Membrane Phospholipid PLA2 and Inflammation
��
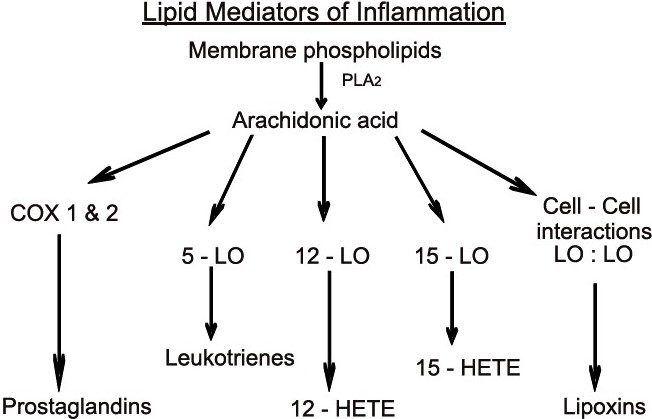
1. rising intracellular Ca2+ activates directly the phospholipase A2 and lipoxygenase pathway.
2. inhibitors of PLA2:glycyrrhzin from locorice/DHA,EPA,ALA,vitamin A, vitamin E,alpha-lipoic acid, bilirubin, chloroquine
��
http://www.jisponline.com/viewimage.asp?img=JIndianSocPeriodontol_2010_14_1_3_65426_f1.jpg
��
��
��Flavay® is Unique
Flavay® consists of molecules known as flavanols (note the "a")��and only in a highly specific form. Flavay® consists of organic groups of one flavanol molecule bonded together in groups of three, known as "flavan-3-ols," which have bonded into small complexes of two, three, four and five (but no larger) clusters, and retained in their naturally-occurring acid nutrients.
��
http://www.flavay.com/anti-inflammatory.html
��
��
��
Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation
Beatrice Oehler, Katrin Kistner, Corinna Martin, J��rgen Schiller, Rafaela Mayer, Milad Mohammadi, Reine-Solange Sauer, Milos R. Filipovic, Francisco R. Nieto, Jan Kloka, Diana Pfl��cke, Kerstin Hill, Michael Schaefer, Marzia Malcangio, Peter W. Reeh, Alexander Brack, Robert Blum & Heike L. Rittner
Scientific Reports volume 7, Article number: 5447 (2017) Cite this article
Abstract
Phospholipids occurring in cell membranes and lipoproteins are converted into oxidized phospholipids (OxPL) by oxidative stress promoting atherosclerotic plaque formation. Here, OxPL were characterized as novel targets in acute and chronic inflammatory pain. Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC) and its derivatives were identified in inflamed tissue by mass spectrometry and binding assays. They elicited calcium influx, hyperalgesia and induced pro-nociceptive peptide release. Genetic, pharmacological and mass spectrometric evidence in vivo as well as in vitro confirmed the role of transient receptor potential channels (TRPA1 and TRPV1) as OxPAPC targets. Treatment with the monoclonal antibody E06 or with apolipoprotein A-I mimetic peptide D-4F, capturing OxPAPC in atherosclerosis, prevented inflammatory hyperalgesia, and in vitro TRPA1 activation. Administration of D-4F or E06 to rats profoundly ameliorated mechanical hyperalgesia and inflammation in collagen-induced arthritis. These data reveal a clinically relevant role for OxPAPC in inflammation offering therapy for acute and chronic inflammatory pain treatment by scavenging OxPAPC.
Introduction
Hallmarks of inflammation �C occurring, for example, acutely in wounds after surgery or chronically in rheumatoid arthritis - are pain and hyperalgesia. Proalgesic inflammatory mediators include prostaglandins, bradykinin, reactive oxygen species (ROS) and their downstream products1. Non-steroidal anti-inflammatory drugs target enzymes responsible for the formation of prostaglandins and are among the most widely used painkillers worldwide. Despite their unequivocal effectiveness serious side effects like gastric bleeding, renal failure or heart attacks restrict non-steroidal anti-inflammatory drug usage indicating the need for alternative treatment options.
