. Thiourea caused almost 85%inhibition of hemolysis suggesting that ·OH is the major ROS involved in the reaction. Using spectral studies and other observations, we propose that when NaN3 is added to the riboflavin-Cu(II) system, it inhibits the photodegradation of riboflavin resulting in increased ·OH generation. Also, the possibility of azide radical formation and its involvement in the reaction could not be ruled out.
Key words
riboflavin copper sodium azide reactive oxygen species RBC hemolysis
Biochemistry (Moscow)
September 2005, Volume 70, Issue 9, pp 1011–1014 | Cite as
Authors
Authors and affiliations
I. AliEmail authorN. SakhniniI. Naseem
Hemolysis of Human Red Blood Cells by Riboflavin-Cu(II) System: Enhancement by Azide | Springer for Research & Development https://rd.springer.com/article/10.1007%2Fs10541-005-0217-x
核黄素-Cu(II)系统对人红细胞的溶血作用
Hemolysis of human red blood cells by riboflavin-Cu(II) system
核黄素的光动力作用通常被认为涉及活性氧物质的产生,当反应中存在Cu(II)时,其活性增加。
在本研究中,我们报道光活化核黄素以时间依赖性方式导致新鲜人红细胞(RBC)K(+)损失。 Cu(II)的加入进一步增强了K(+)的损失并且还导致显着的溶血。与Cu(II)以2:1化学计量的核黄素导致最大K(+)损失和高达45%的溶血。当存在于反应中时,浴铜灵(一种特定的Cu(I) - 多价螯合剂)完全抑制溶血。自由基清除剂如超氧化物歧化酶,碘化钾和甘露醇抑制溶血达55%或更多。然而,硫脲是最有效的清除剂,显示出90%的抑制作用。这些结果表明人类RBC的K(+)渗漏和溶血基本上是自由基介导的反应。
Hemolysis of human red blood cells by riboflavin-Cu(II) system
The photodynamic action of riboflavin is generally considered to involve the generation of reactive oxygen species, whose production is enhanced when Cu(II) is present in the reaction. In the present study we report that photoactivated riboflavin causes K(+) loss from fresh human red blood cells (RBC) in a time dependent manner. Addition of Cu(II) further enhances the K(+) loss and also leads to significant hemolysis. Riboflavin in a 2:1 stoichiometry with Cu(II) leads to maximum K(+) loss and up to 45% hemolysis. Bathocuproine, a specific Cu(I)-sequestering agent, when present in the reaction, inhibits the hemolysis completely. Free radical scavengers like superoxide dismutase, potassium iodide and mannitol inhibited the hemolysis up to 55% or more. However, thiourea was the most effective scavenger showing 90% inhibition. These results suggest that K(+) leakage and hemolysis of human RBC are basically free radical mediated reactions.
Article in Biochimica et Biophysica Acta 1523(2-3):225-9 · November 2000 with 49 Reads
DOI: 10.1016/S0304-4165(00)00126-4 · Source: PubMed
Hemolysis of human red blood cells by riboflavin-Cu(II) system | Request PDF https://www.researchgate.net/publication/12282848_Hemolysis_of_human_red_blood_cells_by_riboflavin-CuII_system
光致发光的核黄素/核黄素-Cu(II)使胰蛋白酶失活:Cu(II)使平衡倾向
Photoilluminated riboflavin/riboflavin-Cu(II) inactivates trypsin: Cu(II) tilts the balance.
