OXYGEN DELIVERY AND DIFFUSION INTO THE TISSUE AND CELLLS
1.血液循环是细胞获得氧气和养分、排出代谢废物的唯一途径。
2.氧气进入细胞的方式是被动运输~顺浓度梯度从血液通过组织液进入细胞。
3.代谢废物二氧化碳(CO2)有扩张血管、促进血红蛋白释放氧气的作用。
4.OTTO WARBURG在1923年发现正常细胞在氧气减少35%持续48小时条件下,会转变为癌细胞。
5.缺氧细胞会产生缺氧诱导因子HIF-1和NFkB因子,启动适应性生存策略包括有氧糖酵解的代谢方式。
6.肿瘤组织普遍循环不良缺氧缺血,远离血管400微米的区域存在坏死组织,吸引巨噬细胞和单核细胞进驻。
7.OTTO WARBURG发现,对比正常组织,从肿瘤组织流出的静脉血中的葡萄糖浓度显著降低,而乳酸水平显著上升。
8.FDG-PET通过分子影像追踪定位放射性葡萄糖在体内的分布,准确发现和定位恶性肿瘤。
9.癌细胞表面有超出正常细胞数量10~20倍的胰岛素受体和葡萄糖转运载体。
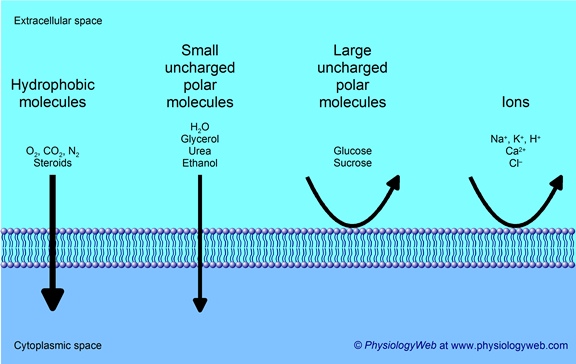
Oxygen crosses a plasma membrane by passive transport

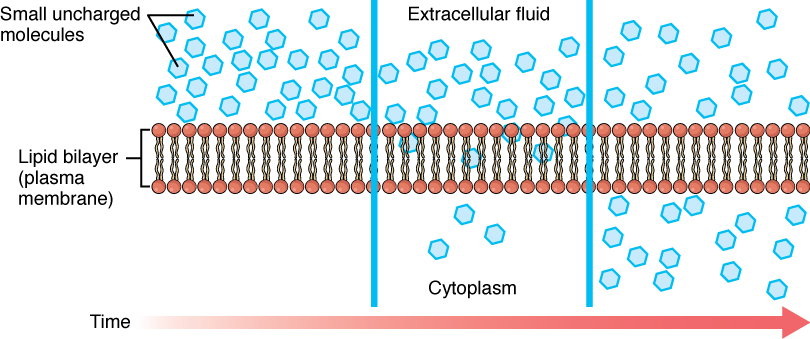
Figure 3.1.3 – Simple Diffusion Across the Cell (Plasma) Membrane: The structure of the lipid bilayer allows small, uncharged substances such as oxygen and carbon dioxide, and hydrophobic molecules such as lipids, to pass through the cell membrane, down their concentration gradient, by simple diffusion.
Passive transport is the transfer of molecules or ions without using energy, the
transfers of these molecules occur spontaneously, from high to low
concentrations, so passive transport it’s not like active transport that
requires energy to work, passive transport includes diffusion and osmosis
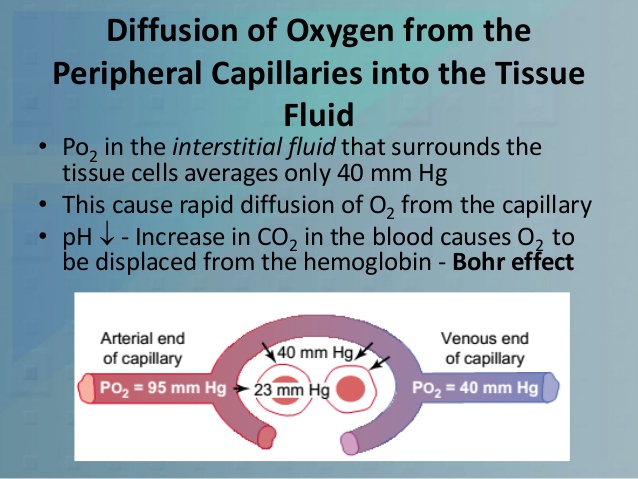
Diffusion of Oxygen from the Peripheral Capillaries into the Tissue Fluid
When the arterial blood reaches the peripheral tissues, its Po2 in the
capillaries is still 95 mm Hg.Yet, as shown in Figure 40-3, the Po2 in the
interstitial fluid that surrounds the tissue cells averages only 40 mm Hg. Thus,
there is a tremendous initial pressure difference that causes oxygen to diffuse
rapidly from the capillary
Diffusion of oxygen from a tissue capillary to the cells. (Po2 in interstitial fluid = 40 mm Hg, and in tissue cells = 23 mm Hg.)
