MAPK Pathway (RAS-RAF-MEK-ERK signaling pathway)
Vitamin C inhibits TNF-alpha induced activation of MARK and NF-kB signaling pathway
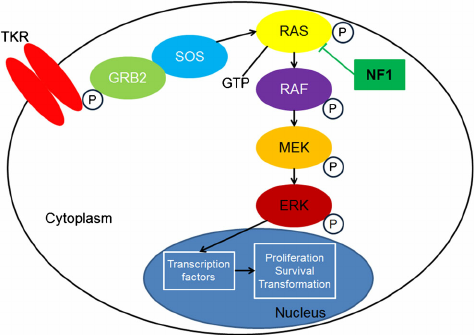
ERK Extracellular signal-regulated kinase (ERK) is a protein kinase that is a part of the Ras-Raf pathway. ERK is a type of MAPK. Activation of ERK results in the activation of transcription factors that lead to the expression of genes. These genes regulate cell proliferation and survival.
Roche - The MAPK pathway, revisited
www.roche.com/research_and_development/what_we_are_working_on/oncology/mapk.htm
The MAPK (ERK) Pathway - PubMed Central (PMC)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3628180
The RAS-RAF-MEK-ERK pathway is far more complex than once thought. Figure 1 presents a simplified description of its basic components. The pathway’s general structure includes a small G protein (RAS) and three protein kinases (RAF, MEK, ERK). (A kinase is an enzyme that catalyzes transfer of a phosphate group from a donor molecule to an acceptor.)
The KRAS gene provides instructions for making a protein called K-Ras that is part of a signaling pathway known as the RAS/MAPK pathway. The protein relays signals from outside the cell to the cell's nucleus. These signals instruct the cell to grow and divide (proliferate) or to mature and take on specialized functions (differentiate).
BRAF explanation
The BRAF gene provides instructions for making a protein that helps transmit chemical signals from outside the cell to the cell's nucleus. This protein is part of a signaling pathway known as the RAS/MAPK pathway, which controls several important cell functions.
MEK-1 is a dual threonine and tyrosine recognition kinase that phosphorylates and activates mitogen-activated protein kinase (MAPK). MEK-1 is in turn activated by phosphorylation. Raf and MAPK/extracellular signal-regulated kinase kinase (MEKK) independently phosphorylate and activate MEK-1.Extracellular signal-regulated kinases - Wikipedia
https://en.wikipedia.org/wiki/Extracellular_signal-regulated_kinases
Mitogen-activated protein kinase 1 (MAPK1) is also known as "extracellular signal-regulated kinase 2" (ERK2). Two similar (85% sequence identity) protein kinases were originally called ERK1 and ERK2. They were found during a search for protein kinases that are rapidly phosphorylated after activation of cell surface tyrosine kinases such as the epidermal growth factor receptor.
NORMAL MOLECULAR PATHWAYS AND ASSOCIATED MUTATIONS
The RAS-RAF-MEK-ERK signaling pathway (MAPK pathway) is a classical intracellular pathway that plays a crucial role in the homeostasis of normal cell turnover, cellular proliferation, differentiation, survival, and apoptosis. When activated aberrantly, this signaling pathway can induce tumorigenesis and has been associated with various malignancies.
EGFR
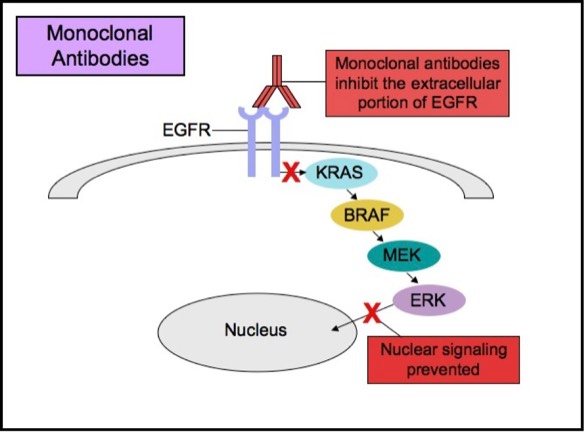
The Epidermal Growth Factor Receptor (EGFR), also known as HER1 and ERBB1, is an important trans-membrane tyrosine kinase receptor involved in the initiation of the MAPK pathway. Activating mutations in EGFR can lead to aberrant cellular proliferation, inhibition of apoptosis, angiogenesis, increased cell survival, and gene transcription.