抽象
核黄素(RF)在用荧光照射时产生活性氧物质,如超氧阴离子,单线态和三线态氧,黄素基团和大量过氧化氢(H 2 O 2)。
H2O2可以自由地穿透细胞膜并与过渡金属离子如Cu(II)反应,通过改性金属催化的Haber-Weiss反应产生羟基自由基。
早些时候,据报道胰蛋白酶 - 胰凝乳蛋白酶混合物作为间接抗氧化剂并减少自由基的产生。因此,在本研究中,我们使用光照射RF作为ROS的来源,以研究自由基对胰蛋白酶活性的影响。我们还比较了使用胰蛋白酶作为靶分子的光致发光RF和RF-Cu(II)系统的破坏作用。当Cu(II)加入到反应中时,RF引起胰蛋白酶的片段化,并且效果进一步增强。用各种ROS清除剂获得的结果表明,超氧化物自由基,单线态和三线态氧主要是由光照射RF引起的胰蛋白酶损伤的原因。另一方面,当Cu(II)加入到反应中时,羟基自由基是胰蛋白酶损伤的主要原因。还提出了在反应中产生各种ROS的机理。胰蛋白酶未单独使用RF或使用RF-Cu(II)组合显示出任何抗氧化作用。
Photoilluminated riboflavin/riboflavin-Cu(II) inactivates trypsin: Cu(II) tilts the balance.
Abstract
Riboflavin (RF) upon irradiation with fluorescent light generates reactive oxygen species like superoxide anion, singlet and triplet oxygen, flavin radicals and substantial amounts of hydrogen peroxide (H2O2). H2O2 can freely penetrate cell membrane and react with a transition metal ion like Cu(ll), generating hydroxyl radical via the modified metal-catalyzed Haber-Weiss reaction. Earlier, it was reported that trypsin-chymotrypsin mixture served as an indirect antioxidant and decreased free radical generation. Thus, in the present study, we used photoilluminated RF as a source of ROS to investigate the effect of free radicals on the activity of trypsin. We also compared the damaging effect of photoilluminated RF and RF-Cu(ll) system using trypsin as a target molecule. RF caused fragmentation of trypsin and the effect was further enhanced, when Cu(II) was added to the reaction. Results obtained with various ROS scavengers suggested that superoxide radical, singlet and triplet oxygen were predominantly responsible for trypsin damage caused by photoilluminated RF. On the other hand, when Cu(ll) was added to the reaction, hydroxyl radical was mainly responsible for trypsin damage. A mechanism of generation of various ROS in the reaction is also proposed. Trypsin did not show any antioxidant effect with RF alone or with RF-Cu(II) combination.
Published at:Indian J Biochem Biophys
Indian J Biochem Biophys. 2006 Oct;43(5):312-8.
Photoilluminated riboflavin/riboflavin-Cu(II) inactivates trypsin: Cu(II) tilts the balance.
Husain E, Fatima RA, Ali IA, Naseem I.
Department of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh 202 002 (U.P.), India.
An-Najah Blogs : Photoilluminated riboflavin/riboflavin-Cu(II) inactivates trypsin: Cu(II) tilts the balance. https://blogs.najah.edu/staff/iyadali/article/Photoilluminated-riboflavinriboflavin-CuII-inactivates-trypsin-CuII-tilts-the-balance
Blue light induced free radicals from riboflavin on E. coli DNA damage
Abstract
The micronutrients in many cellular processes, riboflavin (vitamin B2), FMN, and FAD are photo-sensitive to UV and visible light to generate reactive oxygen species (ROS). The riboflavin photochemical treatment with UV light has been applied for the inactivation of microorganisms to serve as an effective and safe technology. Ultra-violet or high-intensity radiation is, however, considered as a highly risky practice. This study was working on the application of visible LED lights to riboflavin photochemical reactions to development an effective antimicrobial treatment. The photosensitization of bacterial genome with riboflavin was investigated in vitro and in vivo by light quality and irradiation dosage. The riboflavin photochemical treatment with blue LED light was proved to be able to inactivate E. coli by damaging nucleic acids with ROS generated. Riboflavin is capable of intercalating between the bases of bacterial DNA or RNA and absorbs lights in the visible regions. LED light illumination could be a more accessible and safe practice for riboflavin photochemical treatments to achieve hygienic requirements in vitro.