In inflammation, macrophages and neutrophils are major sources of ROS2, 3. Oxidized phospholipids (OxPL) are generated from the plasma membrane and from circulating lipoproteins through either enzymatic or non-enzymatic mechanisms. They are highly reactive and promote pro- and anti-inflammatory pathways. In atherosclerosis OxPL support adhesion of monocytes to endothelial cells, transformation of macrophages into foam cells containing oxidized low density lipoprotein (OxLDL), plaque formation, and chemokine production4,5,6. On the other hand, OxPL block lipopolysaccharide-induced activation of toll-like receptors7 or simply activate toll-like receptors possibly in complexes with CD368. Thus, OxPL are well known to shape the pathophysiology of atherosclerosis, but might also play a role in acute inflammatory (acute lung injury or sepsis)9, chronic inflammatory or neurodegenerative diseases (Alzheimer��s and Parkinson��s)10, 11. One of the commercially available OxPL is oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC) which consists of a mixture of oxidized, chain-shortened phospholipids including 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) and 1-palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine (PGPC) as well as oxygenated phospholipids such as 1-palmitoyl-2-(5,6)-epoxyisoprostaneE2-sn-glycero-3-phosphocholine (PEIPC)12, 13. OxPAPC species have been previously detected in inflammation, but its role in inflammatory pain is entirely unknown7.
Atherosclerotic plaque formation and OxPL are reduced by apolipoprotein A-I (ApoA-I), a structural protein of the high density lipoproteins14. Indeed, the ApoA-I mimetic peptide D-4F reduces plaque size in early vascular lesions as well as the pro-inflammatory reactions of low density lipoproteins (LDL). Furthermore, it might improve symptoms of other autoimmune diseases such as scleroderma, collagen-induced arthritis or systemic lupus15, 16. A second approach to neutralize OxPL is an antibody. Using sera of ApoE-deficient mice, autoantibodies against oxidized phospholipids have been extracted of which subtype E06, also known as T15, most efficiently binds copper-oxidized LDL17, 18.
ROS belong to the group of pro-nociceptive mediators and are generated by resident or infiltrating leukocytes via e.g. NADPH oxidase complexes. Besides oxidation of reactive sites of proteins, particularly free sulfhydryl groups, ROS can also oxidize lipids and phospholipids in plasma membranes. ROS and their downstream products themselves are pro-nociceptive through activation of transient receptor potential vanilloid 1 (TRPV1) or ankyrin 1 (TRPA1) receptors in sensory neurons. TRPA1 and TRPV1 channels are receptors of chemical stimuli in nociceptors and act as sensors in the pain pathway1. TRPA1 and TRPV1 are both expressed in peptidergic nociceptors containing calcitonin gene-related peptide (CGRP) as well as in non-peptidergic isolectin 4-positive nociceptors19, 20. TRPA1-expressing dorsal root ganglion neurons (DRGs) overlap with TRPV1-expressing neurons, but TRPV1 is more abundantly expressed21. TRPV1 is activated by capsaicin, the pungent ingredient of chili peppers, and by oxidized linoleic acid metabolites like 9-hydroxy-10E- and 13-hydroxy-10E,12Z-octadecadienoic acid (9-HODE and 13-HODE) released in inflammation or after exposure to heat22. TRPA1 responds to endogenous and exogenous irritants like allyl isothiocyanate (AITC)21 and also to 4-hydroxynonenal, a downstream metabolite of OxPL found in complete Freund��s adjuvant (CFA)-induced hindpaw inflammation3. OxPL in turn are generated by ROS and present in inflamed tissues9.
