光动力疗法 - 单线态氧疗法作为广谱包膜病毒进入抑制剂
Photodynamic therapy-Singlet Oxygen Therapy
as broad-spectrum enveloped virus entry inhibitor
什么是单线态氧?
单线态氧(singlet oxygen)是分子氧分子(dioxygen molecule)的最低激发态。其在溶液中的寿命在微秒范围内(在水中3微秒至C6D6中约700微秒)。它与有机分子发生几种反应(Ene-Reaction,Diels-Alder Reaction)。这些反应已经研究了很多年。
在溶液中,单线态氧通常通过称为光敏化(photosensitization)的过程制备。将光敏剂照射到其单重激发态,然后转换(称为系统间交叉)至其三重激发态。三重激发的敏化剂可以进行自由基反应(I型过程)或产生单线态氧singlet oxygen (II型过程)。超氧化物(superoxide)形成(另一种活性氧物质)也是可能的,但是很少见。在低底物浓度和高氧浓度下,II型方法通常是优选的。
在其衰变回基态期间,单线态氧分子在近红外区域(1270nm)发射一些辐射。这种发光很弱,但可以检测到。
光动力疗法 - 单线态氧疗法,作为广谱包膜病毒进入抑制剂
Photodynamic therapy-Singlet Oxygen Therapy
as broad-spectrum enveloped virus entry inhibitor
什么是单线态氧?
单线态氧(singlet oxygen)是分子氧分子的最低激发态。其在溶液中的寿命在微秒范围内(在水中3微秒至C6D6中约700微秒)。它与有机分子发生几种反应(Ene-Reaction,Diels-Alder Reaction)。这些反应已经研究了很多年。
在溶液中,单线态氧通常通过称为光敏化的过程制备。将光敏剂照射到其单重激发态,然后转换(称为系统间交叉)至其三重激发态。三重激发的敏化剂可以进行自由基反应(I型过程)或产生单线态氧(II型过程)。超氧化物形成(另一种活性氧物质)也是可能的,但是很少见。在低底物浓度和高氧浓度下,II型方法通常是优选的。
在其衰变回基态期间,单线态氧分子在近红外区域(1270nm)发射一些辐射。这种发光很弱,但可以检测到。
Photodynamic therapy-Singlet Oxygen Therapy
as broad-spectrum enveloped virus entry inhibitor
What is Singlet Oxygen?
Singlet oxygen is the lowest excited state of the dioxygen molecule. Its lifetime in solution is in the microsecond range (3 µsec in water to about 700 µsec in C6D6). It undergoes several reactions with organic molecules (Ene-Reaction, Diels-Alder Reaction). These reactions have been studied for many years.
In solution, the singlet oxygen is often prepared by a process called photosensitization. A photosensitizer is irradiated to its singlet excited state, followed by conversion (called intersystem crossing) to its triplet excited state. The triplet excited sensitizer may undergo radical reactions (Type I process) or produce singlet oxygen (Type II process). Superoxide formation (another reactive oxygen species) is also possible, but rare. The type II process is usually preferred at low substrate concentration and high oxygen concentration.
During its decay back to the ground state, the singlet oxygen molecule emits some radiation in the near IR region(1270nm). This luminescence is very weak, but can be detected.
http://www.calstatela.edu/dept/chem/selke/r-1.htm
Singlet Oxygen in Nature
Throughout billions of years leaves of the tree have produced the oxygen we breath everyday. Singlet oxygen is formed in nature on the surface on leaves in a process called photosensitization. Plants produce singlet oxygen when the light from the sun hits their leaves and chlorophyll acts as a catalyst to transfer light into life energy producing singlet oxygen in the process.
Singlet oxygen is highly reactive so that within a very brief fraction of a second, the oxygen reverts to its ground state, releasing the energy into the water vapor in the air. When this humidified, activated air enters the lungs and is absorbed by cells in the body it triggers our own natural processes of healing, cell repair, stress reduction, and detoxification. This results in the reduction of free radical and stress induced aging processes and subsequent increased vitality, health, beauty, and well-being.
In the words of renown German Physician, Dr. Klaus Erpenbach:
“Oxygen is the most important element in our life. Without food we can survive weeks, without drinking up to 5 days, but without oxygen only minutes! Oxygen is the central part in each and every cell of our body.
In our cellular power stations the oxygen is transformed into energy and so used for regeneration, repair and power – in every cell. Thus an optimisation of the oxygen utilisation by applying the Singlet Oxygen Energy technology can lead to enhanced performance in every cell.
