iNOS, Arginases and Macrophage
1. M1 macrophage produces antimicrobial nitric oxide
2. Nitric oxide (NO) has been shown to inhibit Giardia lamblia in vitro and in vivo.
3. M1 macrophages being involved in pathogen control and M2 macrophages serving to limit excessive NO production and support healing.
4. Heme oxygenase-1 dysregulates macrophage polarization and the immune response to Helicobacter pylori.
5.Macrophages expressing arginase 1 (M2)and nitric oxide synthase 2 (M1) accumulate in the small intestine during Giardia lamblia infection. Arginase 1 in liver for urea cycle to remove amine.
6. arginine is the sole amino acid substrate for NO production
7.Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages.In mammals, two arginase isoforms are expressed: the cytosolic Arg-1 and the mitochondrial Arg-2 (11). ref: schistosome eggs, a potent Th2-inducing stimulus
8.Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II.
9.Heme oxygenase-1 (HO-1) is an inducible enzyme that exhibits anti-inflammatory functions.
10.monocyte-decrived macrophage accounts for more than 50% of instestinal macrophage
11. inhibitors of arginase 2: N‐hydroxy‐L‐arginine (3, NOHA),polyphenols, norvaline,
http://www.jimmunol.org/content/167/11/6533
Biosynthesis of nitric oxide
NO biosynthesis is carried out from L-arginine and is catalyzed by NO-synthases, NOS, an heminic enzyme whose structure resembles that of P-450 cytochrome. In the presence of cofactors ( NADPH, oxygen, iron, tetrahydrobiopterine, FAD and FMN), enzyme NO-synthases, convert arginine into hydroxyarginine and finally into citrulline and NO according to the following reactions:
Biosynthesis of NO starting from L-arginine
Biosynthesis of NO starting from L-arginine
Citrulline, in presence of arginosuccinate synthethase and aspartate, is then converted into arginosuccinate, fumarate and arginine. Thus, arginine comes from an endogenous renewal and an exogenous source, food.
Nitric oxide - Pharmacorama
https://www.pharmacorama.com/en/Sections/NoNitric_2.php
Role of the metabolism of branched-chain amino acids in the development of Alzheimer's disease and other metabolic disorders
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7059578/
http://jpet.aspetjournals.org/content/318/3/1368
Human Gastric Epithelium Produces IL-4 and IL-4δ2 Isoform Only upon Helicobacter Pylori Infection
https://journals.sagepub.com/doi/abs/10.1177/039463200702000417
The Immunobiology of Schistosomiasis
University of Manchester
Macrophages function as important effector cells during most immune responses; therefore, their activity can have a major impact on the duration, magnitude, and overall character of inflammatory reactions. There is accumulating evidence that the effector function of macrophages is controlled by specific triggering signals that stimulate their differentiation into classically activated or alternatively activated cells (28, 29). In this regard, cytokines, glucocorticoids, and catecholamines have proven highly effective at controlling the functional diversity of macrophages (28, 30, 31). Signals such as LPS, unmethylated CpG oligodeoxynucleotides, the phagocytosis of necrotic cells, and triggering of specific toll-like receptors can also influence the effector phenotype of activated macrophages and dendritic cells (32, 33). In mice IFN-γ, IL-1, and TNF-α stimulate NO production by macrophages and therefore serve as the key signals that promote the development of classically activated macrophages (7). In contrast, recent in vitro experiments demonstrate that several Th2-associated cytokines, including IL-4, IL-13, and IL-10, are involved in the generation of alternatively activated cells, characterized by up-regulation of Arg-1, the hepatic isoform of arginase (8, 9, 10).
proline production by alternatively activated macrophages is blocked by l-hydroxyarginine (Fig. 9⇑A) and is regulated exclusively by the availability of l-ornithine. IL-4/IL-13-activated macrophages are an important source of proline.
macrophages as well as other NOS-2/Arg-1-expressing cells play key roles in the pathogenesis of chronic inflammatory diseases
while Th1 cytokines are critical, the downstream functional activity of NO-producing cells is equally important. Thus, NOS-2 is important not only for its antimicrobial activities (7), but also because it can serve as a potent anti-inflammatory and antifibrotic mediator.
Differential Regulation of Nitric Oxide Synthase-2 and Arginase-1 by Type 1/Type 2 Cytokines In Vivo: Granulomatous Pathology Is Shaped by the Pattern of l-Arginine Metabolism | The Journal of Immunology
https://www.jimmunol.org/content/167/11/6533Macrophages as effector cells.Macrophages constitute a potentially powerful line of defense against H. pylori through their own effector function, yet, intriguingly, these capabilities fail the host. One such pathway is the generation of nitric oxide (NO) derived from the enzyme inducible NO synthase (iNOS or NOS2), which has been shown to be upregulated by H. pylori in macrophages in vitro (42, 117, 118, 339) and in vivo (103, 192) (Fig. 3). Coculture studies demonstrate that H. pylori organisms can be eliminated by macrophages even when the bacteria are physically separated from these cells and that this antimicrobial defense is NO dependent (42, 117). The arginase enzyme possessed by H. pylori, encoded by the rocF gene, can compete sufficiently with macrophages for the iNOS substrate l-arginine (l-Arg) that host NO production is impaired, leading to enhanced survival of the bacterium through this mechanism (117). Moreover, this competition can deplete l-Arg sufficiently to impair the synthesis of iNOS protein, since its translation is highly dependent on l-Arg availability inside the macrophage (46). Bacterial arginase serves to generate urea from l-Arg, which is then utilized by urease to synthesize ammonia that is required to neutralize gastric acid.