Here, we report that OxPAPC is an important endogenous player in inflammatory pain. OxPAPC acts through TRPA1 and TRPV1 ion channels on nociceptors. The ApoA-I mimetic peptide D-4F or the anti-OxPL antibody E06 antagonize OxPAPC-induced hyperalgesia. Both agents constitute novel therapeutic options for the treatment of hyperalgesia in acute and chronic inflammation.Inflammatory pain control by blocking oxidized phospholipid-mediated TRP channel activation | Scientific Reports
https://www.nature.com/articles/s41598-017-05348-3��
Phospholipid oxidation generates potent anti�\inflammatory lipid mediators that mimic structurally related pro�\resolving eicosanoids by activating Nrf2Peter Bretscher Julian Egger Abdijapar Shamshiev Martin Trötzm��ller Harald Köfeler Erick M Carreira Manfred Kopf Stefan Freigang
Institute of Molecular Health Sciences, ETH Zurich, Zurich, Switzerland
2Laboratory of Organic Chemistry, Department of Chemistry, ETH Zurich, Zurich, Switzerland
3Core Facility for Mass Spectrometry, Medical University of Graz, Graz, Austria
†Present address: Delenex Therapeutics AG, Schlieren, Switzerland
†Present address: Institute of Pathology, University of Bern, Bern, Switzerland
Abstract
Exposure of biological membranes to reactive oxygen species creates a complex mixture of distinct oxidized phospholipid (OxPL) species, which contribute to the development of chronic inflammatory diseases and metabolic disorders. While the ability of OxPL to modulate biological processes is increasingly recognized, the nature of the biologically active OxPL species and the molecular mechanisms underlying their signaling remain largely unknown. We have employed a combination of mass spectrometry, synthetic chemistry, and immunobiology approaches to characterize the OxPL generated from the abundant phospholipid 1�\palmitoyl�\2�\arachidonoyl�\sn�\glycero�\3�\phosphocholine (PAPC) and investigated their bioactivities and signaling pathways in vitro and in vivo. Our study defines epoxycyclopentenones as potent anti�\inflammatory lipid mediators that mimic the signaling of endogenous, pro�\resolving prostanoids by activating the transcription factor nuclear factor E2�\related factor 2 (Nrf2). Using a library of OxPL variants, we identified a synthetic OxPL derivative, which alleviated endotoxin�\induced lung injury and inhibited development of pro�\inflammatory T helper (Th) 1 cells. These findings provide a molecular basis for the negative regulation of inflammation by lipid peroxidation products and propose a novel class of highly bioactive compounds for the treatment of inflammatory diseases.
Synopsis
This study characterizes the potent anti�\inflammatory bioactivity of oxidized phospholipids and identifies a fatty acid epoxycyclopentenone as the active component that triggers Nrf2 signaling in myeloid cells and alleviates inflammation and endotoxin�\induced lung injury in a mouse model.
Epoxycyclopentenone�\containing phospholipids broadly inhibit pro�\inflammatory cytokine and chemokine responses of myeloid cells and prevent the development of inflammatory Th1 cells.
Epoxycyclopentenone and endogenous pro�\resolving lipid mediators sharing the cyclopentenone motif activate the oxidative stress�\responsive transcription factor Nrf2 to dampen inflammation in vitro and in vivo.
Administration of epoxycyclopentenone activates Nrf2 signaling in vivo and mitigates endotoxin�\induced lung injury in the mouse model.
Structure�Cfunction studies identified critical molecular determinants of this anti�\inflammatory bioactivity and facilitated the development of an epoxycyclopentenone�\derived lipid variant with greatly increased potency.Phospholipid oxidation generates potent anti�\inflammatory lipid mediators that mimic structurally related pro�\resolving eicosanoids by activating Nrf2 | EMBO Molecular Medicine
https://www.embopress.org/doi/full/10.15252/emmm.201404702��
Oxid Med Cell Longev. 2016; 2016: 7432797.
Oxidative Stress and Inflammation: What Polyphenols Can Do for Us?