Scientific studies proved that stress hormones are normalised under Singlet Oxygen Energy application, you become more stress resistant, sleep better, are more relaxed in the mornings, and can start the day actively: well-tempered and with more power. Simultaneously the stress resistance affects the heart: by an increased heart variability and therefore improved performance.
Another clinical trial showed that the use of SOE technology resulted in a normalisation of the blood pressure after 4 weeks. Also based on scientific evidence, the lung function and the physical fitness of patients with COPD or bronchial asthma could be clearly improved after 4 weeks; the patients even experienced better sleep patterns and lower blood pressure.”
-Dr. Klaus Erpenbach
Singlet Oxygen | Essential Medicine https://www.essentialmedi.com/singlet-oxygen-therapy/
Harvard Univerisity: Singlet oxygen in natural waters
MANY studies have shown that singlet molecular oxygen can oxidise a variety of organic substances1-7. These studies have included biologically important compounds present in the aquatic environment such as amino acids4,7 and pollutants like polycyclic aromatic hydrocarbons5 and pesticides6. No data, however, have been obtained to demonstrate that reactions involving singlet oxygen (often called oxygenations) are sufficiently rapid to be significant in natural waters. The most likely mechanism for oxygenation in the environment was originally proposed by Kautsky8 by which light energy absorbed by a sensitiser is transferred to ground-state oxygen to form singlet oxygen, which in turn reacts with the organic substance, or `acceptor' (A), to form a peroxide. Here we present evidence that rapid photochemical generation of singlet oxygen occurs in inland and coastal water bodies of the south-eastern United States.
Singlet oxygen in natural waters http://adsabs.harvard.edu/abs/1977Natur.267..421Z
Singlet Oxygen Biochemistry
Reactions
Because of differences in their electron shells, singlet and triplet oxygen differ in their chemical properties; singlet oxygen is highly reactive.[22] The lifetime of singlet oxygen depends on the medium. In normal organic solvents, the lifetime is only a few microseconds whereas in solvents lacking C-H bonds, the lifetime can be as long as seconds.[21]
In photosynthesis, singlet oxygen can be produced from the light-harvesting chlorophyll molecules. One of the roles of carotenoids in photosynthetic systems is to prevent damage caused by produced singlet oxygen by either removing excess light energy from chlorophyll molecules or quenching the singlet oxygen molecules directly.
In mammalian biology, singlet oxygen is one of the reactive oxygen species, which is linked to oxidation of LDL cholesterol and resultant cardiovascular effects. Polyphenol antioxidants can scavenge and reduce concentrations of reactive oxygen species and may prevent such deleterious oxidative effects.[29]
Ingestion of pigments capable of producing singlet oxygen with activation by light can produce severe photosensitivity of skin (see phototoxicity, photosensitivity in humans, photodermatitis, phytophotodermatitis). This is especially a concern in herbivorous animals (see Photosensitivity in animals).
Singlet oxygen is the active species in photodynamic therapy.
Singlet oxygen - Wikipedia https://en.wikipedia.org/wiki/Singlet_oxygen
Imaging singlet oxygen luminescence in blood vessels
Photodynamic therapy (PDT) is a minimally invasive treatment for cancer and other diseases that uses light-activated compounds to attack malignant cells and tissues. Using a photosensitizer (an agent that transfers energy from incident light), PDT produces reactive oxygen species—predominantly singlet oxygen (1O2)—that are toxic to the targeted cells. Direct imaging of singlet oxygen near-IR (NIR) luminescence at around 1270nm reveals the spatial and temporal heterogeneity of tumors and their response to PDT. However, the imaging process is technically challenging because of the extremely high reactivity of singlet oxygen and its short lifetime in biological microenvironments.1–3 This can limit the chances of luminescence emission. Furthermore, current NIR detectors have very low quantum efficiency (the ratio of incident photons to converted electrons).
.png)
In summary, the novel configuration of an NIR-sensitive InGaAs camera and optimized light collection enables direct imaging of the singlet oxygen luminescence generated in blood vessels during PDT. With a 2s image integration time, the system is practical for many in vivo studies.
SOURCE:
Lisheng Lin, Huiyun Lin, Shusen Xie, Buhong Li, Defu Chen, Ying Gu and Brian C. Wilson
A novel configuration of a near-IR-sensitive camera and adaptive optics for fast imaging of singlet oxygen luminescence in vivo predicts the effectiveness of light-based therapies.