Biochim Biophys Acta. Author manuscript; available in PMC 2007 Nov 23.
Department of Biophysics, Medical College of Wisconsin, Milwaukee, Wisconsin
53226, USA
Oxygen permeability of the lipid bilayer membrane made of calf
lens lipids
Abstract
The oxygen permeability coefficient across the membrane made of the total lipid
extract from the plasma membrane of calf lens was estimated from the profile of
the oxygen transport parameter (local oxygen diffusion-concentration product)
and compared with those estimated for membranes made of an equimolar
1-palmitoyl-2-oleoylphosphatidylcholine/cholesterol (POPC/Chol) mixture and of
pure POPC. Profiles of the oxygen transport parameter were obtained by observing
the collision of molecular oxygen with nitroxide radical spin labels placed at
different depths in the membrane using the saturation-recovery EPR technique and
were published by us earlier (J. Widomska, M. Raguz, J. Dillon, E. R. Gaillard,
W. K. Subczynski, Biochim. Biophys. Acta. Epub 2007 March 20). At 35°C, the
estimated oxygen permeability coefficients were 51.3, 49.7, and 157.4 cm/s for
lens lipid, POPC/Chol, and POPC membranes, respectively (compared with 53.3 cm/s
for a water layer with the same thickness as a membrane). Membrane permeability
significantly decreases at lower temperatures. In the lens lipid membrane,
resistance to the oxygen transport is located in and near the polar headgroup
region of the membrane to the depth of the ninth carbon, which is approximately
where the steroid-ring structure of cholesterol reaches into the membrane. In
the central region of the membrane, oxygen transport is enhanced, significantly
exceeding that in bulk water. It is concluded that the high level of cholesterol
in lens lipids is responsible for these unique membrane properties.
Keywords: oxygen permeation, lens lipids, lipid bilayer, cholesterol,
hydrophobic barrier, membrane rigidity, EPR
Oxygen permeability of the lipid bilayer membrane made of calf lens lipids
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2093700/
Chem Phys Lipids. 1981 May;28(3):269-79.
Effect of fatty acids and monoglycerides on permeability of
lipid bilayer.
Abstract
The effect of fatty acids and monoglycerides on barrier properties of liposomal
membranes prepared from egg phosphatidylcholine was investigated. The
incorporation of these lipids as liposomal membrane components induced the
alteration of the permeability to less permeable liposomally entrapped drugs,
sulfanilic acid and procainamide ethobromide (PAEB). Monoolein caused greatly
increased permeability of both drugs and unsaturated fatty acids markedly
enhanced the release rate of PAEB, while saturated fatty acids caused a small
increase in the release rate. Electron spin resonance (ESR) investigation with
5-nitroxide stearic acid showed that fatty acids disordered the hydrophobic
region of the lipid bilayer and the disordering effect of unsaturated fatty
acids was greater than that of saturated ones. It was demonstrated that the
incorporated fatty acids and monoglycerides interacted with the polar region of
the membranes by ESR study with cholestane label and 1H-NMR study. These results
indicated that the increase in the membrane permeability caused by fatty acids
and monoglycerides associated with the disorder in the membranes' interior and
the interaction of the incorporated lipid with the polar head group of
phospholipid.
Effect of fatty acids and monoglycerides on permeability of lipid bilayer. -
PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/6263505
Effect of fatty acids on the permeability barrier of model
and biological membranes
Highlights
•
In general, membrane perturbing effect of saturated fatty acids increases with
acyl chain length.
•
C12, C14 and C16 saturated fatty acids possess the highest cytotoxicity on MCF-7
cell line.