Highlights
► Application of visible LED lights to riboflavin photochemical reactions to development an antimicrobial method. ► The riboflavin photochemical treatment with blue LED light is able to inactivate Escherichia coli. ► Nucleic acids of E. coli are damaged with ROS generated after illumination by blue LED light. ► LED light illumination could be practical to riboflavin photochemical treatments for hygienic requirements in vitro.
Journal of Photochemistry and Photobiology B: Biology
Volume 119, 5 February 2013, Pages 60-64
Author links open overlay panelJi-YuanLiangJeu-Ming P.YuannChien-WeiChengHong-LinJianChin-ChangLinLiang-YuChen
Show more
https://doi.org/10.1016/j.jphotobiol.2012.12.007Get rights and content
Blue light induced free radicals from riboflavin on E. coli DNA damage - ScienceDirect https://www.sciencedirect.com/science/article/pii/S1011134412002631
An Action Spectrum of the Riboflavin Photosensitized Inactivation of Lambda Phage
Article in Photochemistry and Photobiology 81(2):474-80 · November 2004 with 43 Reads
DOI: 10.1562/2004-08-25-RA-292 · Source: PubMed
The Action Spectrum of riboflavin (RB) sensitized inactivation of lambda phage was determined between 266 and 575 nm. Below 304 nm, RB depresses the phage reduction by screening phage from radiation that it would otherwise absorb directly. Between 308 and 525 nm, RB sensitizes the inactivation of phage. Enhanced phage reduction is observed at 320 and 500 nm because of binding of RB to the phage and the shifting of the absorption curve of the phage-bound flavin relative to free flavin in phosphate-buffered saline. Enhanced inactivation at 320 and 500 nm and depressed phage inactivation between 360 and 410 nm is also influenced by the inner filter effect.
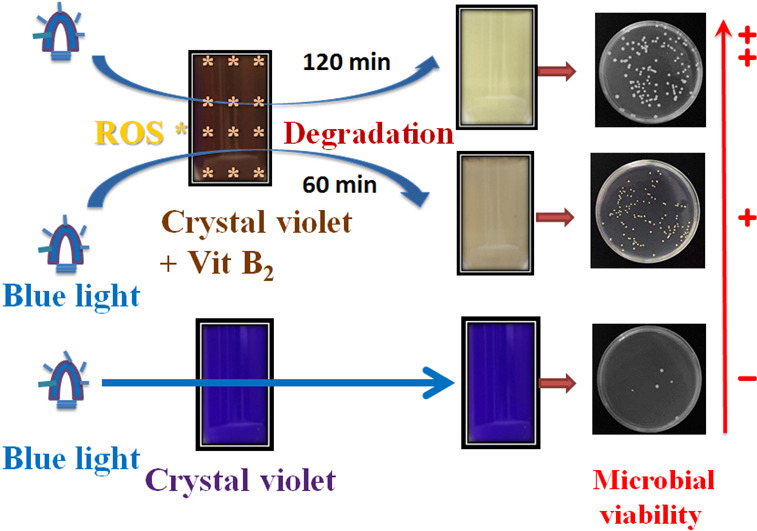
Blue light induced free radicals from riboflavin in degradation of crystal violet by microbial viability evaluation
Highlights
• Riboflavin (RF) treated with blue light illumination can degrade CV.
• The structure of degraded CV was changed by ROS generated from RF photolysis.
• The antimicrobial ability of degraded CV was greatly reduced by RF photolysis.