3 July 2014, SPIE Newsroom. DOI: 10.1117/2.1201406.005511
Imaging singlet oxygen luminescence in blood vessels | SPIE Homepage: SPIE http://spie.org/newsroom/5511-imaging-singlet-oxygen-luminescence-in-blood-vessels?SSO=1
The livestock photosensitizer, phytoporphyrin (phylloerythrin), is a substrate of the ATP-binding cassette transporter ABCG2
Article in Research in Veterinary Science 81(3):345-9 · January 2007 with 13 Reads
DOI: 10.1016/j.rvsc.2006.04.003 · Source: PubMed
Hepatogenous photosensitization occurs in livestock following damage to the liver or biliary apparatus that results in impaired excretion of phytoporphyrin (phylloerythrin), a photosensitizer. Based on earlier observations that porphyrin-based photosensitizers are substrates of the ATP-binding cassette transporter ABCG2, we examined the ability of the hepatic transporters ABCB1 (P-glycoprotein) and ABCG2 to transport phytoporphyrin. Transport of phytoporphyrin was blocked by the ABCG2-specific inhibitor fumitremorgin C (FTC) in human embryonic kidney cells transfected with full length human ABCG2, while no transport by cells transfected with human ABCB1 was noted. FTC-inhibited transport of phytoporphyrin was also demonstrated in ABCG2-expressing LLC-PK1 pig kidney cells, consistent with the idea that the pig orthologue, like human ABCG2, transports the photosensitizer. ABCG2 expression was confirmed by immunohistochemistry in the hepatocytes of cow, pig and sheep livers. We conclude that phytoporphyrin is a substrate for ABCG2 and that the transporter is likely responsible for its biliary excretion.
The livestock photosensitizer, phytoporphyrin (phylloerythrin), is a substrate of the ATP-binding cassette transporter ABCG2 | Request PDF https://www.researchgate.net/publication/6975697_The_livestock_photosensitizer_phytoporphyrin_phylloerythrin_is_a_substrate_of_the_ATP-binding_cassette_transporter_ABCG2
April 2010, Volume 34, Issue 4, pp 347–357 | Cite as
Detection of singlet oxygen in blood serum samples of clinically healthy lambs and lambs suffering from alveld disease
Authors and affiliations
Hanne Hjorth TønnesenEmail authorIvar MysterudJan KarlsenOlav M. SkulbergCarl M. M. LaaneTrond Schumacher
Abstract
Alveld is a disease in lambs of domestic sheep (Ovis aries L.), characterized by a combination of photosensitivity and liver damage. Generation of singlet oxygen play a major role in phototoxicity reactions. The compound phylloerythrin (phytoporphyrin) is so far assumed to be the main photodynamic agent in hepatogenous photosensitivity diseases in sheep. Phylloerythrin is a potent photosensitizer and an efficient source of singlet oxygen. The compound accumulates in the peripheral circualtion upon liver damage. Liver dysfunction is also likely to cause an increase in the blood level of bilirubin. Formation of singlet oxygen by bilirubin is reported. In the present work the photosensitizing potential of serum has been measured and related to the bilirubin- and phylloerythrin levels in lambs suffering from alveld and in clinically healthy controls. The singlet oxygen level of the serum was taken as a measure of the photosensitizing potential. The observed singlet oxygen values in serum from alveld lambs were significantly higher than the corresponding values observed in clinically healthy control lambs. This indicates that the serum of the alveld lambs contains an elevated concentration of photosensitizer. The singlet oxygen level was not correlated to the concentration of bilirubin or phylloerythrin. The results indicate that the photosensitizing mechanism is quite complex and may involve other sensitizer(s) than phylloerythrin.