Pathways involved in regulation of macrophage iNOS synthesis and NO production in response to H. pylori. The translation of iNOS protein depends on the availability of l-arginine (l-Arg). Pathogenic mechanisms that inhibit l-Arg availability for iNOS include (i) the consumption of extracellular l-Arg by H. pylori itself, through its bacterial arginase activity; (ii) the upregulation of macrophage arginase II, which depletes intracellular l-Arg; and (iii) induction of ODC that generates the polyamine spermine, which blocks uptake of l-Arg into macrophages by CAT2. The resulting effect is limitation of iNOS protein synthesis and NO production, despite high levels of iNOS mRNA. Arginase and ODC are novel targets for therapeutic intervention to enhance antimicrobial NO production and hence reduce persistent colonization that leads to chronic inflammation and cancer risk.
FIG. 3. | Clinical Microbiology Reviews
https://cmr.asm.org/content/23/4/713/F3
Mechanism of macrophage apoptosis caused by H. pylori. This pathway is dependent on the activities of the enzymes arginase II, ODC, and SMO. Induction of arginase II enhances synthesis of l-ornithine, which is converted into polyamines by ODC via a process that requires both H. pylori activation of the ODC promoter and c-Myc as a transcriptional enhancer. Production of the polyamine spermine provides a substrate for SMO, which is also upregulated by H. pylori. SMO generates H2O2, which causes mitochondrial membrane depolarization, cytochrome c release from mitochondria to the cytosol, and caspase-3 activation, followed by apoptosis. Induction of macrophage apoptosis leads to impairment of mucosal immunity to H. pylori, chronic inflammation, and cancer risk (48, 50, 116).
Relationships between H. pylori, inflammation, and acid secretion. H. pylori infection can reduce acid secretion and increase inflammation via multiple intermediates. Increased production of IL-1β and TNF-α from inflammatory cells inhibits acid secretion from parietal cells. Acid secretion is also inhibited by repression of H+K+ ATPase α-subunit promoter activity, in addition to VacA-induced proteolysis of ezrin.
Chronic H. pylori infection may result in hypochlorhydria or hyperchlorhydria, depending on the severity and distribution of gastritis. Most patients infected long-term develop pangastritis associated with hypochlorydria, which may progress to gastric ulceration and/or adenocarcinoma. Conversely, antral predominant gastritis occurs in approximately 12% of chronically infected patients and is characterized by hyperchlorhydria, which may lead to duodenal ulcer disease (23).Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk | Clinical Microbiology Reviews
https://cmr.asm.org/content/23/4/713
IL-13 and IFN-/IL-12 counter-regulate macrophage-activation status and control fibrosis.The fibrogenic role of interleukin-13 (IL-13) seems to stem from its ability, together with IL-4, to induce the expression of arginase in macrophages18. Arginase uses l-arginine as a substrate to make l-ornithine, which is converted to proline by ornithine-aminotransferase. Proline is an essential amino acid that is involved in collagen production and, therefore, in the development of fibrosis. Fibrosis is inhibited in mice that are immunized with egg antigens plus IL-12; cytokines that are produced as components of the induced TH1 response (such as IFN- and TNF) prevent TH2-response development (and, so, IL-13 production) and also activate macrophages to express inducible nitric oxide synthase (iNOS), rather than arginase. This immunization protocol is ineffective in iNOS-knockout mice, despite the induction of excellent TH1 responses in these animals. This seems to be due to the fact that iNOS uses arginine to make nitric oxide (NO) and citrulline — an intermediate in this pathway is l-hydroxyarginine, which inhibits arginase, effectively reducing the amount of proline that is available for collagen synthesis. These findings fit nicely with early work in this area, which showed that IFN- could have an anti-fibrogenic role in the schistosome granuloma, as well as in other conditions. Adapted from Ref. 18. IFN-, interferon-; TNF, tumour-necrosis factor.Schistosomes are parasitic worms that are a prime example of a complex multicellular pathogen that flourishes in the human host despite the development of a pronounced immune response. Understanding how the immune system deals with such pathogens is a daunting challenge. The past decade has seen the use of a wide range of new approaches to determine the nature and function of the immune response to schistosomes. Here, we attempt to summarize advances in our understanding of the immunology of schistosomiasis, with the bulk of the review reflecting the experimental focus on Schistosoma mansoni infection in mice.