Tarique Hussain, 1 , 2 Bie Tan, 1 , 3 , * Yulong Yin, 1 Francois Blachier, 4 Myrlene C. B. Tossou, 1 , 2 and Najma Rahu 5
Abstract
Oxidative stress is viewed as an imbalance between the production of reactive oxygen species (ROS) and their elimination by protective mechanisms, which can lead to chronic inflammation. Oxidative stress can activate a variety of transcription factors, which lead to the differential expression of some genes involved in inflammatory pathways. The inflammation triggered by oxidative stress is the cause of many chronic diseases. Polyphenols have been proposed to be useful as adjuvant therapy for their potential anti-inflammatory effect, associated with antioxidant activity, and inhibition of enzymes involved in the production of eicosanoids. This review aims at exploring the properties of polyphenols in anti-inflammation and oxidation and the mechanisms of polyphenols inhibiting molecular signaling pathways which are activated by oxidative stress, as well as the possible roles of polyphenols in inflammation-mediated chronic disorders. Such data can be helpful for the development of future antioxidant therapeutics and new anti-inflammatory drugs.
1. Introduction
Oxidative stress refers to the excessive production of reactive oxygen species (ROS) in the cells and tissues and antioxidant system cannot be able to neutralize them. Imbalance in this protective mechanism can lead to the damage of cellular molecules such as DNA, proteins, and lipids [1]. Reactive oxygen species are normally produced within the body in limited quantity and are important compounds involved in the regulation of processes involving the maintaining of cell homeostasis and functions such as signal transduction, gene expression, and activation of receptors [2]. Mitochondrial oxidative metabolism in cells produces ROS species and organic peroxides in the process of cell respiration [3]. In addition, in hypoxic conditions, nitric oxide may also be produced during the respiratory chain reaction [4]. This latter reactive nitrogen species (RNS) may further lead to the production of reactive species such as reactive aldehydes, malondioaldehyde, and 4-hydroxynonenal [5]. Main targets of oxidative stress are proteins, lipids, and DNA/RNA, and modifications in these molecules may increase the chances of mutagenesis. ROS/RNS overproduction notably over a prolonged period of time can cause damage of the cellular structure and functions and may induce somatic mutations and preneoplastic and neoplastic transformations. Then, excessive production of ROS in cells and tissues may be deleterious if not removed quickly [6]. Indeed, excessive ROS/RNS production may cause irreversible damage to cells resulting in cell death by the necrotic and apoptotic processes [7].
Polyphenols are natural compounds present in plants with numerous biological activities. Phenolic compounds and flavonoids can interact with ROS/RNS and thus terminate chain reaction before cell viability is seriously affected [21].
Various inflammatory stimuli such as excessive ROS/RNS produced in the process of oxidative metabolism and some natural or artificial chemicals have been reported to initiate the inflammatory process resulting in synthesis and secretion of proinflammatory cytokines. Activation of nuclear factor-kappa B/active protein-1 (NF-��B/AP-1) and production of tumor necrosis factor-alpha (TNF-��) have been for instance documented to play critical role in the inflammatory process resulting in several chronic diseases. Phytochemicals such as polyphenols have been reported to be able to modulate the inflammatory processes [22].
This review paper was designed to highlight the biological effects of the polyphenols and their potential to act as compounds with anti-inflammatory properties.Oxidative Stress and Inflammation: What Polyphenols Can Do for Us?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5055983/��
Biochem Pharmacol. 2012 Nov 1;84(9):1113-22. doi: 10.1016/j.bcp.2012.07.017. Epub 2012 Jul 25.
The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases.
Mitjavila MT1, Moreno JJ.
Author information
Abstract
Redox state unbalance and the activation of the arachidonic acid (AA) cascade, contribute to the pathogenesis of cardiovascular disease (CVD) and cancer. Inflammatory cells that infiltrate the atheroma plaque or tumor are a major source of reactive oxygen species and eicosanoids. The human antioxidant defense network is complex and interlocking and there is controversy surrounding the beneficial effects of diet-derived antioxidants in vivo. However, epidemiological studies indicate that populations that consume high levels of plant-derived foods containing phenolic compounds have low rates of CVD and cancer. The molecular mechanisms for these effects are multi-faceted. They include the regulation of transcription factors and consequently the modulation of genes (cytokines, growth factors and adhesion molecules), and growth factor-receptor interactions and cell signaling cascades, which determine the expression of genes involved in cell cycle, cell survival and apoptosis, as well as adhesiveness/invasiveness and angiogenesis. The present paper also focuses on the effects of phenolic compounds on AA cascade enzymes (cyclooxygenases and lipoxygenases) and the subsequent synthesis of eicosanoids, which are involved in CVD and cancer. A better understanding of these processes could explain the beneficial effects of polyphenols on the most prevalent diseases in Western societies. This commentary shows that antioxidants under evaluation include structural modifications of low-molecular-mass polyphenols, which could lead to a valuable strategy for modulating the generation of inflammatory mediators involved in these chronic diseases.
