Cancer Stem Cells Model
1. 肿瘤干细胞的代谢特征 metabolic traits of cancer stem cells
2. 肿瘤干细胞铁代谢的异常 Dysregulation of iron metabolism in cancer stem cells
3. 日本发现 肿瘤微环境中的血管内皮细胞、成纤维细胞起源于肿瘤干细胞-Cancer stem cells model as point of orgin of cancer-associated fibroblasts in tumor microenvionment
A Model of Cancer Stem Cells Derived from Mouse Induced Pluripotent Stem Cells
Ling Chen, Tomonari Kasai, Yueguang Li, Yuh Sugii, Guoliang Jin, Masashi Okada, Arun Vaidyanath, Akifumi Mizutani, Ayano Satoh, Takayuki Kudoh, Mary J. C. Hendrix, David S. Salomon, Li Fu , Masaharu SenoOkayama University, Japan
Published: April 12, 2012https://doi.org/10.1371/journal.pone.0033544Abstract
Cancer stem cells (CSCs) are capable of continuous proliferation and self-renewal and are proposed to play significant roles in oncogenesis, tumor growth, metastasis and cancer recurrence. CSCs are considered derived from normal stem cells affected by the tumor microenvironment although the mechanism of development is not clear yet. In 2007, Yamanaka's group succeeded in generating Nanog mouse induced pluripotent stem (miPS) cells, in which green fluorescent protein (GFP) has been inserted into the 5′-untranslated region of the Nanog gene. Usually, iPS cells, just like embryonic stem cells, are considered to be induced into progenitor cells, which differentiate into various normal phenotypes depending on the normal niche. We hypothesized that CSCs could be derived from Nanog miPS cells in the conditioned culture medium of cancer cell lines, which is a mimic of carcinoma microenvironment. As a result, the Nanog miPS cells treated with the conditioned medium of mouse Lewis lung carcinoma acquired characteristics of CSCs, in that they formed spheroids expressing GFP in suspension culture, and had a high tumorigenicity in Balb/c nude mice exhibiting angiogenesis in vivo. In addition, these iPS-derived CSCs had a capacity of self-renewal and expressed the marker genes, Nanog, Rex1, Eras, Esg1 and Cripto, associated with stem cell properties and an undifferentiated state. Thus we concluded that a model of CSCs was originally developed from miPS cells and proposed the conditioned culture medium of cancer cell lines might perform as niche for producing CSCs. The model of CSCs and the procedure of their establishment will help study the genetic alterations and the secreted factors in the tumor microenvironment which convert miPS cells to CSCs. Furthermore, the identification of potentially bona fide markers of CSCs, which will help the development of novel anti-cancer therapies, might be possible though the CSC model.
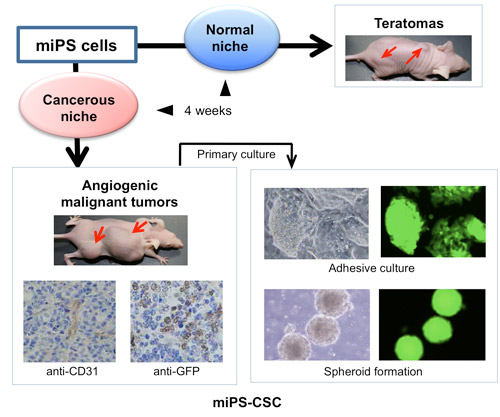
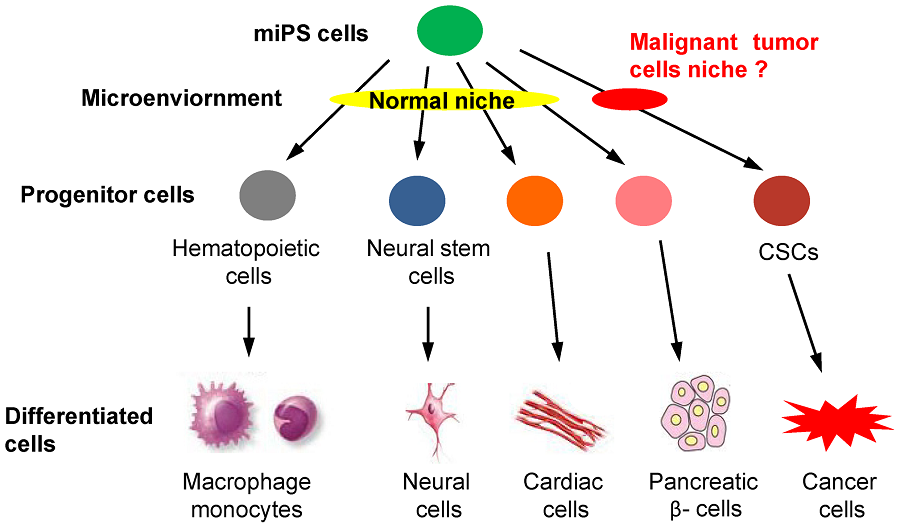
Figure 1. The hypothesis of miPS differentiation when exposed to normal or malignant niche.
miPS cells should be induced to some kinds of progenitor cells, such as hematopoietic cells and neural stem cells, differentiating into various phenotypes, such as macrophage, monocytes, neural cells, cardiac cells and pancreatic β- cells, when exposed to the normal niche. We hypothesized that CSCs may also be derived from miPS cells only when exposure to a malignant niche.A Model of Cancer Stem Cells Derived from Mouse Induced Pluripotent Stem Cells
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0033544
Current Issue
JSRM Vol.10; Iss.1
Cancer Stem Cells Converted from Pluripotent Stem Cells and the Cancerous Niche
Kasai T1, Chen L2, Mizutani AZ1, Kudoh T1, Murakami H1, Fu L3, Seno M1
Author Names in full: Tomonari Kasai1, Ling Chen2, Akifumi Mizutani1, Takayuki Kudoh1, Hiroshi Murakami1, Li Fu3, Masaharu Seno1
1 Department of Biotechnology, Graduate School of Natural Science and Technology, Okayama University, Okayama, Japan;
2 Department of Pathology, Tianjin Central Hospital of Gynecology Obstetrics, Tianjin, China;
3 Department of Breast Cancer Pathology and Research laboratory of Cancer Hospital, Tianjin Medical University, Tianjin, China.
Abstract
Nowadays, the cancer stem cells are considered to be significantly responsible for growth, metastasis, invasion and recurrence of all cancer. Cancer stem cells are typically characterized by continuous proliferation and self-renewal as well as by differentiation potential, while stem cells are considered to differentiate into tissue- specific phenotype of mature cells under the influence of micro-environment. Cancer stem cells should be traced to the stem cells under the influence of a micro-environment, which induces malignant tumors. In this review, we propose this micro-environment as a 'cancerous niche' and discuss its importance on the formation and maintenance of cancer stem cells with the recent experimental results to establish cancer stem cell models from induced pluripotent stem cells. These models of cancer stem cell will provide the great advantages in cancer research and its therapeutic applications in the future.