Detection of singlet oxygen in blood serum samples of clinically healthy lambs and lambs suffering from alveld disease | SpringerLink https://link.springer.com/article/10.1007/s11259-010-9362-9
广谱病毒融合抑制剂产生的单线态氧对膜纳米结构的影响
Effects of singlet oxygen generated by a broad-spectrum viral fusion inhibitor on membrane nanoarchitecture
抽象
包膜病毒的靶向膜是探索广谱抗病毒药物开发的一个令人兴奋的新范例。最近,描述了广谱小分子抗病毒药物,在病毒结合后但在病毒 - 细胞融合之前防止包膜病毒进入中间步骤。这些化合物,包括名为JL103的恶唑烷-2,4-二硫酮,具有最有希望的结果,对病毒包膜有害但对细胞膜无影响。在这项工作中,通过使用原子力显微镜(AFM),我们旨在揭示JL103能够在纳米尺度的脂质膜结构中诱导的效应。我们的结果表明,JL103产生的单线态氧通过攻击不饱和磷脂的双键降低膜厚度,并且每个磷脂的面积扩大。这种膜重组阻止了包膜病毒和靶细胞膜之间的融合,阻止病毒进入细胞。
背景
最近,1-3中描述了一系列创新的广谱小分子抗病毒药物。这些分子防止包膜病毒在病毒结合和病毒 - 细胞融合之间的中间步骤进入。这些化合物对病毒包膜有害,但不影响细胞膜。后来,我们发现LJ001 1,3及其恶唑烷对应物(JL103)都属于这一新类别,可作为膜靶向光敏剂。它们在膜中产生单线态氧(1O2),改变其生物物理特性并导致靶细胞感染所需的病毒 - 细胞膜融合的抑制。1O2介导的脂质氧化靶向不饱和的C = C双键。磷脂酰基链,在脂质双层的疏水区域引入极性氢过氧化物基团。这些氧化磷脂的存在导致几种膜性质变化,这对其经历病毒 - 细胞融合所必需的极端膜曲率转变的能力产生负面影响。这些化合物仅影响病毒膜,没有明显的细胞毒性,也没有影响细胞 - 细胞融合。这与细胞质膜的生物学特性和静态病毒膜的生物学特性之间的差异有关。代谢活跃的宿主细胞具有修复由活性氧物质诱导的脂质损伤的内在机制,而病毒缺乏这种恢复膜改变的能力。
关键词:广谱抗病毒,单线态氧,原子力显微镜,膜组织
Effects of singlet oxygen generated by a broad-spectrum viral fusion inhibitor on membrane nanoarchitecture
Abstract
Targeting membranes of enveloped viruses represents an exciting new paradigm to explore on the development of broad-spectrum antivirals. Recently, broad-spectrum small-molecule antiviral drug were described, preventing enveloped virus entry at an intermediate step, after virus binding but before virus–cell fusion. Those compounds, including an oxazolidine-2,4-dithione named JL103 that presented the most promissing results, act deleteriously on the virus envelope but not at the cell membrane level. In this work, by using atomic force microscopy (AFM), we aimed at unraveling the effects that JL103 is able to induce in the lipid membrane architecture at the nanoscale. Our results indicate that singlet oxygen produced by JL103 decreases membrane thickness, with and expansion of the area per phospholipid, by attacking the double bonds of unsaturated phospholipids. This membrane reorganization prevents the fusion between enveloped virus and target cell membranes, resulting in viral entry inhibition.
Background
Recently, a family of innovative broad-spectrum small-molecule antiviral drugs was described 1–3. These molecules prevent enveloped viruses entry at an intermediate step, between virus binding and virus–cell fusion. These compounds act deleteriously on the virus envelope, but not at the cell membrane level. Later, we showed that LJ001 1, 3 and its oxazolidine counterpart (JL103) 2, 4, all belonging to this new class, act as membrane-targeted photosensitizers. They generate singlet oxygen (1O2) in the membrane, changing its biophysical properties and leading to the inhibition of the viral-cell membrane fusion necessary for target cell infection 4, 5. 1O2-mediated lipid oxidation targets the C=C double bonds of unsaturated phospholipid acyl chains, introducing polar hydroperoxide groups in the hydrophobic region of the lipid bilayer 6. The presence of these oxidized phospholipids results in several membrane properties changes, which negatively impact on its ability to undergo the extreme membrane curvature transitions necessary for virus-cell fusion. These compounds only affect the viral membrane, showing no significant cytotoxicity and not affecting cell–cell fusion1, 2, 4. This is related to the differences between the biogenic properties of cell plasma membranes and those of static viral membranes. Metabolically active host cells have intrinsic mechanisms to repair the lipid damage induced by reactive oxygen species, while viruses lack this ability to revert membrane alterations.
Keywords: Broad-spectrum antiviral, singlet oxygen, AFM, membrane organization
Axel Hollmann, PhD,1 Sónia Gonçalves, PhD,1 Marcelo T. Augusto, MSc,1 Miguel A. R. B. Castanho, PhD,1 Benhur Lee, PhD,2 and Nuno C. Santos, PhDcorresponding author1
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4476930/
Singlet oxygen effects on lipid membranes: implications for the mechanism of action of broad-spectrum viral fusion inhibitors.