血吸虫是一种寄生蠕虫,它是一种复杂的多细胞病原体的主要例子,尽管在人类宿主体内有明显的免疫反应,但这种病原体仍会大量繁殖。了解免疫系统如何应对这些病原体是一项艰巨的挑战。在过去的十年中,人们使用了大量的新方法来确定对血吸虫的免疫反应的性质和功能。在这里,我们试图总结我们对血吸虫病免疫学的理解的进展,大部分综述反映了对小鼠曼氏血吸虫感染的实验重点。
L-正缬氨酸增强活化巨噬细胞产生 NO
L -正缬氨酸 arginase inhibitor | Sigma-Aldrich
https://www.sigmaaldrich.com/catalog/product/sigma/n7627?lang=zh®ion=CN
http://journal.frontiersin.org/article/10.3389/fimmu.2013.00174/full
http://vetmed.tamu.edu/faculty/zhou-lab/research
IJMS | Free Full-Text | Pro-Resolving Molecules—New Approaches to Treat Sepsis? | HTML
https://www.mdpi.com/1422-0067/18/3/476/htm
M1: expression of iNOS(NO,citrunine), M2:expression of Arg2 (ornithine,a precursor of polyamines and collagen)
IJMS | Free Full-Text | Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing
https://www.mdpi.com/1422-0067/18/7/1545
Immune Effectors » Protozoan and Helminth Parasites » Pathogen Profile Dictionary
https://ppdictionary.com/parasites_2.htm
Frontiers | Functions of Arginase Isoforms in Macrophage Inflammatory Responses: Impact on Cardiovascular Diseases and Metabolic Disorders | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2014.00533/full
https://openi.nlm.nih.gov/detailedresult.php?img=PMC4629055_MI2015-652035.002&req=4
https://openi.nlm.nih.gov/detailedresult.php?img=PMC4629055_MI2015-652035.002&req=4
Dendritic cell interaction with T-lymphocytes | Download Scientific Diagram
https://www.researchgate.net/figure/Dendritic-cell-interaction-with-T-lymphocytes_fig4_257837657
mouse macrophage undergoing pyroptosis, Washington Unversity
Pyroptosis - an overview | ScienceDirect Topics
https://www.sciencedirect.com/topics/neuroscience/pyroptosisPyroptosis is an inflammatory form of programmed cell death that occurs most frequently upon infection with intracellular pathogens (Le and Harton, 2013). In contrast to apoptosis and necrosis, pyroptosis requires the function of the enzyme caspase-1.
https://www.science20.com/news_releases/pyroptosis_how_a_dying_cell_sounds_an_alarm
https://www.nature.com/articles/nrmicro2070
http://www.mdpi.com/1422-0067/17/12/2068/htm
Microbes Infect. Author manuscript; available in PMC 2016 Jun 1.
Macrophages expressing arginase 1 and nitric oxide synthase 2 accumulate in the small intestine during Giardia lamblia infection
Jenny Maloney, Aleksander Keselman, Erqiu Li, and Steven M. Singer*
Abstract
Nitric oxide (NO) has been shown to inhibit Giardia lamblia in vitro and in vivo. This study sought to determine if Giardia infection induces arginase 1 (ARG1) expression in host macrophages to reduce NO production. Stimulations of RAW 264.7 macrophage-like cells with Giardia extract induced arginase activity. Real-time PCR and immunohistochemistry showed increased ARG1 and nitric oxide synthase 2 (NOS2) expression in mouse intestine following infection. Flow cytometry demonstrated increased numbers of macrophages positive for both ARG1 and NOS2 in lamina propria following infection, but there was no evidence of increased expression of ARG1 in these cells.
Keywords: Giardia lamblia, arginase 1, macrophages
1. Introduction
The protozoan parasite Giardia lamblia (syn. G. intestinalis, G. duodenalis) is a common cause of diarrheal disease worldwide. G. lamblia can infect humans and many other mammals, with prevalence rates in humans that range from 2–7% in developed countries to 20–30% in developing countries [1]. G. lamblia is transmitted through faecal contamination of food or water. As such infection rates are highest in countries where water purification is limited, leading to G. lamblia being included in the World Health Organization’s Neglected Disease Initiative since 2004 [2]. Intestinal pathology during infection is driven in part by the host immune system [3]. Yet the immunological processes that control infection and pathology are not entirely identified.
Arginine has diverse functions in mammalian physiology and plays an important role in host immunity. The production of Nitric Oxide (NO) by Nitric Oxide Synthase 2 (NOS2) from arginine is a key element of the innate immune response as NO is toxic to many pathogens. In fact arginine is the sole amino acid substrate for NO production [4]. The depletion of arginine as a means of limiting NO production is a survival strategy employed by many pathogenic organisms including viruses, bacteria, fungi, and protists [5]. Arginine limitation can be achieved through a number of pathways that include pathogen production of arginase (ARG), pathogen mediated induction of host ARG in macrophages, or consumption of host arginine by the pathogen [6, 7]. Nitric oxide is known to be cytostatic and potentially cytotoxic to G. lamblia in culture [8–11]. Yet the activation of host ARG as an evasive mechanism of G. lamblia has not been explored in vivo.
Host macrophages are often divided into two classes based on the expression of ARG1 and NOS2: classically activated M1 macrophages which express NOS2 and alternatively activated M2 macrophages which express ARG1 [5, 12]. These cells are thought to have antagonistic roles in the immune response with M1 macrophages being involved in pathogen control and M2 macrophages serving to limit excessive NO production and support healing. The ability of some pathogens to influence the expression of ARG1 and NOS2 can play an important role in immune subversion and parasite survival.