Copyright © 2012 Elsevier Inc. All rights reserved.
PMID: 22858365 DOI: 10.1016/j.bcp.2012.07.017The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence disea... - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/22858365��
Arachidonic Acid Cascade
Lipids in Cell Signaling
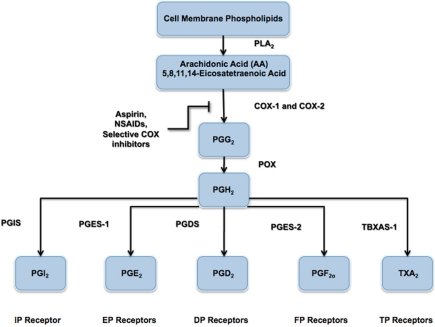
Many of the lipids involved as second messengers in cell signaling pathways arise from the arachidonic acid (AA) pathway.AA is an unsaturated fatty acid that is a normal constituent of membrane phospholipids and is released from the phospholipids by the actions of phospholipase A2 (PLA2).Prostaglandins (PG) arise from a cyclic endoperoxide generated by the enzyme system PG synthetase. This is a complex of enzymes, including cyclooxygenase (COX), required to produce the key intermediate, the cyclic endoperoxide derivative of AA. There is a constitutive (COX-1) and an inducible cyclooxygenase (COX-2). The cyclic endoperoxide is also a precursor of prostacyclin (PGI2) and thromboxane (TXA3). Other groups of compounds in this class, leukotrienes (LT) and lipoxins (LP) are derived directly from AA without the mediation of a cyclic endoperoxide.
Lipoxygenase acts on AA to produce 5-hydroperoxyeicosatetraenoic acid (5-HPETE) that is converted to LTA4. LTA4 is the precursor of LTB4, that induces inflammation by its chemotactic and degranulating actions on polymorphonuclear lymphocytes (PML), and of LTC4, LTD4, and LTE4, the amino acid-containing LTs that induce vasoconstriction and bronchoconstriction and are involved in asthma and anaphylaxis.
References:
Heller, A., et al., Lipid mediators in inflammatory disorders. Drugs, 55, 487-496(1998).
Cook, J.A., et al., Prostaglandins, thromboxanes, leukotrienes, and cytochrome P-450 metabolites of arachidonic acid. New Horiz. 1,60-69(1993).
Dubois, R.N., et al., Cyclooxygenase in biology and disease. FASEB J. 12, 1063-1073(1998).Arachidonic Acid Cascade - Lipids in Cell Signaling | China mainland | Sigma-Aldrich
https://www.sigmaaldrich.com/life-science/cell-biology/cell-biology-products.html?TablePage=9561026��
Cancers (Basel). 2014 Sep; 6(3): 1500�C1521.
Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked
Rosalina Wisastra and Frank J. Dekker*
Pharmaceutical Gene Modulation, Groningen Research Institute of Pharmacy, University of Groningen, Antonius Deusinglaan 1, 9713 AV Groningen, The Netherlands; E-Mail: moc.liamg@artsasiw.r
Abstract
Cancer and inflammation are intimately linked due to specific oxidative processes in the tumor microenvironment. Lipoxygenases are a versatile class of oxidative enzymes involved in arachidonic acid metabolism. An increasing number of arachidonic acid metabolites is being discovered and apart from their classically recognized pro-inflammatory effects, anti-inflammatory effects are also being described in recent years. Interestingly, these lipid mediators are involved in activation of pro-inflammatory signal transduction pathways such as the nuclear factor ��B (NF-��B) pathway, which illustrates the intimate link between lipid signaling and transcription factor activation. The identification of the role of arachidonic acid metabolites in several inflammatory diseases led to a significant drug discovery effort around arachidonic acid metabolizing enzymes.Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4190552/��
��-lipoic acid: An inhibitor of secretory phospholipase A2 with anti-inflammatory activity
University of Mysore
Alpha-lipoic acid (ALA) and its reduced form dihydrolipoic acid (DHLA) are powerful antioxidants both in hydrophilic and lipophylic environments with diverse pharmacological properties including anti-inflammatory activity. The mechanism of anti-inflammatory activity of ALA and DHALA is not known. The present study describes the interaction of ALA and DHALA with pro-inflammatory secretory PLA(2) enzymes from inflammatory fluids and snake venoms. In vitro enzymatic inhibition of sPLA(2) from Vipera russellii, Naja naja and partially purified sPLA(2) enzymes from human ascitic fluid (HAF), human pleural fluid (HPF) and normal human serum (HS) by ALA and DHLA was studied using (14)C-oleate labeled Escherichia coli as the substrate. Biophysical interaction of ALA with sPLA(2) was studied by fluorescent spectral analysis and circular dichroism studies. In vivo anti-inflammatory activity was checked using sPLA(2) induced mouse paw edema model.ALA but not DHLA inhibited purified sPLA(2) enzymes from V. russellii, N. naja and partially purified HAF, HPF and HS in a dose dependent manner. This data indicated that ALA is critical for inhibition. IC(50) value calculated for these enzymes ranges from 0.75 to 3.0 microM. The inhibition is independent of calcium and substrate concentration. Inflammatory sPLA(2) enzymes are more sensitive to inhibition by ALA than snake venom sPLA(2) enzymes. ALA quenched the fluorescence intensity of sPLA(2) enzyme in a dose dependent manner. Apparent shift in the far UV-CD spectra of sPLA(2) with ALA indicated change in its alpha-helical confirmation and these results suggest its direct interaction with the enzyme. ALA inhibits the sPLA(2) induced mouse paw edema in a dose dependent manner and confirms the sPLA(2) inhibitory activity in vivo also. These data suggest that ALA may act as an endogenous regulator of sPLA(2) enzyme activity and suppress inflammatory reactions.
��-lipoic acid: An inhibitor of secretory phospholipase A2 with anti-inflammatory activity | Request PDF
https://www.researchgate.net/publication/6782502_a-lipoic_acid_An_inhibitor_of_secretory_phospholipase_A2_with_anti-inflammatory_activity��
Chloroquine is a potent inhibitor of SARS coronavirus ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1232869
Aug 22, 2005 �� Chloroquine is a potent inhibitor of SARS coronavirus infection and spread Martin J Vincent , 1 Eric Bergeron , 2 Suzanne Benjannet , 2 Bobbie R Erickson , 1 Pierre E Rollin , 1 Thomas G Ksiazek , 1 Nabil G Seidah , 2 and Stuart T Nichol 1
Cited by: 102
Publish Year: 2005
Author: Martin J Vincent, Eric Bergeron, Suzanne Benjannet, Bobbie R Erickson, Pierre E Rollin, Thomas G Ksi...
The Nine Lives of Hydroxychloroquine | RheumNow ...
rheumnow.com/blog/nine-lives-hydroxychloroquine
Nov 05, 2019 �� The hydroxychloroquine story begins in 1638 when the wife of the Viceroy of Peru, Countess Cinchona, acquired malaria while living in the New World. The Nine Lives of Hydroxychloroquine | RheumNow - Rheumatology News & Information��
Anti-inflammatory therapy for cardiovascular disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6511577
Feb 13, 2019 �� Hydroxychloroquine (HCQ) HCQ, a disease-modifying antirheumatic drug (DMARD), is an immunosuppressant agent used in the management of certain autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), as well for the prevention and treatment of certain types of malaria ( 18 ).
Cited by: 1
Publish Year: 2019
Author: Constantine E. Kosmas, Delia Silverio, Andreas Sourlas, Peter��
Ann Transl Med. 2019 Apr; 7(7): 147.