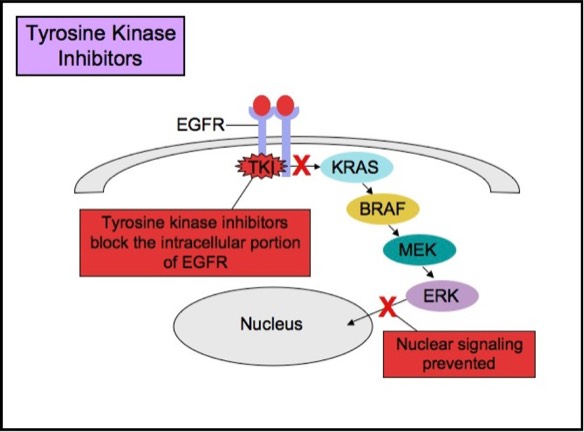
Some patients with tumors carrying EGFR mutations may respond to selective anti-EGFR therapies. EGFR has an extracellular ligand-binding region and a cytoplasmic tyrosine kinase-containing domain. Currently, there are two major types of anti-EGFR drug therapies. The first drug type (cetuximab, panitumumab) is a monoclonal antibody that inhibits EGFR by binding to an extracellular portion of the EGFR protein. The second type (gefitinib, erlotinib) is an intracellular tyrosine kinase inhibitor that inhibits EGFR by crossing the cell membrane and blocking the receptor’s active site.
Studies show that patients whose lung cancers exhibit EGFR mutations may benefit from anti-EGFR therapies. Tumor subtypes most likely to respond to anti-EGFR therapies include adenocarcinoma, non-mucinous bronchioloalveolar carcinoma (BAC), and adenosquamous carcinoma. EGFR mutations are rare in other histologic types of lung cancer (small cell carcinoma, squamous cell carcinoma, and large cell carcinoma). Thus, anti-EGFR therapies are most commonly employed for treatment of adenocarcinoma. The two most common EGFR mutations, comprising about 90% of all EGFR mutations in lung adenocarcinomas, are on exon 19 and 21 (L858R). Patients with these mutations are likely to respond to anti-EGFR therapies. There are also less common mutations (exon 20 insertion), including those that actually cause poor response to anti-EGFR therapies, but these mutations are relatively uncommon.
KRAS
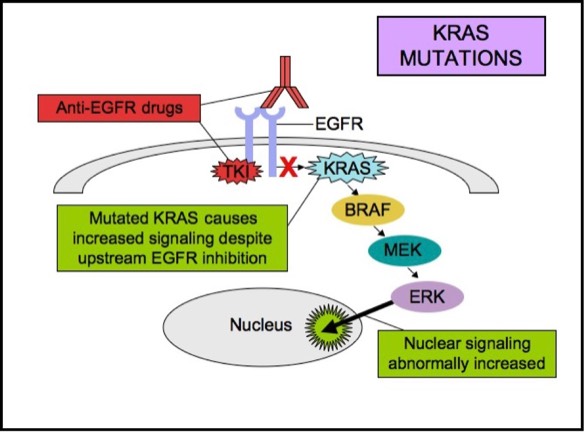
KRAS is a small G-protein that is important for EGFR signaling. KRAS mutations activate downstream signaling cascade despite the upstream EGFR regulation. Anti-EGFR therapies are ineffective in tumors with such activating KRAS mutations, because the KRAS mutations trigger the EGFR-signaling cascade at a point downstream of the therapy’s target. Therefore, both monoclonal antibodies and tyrosine kinase inhibitors may fail to respond in the presence of KRAS mutations.
KRAS mutations are seen in about 15-20% of non-small cell lung cancers and 30-50% of lung adenocarcinomas. EGFR and KRAS mutations are essentially mutually exclusive in lung adenocarcinomas. The presence of both EGFR and KRAS mutations in lung adenocarcinomas is rare, but if they do occur together, EGFR inhibitors are less likely to be effective. KRAS mutation is a poor prognostic indicator. The presence of KRAS mutation usually means failure to respond to anti-EGFR therapy. Anti-EGFR therapies are likely to be ineffective in patients with advanced or metastatic lung adenocarcinomas when a KRAS mutation is present.
In patients with lung adenocarcinoma, non-mucinous BAC, or adenosquamous carcinoma, EGFR mutation analysis by polymerase chain reaction (PCR) is recommended to detect those patients who may benefit from anti-EGFR therapy. Laboratories often offer EGFR mutation PCR test, with reflex to KRAS (if EGFR is negative), since the two mutations are mutually exclusive.