Previous work has shown that host NO can inhibit G. lamblia survival in vitro, and that arginine limitation negates this effect [9]. Recent studies on the influence of G. lamblia on epithelial cell ARG activity report a down regulation of host ARG in response to G. lamblia [13]. However these studies exclusively used in vitro culture systems of non-immune cells. In this study we aimed to determine if G. lamblia can directly induce macrophage ARG1 in vitro and if infection can lead to an increase in ARG1 expression in host macrophages in vivo.Macrophages expressing arginase 1 and nitric oxide synthase 2 accumulate in the small intestine during Giardia lamblia infection
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4461514/
Crit Rev Immunol. 2001;21(5):399-425.
Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue.
Mills CD1.
Author information
1
Department of Surgery and Diabetes Institute for Immunology and Transplantation, University of Minnesota Hospitals and Clinics, Minneapolis 55455, USA. mills002@tc.umn.edu
Abstract
Macrophages can metabolize arginine to nitric oxide in quantities that inhibit pathogens or nearby host cells. They can instead metabolize arginine to ornithine (a precursor of polyamines and collagen) in quantities that stimulate pathogens or nearby host cells.Macrophages are essentially the only circulating cells that can make these life or death decisions with arginine. Macrophages expressing these destructive or constructive phenotypes have been termed M-1 or M-2 because they also stimulate TH1 or TH2 responses, respectively.
Factors that influence whether a macrophage expresses the M-1 or M-2 phenotype and the real or potential impact on immune responses and other host processes are discussed.
PMID: 11942557
[Indexed for MEDLINE]https://www.ncbi.nlm.nih.gov/pubmed/11942557
J Immunol. 2010 Mar 1;184(5):2572-82. doi: 10.4049/jimmunol.0902436. Epub 2010 Jan 22.
Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages.
Lewis ND1, Asim M, Barry DP, Singh K, de Sablet T, Boucher JL, Gobert AP, Wilson KT.
Author information
Division of Gastroenterology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN 37240, USA.
Abstract
Helicobacter pylori infection of the stomach causes peptic ulcer disease and gastric cancer. Despite eliciting a vigorous immune response, the bacterium persists for the life of the host.An important antimicrobial mechanism is the production of NO derived from inducible NO synthase (iNOS). We have reported that macrophages can kill H. pylori in vitro by an NO-dependent mechanism, but supraphysiologic levels of the iNOS substrate l-arginine are required. Because H. pylori induces arginase activity in macrophages, we determined if this restricts NO generation by reducing l-arginine availability.
Inhibition of arginase with S-(2-boronoethyl)-l-cysteine (BEC) significantly enhanced NO generation in H. pylori-stimulated RAW 264.7 macrophages by enhancing iNOS protein translation but not iNOS mRNA levels. This effect resulted in increased killing of H. pylori that was attenuated with an NO scavenger.
In contrast, inhibition of arginase in macrophages activated by the colitis-inducing bacterium Citrobacter rodentium increased NO without affecting iNOS levels. H. pylori upregulated levels of arginase II (Arg2) mRNA and Arg2 protein, which localized to mitochondria, whereas arginase I was not induced.
Increased iNOS protein and NO levels were also demonstrated by small interfering RNA knockdown of Arg2 and in peritoneal macrophages from C57BL/6 Arg2(-/-) mice.
In H. pylori-infected mice, treatment with BEC or deletion of Arg2 increased iNOS protein levels and NO generation in gastric macrophages, but treatment of Arg2(-/-) mice with BEC had no additional effect.
These studies implicate Arg2 in the immune evasion of H. pylori by causing intracellular depletion of l-arginine and thus reduction of NO-dependent bactericidal activity.
PMID: 20097867 PMCID: PMC2841360 DOI: 10.4049/jimmunol.0902436
[Indexed for MEDLINE] Free PMC ArticleArginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/20097867
J Immunol. 2011 Mar 15;186(6):3632-41. doi: 10.4049/jimmunol.1003431. Epub 2011 Feb 4.
Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II.
Lewis ND1, Asim M, Barry DP, de Sablet T, Singh K, Piazuelo MB, Gobert AP, Chaturvedi R, Wilson KT.
Author information
1
Division of Gastroenterology, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN 37232, USA.
Abstract
Helicobacter pylori infection persists for the life of the host due to the failure of the immune response to eradicate the bacterium. Determining how H. pylori escapes the immune response in its gastric niche is clinically important.We have demonstrated in vitro that macrophage NO production can kill H. pylori, but induction of macrophage arginase II (Arg2) inhibits inducible NO synthase (iNOS) translation, causes apoptosis, and restricts bacterial killing.
Using a chronic H. pylori infection model, we determined whether Arg2 impairs host defense in vivo. In C57BL/6 mice, expression of Arg2, but not arginase I, was abundant and localized to gastric macrophages. Arg2(-/-) mice had increased histologic gastritis and decreased bacterial colonization compared with wild-type (WT) mice. Increased gastritis scores correlated with decreased colonization in individual Arg2(-/-) mice but not in WT mice.
When mice infected with H. pylori were compared, Arg2(-/-) mice had more gastric macrophages, more of these cells were iNOS(+), and these cells expressed higher levels of iNOS protein, as determined by flow cytometry and immunofluorescence microscopy.