Figure 1: Hierarchy of differentiation of embryonic stem cells (ESCs) and induced stem cells (iPSCs). Stem cells are considered to undergo differentiation depending on unique microenvironment, so called niche, as well as self-renewal. Normal tissue type cells are produced under the effect of "Normal niche". Cancer stem cells (CSCs) are hypothetically considered induced from ESCs/iPSCs to produce cancer cells.
Figure 2 – A model of cancer stem cells. iPSCs were converted to CSCs (miPS-CSCs) under the treatment with conditioned medium from cancer derived cells regarding as "Cancerous niche" while normal culture condition as "Normal niche" (Chen et al., 2012). miPS-LLCcm cells, which was developed with the conditioned medium of Lewis lung carcinoma cells, showed angiogenic malignant tumor in nude mice in vivo. Primary culture from the tumor exhibited both potential of self-renewal and differentiation. Since GFP expression was directed under the control of Nanog promoter, the cells expressing GFP are judged undifferentiated.
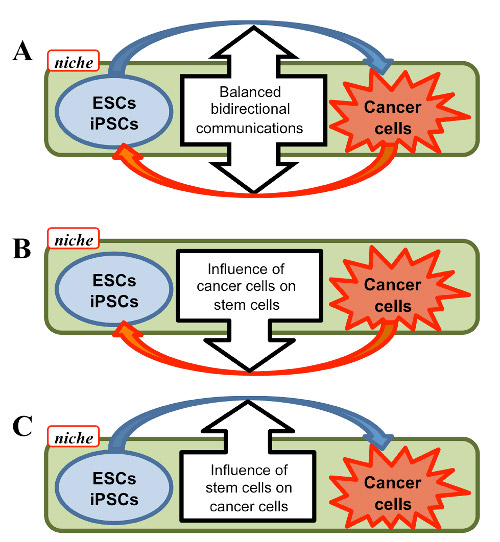
Figure 3 – Proposed cellular communication between ESCs/iPSCs and Cancer cells. cancer therapy and/or EMT. A, bidirectional communication balanced between stem and cancer cells. B, one-way influence of cancer cells on stem cells. C, one-way influence of stem cells on cancer cells. See text in detail.
Seno M et. al. - Cancer Stem Cells Converted from Pluripotent Stem Cells and the Cancerous Niche - Journal of Stem cells & Regenerative Medicine; JSRM- ISSN Number 0973-7154; a free online journal for students in medicine, life sciences and biotechnology
https://www.pubstemcell.com/monthly/010010200002.htm
iPSC-derived cancer stem cells provide a model of tumor ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5043102
Yan T, Mizutani A, Chen L, Sugii Y, Jin G, Okada M, Vaidyanath A, Mizutani A, Satoh A, Kudoh T, Hendrix MJC, Salomon DS, Fu L, Seno M. Characterization of cancer stem-like cells derived from mouse induced pluripotent stem cells transformed by tumor-derived extracellular vesicles.
Cited by: 5
Publish Year: 2016
Author: Marta Prieto-Vila, Ting Yan, Anna Sanchez Calle, Neha Nair, Laura Hurley, Tomonari Kasai, Hiroki Kak...
A three-dimensional model of human ... - Nature Cell Biology
https://www.nature.com/articles/ncb3510
Apr 24, 2017 · Chen et al. generate lung bud organoids from human pluripotent stem cells that recapitulate early lung development, such as branching airway formation …
Cited by: 121
Publish Year: 2017
Author: Ya-Wen Chen, Sarah Xuelian Huang, Ana Luisa Rodrigues Toste de Carvalho, Siu-Hong Ho, Mohammad Naimu...
Author: Ya-Wen Chen
Cancer Stem Cells: Implications for Cancer Therapy ...
https://www.cancernetwork.com/oncology-journal/cancer-stem-cells-implications-cancer...
Oct 28, 2019 · The cancer stem cell model proposes that cancer stem cells, which form a subset of the tumor cells, are ultimately responsible for tumor initiation, progression, and recurrence.[27] Through self-renewal and differentiation, cancer stem cells are responsible for the production of various tumor cells and contribute to tumor heterogeneity.
Cited by: 121
Publish Year: 2014
Author: Shaheenah Dawood, Laura Austin, Massimo COvarian cancer stem cells: still an elusive entity ...
https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-017-0638-3
Mar 20, 2017 · The cancer stem cell (CSC) model proposes that tumor development and progression are fueled and sustained by undifferentiated cancer cells, endowed with self-renewal and tumor-initiating capacity. Ovarian carcinoma, based on its biological features and clinical evolution, appears as a prototypical example of CSC-driven disease.
Cited by: 26
Publish Year: 2017
Author: Michela Lupia, Ugo Cavallaro
Anticancer effect of curcumin on breast cancer and stem cells
https://www.sciencedirect.com/science/article/pii/S2213453018300533
Curcumin treatment decreases the IC50 of cancer therapy drugs and the number of stem cells simultaneously. Zhou et al. treated the breast cancer cell lines MBA-MB-231 and MCF-7 with paclitaxel, cisplatin, or doxorubicin alone or combined with curcumin or other natural compounds.
Cited by: 10
Publish Year: 2018
Author: Hui-Tien Liu, Yuan-Soon HoThe Stem Cell Theory of Cancer | Ludwig Center | Stanford ...
https://med.stanford.edu/ludwigcenter/overview/theory.html
The Stem Cell Theory of Cancer. Research has shown that cancer cells are not all the same. Within a malignant tumor or among the circulating cancerous cells of a leukemia, there can be a variety of types of cells. The stem cell theory of cancer proposes that among all cancerous cells, a few act as stem cells that reproduce themselves and sustain...
Metabolic Traits of Cancer Stem Cells
Frontiers | Stem Cell Metabolism in Cancer and Healthy Tissues: Pyruvate in the Limelight | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2017.00958/full
How does Metformin Work?