Abstract
Crystal violet (CV) is applied in daily use mainly as a commercial dye and antimicrobial agent. Waste water containing CV may affect aquatic ecosystems. Riboflavin, also known as vitamin B2, is non-toxic and an essential vitamin required for the functions of the human body. Riboflavin is photosensitive to UV and visible light in terms of generating reactive oxygen species. This study investigated the potential application of blue light on riboflavin, so as to come up with an effective way of degrading CV during its treatment. Photosensitivity of CV leading to degradation in the presence of riboflavin was investigated by light intensity, exposure time, and irradiation dosage. The degradation of CV during riboflavin photolysis treatment was studied by a UV/vis spectrometry and chromatography. The effects of CV degradation on microbial viability are relevant when considering the influences on the ecosystem. This study proved that riboflavin photochemical treatment with blue light degrades CV dye by ROS formation. The riboflavin photolysis-treated CV solution appeared to be transparent during conformational transformations of the CV that was rearranged by free radical species generated from riboflavin photolysis. After riboflavin photolysis, colony-forming units (CFUs) were determined for each CV solution. CFU preservation was 85.2% for the CV dissolved riboflavin solution treated with blue light irradiation at 2.0 mW/cm2 for 120 min. Degradation of CV by riboflavin photochemical procedures can greatly reduce antimicrobial ability and serve as an environmental friendly waste water treatment method. Our results presented here concerning riboflavin photolysis in degradation of CV provide a novel technique, and a simple and safe practice for environmental decontamination processes.
Author links open overlay panelJi-YuanLiangaJeu-Ming P.YuannaZong-JheHsieaShiuh-TsuenHuangbChiing-ChangChenb
https://doi.org/10.1016/j.jphotobiol.2017.08.018
Keywords
Blue lightCrystal violetFree radicalRiboflavin
Blue light induced free radicals from riboflavin in degradation of crystal violet by microbial viability evaluation - ScienceDirect https://www.sciencedirect.com/science/article/pii/S1011134417305602
BLOOD COMPONENTS
Inactivation of Plasmodium falciparum in whole blood by riboflavin plus irradiation
Abstract
Background
Malaria parasites are frequently transmitted by unscreened blood transfusions in Africa. Pathogen reduction methods in whole blood would thus greatly improve blood safety. We aimed to determine the efficacy of riboflavin plus irradiation for treatment of whole blood infected with Plasmodium falciparum.
Study Design and Methods
Blood was inoculated with 104 or 105 parasites/mL and riboflavin treated with or without ultraviolet (UV) irradiation (40‐160 J/mL red blood cells [mLRBCs]). Parasite genome integrity was assessed by quantitative amplification inhibition assays, and P. falciparum viability was monitored in vitro.
Results
Riboflavin alone did not affect parasite genome integrity or parasite viability. Application of UV after riboflavin treatment disrupted parasite genome integrity, reducing polymerase‐dependent amplification by up to 2 logs (99%). At 80 J/mLRBCs, riboflavin plus irradiation prevented recovery of viable parasites in vitro for 2 weeks, whereas untreated controls typically recovered to approximately 2% parasitemia after 4 days of in vitro culture. Exposure of blood to 160 J/mLRBCs was not associated with significant hemolysis.
Conclusions
Riboflavin plus irradiation treatment of whole blood damages parasite genomes and drastically reduces P. falciparum viability in vitro. In the absence of suitable malaria screening assays, parasite inactivation should be investigated for prevention of transfusion‐transmitted malaria in highly endemic areas.
Mira El Chaar Sharan Atwal Graham L. Freimanis Bismarck Dinko Colin J. Sutherland Jean‐Pierre Allain
First published: 09 May 2013 https://doi.org/10.1111/trf.12235 Cited by: 16
This work was supported by a grant from TerumoBCT to CS and JPA.