http://www.apmggroup.net/innovation/molecular_testing/Lung_Pathways/lung.html
BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies - Clarke - Journal of Gastrointestinal Oncology
http://jgo.amegroups.com/article/view/4881/html
Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer
C Alberti, P Pinciroli, B Valeri, R Ferri, A Ditto, K Umezawa, M Sensi, S Canevari & A Tomassetti
Oncogene volume 31, pages4139–4149(2012)Cite this article
Abstract
The epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinases, is expressed in up to 70% of epithelial ovarian cancers (EOCs), where it correlates with poor prognosis. The majority of EOCs are diagnosed at an advanced stage, and at least 50% present malignant ascites. High levels of IL-6 have been found in the ascites of EOC patients and correlate with shorter survival. Herein, we investigated the signaling cascade led by EGFR activation in EOC and assessed whether EGFR activation could induce an EOC microenvironment characterized by pro-inflammatory molecules. In vitro analysis of EOC cell lines revealed that ligand-stimulated EGFR activated NFkB-dependent transcription and induced secretion of IL-6 and plasminogen activator inhibitor (PAI-1). IL-6/PAI-1 expression and secretion were strongly inhibited by the tyrosine kinase inhibitor AG1478 and EGFR silencing. A significant reduction of EGF-stimulated IL-6/PAI-1 secretion was also obtained with the NFkB inhibitor dehydroxymethylepoxyquinomicin. Of 23 primary EOC tumors from advanced-stage patients with malignant ascites at surgery, 12 co-expressed membrane EGFR, IL-6 and PAI-1 by immunohistochemistry; both IL-6 and PAI-1 were present in 83% of the corresponding ascites. Analysis of a publicly available gene-expression data set from 204 EOCs confirmed a significant correlation between IL-6 and PAI-1 expression, and patients with the highest IL-6 and PAI-1 co-expression showed a significantly shorter progression-free survival time (P=0.028). This suggests that EGFR/NFkB/IL-6-PAI-1 may have a significant impact on the therapy of a particular subset of EOC, and that IL-6/PAI-1 co-expression may be a novel prognostic marker.
Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer | Oncogene
https://www.nature.com/articles/onc2011572
3015 例真实世界患者数据,再次验证奥希替尼疗效
康健老师聊健康
研究背景
一代/二代 EGFR-TKI 治疗进展后约有 50% 的患者会出现 EGFR T790M 突变。第三代 EGFR-TKI 奥希替尼可精准靶向 EGFR 敏感突变和 EGFR T790M 耐药突变。
一项 III 期随机对照研究(AURA3)显示:奥希替尼或含铂双药化疗治疗既往 EGFR-TKI 治疗失败且明确 EGFR T790M 突变阳性的晚期 NSCLC 患者,达到主要研究终点,PFS 分别为 10.1 个月和 4.4 个月,HR=0.3,P<0.001,ORR 分别为 71% 和 31%。
也是基于 AURA3 研究的成功,奥希替尼在 2017 年 3 月被 CFDA 批准上市,2018 年为了响应国家号召,奥希替尼主动降价、积极谈判,顺利进入国家乙类医保目录。
研究目的
为了在更广泛的人群以及在真实世界中评估奥希替尼治疗既往 EGFR-TKI治疗失败且明确 EGFR T790M 突变阳性晚期 NSCLC 患者的疗效和安全性。
研究设计
这是一项国际、多中心、单臂、真实世界研究,NCT02474355,至数据截止时,共纳入 3015 例,既往接受过 ≥1 线 EGFR-TKI 治疗失败且经检测明确有 EGFR T790M 突变的晚期非小细胞肺癌成年患者,使用奥希替尼 80mg po QD 进行治疗,每 6 周进行一次疗效评价,主要研究终点是 OS,次要研究终点包括研究者评估的 ORR、PFS 和至治疗终止时间。
研究特点如图:
研究结果
数据截止时,96%(2900 例)的患者有至少 1 次疗效评估,其中 :
1655 例可被定义为 CR 或 PR,即研究者评估的 ORR=57.1%(95% CI 55.2%-58.9%);
69%(2071 例)的患者报告疾病进展或死亡的事件,即中位 PFS=11.1 个月(95% CI 11.0 个月-12.0 个月);
共 64%(1930 例)的患者已经终止治疗,即中位至治疗终止时间=13.5 个月(95% CI 12.6 个月-13.9 个月);
41%(1237 例)的患者已经报告死亡,但OS结果尚未成熟,12 个月的 OS 率为74%(95% CI 72.5%-75.7%);
间质性肺炎(ILD)/肺炎样事件发生率为 1%(共 28 例患者)。
全分析集:中位 PFS=11.1 个月(95% CI 11.0 个月-12.0 个月)
安全性:共 30%(901 例)的患者发生至少 1 次方案定义的不良事件
研究结论
奥希替尼在真实世界中展现出来的疗效与既往临床研究结果相似,且没有新的药物安全性警示。
文章出处:Future Oncol. 2019 Sep;15(26):3003-3014.
doi: 10.2217/fon-2019-0324. Epub 2019 Jul 24.
校稿 / 审核专家