Metformin accumulates inside the mitochondria, the little energy producing organelles in our cells. Once inside , metformin inhibits complex I of the mitochondrial electron transport chain. This in turn activates AMP-Kinase(AMPK), which then inhibits the mTOR signal pathway which reduces cancer cell proliferation.(4) Left Image Electron Microscopic Image of Mitochondria.Metformin Repurposed Anti-Cancer Drug - Jeffrey Dach MD
https://jeffreydachmd.com/2017/07/metformin-repurposed-anti-cancer-drug/
Published: 28 July 2017
A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment
Neha Nair, Anna Sanchez Calle, Maram Hussein Zahra, Marta Prieto-Vila, Aung Ko Ko Oo, Laura Hurley, Arun Vaidyanath, Akimasa Seno, Junko Masuda, Yoshiaki Iwasaki, Hiromi Tanaka, Tomonari Kasai & Masaharu Seno
Abstract
Cancer-associated fibroblasts (CAFs) are one of the most prominent cell types in the stromal compartment of the tumor microenvironment. CAFs support multiple aspects of cancer progression, including tumor initiation, invasion, and metastasis. The heterogeneous nature of the stromal microenvironment is attributed to the multiple sources from which the cells in this compartment originate. The present study provides the first evidence that cancer stem cells (CSCs) are one of the key sources of CAFs in the tumor niche. We generated CSC-like cells by treating mouse induced pluripotent stem cells with conditioned medium from breast cancer cell lines. The resulting cell population expressed both CSC and pluripotency markers, and the sphere-forming CSC-like cells formed subcutaneous tumors in nude mice. Intriguingly, these CSC-like cells always formed heterogeneous populations surrounded by myofibroblast-like cells. Based on this observation, we hypothesized that CSCs could be the source of the CAFs that support tumor maintenance and survival. To address this hypothesis, we induced the differentiation of spheres and purified the myofibroblast-like cells. The resulting cells exhibited a CAF-like phenotype, suggesting that they had differentiated into the subpopulations of cells that support CSC self-renewal. These findings provide novel insights into the dynamic interplay between various microenvironmental factors and CAFs in the CSC niche.A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment | Scientific Reports https://www.nature.com/articles/s41598-017-07144-5
Dis Model Mech. 2018 Aug 1; 11(8): dmm033464.
Published online 2018 Jun 28. doi: 10.1242/dmm.033464
Metabolic traits of cancer stem cells
ABSTRACT
Cancer stem cells are a subpopulation of cells within a tumour believed to confer resistance to standard cancer therapies. Although many studies have addressed the specific mechanisms of tumour recurrence driven by cancer stem cells, cellular metabolism is an often-neglected attribute. The metabolic features of cancer stem cells are still poorly understood, and they thus constitute a promising field in cancer research. The findings published so far point to a distinct metabolic phenotype in cancer stem cells, which might depend on the cancer type, the model system used or even the experimental design, and several controversies still need to be tackled. This Review describes the metabolic phenotype of cancer stem cells by addressing the main metabolic traits in different tumours, including glycolysis and oxidative, glutamine, fatty acid and amino acid metabolism. In the context of these pathways, we also mention the specific alterations in metabolic enzymes and metabolite levels that have a role in the regulation of cancer stemness. Determining the role of metabolism in supporting resistance to therapy driven by cancer stem cells can raise the opportunity for novel therapeutic targets, which might not only eliminate this resistant population, but, more importantly, eradicate the whole tumour in a relapse-free scenario.
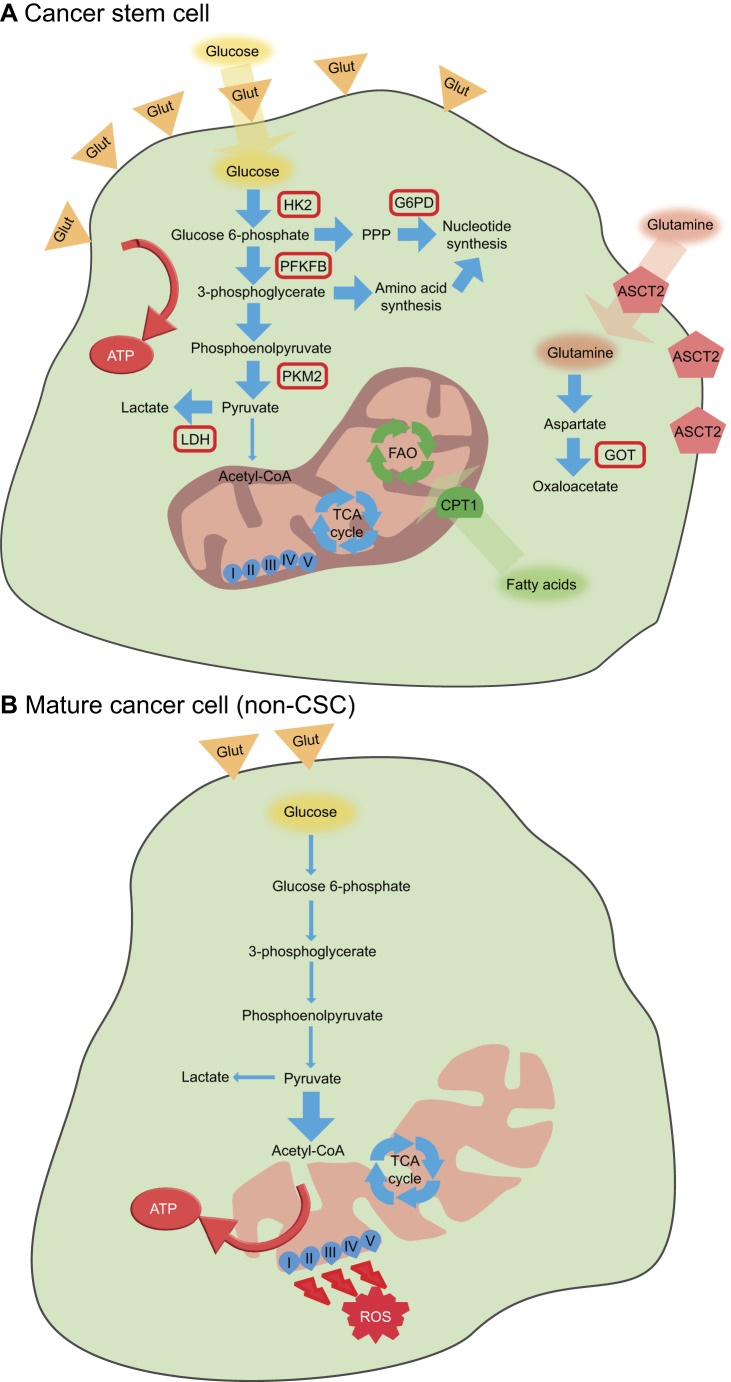
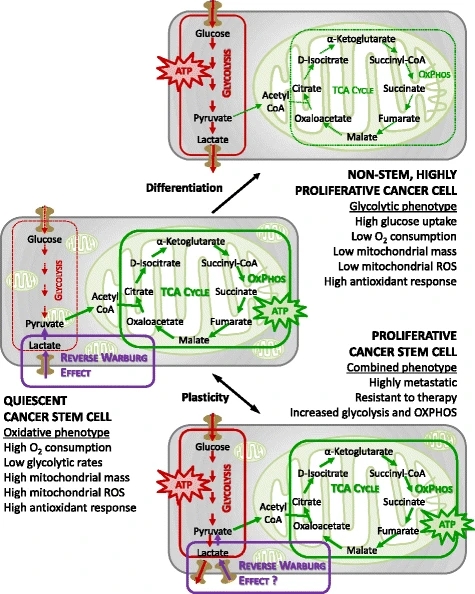
Fig. 1.