Inactivation of Plasmodium falciparum in whole blood by riboflavin plus irradiation - El Chaar - 2013 - Transfusion - Wiley Online Library https://onlinelibrary.wiley.com/doi/abs/10.1111/trf.12235
Treatment of Whole Blood With Riboflavin and UV Light: Impact on Malaria Parasite Viability and Whole Blood Storage
ABSTRACT
Background: Sub-Saharan African countries utilize whole blood (WB) to treat severe anemia secondary to severe blood loss or malaria on an emergency basis. In many areas with high prevalence of transfusion-transmissible agents, blood safety measures are insufficient. Pathogen reduction technology applied to WB might considerably improve blood safety. Methods: Whole blood from 40 different donors were treated with riboflavin and UV light (pathogen reduction technology) in order to inactivate malaria parasite replication. The extent of parasite inactivation was determined using quantitative polymerase chain reaction methods and was correlated to studies evaluating the replication of malaria parasites in culture. Products were also stored for 21 days at +4°C and monitored for cell quality throughout storage. Results: Plasmodium amplicon was present in 21 samples (>100 copies/mL), doubtful in four (10–100 genome equivalents [gEq]/mL), and negative in 15 U. The majority of asymptomatic parasitemic donors carried low parasite levels, with only six donors above 5,000 copies/mL (15%). After treatment with riboflavin and UV light, these six samples demonstrated a 0.5 to 1.2 log reduction in quantitative polymerase chain reaction amplification. This correlated to equal to or greater than 6.4 log reductions in infectivity. In treated WB units, cell quality parameters remained stable; however, plasma hemoglobin increased to 0.15 g/dL. All markers behaved similarly to published data for stored, untreated WB. Conclusions: Pathogen reduction technology treatment can inactivate malaria parasites in WB while maintaining adequate blood quality during posttreatment cold storage for 21 days.
KEYWORDS: Whole blood, pathogen reduction, cold storage, Sub-Saharan Africa
Shock. 2015 Aug; 44(Suppl 1): 33–38.
Shirley Owusu-Ofori,* Joseph Kusi,* Alex Owusu-Ofori,† Graham Freimanis,‡ Christine Olver,§ Caitlyn R. Martinez,§ Shilo Wilkinson,∥ Janna M. Mundt,∥ Shawn D. Keil,∥ Raymond P. Goodrich,∥ and Jean-Pierre Allain‡
*Transfusion Medicine Unit and †Department of Clinical Microbiology, Kwame Nkrumah University of Science and Technology/Komfo Anokye Teaching Hospital, Kumasi, Ghana; ‡Dept of Haematology, University of Cambridge, Cambridge, UK; and §Colorado State University, Ft Collins; and ∥Terumo BCT, Lakewood, Colorado
Address reprint requests to Raymond P. Goodrich, PhD, Terumo BCT, Lakewood, CO 80227. E-mail: moc.tcbomuret@hcirdoog.yaR.
Treatment of Whole Blood With Riboflavin and UV Light: Impact on Malaria Parasite Viability and Whole Blood Storage https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4498649/
Iron ion induced haemolysis: effect of caeruloplasmin, albumin and ascorbate (vitamin C)
Abstract
1. Human caeruloplasmin (ferroxidase), bovine serum albumin and ascorbate protected washed rat erythrocytes against iron ion stimulated haemolysis, while Superoxide dismutase, catalase and other scavengers of “activated oxygen” species had little or no effect.
2. Caeruloplasmin retained its protective action when its oxidase activity was completely inhibited by azide, and when its copper ions had been removed.
3. The effect of caeruloplasmin, apocaeruloplasmin and albumin could not be attributed to a binding of iron ions to protein molecules.
International Journal of Biochemistry
Volume 15, Issue 8, 1983, Pages 1067-1071
Rolf A.Løvstad
Institute of Medical Biochemistry, University of Oslo, Sognsvannsveien 9, Oslo 3, Norway
Iron ion induced haemolysis: effect of caeruloplasmin, albumin and ascorbate (vitamin C) - ScienceDirect https://www.sciencedirect.com/science/article/pii/0020711X83900447
Effect of metabolic inhibitors on lauric acid-induced hemolysis
Abstract
Lauric acid reduced osmotic RBC fragility at low salt concentrations and caused hemolysis under normotonic conditions. KCN inhibited the hemolytic effect of l.a. under normotonic but did not influence its action under hypotonic conditions. It is concluded that l.a.-induced hemolysis requires penetration of the f.a. into a deep membrane pool whereas binding of l.a. in the superficial RBC-membrane pool is sufficient to reduce osmotic fragility of RBC at low salt concentrations.