There was enhanced nitrotyrosine staining in infected Arg2(-/-) versus WT mice, indicating increased NO generation. Infected Arg2(-/-) mice exhibited decreased macrophage apoptosis, as well as enhanced IFN-γ, IL-17a, and IL-12p40 expression, and reduced IL-10 levels consistent with a more vigorous Th1/Th17 response.
These studies demonstrate that Arg2 contributes to the immune evasion of H. pylori by limiting macrophage iNOS protein expression and NO production, mediating macrophage apoptosis, and restraining proinflammatory cytokine responses.
PMID: 21296975 PMCID: PMC3069806 DOI: 10.4049/jimmunol.1003431
[Indexed for MEDLINE] Free PMC ArticleImmune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/21296975
Amino Acids. 2016 Oct;48(10):2375-88. doi: 10.1007/s00726-016-2231-2. Epub 2016 Apr 13.
Arginase 2 deletion leads to enhanced M1 macrophage activation and upregulated polyamine metabolism in response to Helicobacter pylori infection.
Hardbower DM1,2, Asim M2, Murray-Stewart T3, Casero RA Jr3, Verriere T2, Lewis ND2, Chaturvedi R2,4, Piazuelo MB2, Wilson KT5,6,7,8,9.
Author information
1
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Vanderbilt University School of Medicine, 2215 Garland Avenue, 1030C Medical Research Building IV, Nashville, TN, 37232, USA.
2
Division of Gastroenterology, Hepatology and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA.
3
The Sydney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
4
School of Biotechnology, Jawaharlal Nehru University, New Delhi, India.
5
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Vanderbilt University School of Medicine, 2215 Garland Avenue, 1030C Medical Research Building IV, Nashville, TN, 37232, USA. keith.wilson@vanderbilt.edu.
6
Division of Gastroenterology, Hepatology and Nutrition, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN, USA. keith.wilson@vanderbilt.edu.
7
Department of Cancer Biology, Vanderbilt University Medical Center, Nashville, TN, USA. keith.wilson@vanderbilt.edu.
8
Center for Mucosal Inflammation and Cancer, Vanderbilt University Medical Center, Nashville, TN, USA. keith.wilson@vanderbilt.edu.
9
Veterans Affairs Tennessee Valley Healthcare System, Nashville, TN, USA. keith.wilson@vanderbilt.edu.
Abstract
We reported that arginase 2 (ARG2) deletion results in increased gastritis and decreased bacterial burden during Helicobacter pylori infection in mice. Our studies implicated a potential role for inducible nitric oxide (NO) synthase (NOS2), as Arg2 (-/-) mice exhibited increased NOS2 levels in gastric macrophages, and NO can kill H. pylori.We now bred Arg2 (-/-) to Nos2 (-/-) mice, and infected them with H. pylori. Compared to wild-type mice, both Arg2 (-/-) and Arg2 (-/-) ;Nos2 (-/-) mice exhibited increased gastritis and decreased colonization, the latter indicating that the effect of ARG2 deletion on bacterial burden was not mediated by NO. While Arg2 (-/-) mice demonstrated enhanced M1 macrophage activation, Nos2 (-/-) and Arg2 (-/-) ;Nos2 (-/-) mice did not demonstrate these changes, but exhibited increased CXCL1 and CXCL2 responses.
There was an increased expression of the Th1/Th17 cytokines, interferon gamma and interleukin 17, in gastric tissues and splenic T-cells from Arg2 (-/-), but not Nos2 (-/-) or Arg2 (-/-) ;Nos2 (-/-) mice. Gastric tissues from infected Arg2 (-/-) mice demonstrated increased expression of arginase 1, ornithine decarboxylase, adenosylmethionine decarboxylase 1, spermidine/spermine N (1)-acetyltransferase 1, and spermine oxidase, along with increased spermine levels.
These data indicate that ARG2 deletion results in compensatory upregulation of gastric polyamine synthesis and catabolism during H. pylori infection, which may contribute to increased gastric inflammation and associated decreased bacterial load. Overall, the finding of this study is that ARG2 contributes to the immune evasion of H. pylori by restricting M1 macrophage activation and polyamine metabolism.
KEYWORDS:
Helicobacter pylori; Immune evasion; Macrophage activation; Polyamines
PMID: 27074721 PMCID: PMC5042810 DOI: 10.1007/s00726-016-2231-2
[Indexed for MEDLINE] Free PMC ArticleArginase 2 deletion leads to enhanced M1 macrophage activation and upregulated polyamine metabolism in response to Helicobacter pylori infection. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/27074721
Absolute Monocytes Low, High, Normal Range, Blood Test
Learn all about absolute monocytes low, high, normal range and blood test.
Monocytes are produced in the bone marrow and then enter the blood, where they account for about 1 to 10% of the circulating white blood cells (200 to 600 monocytes per microliter of blood). After a few hours in the blood, monocytes migrate to tissues (such as spleen, liver, lungs, and bone marrow tissue), where they mature into macrophages.