General metabolic features of cancer stem cells and mature cancer cells (non-CSCs).(A) Cancer stem cells tend to rely more on glycolysis for ATP synthesis, with overexpression of the glucose transporters GLUT1 and GLUT3, and increased expression of hexokinase 2 (HK2), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB), pyruvate kinase isozyme M2 (PKM2) and lactate dehydrogenase (LDH). Nucleotide biosynthesis is often increased in cancer stem cells owing to overexpression of glucose-6-phosphate dehydrogenase (G6PD) and amino acid synthesis. Glutamine uptake and metabolization to oxaloacetate, together with fatty acid oxidation, also appear to be important mechanisms in cancer stem cells.
(B) In contrast, mature cancer cells tend to rely more on OXPHOS for adenosine triphosphate (ATP) production, leading to increased levels of reactive oxygen species (ROS); these cells show low levels of glycolysis and nucleotide synthesis, although this can vary. ASCT2, alanine, serine, cysteine-preferring transporter 2; CPT1, carnitine-dependent transporter 1; FAO, fatty acid oxidation; Glut, glucose transporter (GLUT1 or GLUT3); GOT, glutamate-oxaloacetate transaminase; PPP, pentose phosphate pathway; TCA, tricarboxylic acid.
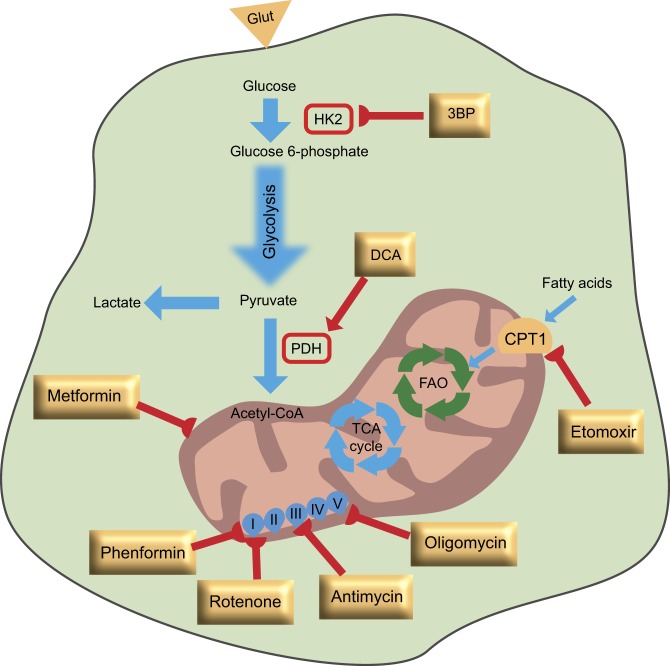
Fig. 2.
Metabolic targets of cancer stem cells. In general, metabolic inhibitors can sensitize cancer stem cells to standard anticancer therapies (highlighted in yellow rectangles), leading to their eradication. Specifically, in models in which cancer stem cells are more reliant on glycolysis, 3-bromopyruvate (3BP) or dichloroacetate (DCA) can reprogram the metabolism of these cells and sensitize them to chemotherapeutic agents. In cancer stem cells that exhibit increased oxidative phosphorylation (OXPHOS), inhibition of mitochondrial respiration by metformin, phenformin, rotenone, oligomycin or antimycin can trigger apoptosis. Inhibition of fatty acid oxidation (FAO) by etomoxir, which inhibits the carnitine-dependent transporter 1 (CPT1), leads to sensitization of cancer stem cells to apoptosis-inducing agents. Glut, glucose transporter; HK2, hexokinase 2; PDH, pyruvate dehydrogenase; I-V, mitochondrial respiratory chain complex I (NADH dehydrogenase subunit), complex II (succinate dehydrogenase subunit), complex III (ubiquinol-cytochrome c reductase complex subunit), complex IV (cytochrome c oxidase subunit) and complex V (ATP synthase subunit).
KEY WORDS: Cancer metabolism, Cancer stem cells, Therapy resistance, Tumour heterogeneity
Metabolic traits of cancer stem cells
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6124552/
Cancer stem cell metabolism
Maria Peiris-Pagès, Ubaldo E. Martinez-Outschoorn, Richard G. Pestell, Federica Sotgia & Michael P. Lisanti
Breast Cancer Research volume 18, Article number: 55 (2016) Cite this article
Abstract
Cancer is now viewed as a stem cell disease. There is still no consensus on the metabolic characteristics of cancer stem cells, with several studies indicating that they are mainly glycolytic and others pointing instead to mitochondrial metabolism as their principal source of energy. Cancer stem cells also seem to adapt their metabolism to microenvironmental changes by conveniently shifting energy production from one pathway to another, or by acquiring intermediate metabolic phenotypes. Determining the role of cancer stem cell metabolism in carcinogenesis has become a major focus in cancer research, and substantial efforts are conducted towards discovering clinical targets.
Cancer, above all other diseases, has countless secondary causes.
But even for cancer, there is only one prime cause …: metabolism.
Otto Warburg
The cancer stem cell model: Omnis cellula e cellula
Adult stem cells, in contrast to most cells in our body, which are differentiated and have a specific role, are rare cells that harbour unique biological properties such as a lack of differentiation and indefinite self-renewal. Stem cell asymmetrical division into one new stem cell and a committed progenitor, which can give rise to a functionally mature progeny, helps maintain tissue homeostasis [1].
Cancer is characterized by an unrestrained proliferation of malignant cells that are morphologically and functionally different. Two models have been proposed in order to explain this cellular diversity within tumours. The traditional, stochastic way of explaining cancer initiation and development is through sequential accumulation of mutations, each of which promotes the loss of specific tissue traits until dedifferentiation and regression into a more primitive phenotype occurs. According to this clonal evolution model, each cancer cell has a similar potential to grow a tumour. A second model, the cancer stem cell (CSC) hypothesis, postulates that a reduced group of stem-like cells is responsible for the development of the disease. Accordingly, tumours are hierarchically organized and sustained by a distinct self-renewal subpopulation of cancer cells. These tumour-initiating cells (TICs) with stemness properties are located at the apex of a pyramid and are responsible for the generation of a varied progeny of highly proliferative cells forming the bulk of the tumour [1, 2]. Both models are not mutually exclusive and can be viewed as integrated processes because CSCs can themselves undergo clonal evolution, through which a second more dominant population of CSCs may emerge. In addition, recent reports add more complexity to this scenario by demonstrating that cancer cells have a remarkable degree of plasticity. Indeed, it is thought that CSCs may arise from different cell types such as normal adult stem cells or differentiated cancer cells [2, 3].