Keywords
Salt Concentration Lauric Acid Metabolic Inhibitor Osmotic Fragility Hemolytic Effect
Agents and Actions
March 1973, Volume 3, Issue 1, pp 45–47 | Cite as
Authors
Authors and affiliations
Elisabeth BachmannGerhard Zbinden
1.
Models in Toxicology
Effect of metabolic inhibitors on lauric acid-induced hemolysis | Springer for Research & Development https://rd.springer.com/article/10.1007%2FBF02023851
Degradation of Hyaluronic Acid by Photosensitized Riboflavin In Vitro. Modulation of the Effect by Transition Metals, Radical Quenchers, and Metal Chelators
The effect of photoexcited riboflavin (RF) on the viscosity of hyaluronic acid (HA) solutions has been investigated.
UV irradiation of RF causes under aerobic conditions fragmentation of HA and a decrease in the viscosity of its solutions. A decrease of HA viscosity occurs in PO4-buffered solutions and is accelerated by high pH, Fe2+ (but much less so by Fe3+), certain metal chelators, and horseradish peroxidase (HRP); it is partially inhibited by catalase and less so by superoxide dismutase (SOD). The reactivity of the system was completely blocked by Tris, ethanol, aspirin, d-manitol, dimethylthiourea (DMTU), dimethylsulfoxide (DMSO), and sodium azide.
These results indicate that the most likely chemical species involved in the reaction is the hydroxyl radical. Singlet oxygen (102) generation is suggested by the ability of NaN3 and DMSO to completely inhibit the reactivity of the system. These two agents, however, may also interact with OH radical, as well and suppress the reactivity of the system. H2O2 and O2/̇016/− seem also to be produced in significant amounts, because catalase and SOD partially block the reactivity of the system. The effect of HRP may be due to hydrogen subtraction from HA and H2O2 reduction to water. Photoexcitation of RF may potentially occur in vitro and in vivo in the organs and tissues that are permeable to light, such as the eye or skin, and damage HA and other cell-matrix components causing inflammation and accelerating aging. © 1997 Elsevier Science Inc.
Free Radical Biology and Medicine
Volume 22, Issue 7, 1997, Pages 1139-1144
Author links open overlay panelElenaFratiabAbdel-MajidKhatibaPhilippeFrontaAndrejPanasyukacFranceAprileaDragoslav R.Mitrovica