The presence of a large amount of monocytes in your complete blood count may indicate a chronic inflammatory process like autoimmune conditions, or TB, viral infections such as mumps, mono, measles, or a parasitic infection. The monocytes do not cause these issues but they may lead your practitioner to look for further clarification by having other tests ordered. They are a product of your body’s immune response.Absolute Monocytes Low, High, Normal Range, Blood Test
http://healthncare.info/absolute-monocytes-low-high-normal-range-blood-test/
Research Highlight
Published: 10 July 2017
Macrophage’s little helper: vitamin A directs alternatively activated monocyte-derived macrophages to tissue-resident macrophages
Dirk Schlüter & Florian H Heidel
Cellular & Molecular Immunology volume 14, pages805–808(2017)Cite this article
Institute of Medical Microbiology and Hospital Hygiene, Otto-von-Guericke University Magdeburg, Magdeburg, 39120, Germany
...
Tissue-resident macrophages originate from the yolk sac in a Myb-independent manner and populate all organs during embryogenesis. These macrophages are a heterogeneous self-renewing population that adapt to the organ-specific local environment to contribute to tissue homeostasis. Depending on the age, organ and inflammatory conditions, macrophages that are derived from hematopoietic stem cells (HSCs) in a Myb-dependent manner may also infiltrate organs and develop into tissue-resident macrophages. The factors and mechanism driving the conversion of HSC-derived monocytes into tissue-resident macrophages are incompletely understood but may potentially offer the opportunity to therapeutically modulate tissue-specific inflammation. Recently, Gundra et al. presented the first evidence that activated monocytes recruited to the peritoneal cavity by a type 2 immune reaction can convert them into macrophages with the transcriptional profile of resident peritoneal macrophages.1 In addition, monocyte-derived macrophages can convert into tissue macrophages in Schistosoma mansoni-induced T helper cell type 2 (TH2)-dependent granuloma of the liver.
Most notably, in the peritoneum and liver, the conversion of monocytes to tissue-resident macrophages lasts weeks and is vitamin A-dependent.
Tissue-resident macrophages are highly adapted to the local environment of their respective tissue. This functional adaptation is reflected at both the genetic and epigenetic (enhancer landscape) levels of different tissue-resident macrophages.2, 3 Reprogramming of AAMmono to tissue-resident AAMconv resulted in change to a similar transcriptional profile in AAMconv compared with AAMres.
In some tissues, particularly intestine, monocyte-derived macrophages contribute substantially to the number of resident macrophages (Figures 1 and 2). These HSC-derived resident macrophages self-renew and acquire a tissue-specific transcriptional profile under homeostatic conditions. In addition, HSC-derived macrophages can fill the niche of depleted tissue-resident macrophages.
In the lung, kidney, peritoneal cavity and, particularly, intestine, the contribution of HSC to tissue-resident macrophages is up to 50%, depending on the age and gender.4, 5 By contrast, in the brain and liver, replacement of tissue-resident macrophages by HSC-derived macrophages under homeostatic conditions is negligible.4, 5.
...
The environment is an important factor that regulates the function and fate of tissue-resident macrophages under both homeostatic and inflammatory conditions.
...
Taken together, vitamin A and retinoic acid are important regulators of the functional plasticity of AAMmono and tissue-resident macrophages during steady state conditions and in ongoing immune reactions.Macrophage’s little helper: vitamin A directs alternatively activated monocyte-derived macrophages to tissue-resident macrophages | Cellular & Molecular Immunology
https://www.nature.com/articles/cmi201758
Pro-Resolving Molecules—New Approaches to Treat Sepsis?
by Christa Buechler 1,*OrcID, Rebekka Pohl 1 and Charalampos Aslanidis 2
1 Department of Internal Medicine I, Regensburg University Hospital, 93042 Regensburg, Germany
2 Institute of Clinical Chemistry and Laboratory Medicine, Regensburg University Hospital, 93042 Regensburg, Germany
Int. J. Mol. Sci. 2017, 18(3), 476; https://doi.org/10.3390/ijms18030476
Abstract
Inflammation is a complex response of the body to exogenous and endogenous insults. Chronic and systemic diseases are attributed to uncontrolled inflammation. Molecules involved in the initiation of inflammation are very well studied while pathways regulating its resolution are insufficiently investigated. Approaches to down-modulate mediators relevant for the onset and duration of inflammation are successful in some chronic diseases, while all of them have failed in sepsis patients.Inflammation and immune suppression characterize sepsis, indicating that anti-inflammatory strategies alone are inappropriate for its therapy.
Heme oxygenase 1 is a sensitive marker for oxidative stress and is upregulated in inflammation. Carbon monoxide, which is produced by this enzyme, initiates multiple anti-inflammatory and pro-resolving activities with higher production of omega-3 fatty acid-derived lipid metabolites being one of its protective actions. Pro-resolving lipids named maresins, resolvins and protectins originate from the omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid while lipoxins are derived from arachidonic acid.
These endogenously produced lipids do not simply limit inflammation but actively contribute to its resolution, and thus provide an opportunity to combat chronic inflammatory diseases and eventually sepsis.