CSCs share numerous properties with normal stem cells besides their ability to renew themselves by remaining in an undifferentiated state: the expression of surface markers, such as CD44, CD133 or the enzyme aldehyde dehydrogenase (ALDH), the activation of particular cell signalling pathways, such as Wnt, Notch or Hedgehog, a relative quiescence or an active DNA repair capacity [2]. Given that CSCs are considered to be the source from which cancer cells arise, are therapy resistant and are responsible for metastatic dissemination, eliminating them could potentially achieve a permanent cure for the patient. In addition, if conventional therapy fails to kill CSCs, acting only against differentiated cancer cells, the tumour can eventually relapse [2]. The specific elimination of CSCs may thus represent one of the most important challenges of current cancer research (Fig. 1). Because of their similarity, an accurate distinction between CSCs and normal stem cells is needed, and once these differences are identified new therapies can be developed to eliminate CSCs without damaging normal cells. In particular, the metabolic features of CSCs might represent a promising target. In this review we summarize the latest findings and most significant discoveries on CSC metabolism.
Bioenergetic pathways underlying CSC metabolism. In more differentiated cancer cells, the glycolytic phenotype might predominate over oxidative phosphorylation (OXPHOS). CSCs instead might rely more on an oxidative metabolism for their energy production. CSCs also appear to be metabolically plastic: when OXPHOS is blocked they can eventually develop resistance by acquiring an intermediate glycolytic/oxidative phenotype. ROS reactive oxygen species, TCA tricarboxylic acid
Cancer stem cell metabolism | Breast Cancer Research | Full Text
https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-016-0712-6
Stem Cell Metabolism in Cancer and Healthy Tissues: Pyruvate in the Limelight
Cyril Corbet*
Pole of Pharmacology and Therapeutics, Institut de Recherche Expérimentale et Clinique, Université catholique de Louvain, Brussels, Belgium
Normal and cancer stem cells (CSCs) share the remarkable potential to self-renew and differentiate into many distinct cell types. Although most of the stem cells remain under quiescence to maintain their undifferentiated state, they can also undergo cell divisions as required to regulate tissue homeostasis. There is now a growing evidence that cell fate determination from stem cells implies a fine-tuned regulation of their energy balance and metabolic status. Stem cells can shift their metabolic substrate utilization, between glycolysis and mitochondrial oxidative metabolism, during specification and/or differentiation, as well as in order to adapt their microenvironmental niche. Pyruvate appears as a key metabolite since it is at the crossroads of cytoplasmic glycolysis and mitochondrial oxidative phosphorylation. This Review describes how metabolic reprogramming, focusing on pyruvate utilization, drives the fate of normal and CSCs by modulating their capacity for self-renewal, clonal expansion/differentiation, as well as metastatic potential and treatment resistance in cancer. This Review also explores potential therapeutic strategies to restore or manipulate stem cell function through the use of small molecules targeting the pyruvate metabolism.
IGURE 1. Metabolic requirements in pluripotent stem cells and differentiated cells. Most of the quiescent stem cell populations rely on glycolysis to provide ATP and building blocks necessary for the synthesis of lipids, proteins or nucleotides. Along differentiation, energy metabolism shifts toward mitochondrial OXPHOS to support the increasing energy demands. Glycolysis-associated enzymes, such as hexokinase (HK), pyruvate kinase (PK) and lactate dehydrogenase A (LDHA), are highly expressed in PSCs. Pyruvate import into mitochondria is repressed by high expression of uncoupling protein 2 (UCP2). UCP2 is upregulated by accumulation of phosphatidylinositol phosphate (PIP) substrates following the downregulation of the mitochondrial phosphatase PTPMT1. αKG, α-ketoglutarate; ATP, adenosine triphosphate; CS, citrate synthase; G6P, glucose 6-phosphate; HIF, hypoxia-inducible factor; MPC, mitochondrial pyruvate carrier; PDH, pyruvate dehydrogenase; PDK, PDH kinase; OAA, oxaloacetate. Elevated metabolic pathways are indicated with bold arrows.
Frontiers | Stem Cell Metabolism in Cancer and Healthy Tissues: Pyruvate in the Limelight | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2017.00958/full
Cancer Stem Cells Under Hypoxia as a Chemoresistance Factor in the Breast and Brain
Over the last 15 years, basic science and clinical studies have been conducted to identify cancer stem cells (CSCs) in several types of cancer to reveal their mechanistic involvement in cancer recurrence for therapeutic exploitation. Exposure of cancer cells and tissues to hypoxia, or sub-atmospheric concentrations of oxygen (<21 % O2), stimulates a variety of stress responses that bias the cells toward a self-preserving, anti-apoptotic phenotype, properties that are found in CSCs. Despite major advances in our understanding of hypoxia, CSCs, and their interrelated nature, some of the most promising cancer therapy has been of limited clinical efficacy, in part because of the inherently hypoxic nature of growing tumors. In this paper we discuss recent findings regarding the behavior of breast and brain CSCs under hypoxia, and the mechanisms of their chemo and/or radioresistance and metastatic potential.
Keywords
Cancer stem cells Hypoxia Chemoresistance Metastasis Breast cancer Brain cancer
Introduction
Cancer stem cells (CSCs) are slow-dividing subpopulations of tumors with the ability to repopulate eradicated cancer cells by asymmetric cell division, resulting in cancer recurrence and patient mortality. CSCs have the same intrinsic properties as healthy stem cell populations found throughout the body, including the ability for self-renewal and capacity for multilineage differentiation [1]. Over the last 15 years, basic science and clinical studies have been conducted to identify CSCs in several types of cancer to reveal the complexities of their behavior for therapeutic exploitation.
Exposure of cells and tissues to hypoxia, or sub-atmospheric concentrations of oxygen (<21 % O2), stimulates a variety of stress response pathways that bias the cells toward a self-preserving, anti-apoptotic phenotype. As early as 1906, evidence for the protective effects of hypoxia against radiation, in both cancer and healthy tissue, began to accumulate in the literature [2, 3]; however, dissection of hypoxia-dependent pathways has come to the forefront of cancer and radiation research only in the past few decades. Exposure of cells and tissues to hypoxia has revealed a tightly-regulated cascade of events that lead to increased expression of pro-survival factors that impart chemo and/or radioresistance [4, 5, 6]. In short, hypoxia stabilizes the hypoxia inducible factor-α proteins, which form a heterodimeric transcription factor complex. This complex binds the hypoxia-responsive elements of transcriptional targets involved in various pro-survival processes, for example angiogenesis, metabolism, and proliferation [7]. Because of this, the hypoxic state and the expression level of HIF1α tend to correlate with more aggressive tumors and poor patient prognosis [8, 9, 10]. The exact mechanisms by which hypoxia induces its protective effects are complex and remain under investigation, but are broadly recognized to include:
increased genomic instability and aberrant cell cycling [8, 11, 12, 13, 14, 15, 16];
dysregulated reactive oxygen species and redox mechanisms [17, 18, 19, 20, 21, 22];
a metabolic shift toward aerobic glycolysis [23, 24, 25, 26, 27]; and
mitigated expression of pro-apoptotic factors [28, 29, 30, 31, 32].