•
Saturated fatty acids in sub-cytotoxic concentrations cannot reduce the
permeability barrier of cell membranes.
•
The membrane perturbing effect of fatty acids on model membranes cannot simply
be carried over to biological membranes of live cells.
Abstract
Because of the amphipathicity and conical molecular shape of fatty acids, they
can efficiently incorporate into lipid membranes and disturb membrane integrity,
chain packing, and lateral pressure profile. These phenomena affect both model
membranes as well as biological membranes. We investigated the feasibility of
exploiting fatty acids as permeability enhancers in drug delivery systems for
enhancing drug release from liposomal carriers and drug uptake by target cells.
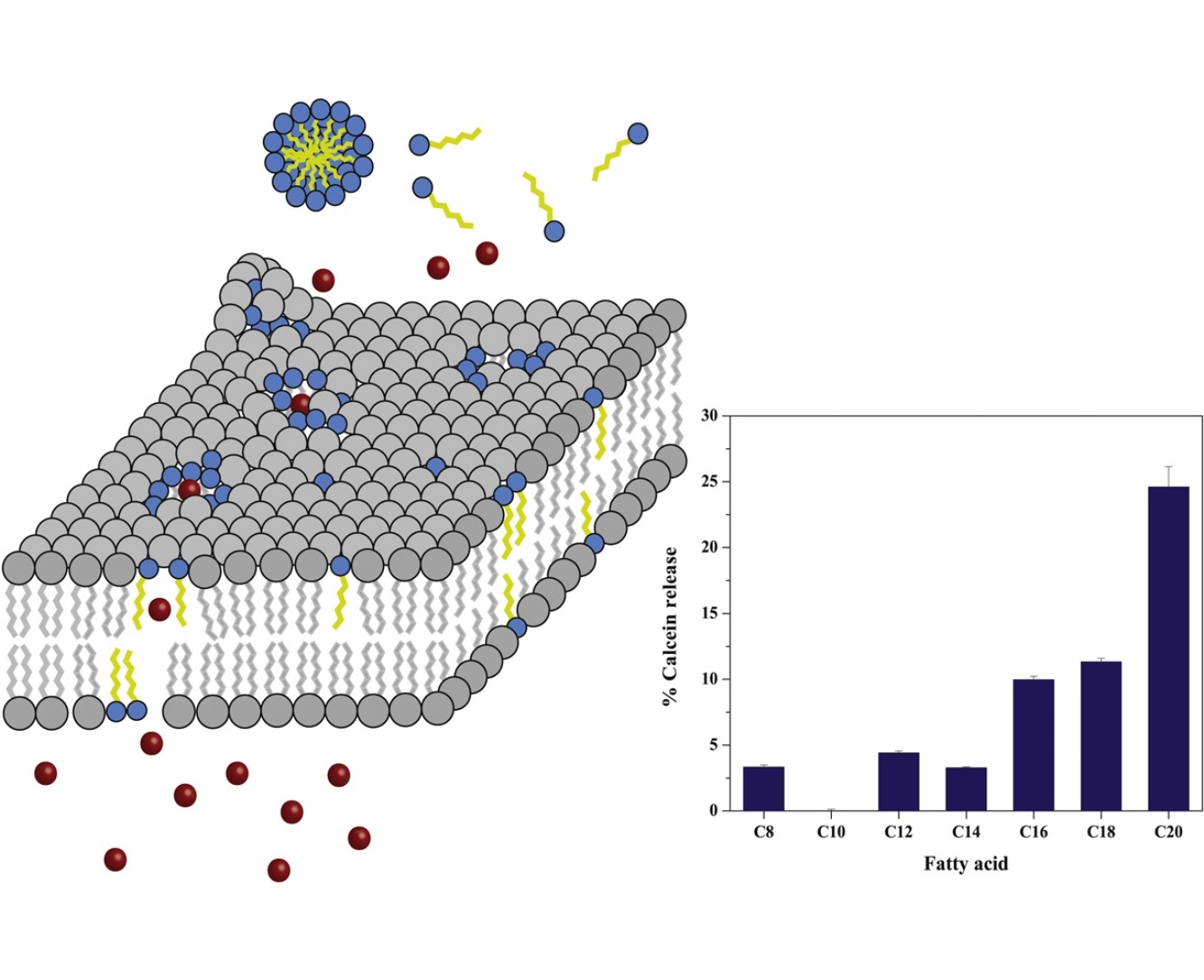
Saturated fatty acids, with acyl chain length from C8 to C20, were tested using
model drug delivery liposomes of 1,2- dipalmitoyl-sn-glycero-3-phosphocholine
(DPPC) and the breast cancer MCF-7 cell line as a model cell. A calcein release
assay demonstrated reduction in the membrane permeability barrier of the DPPC
liposomes, proportionally to the length of the fatty acid. Differential scanning
calorimetry (DSC) and dynamic light scattering (DLS) experiments revealed that
C12 to C20 fatty acids can stabilize DPPC liposomal bilayers and induce the
formation of large structures, probably due to liposome aggregation and bilayer
morphological changes. On the other hand, the short fatty acids C8 and C10 tend
to destabilize the bilayers and only moderately cause the formation of large
structures. The effect of fatty acids on DPPC liposomes was not completely
transferrable to the MCF-7 cell line. Using cytotoxicity assays, the cells were
found to be relatively insensitive to the fatty acids at apoptotic
sub-millimolar concentrations. Increasing the fatty acid concentration to few
millimolar substantially reduced the viability of the cells, most likely via the
induction of necrosis and cell lysis. A bioluminescence living-cell-based
luciferase assay showed that saturated fatty acids in sub-cytotoxic
concentrations cannot reduce the permeability barrier of cell membranes. Our
results confirm that the membrane perturbing effect of fatty acids on model
membranes cannot simply be carried over to biological membranes of live cells.
Effect of fatty acids on the permeability barrier of model and biological
membranes - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0009308416301232
Is the mammalian cell plasma membrane a barrier to oxygen
transport?
Oxygen transport in the Chinese hamster ovary (CHO) plasma membrane has been
studied by observing the collision of molecular oxygen with nitroxide radical