Anti-inflammatory therapy for cardiovascular disease
Constantine E. Kosmas,corresponding author1 Delia Silverio,2 Andreas Sourlas,3 Peter D. Montan,2 Eliscer Guzman,1 and Mario J. Garcia1
Author information Article notes Copyright and License information Disclaimer
1Department of Medicine, Montefiore Medical Center, Bronx, NY, USA;
2Cardiology Clinic, Cardiology Unlimited, PC, New York, NY, USA;
3School of Medicine, University of Crete, Heraklion, GreecAbstract
Chronic subclinical inflammation is a central process in the pathogenesis of cardiovascular disease (CVD) and it has been linked with both the initiation and progression of atherosclerosis. Several pro-inflammatory cytokines, such as the C-reactive protein (CRP), tumor necrosis factor-�� (TNF-��) and interleukin-6 (IL-6) have been described as independent risk factors for coronary heart disease and promoters of atherogenesis. Thus, extensive research is being conducted to assess the role of anti-inflammatory therapy in the primary and secondary prevention of CVD. Our review aims to provide the clinical and scientific data pertaining to the effects of different anti-inflammatory agents administered in patients with CVD.
Keywords: Inflammation, pro-inflammatory cytokines, anti-inflammatory agents/therapy, cardiovascular disease (CVD)Hydroxychloroquine (HCQ)
HCQ, a disease-modifying antirheumatic drug (DMARD), is an immunosuppressant agent used in the management of certain autoimmune diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), as well for the prevention and treatment of certain types of malaria (18).
It exerts its immunomodulatory properties by suppressing inflammatory pathways through the prevention of toll-like receptor activation, which is needed for the expression of interferon-regulated genes and for the production of TNF-��, a major component of the cell-mediated inflammatory response (18,19).
High-dose HCQ therapy (400 mg daily), has been independently associated with a 56.8% reduced risk for CV morbidity in patients with RA (20).
In a retrospective study, which included 1,266 patients with incident RA (excluding patients with CVD prior to RA diagnosis), HCQ use was associated with a 72% reduction in the risk of incident CVD and a 70% reduction in the risk of the composite incident CAD, stroke, and transient ischemic attack (TIA) (21).
Furthermore, there is evidence that HCQ exhibits hypolipidemic, hypoglycemic and antithrombotic properties when administered in patients with autoimmune diseases, such as RA or SLE (19,22-24), which may significantly contribute in the reduction of the risk for CVD conferred by HCQ in these patients.
Phospholipase A2 (PLA2) inhibitors
PLA2 is a widely distributed group of enzymes found in many isoforms, including the lipoprotein-associated phospholipase A2 (Lp-PLA2), cytosolic phospholipase A2 (cPLA2) and secretory phospholipase A2 (sPLA2) (42).
PLA2 enzymes hydrolyze phospholipids to generate free fatty acids and lysolipids, which are key components for the biosynthesis of eicosanoids and platelet-activating factor (PAF), thus potently promoting inflammation and atherogenesis (43,44). Both, Lp-PLA2 and sPLA2 are expressed primarily in pro-atherogenic inflammatory cells including macrophages, monocytes and lymphocytes (42,44). Furthermore, several sPLA2 are expressed with various patterns in all stages of atherosclerosis development (44,45).
In addition, both Lp-PLA2 and sPLA2, to a different extent, are carried by LDL and generate lysophosphatidylcholine (Lyso-PC) and oxidized fatty acid (oxFA), two pro-inflammatory mediators promoting cell activation and production of inflammatory cytokines. Furthermore, Lyso-PC may also perpetuate vascular inflammation and promote necrotic core formation in atheromatous plaques, thus making plaques susceptible to rupture (46).
Given the above described pro-inflammatory and pro-atherogenic properties of PLA2 enzymes, it becomes well understandable why PLA2 inhibitors have received considerable attention in the medical research field as drug targets for the prevention and management of CVD. Two PLA2 inhibitors have been developed, varespladib and darapladib.Anti-inflammatory therapy for cardiovascular disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6511577/��