Despite major advances in our understanding of hypoxia, CSCs, and their interrelated nature, some of the most promising cancer therapy has been of limited clinical efficacy over the past few years, in part because of the inherently hypoxic nature of growing tumors. In this paper we discuss recent findings regarding the behavior of breast and brain CSCs under hypoxia, and the mechanisms of their chemo and/or radioresistance and metastatic potential.
Breast CSCs
The involvement of CSCs in breast cancer was first discovered in 2003 when a CD44+CD24−/low Lineage− subpopulation of tumor cells from human patient samples were shown to have tumor-initiating capacity in immunodeficient mice [33]. Interestingly, injection of as few as 100 of these tumorigenic cells gave rise to palpable tumors within 12 weeks. Continual serial passaging of these cells yielded a similar subpopulation. CD44− cells from the patient tumor samples were non-tumorigenic, even when 10,000 cells were injected. In the years since, there has been an exponential increase in the number of breast cancer research studies attempting to identify the mechanisms by which breast CSCs resist classical chemotherapy and/or radiation and to identify the signaling that drives tumor recurrence.
Because tumors require blood perfusion to maintain viability and proliferation, anti-angiogenic therapy (i.e. therapy that prevents vascularization of the tumor) has been regarded as promising intervention for cancer treatment [34]. However, limited efficacy of anti-angiogenic therapy has been observed in clinical and pre-clinical studies, including studies of mouse models that demonstrated increased invasiveness and metastasis after inhibition of the vascular endothelial growth factor (VEGF) pathway [35, 36]. In 2009, Conley et al. [37] demonstrated that treatment with an anti-angiogenic agent, sunitinib, increased intratumoral hypoxia, which stimulated an increase in the number of aldehyde dehydrogenase (ALDH)-positive CSCs in vitro through a HIF1α-dependent mechanism. Furthermore, the tumors that developed after in vivo implantation of immortalized cancer cells were much larger in animals treated with sunitinib than in vehicle-treated controls.
Another recent study demonstrated an inverse correlation between the number of CSCs present in a given cell population and the enhanced chemoprotective effect of hypoxia in four immortalized cell lines and two samples derived from patients with recurring breast cancer [38]. Specifically, the resistance to radiation of cell populations in which the percentage of CSCs was low was greatly enhanced by culture under hypoxic conditions; however, for populations with a high percentage of CSCs, or for any cell population cultured in CSC-enriched mammospheres, no additional protective effect against radiation damage was observed when the cells were cultured under hypoxic, as compared with normoxic, conditions. The authors conducted gene-level screening of relevant antioxidative enzymes and concluded that expression of superoxide dismutase 2 (SOD2), but not SOD1, proportionally correlated with the number of breast CSCs in the population. These results suggest that breast CSCs have resistance to radiation via an SOD2-mediated, oxygen concentration-independent mechanism.
The hypoxia-inducible protein carbonic anhydrase IX (CAIX) regulates tumor pH and cell survival by improving the transport of acids that accumulate within the tumor because of the large distances between cells and capillaries [39]. In a recent study, inhibition of CAIX gene expression or functional activity inhibited breast CSC expansion under hypoxia, an effect that was mediated downstream by the mammalian target of rapamycin complex 1 (mTORC1) [40]. Defined markers of tumorigenic breast CSCs (CD44+CD24−/low) were used to show that gene-level knockdown of CAIX reduced the number of breast CSCs in vitro, and their potential to undergo the epithelial-to-mesenchymal transition (EMT). Furthermore, treatment with a specific, small-molecule inhibitor of CAIX, U-104, reduced the volume of orthotopic tumors in mice and virtually eliminated metastatic capability of primary tumors to the lungs, indicating the potential for CAIX as a novel therapeutic target for breast cancer treatment.
The status of estrogen receptor (ER) expression is also considered a strong indicator of the hypoxic response of breast CSCs, including mammosphere formation capacity and resistance to anti-angiogenic therapies [41•]. In a recent article by Harrison et al. [41•], 13 patient-derived samples and four immortalized cell lines were classified as either ER-α-positive or negative, and their response to hypoxia was characterized. Mammosphere formation in vitro and tumor formation in vivo of ER-α-positive cells, but not ER-α-negative cells, was enhanced under 1 % oxygen, as compared with 21 % oxygen, through a hypoxia inducible factor 1-alpha (HIF1α)-dependent mechanism. Because Notch1 is a downstream mediator of ER-α and has been shown to be involved in breast CSC maintenance and proliferation [42], the authors further demonstrated that hypoxic culture of ER-α-positive cells stimulated upregulation of Notch genes, and that mammosphere formation capacity under hypoxic conditions could be reduced by specifically blocking Notch activity with either gamma secretase inhibitor (GSI) or shRNA. Finally, the authors used an in-vivo xenograft model to demonstrate that the size of ER-α-positive tumors correlated with the proportion of CSCs in the tumor, but an inverse correlation was observed for ER-α-negative tumors. Taken together, this study reveals that ER status regulates the response to hypoxia in breast CSCs via Notch- and HIF1α-dependent pathways, and might provide new insights leading to anti-angiogenic clinical therapy.
To elucidate the specific involvement of Notch signaling in hypoxia-induced tumor metastasis, Xing et al. [43••] analyzed expression of Notch ligands in 779 breast cancer patients and identified a significant correlation between Jagged2 expression and patient survival. Furthermore, Notch and Jagged2 were strongly upregulated at the hypoxic invasive front in immunohistochemistry samples from 61 patients, and in-vitro hypoxic culture induced Jagged2 activation and EMT, an effect that was susceptible to blockage by the Notch inhibitor, GSI. This large-scale study confirmed involvement of the Notch pathway, specifically Jagged2, in hypoxia-driven breast cancer metastasis, and provided a potential prognostic marker for future clinical applications.
Another protein called CD44, a transmemebrane glycoprotein that binds hyaluronic acid, has been associated with aggressive, metastatic breast cancers [44]. A recent study investigated the relationship between hypoxia and a variety of CD44 isoforms in two immortalized breast cancer cell lines, MDA-MB-231 and SUM-149 [45]. Both cell lines were triple-negative (i.e. did not express estrogen receptor (ER), progesterone receptor (PR), or Her2-neu), indicative of the most aggressive, lethal forms of breast cancer. The authors induced hypoxia by exposure to either 0.2 % O2 or 200 μM CoCl2; this stimulated significant upregulation of two CD44 isoforms, and HIF1α and HIF2α. By use of RNA interference techniques, hypoxia-stimulated expression of CD44 variants 6 and 8 was shown to be regulated by HIF1α, but not HIF2α, at both the gene and protein-levels. Finally, CD44 expression was shown to correlate with regions of tumor hypoxia in vivo, confirming the in-vitro findings.