spin labels placed in the lipid bilayer portion of the membrane at various
distances from the membrane surface using the long-pulse saturation-recovery
electron spin resonance (ESR) technique. The collision rate was estimated for
5-, 12-, and 16-doxylstearic acids from spin-lattice relaxation times (T1)
measured in the presence and absence of molecular oxygen. Profiles of the local
oxygen transport parameters across the membrane were obtained showing that the
oxygen diffusion-concentration product is lower than in water for all locations
at 37 degrees C. From oxygen transport parameter profiles, the membrane oxygen
permeability coefficients were estimated according to the procedure developed
earlier by Subczynski et al. (Subczynski, W. K., J. S. Hyde, and A. Kusumi.
1989. Proceedings of the National Academy of Sciences, USA. 86:4474-4478). At 37
degrees C, the oxygen permeability coefficient for the plasma membrane was found
to be 42 cm/s, about two times lower than for a water layer of the same
thickness as the membrane. The oxygen concentration difference across the CHO
plasma membrane at physiological conditions is in the nanomolar range. It is
concluded that oxygen permeation across the cell plasma membrane cannot be a
rate-limiting step for cellular respiration. Correlations of the form PM = cKs
between membrane permeabilities PM of small nonelectrolyte solutes of mol wt
less than 50, including oxygen, and their partition coefficients K into
hexadecane and olive oil are reported. Hexadecane: c = 26 cm/s, s = 0.95; olive
oil: c = 23 cm/s, s = 1.56. These values of c and s differ from those reported
in the literature for solutes of 50 less than mol wt less than 300 (Walter, A.,
and J. Gutknecht. 1986. Journal of Membrane Biology. 90:207-217). It is
concluded that oxygen permeability through membranes can be reliably predicted
from measurement of partition coefficients.
Is the mammalian cell plasma membrane a barrier to oxygen transport? | JGP
http://jgp.rupress.org/content/100/1/69
Clin Cancer Res. Author manuscript; available in PMC 2010 Sep 19.
Blood Flow-Metabolism Mismatch: Good for the Tumor, Bad for the Patient
Departments of Radiology and Medicine, University of Washington and Seattle
Cancer Care Alliance, Seattle, WA
Summary
While tightly coupled in most normal tissues, blood flow and metabolism are
often not well matched in tumors. A flow-metabolism mismatch, specifically high
metabolism relative to blood flow, can be recognized in tumors by functional and
molecular imaging and is associated with poor response to treatment and early
relapse or disease progression.