Degradation of Hyaluronic Acid by Photosensitized Riboflavin In Vitro. Modulation of the Effect by Transition Metals, Radical Quenchers, and Metal Chelators - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0891584996005254
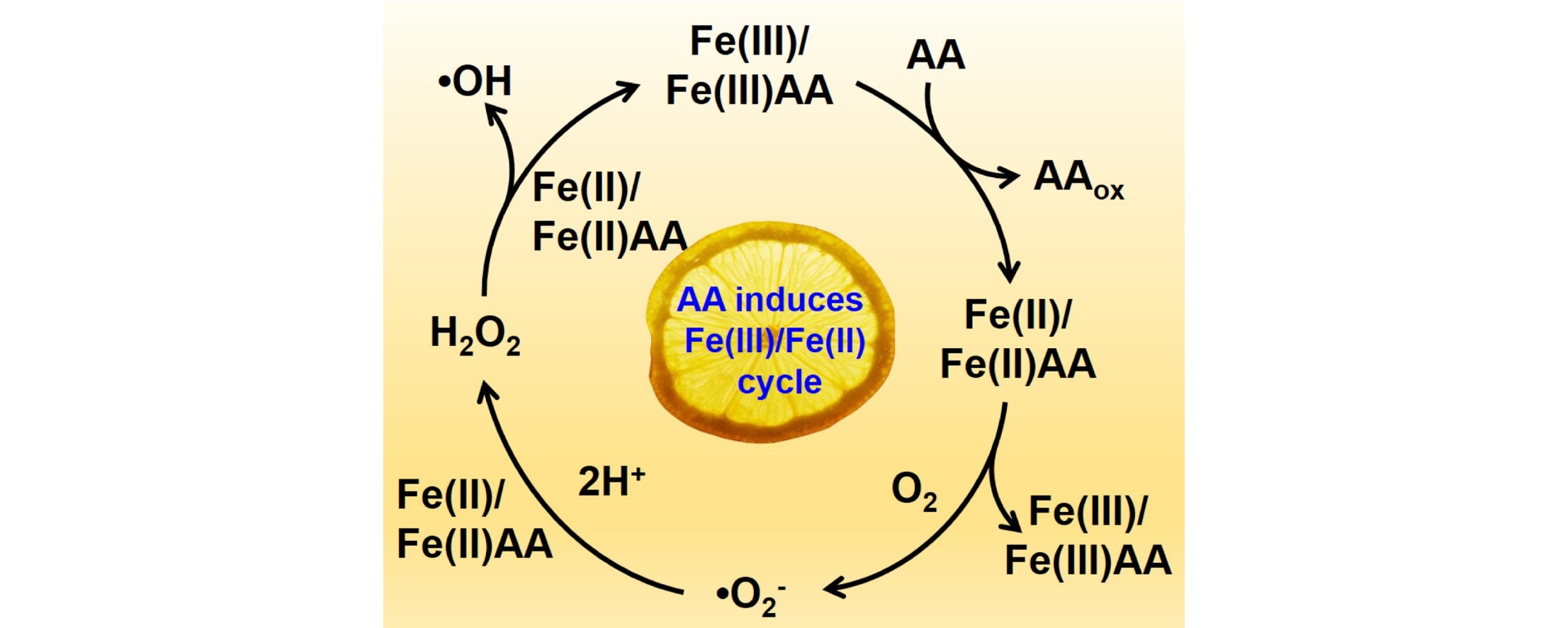
Ascorbic acid enhanced activation of oxygen by ferrous iron: A case of aerobic degradation of rhodamine B
Highlights
• Ascorbic acid could relieve the accumulation of Fe(III) by accelerating Fe(III)/Fe(II) cycle.
• Ascorbic acid could lower the redox potential of Fe(III)/Fe(II) through chelating effect.
• Ascorbic acid enhanced ROS generation for promoting RhB degradation.
Abstract
Molecular oxygen activation by ferrous ions (Fe(II)) in aqueous solution could generate reactive oxygen species (ROS) with high oxidation potential via reaction between Fe(II) and oxygen molecules (Fe(II)/air), however, ROS yielded in the Fe(II)/air process is insufficient for removal of organic pollutants due to the irreversible ferric ions (Fe(III)) accumulation. In this study, we demonstrate that ascorbic acid (AA) could enhance ROS generation via oxygen activation by ferrous irons (AA/Fe(II)/air) and thus improve the degradation of rhodamine (RhB) significantly. It was found that the first-order aerobic degradation rate of RhB in the AA/Fe(II)/air process in the presence of ascorbic acid is more than 4 times that of the Fe(II)/Air system without adding ascorbic acid. The presence of ascorbic acid could relieve the accumulation of Fe(III) by reductive accelerating the Fe(III)/Fe(II) cycles, as well as lower the redox potential of Fe(III)/Fe(II) through chelating effect, leading to enhanced ROS generation for promoting RhB degradation. This study not only sheds light on the effect of ascorbic acid on aerobic Fe(II) oxidation, but also provides a green method for effective remediation of organic pollutants.
Journal of Hazardous Materials
Volume 308, 5 May 2016, Pages 67-74
Key Laboratory of Pesticide & Chemical Biology of Ministry of Education, Institute of Environmental Chemistry, Central China Normal University, Wuhan 430079, PR China
Author links open overlay panelXiaojingHouWenjuanShenXiaopengHuangZhihuiAiLizhiZhang
https://doi.org/10.1016/j.jhazmat.2016.01.031
Ascorbic acid enhanced activation of oxygen by ferrous iron: A case of aerobic degradation of rhodamine B - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0304389416300310
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)