The tumor suppressor Period2 (PER2) is a circadian clock protein that, when lost, promotes invasion, metastasis, and the EMT, and correlates with poor prognosis in breast cancer patients through a mechanism of action that was recently described [46]. PER2 was shown to act as a transcriptional co-repressor by recruiting the polycomb proteins EZH2, SUZ12, and HDAC2 to the OCT4 binding sites of multiple EMT-related genes, including TWIST1 and SLUG, thereby preventing EMT and invasion. Furthermore, exposure to hypoxia resulted in degradation of PER2, which disrupted its repressive activity, and stimulated expression of EMT genes; this relationship was corroborated in clinical samples in which a negative correlation between hypoxia and PER2 was demonstrated.
With advances in general understanding of microRNAs (miRNAs), the involvement of a variety of these in regulating the response of breast CSCs to hypoxia is attracting interest. In 2011 Hwang-Verslues et al. [46] reported that miRNA-495 regulates the aggressiveness and hypoxic response of breast CSCs both in vitro and in vivo. When immortalized breast cancer cells devoid of CSCs were transfected with miRNA-495, significant increases in colony-formation ability and invasive capacity in vitro and tumor formation capacity (i.e. tumor volume) when implanted in vivo were observed. These results were shown to correlate with reduced E-cadherin and REDD1 expression. When cultured under low-oxygen conditions, miRNA-495 inhibited REDD1 expression, which promoted resistance to hypoxia, and stimulated an increase in the number of breast CSCs during the first two days.
Similar to the incorrect functionality of cell cycle regulators (e.g. p53) that leads to cancerous transformation and malignancy, dysregulation of oxygen-dependent stress-response factors can impart detrimental characteristics to cancer cells. For example, aberrant activation of the hypoxia-responsive protein, HIF2α, under normoxic conditions has been shown to impart stem cell-like properties to immortalized cancer cells through an ALDH-dependent pathway [47]. Inhibition of ALDH reduced the proliferation and self-renewal of breast CSCs in vitro, and diminished tumor-initiating capacity and lung metastasis in vivo.Brain CSCs
In addition to the wide-ranging involvement of CSCs in breast cancer, study of CSCs has also been fundamental to our understanding of the growth and metastatic potential of glioblastoma multiforme (GBM). Beginning in 2004, when CSCs in solid tumors were, primarily, known to exist in breast cancer only, two groups were able to separate and identify such CSCs from GBM tumors [48, 49]. Galli et al. [48], using isolated GBM patient lines, identified precursor cells with the functional capabilities of stem cells and determined that these stem cells had the capacity to form tumor masses. Similarly, Singh et al. [49] isolated GBM CD133+ cells with stem cell properties in vitro. When as few as 100 of these cells were implanted in NOD-SCID mouse brains, proliferative tumors were formed, whereas CD133− cells had the potential to engraft on to the brain only. Together, these studies formed the foundation for future work to study the involvement of CSCs in GBM and perhaps develop novel therapy targeting these cells.
In GBM, anti-VEGF treatment has not been effective because of evasive mechanisms. One study determined that GBM resistance was associated with upregulation of genes associated with mesenchymal origin and pro-inflammatory response factors [50]. Prolonged anti-VEGF therapy can also push GBM into mesenchymal and/or stem cell phenotypes that, over time, inhibit the effectiveness of the anti-VEGF therapy [51]. Because of these observed responses, it is no surprise that induced hypoxic environments produce mesenchymal and stem cell phenotypes, as both cell populations are known to be located in low-oxygen environments. Hence, researchers have been focused on understanding the effect of hypoxia, specifically molecular mechanisms, in maintaining GBM CSCs and the resistance of GBM to anti-angiogenic therapy. On the basis of the pioneering studies in 2004, recent work has focused on further characterization of CSCs and their functional properties. One such characterization has been stemness markers and behavior. Li et al. [52] performed an in-vitro study to determine whether hypoxia enhanced stemness of CSCs. Hypoxia treatment of two established cell lines and primary glioma cells for 48 h promoted CD133 expression. OCT4 and SOX2 mRNA levels also increased significantly whereas GFAP (a marker of differentiation in neuronal cell lineages) decreased. When DAPT, an inhibitor of the Notch pathway, was added to the cells, the opposite trends were observed for OCT4, SOX2, and GFAP, leading the authors to speculate that hypoxia in some way dedifferentiates glioma cells into CSC-like populations. Similar trends were also seen in earlier work by a Danish group [53].
Although hypoxic environments have been proved to induce formation of stemness genes, more work has been conducted to study the behavior of crucial molecules that mediate the changes of stemness. Mathieu et al. [54••] took a broad approach by screening cell lines of different cancer types and human embryonic stem cells for overlapping changes of common genes when grown under hypoxic conditions. They showed that OCT4, NANOG, SOX2, KLF4, cMYC, and miRNA 302 were all induced under low oxygen tension in 11 cancer types, including that of the brain. Using OCT4 as a target gene of interest, the group found that when cancer cells were altered to express non-degradable HIF1α and HIF2α and OCT4-GFP, use of hypoxic culture conditions revealed a positive correlation between OCT4-GFP and non-degradable HIF levels. Subsequent experiments using siRNA to knockdown HIF1α and HIF2α showed the opposite trend, with reduced expression of hESC markers. The authors completed their findings by study of primary glioma-derived CD133− cells, which they grew under hypoxic culture conditions. The low-oxygen conditions induced the cells to express the same hESC markers as before but also to form well-defined neurosphere structures associated with GBM. Thus, the authors suggested that HIF targets might act as crucial molecules affecting the dynamic states of stemness in cancer.
Although Mathieu et al. discovered that HIF1α and HIF2α were involved in the transformation of CSCs, their individual functions in the larger hypoxia signaling cascade had not yet been clarified. Bar et al. [55] contributed to this by determining whether HIF1α or HIF2α dominates long term phenotype characterization. Two glioma stem cell lines and three patient lines cultured under hypoxic conditions for 72 h had gene profiles that matched those in previous work by other groups, for example increases in CD133, HIF, KLF4, and SOX2. They also observed more prominent gene expression of LOX, HIG2, and VEGF and of downstream Notch pathway factor Hey2/Hes1 and ligands Jag1/Jag2. FACS confirmed that neurospheres formed under hypoxia comprised, primarily, CD133+ cells. Interestingly, if neurospheres with diameters as small as 100 µm were seeded they would mature into larger neurospheres characteristic of previous in-vitro experiments whereas spheres with diameters less than 100 µm did not mature. When mRNA and protein extraction was performed on these neurospheres, mRNA levels were higher for HIF1α than HIF2α. In addition, only HIF1α was expressed at the protein level. Bar et al. then studied xenograft models of the glioma stem cells (GSCs) and confirmed their in-vitro phenotype findings with one important caveat: hypoxia does not select for the best GSCs that maximize induced stemness transformation. HIF1α may be of central importance in the transformation, but no physiological cutoffs indicating clonal selection from hypoxia were seen in the study.