Abstract
It was reported recently that a new aryl methyldiene rhodanine derivative, LJ001, and oxazolidine-2,4-dithione, JL103, act on the viral membrane, inhibiting its fusion with a target cell membrane. The aim of the present study was to investigate the interactions of these two active compounds and an inactive analogue used as a negative control, LJ025, with biological membrane models, in order to clarify the mechanism of action at the molecular level of these new broad-spectrum enveloped virus entry inhibitors. Fluorescence spectroscopy was used to quantify the partition and determine the location of the molecules on membranes. The ability of the compounds to produce reactive oxygen molecules in the membrane was tested using 9,10-dimethylanthracene, which reacts selectively with singlet oxygen (1O2). Changes in the lipid packing and fluidity of membranes were assessed by fluorescence anisotropy and generalized polarization measurements. Finally, the ability to inhibit membrane fusion was evaluated using FRET. Our results indicate that 1O2 production by LJ001 and JL103 is able to induce several changes on membrane properties, specially related to a decrease in its fluidity, concomitant with an increase in the order of the polar headgroup region, resulting in an inhibition of the membrane fusion necessary for cell infection.
Oxygen-dependent laser inactivation of murine norovirus using visible light lasers
Abstract
Background
Previous work indicated that an ultrashort pulse (USP) 425 nm laser is capable of inactivating murine norovirus (MNV: Virol. J. 11:20), perhaps via an impulsive stimulated Raman scattering (ISRS) mechanism, and does not substantially damage human plasma proteins (PLOS One 9:11). Here, further investigation of virus inactivation by laser light is performed.
Methods
In this study, we evaluate whether inactivation of MNV is specific to the USP wavelength of 425 nm, or if it occurs at other visible wavelengths, using a tunable mode-locked Ti-Sapphire laser that has been frequency doubled to generate femtosecond pulses at wavelengths of 400, 408, 425, 450, 465, and 510 nm. Continuous Wave (CW) lasers are also applied. Singlet oxygen enhancers are used to evaluate the sensitivity of MNV to singlet oxygen and oxygen quenchers are used to evaluate effects on virus inactivation as compared to untreated controls.
Results
> 3 log10 inactivation of MNV pfu occurs after irradiation with an average power of 150 mW at wavelengths of 408, 425 or 450 nm femtosecond-pulsed light for 3 h. Thus results suggest that the mechanism by which a laser inactivates the virus is not wavelength-specific. Furthermore, we also show that irradiation using a continuous wave (CW) laser of similar power at 408 nm also yields substantial MNV inactivation indicating that inactivation does not require a USP. Use of photosensitizers, riboflavin, rose bengal and methylene blue that generate singlet oxygen substantially improves the efficiency of the inactivation. The results indicate a photochemical mechanism of the laser-induced inactivation where the action of relatively low power blue laser light generates singlet oxygen.
Conclusion
Results suggest formation of short-lived reactive oxygen species such as singlet oxygen by visible laser light as the cause of virus inactivation rather than via an ISRS mechanism which induces resonant vibrations.
Oxygen dependence
Inactivation of MNV by the USPL with variable wavelengths of blue light (400–450 nm) and by 408 nm CW indicated the inactivation mechanism was not specific to the 425 nm wavelength and, although more substantial inactivation was achieved with femtosecond pulse light, was not dependent on light pulses. This suggested that inactivation may have been via a non-ISRS mechanism. Given that the MNV samples tested are essentially complex mixtures of organic molecules resulting from viral lysis of host cells, centrifugation, and passage through a 0.2 μm filter, it is conceivable that singlet oxygen, or other reactive oxygen species, were produced directly by the lasers light’s interaction as a result of interplay between complex organic molecules present within the MNV sample and light.