Keywords: carbon monoxide; resolvin; cyclooxygenase; lipoxygenaseIJMS | Free Full-Text | Pro-Resolving Molecules—New Approaches to Treat Sepsis? | HTML
https://www.mdpi.com/1422-0067/18/3/476/htm
Published: 02 February 2015
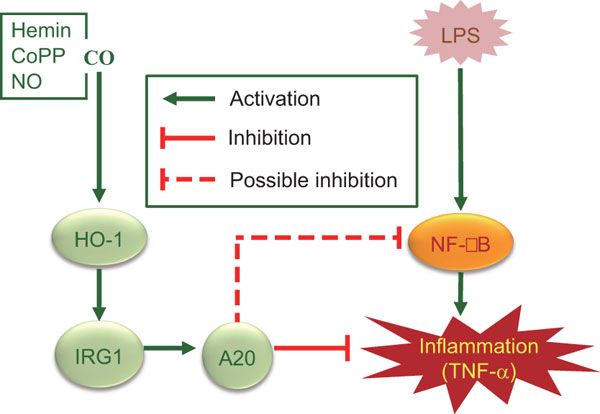
IRG1 induced by heme oxygenase-1/carbon monoxide inhibits LPS-mediated sepsis and pro-inflammatory cytokine production
Md Jamal Uddin, Yeonsoo Joe, Seul-Ki Kim, Sun Oh Jeong, Stefan W Ryter, Hyun-Ock Pae & Hun Taeg Chung
Cellular & Molecular Immunology volume 13, pages170–179(2016)Cite this article
Abstract
The immunoresponsive gene 1 (IRG1) protein has crucial functions in embryonic implantation and neurodegeneration. IRG1 promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species (ROS). The cytoprotective protein heme oxygenase-1 (HO-1), which generates endogenous carbon monoxide (CO), is expressed in the lung during Lipopolysaccharide (LPS) tolerance and cross tolerance. However, the detailed molecular mechanisms and functional links between IRG1 and HO-1 in the innate immune system remain unknown.In the present study, we found that the CO releasing molecule-2 (CORM-2) and chemical inducers of HO-1 increased IRG1 expression in a time- and dose-dependent fashion in RAW264.7 cells. Furthermore, inhibition of HO-1 activity by zinc protoporphyrin IX (ZnPP) and HO-1 siRNA significantly reduced expression of IRG1 under these conditions. In addition, treatment with CO and HO-1 induction significantly increased A20 expression, which was reversed by ZnPP and HO-1 siRNA. LPS-stimulated TNF-α was significantly decreased, whereas IRG1 and A20 were increased by CORM-2 application and HO-1 induction, which in turn were abrogated by ZnPP. Interestingly, siRNA against IRG1 and A20 reversed the effects of CO and HO-1 on LPS-stimulated TNF-α production. Additionally, CO and HO-1 inducers significantly increased IRG1 and A20 expression and downregulated TNF-α production in a LPS-stimulated sepsis mice model. Furthermore, the effects of CO and HO-1 on TNF-α production were significantly reversed when ZnPP was administered.
In conclusion, CO and HO-1 induction regulates IRG1 and A20 expression, leading to inhibition of inflammation in vitro and in an in vivo mice model.
Regulation of LPS-induced inflammation by CO in RAW264.7 macrophages and in mice. CO, in addition to HO-1 inducers including hemin, CoPP and NO, induces HO-1 expression that in turn induces IRG1 and A20 expression, thereby regulating LPS-induced inflammation.
IRG1 induced by heme oxygenase-1/carbon monoxide inhibits LPS-mediated sepsis and pro-inflammatory cytokine production | Cellular & Molecular Immunology
https://www.nature.com/articles/cmi201502
Front. Immunol., 02 July 2013 | https://doi.org/10.3389/fimmu.2013.00174
Therapeutic potential of the nitrite-generated NO pathway in vascular dysfunction
Michael Madigan* and Brian Zuckerbraun
University of Pittsburgh, Pittsburgh, PA, USA
Nitric oxide (NO) generated through L-arginine metabolism by endothelial nitric oxide synthase (eNOS) is an important regulator of the vessel wall. Dysregulation of this system has been implicated in various pathological vascular conditions, including atherosclerosis, angiogenesis, arteriogenesis, neointimal hyperplasia, and pulmonary hypertension. The pathophysiology involves a decreased bioavailability of NO within the vessel wall by competitive utilization of L-arginine by arginase and “eNOS uncoupling.” Generation of NO through reduction of nitrate and nitrite represents an alternative pathway that may be utilized to increase the bioavailability of NO within the vessel wall. We review the therapeutic potential of the nitrate/nitrite/NO pathway in vascular dysfunction.
Introduction
The Nobel Prize in physiology or medicine was awarded to Drs. Furchgott, Ignarro, and Murad in 1998 for their work in identifying nitric oxide (NO), previously recognized as endothelium-derived relaxing factor, as a biologic mediator of the cardiovascular system. Since that time, NO has been extensively researched and has been linked to numerous physiological and pathological processes within the cardiovascular system. Vascular dysfunction is the root cause of a variety of important disease processes, including myocardial infarction, stroke, peripheral vascular disease, pulmonary hypertension, and wound healing. This constellation of pathology imposes a significant financial burden on the healthcare system and produces significant morbidity and mortality in those affected. The underlying pathophysiology of vascular dysfunction occurs in numerous forms, and often involves a combination of dysregulated endothelial cell NO production, increased proliferation and migration of smooth muscle cells, increased formation of intimal and medial plaques, impaired collateral vessel generation, and reduced angiogenesis.