In contrast with Bar et al., Seidel et al. [56] obtained results indicating that HIF2α was the primary mediator of CSC transformation. Initial screening of candidate genes for isolating GSCs was based on three established glioblastoma cell lines grown under hypoxic conditions; this yielded 73 overlapping genes of interest, most of which were involved in the Wnt and TGF-β pathways. Primary glioblastoma cell lines were then grown under 1 % oxygen, and significant upregulation of CD133 and selected candidate genes ASPHD2, NFEL2L, MAML3, NFATc2, and ABL2 was observed. HIF also colocalized with these cells under hypoxia. By use of a Tet-off inducible system, the group determined that only HIF2α led to a significant increase in expression of the candidate marker genes and that a major HIF2α target was OCT4. Knockdown of HIF2α confirmed its function as the primary mediator for glioblastoma tumor stem cell phenotype, because of reduced levels of CD133, ASPHD2, and MAML3. In contrast with reports claiming crucial involvement of HIF1α in hypoxia-mediated CSC phenotypes, Seidel et al. found that HIF1α reduced sphere formation. This observation had been made in other studies of HIF1α [57].
Other research groups have also been interested in how hypoxia affects the ability of CSCs to form tumors. This has meant searching for less conventional downstream targets of HIF, and Heddleston et al. [58•] discovered histone methyl transferase mixed lineage leukemia 1 (MLL1) regulated tumorigenic potential in glioma stem cells. Xenograft-derived non-stem cells of glioblastoma were found to have increased levels of MLL1 under hypoxic conditions. When shRNA knockdown of HIF1α and HIF2α was introduced, MLL1 mRNA levels decreased, indicating MLL1 is a downstream target. When shRNA against MLL1 decreased HIF2α expression and did not effect HIF1α expression, ChIP analysis showed that HIF2α bound to the MLL1 promoter and did not vary when grown under normoxic or hypoxic culture conditions. MLL1 specifically caused a loss of H3K4m3 and gain of H3K27m3, consistent with transcriptional activation of the HIF2α locus. Other targets of MLL1 included PGK1 and VEGF, thus rationalizing MLL1’s functional consequences of participating in some part in tumor angiogenesis. From a phenotypic perspective, loss of MLL1 greatly reduced cell proliferation and self-renewal, thus demonstrating its importance in GSC growth. This was further illustrated when shRNA-transfected glioblastoma cells against MLL1 greatly increased tumor latency in xenografts.
Finally, cancer stem cell researchers have focused on finding molecules responsible for the cells’ interaction with or quasi-similarity to endothelial cell types involved in tumor vasculogenesis. As previously mentioned, tumors require a blood supply to sustain themselves and enable tumor growth, and it is known that GSCs expressing CDH5 can transdifferentiate into endothelial cells. Mao et al. [59] discovered that CDH5 mRNA levels were significantly higher in high-grade tumors than in low-grade tumors and that only tumor grade correlated with CDH5 levels. Compared with normal human brain, in which CDH5 is found on endothelial cells only, GBM samples also had GSCs expressing CDH5 and the proportion of GSCs with CDH5 increased with each passage (GSC5 at 8.4 % and GSC11 at 11.5 %). Bioinformatic analysis of probable CDH5 interactions in these cells included HIF2α, VWF and PECAM-1, but HIF2α was the most reliable predicted gene and known to be specifically expressed in endothelial cells. When cells were grown under hypoxic conditions, CDH5 mRNA increased 2.6 to 17.0-fold. shRNA knockdown of HIF1α and HIF2α also resulted in significant reduction in CDH5 in GSCs, and ChIP confirmed HIF1α and HIF2α interaction with the CDH5 promoter. Given these findings, the group knocked down CDH5 in GSCs grown in matrigel and found their capacity for tubulogenesis was severely diminished.Conclusion and Future Direction
As revealed above, it is increasingly clear that hypoxic conditions stimulate CSCs to undergo metastatic transformation with improved survival by providing protective effects in both brain and breast tumor models. Continuous progress has been made in the elucidation of mechanisms driving the causative involvement of CSCs under hypoxia in cancer recurrence and failure of therapy. However, significant challenges still remain in attempts to understand the complex relationship among mechanistic factors within the context of spatiotemporal and patient-to-patient variations. Moreover, it is difficult to quantitatively investigate the effect of complex microenvironmental factors associated with hypoxia on CSC behavior by use of in-vivo models because of challenges associated with isolating specific microenvironmental interactions as well as discerning the effects of hypoxia on CSC-mediated cancer recurrence and metastasis. One solution the authors suggest is to develop unique models that enable systematic investigation of hypoxic microenvironmental factors regulating CSC behavior and lead to discovery of therapeutic targets to prevent the contribution of CSCs to cancer recurrence and metastasis. For example, we have developed an environmentally relevant in-vitro model of carcinogenic hypoxia in which a metal carcinogen, nickel sulfate, is applied under hypoxic conditions [60]. By using this model we characterized the passage-dependent response of human stem cells in terms of early transformation in vitro, and the in-vivo consequences of implanted human stem cells. The results indicate that carcinogenic hypoxia modulates the activity of three critical transcription factors (c-MYC, p53, and HIF1), resulting in accumulation of reactive oxygen species and causing human stem cells to undergo cancer-like behavioral changes. Also, some promising 3D engineered tissue models have been developed by another group for study of cancer metastasis in vitro [61]. Moore et al. [62] developed an oxygen gradient model for study of vascular tissue remodeling. This type of tissue-engineering model could be used to investigate malignant transformation of CSCs under conditions of different oxygen content (Fig. 1). Considering the natural convergence of tissue engineering, stem cell biology, and cancer biology, collaborative efforts among researchers in these three fields seems a promising means of addressing the challenges.
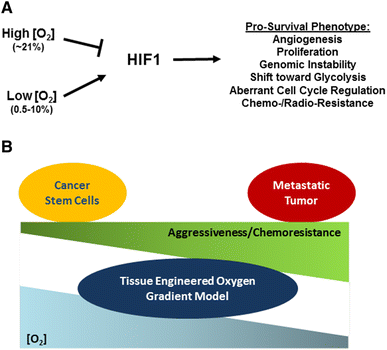
Open image in new windowFig. 1
Fig. 1
a A general summary of the effects of hypoxia-induced HIF expression in CSCs. HIF activity is regulated by oxygen tension and promotes a pro-survival phenotype, including increases in angiogenesis, proliferation, genomic instability, and aberrant cell cycle regulation, and a shift toward glycolysis, all of which contribute to chemo and/or radioresistance. b A experimental model proposed for study of the effects on CSC behavior of different degrees of hypoxia, and how these effects can lead to metastatic potential and patient mortalityCancer Stem Cells Under Hypoxia as a Chemoresistance Factor in the Breast and Brain | SpringerLink
https://link.springer.com/article/10.1007%2Fs40139-013-0035-6
%20and%20induced%20stem%20cells%20(iPSCs)..jpg)