In this issue of Clinical Cancer Research, Komar and colleagues from Turku
University report that malignant pancreatic tumors exhibit decreased blood flow
and increased glucose metabolism compared to the normal pancreas (1). This
mismatch between tumor blood flow and metabolism was associated with poor
survival.
In normal tissues, vascular physiology matches substrate delivery to energy
demand. Energy metabolism and blood flow are tightly coupled through a variety
of local auto-regulatory mechanisms (2), and under equilibrium conditions,
regional rates of metabolism and tissue perfusion are highly correlated. This
results in the efficient delivery and use of energy substrates in normal
tissues.
Unlike normal tissues, tumors have a highly disordered vascular supply (3).
Furthermore, tumor energy metabolism is often aberrant (4). Therefore, in
tumors, metabolism and blood flow may not be well matched. The ratio of glucose
metabolic rate relative to blood flow can be considerably elevated compared to
the tissue of origin for several reasons. The aberrant microvasculature
associated with tumors is ineffective at delivering oxygen, leading to
inefficient use of energy substrates and higher rates of metabolism of
substrates that do not require oxygen, such as glucose (3). Inadequate blood
supply that is unable to meet energy demands results in metabolic stress and low
oxygen levels, i.e, hypoxia. Hypoxia promotes gene expression via the
transcription factor, HIF-1, that leads to accelerated glycolysis (5). Even
under normoxic conditions, increased glycolysis may be favored as part of a
fundamental response to cellular stress that allows tumor cells to avoid cell
death in the face of unregulated growth and meet an extraordinary need for
energy and materials to support such growth (4). Accelerated glucose metabolism
has been recognized as a hallmark of the malignant phenotype dating back to the
early studies of Warburg (6). Through all of these mechanisms, altered
metabolism supports tumor cell survival under environmental stresses and can be
recognized as an aberrantly high rate of glucose metabolism per unit blood flow,
i.e. - a flow-metabolism mismatch.
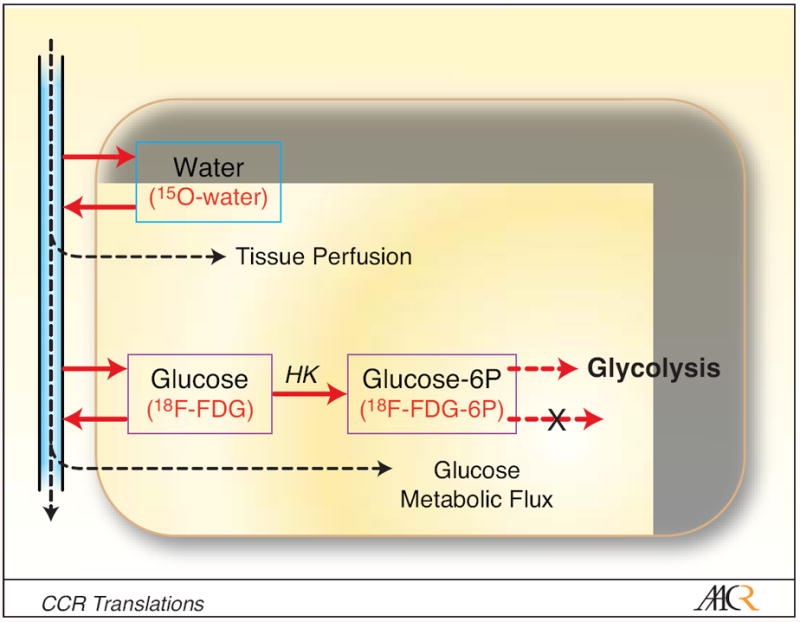
Functional imaging is a unique tool for measuring regional tumor perfusion and
metabolism (7). 18F-fluorodexyglucose positron emission tomography (FDG PET) can
quantify regional tumor glucose metabolism (Figure 1) and is widely used in
clinical oncology. Several quantitative imaging approaches, such as 15O-water
PET, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), and dynamic
contrast-enhanced computed tomography (CT), can measure regional tissue
perfusion. Combinations of FDG PET and perfusion imaging methods can delineate
regional variations in the metabolism/flow ratio. Combined 15O-water/FDG PET is
well suited for this task, since both studies can be performed in the same
imaging session without moving the patient.
Blood Flow-Metabolism Mismatch: Good for the Tumor, Bad for the Patient
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2941711/