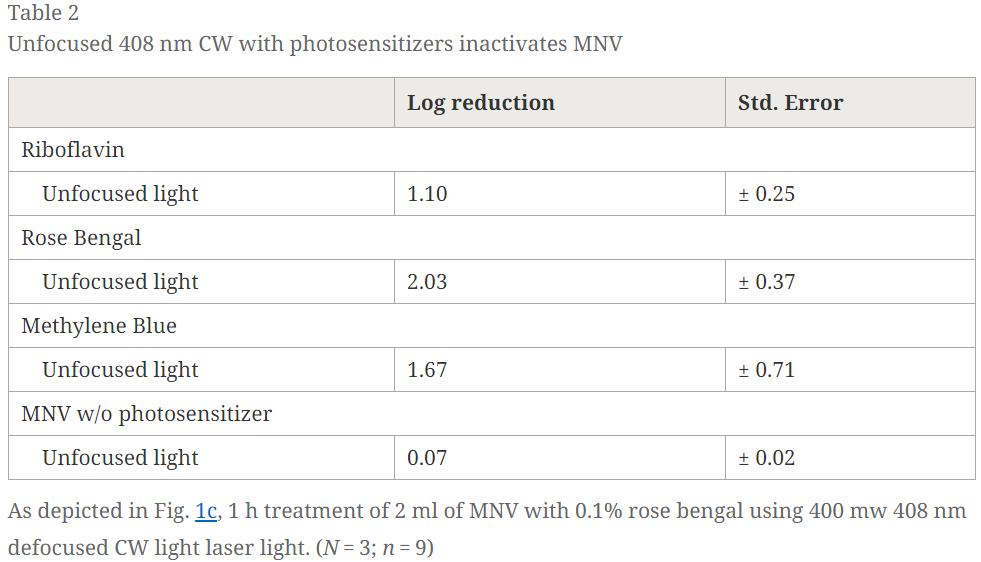
Therefore two different experiments were performed to establish whether photochemistry could be the cause of virus inactivation. First, singlet oxygen generators, also called enhancers, were evaluated for the ability to enhance the inactivation observed in the presence of light. Rose bengal, methylene blue, and riboflavin, substances known to interact with light to generated singlet oxygen and other reactive oxygen species, were added to MNV samples. As shown in Table 2, experiments indicate that the enhancers in the presence of defocused 408 nm CW light do cause substantial inactivation, while defocused 408 CW light alone had a limited effect on MNV. Second, a 532 nm 7 ps laser light, a laser that does not inactivate MNV, was used to evaluate inactivation in the presence of singlet oxygen enhancers. Results are shown in Table 3 and demonstrate that the 532 laser substantially inactivates MNV when 0.1% rose bengal is present. Overall, it is evident that MNV is sensitive to singlet oxygen.
Given that dissolved oxygen within the MNV solution would theoretically be required for visible light to convert dissolved O2 to O2 (a1∆g) or other reactive oxygen species, experiments utilizing sodium bisulfite as an oxygen scavenger were performed. As noted in Table 4, addition of 200 ppm and 1000 ppm sodium bisulfite substantially reduced inactivation observed by the 408 CW laser.
Lack of MNV genome damage
Lack of MNV genome damage
In order to test the effect of laser treatments on the MNV-1 RNA genome, RT-PCR was performed using 10-fold serially diluted RNA samples purified from USPL-treated virus samples. Visualization of 880 bp amplicons generated indicated that untreated, 2 and 4 h USP-treated samples gave identical amplifications, indicating that the viral RNA was not substantially damaged by the USPL treatments (Fig. 2).
DISCUSSION
In H2O, the lifetime of singlet oxygen is short, estimated to be approximately 3.5 microseconds [51], providing limited time for diffusion into the virus capsid. For example, an estimate of O2 (a1∆g) diffusion produced intracellularly was estimated to be only 10 to 160 nm [33]. Thus singlet oxygen’s site of production must be very close to its site of action. Presumably, singlet oxygen generated outside the capsid would not have sufficient time to defuse within the virus capsid to substantially damage the viral RNA.
Overall the implication that reactive oxygen species are induced by light within the visible spectra has substantial bearing on the field of environmental virology, as well as food production. This finding offers a potential explanation as to why norovirus illness was originally termed the “winter vomiting disease.” It has been thought that cooler environmental temperatures permitted the virus to remain viable for longer periods [1, 26]. Results here would seem to support the idea that shorter, less-intense solar irradiation in the winter may also contribute substantially to environmental persistence of noroviruses.
Biochem J. 2014 Apr 1;459(1):161-70. doi: 10.1042/BJ20131058.
Hollmann A1, Castanho MA1, Lee B2, Santos NC1.
Author information
PMID: 24456301 DOI: 10.1042/BJ20131058
Singlet oxygen effects on lipid membranes: implications for the mechanism of action of broad-spectrum viral fusion inhibitors. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/24456301/
Oxygen-dependent laser inactivation of murine norovirus using visible light lasers | Virology Journal | Full Text https://virologyj.biomedcentral.com/articles/10.1186/s12985-018-1019-2
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)