The L-Arginine/Nitric Oxide Pathway
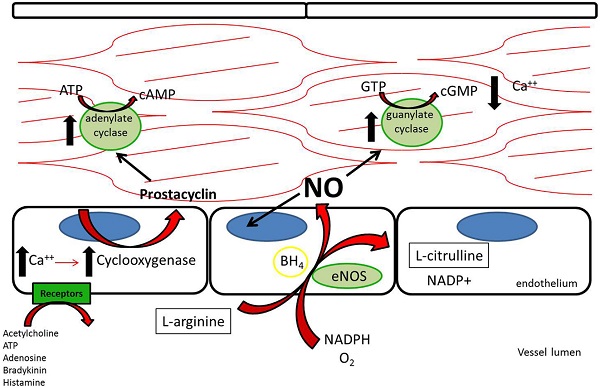
Three nitric oxide synthases (NOSs), nNOS (neuronal), iNOS (inducible), and eNOS (endothelial), were identified and initially thought to be the sole producers of NO within the cardiovascular system (1). Both nNOS and eNOS are calcium-dependent and constitutively active, while iNOS is induced under inflammatory conditions and is calcium-independent. All three isoforms metabolize L-arginine, NADPH, and oxygen to L-citrulline, NADP, and NO (2) (Figure 1). L-arginine may alternatively be metabolized by arginase to L-ornithine and urea. When the supply of L-arginine is limited, metabolism via arginase may effectively reduce production of NO (3).
Figure 1. L-Arginine is metabolized in endothelial cells via endothelial nitric oxide synthase to nitric oxide, which then acts downstream to reduce platelet adhesion, decrease leukocyte adhesion, inhibit smooth muscle proliferation and migration, and induce vasodilation. Acetylcholine, adenosine triphosphate, adenosine, bradykinin, and histamine all act on different receptors to generate downstream prostacyclin, which acts as a redundant system to induce vasodilation and platelet inhibition (112).
It has been suggested that the shunting of L-arginine away from the NOS/NO pathway toward the arginase/L-ornithine pathway contributes to certain vascular pathology (4– 7) (Figure 2). Expression of arginase in the vascular wall is induced under pro-inflammatory conditions, as well as by reactive oxygen species (ROS) and reactive nitrogen species (RNS) (8). Increased arginase activity has been associated with hypertension and coronary vascular dysfunction (9– 11). Also, direct vascular injury induces a local inflammatory response. Arginase is upregulated in the vessel wall after balloon injury in the rat carotid injury model. Polyamines generated through the L-ornithine pathway form the building blocks necessary for smooth muscle cell proliferation and neointimal hyperplasia of the vessel wall (12). Peyton et al. (13) demonstrated that selective inhibitors for arginase attenuate neointimal hyperplasia in the rat carotid injury model.
Figure 2. L-arginine may be competitively metabolized by arginase to L-citrulline and urea, reducing production of nitric oxide and contributing to vascular dysfunction.
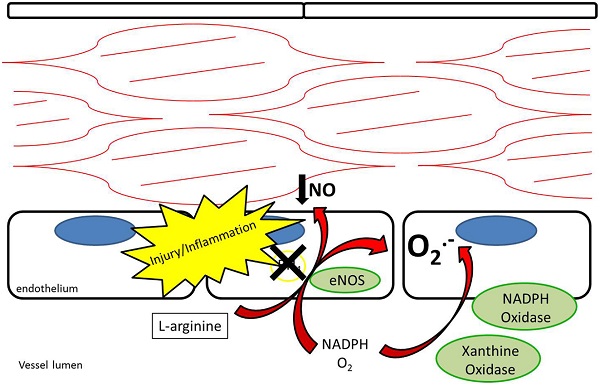
Endothelial NOS is highly expressed in endothelial cells at baseline. Its metabolism of L-arginine to NO is thought to be a major contributor to plasma nitrite levels, which play an important role in baseline vasodilation (14, 15). In addition to regulating baseline vasomotor tone, eNOS is thought to help limit platelet adhesion and thrombosis (16, 17). After vessel injury iNOS is upregulated in arterial smooth muscle cells and eNOS is upregulated in the endothelium resulting in increased NO production (18). Under pathological conditions, the increased NOS activity may not translate into increased NO production. Reduced NO bioavailability through eNOS “uncoupling” is a contributing factor to reduced local NO in atherosclerosis, pulmonary hypertension, and vessel injury (7, 19). Tetrahydrobiopterin (BH4) is an essential cofactor for the enzymatic production of NO via NOSs (20). Uncoupling occurs under conditions of reduced BH4 availability where eNOS produces superoxide anions rather than NO (21, 22) (Figure 3). In addition, ROS are produced by NADPH oxidase and XOR (23, 24). ROS have been recognized as contributing to vascular dysfunction, through mechanisms including endothelial dysfunction, vascular smooth muscle cell growth, lipid peroxidation, and inflammation (25). An alternative source of NO under these conditions may help restore the NO deficiency attributed to uncoupling.
Figure 3. Endothelial nitric oxide synthase uncoupling results in reduced production of nitric oxide as well as production of superoxide anions. NADPH oxidase and xanthine oxidase also contribute to production of superoxide anions.
Nitrate/Nitrite Reduction to Nitric Oxide
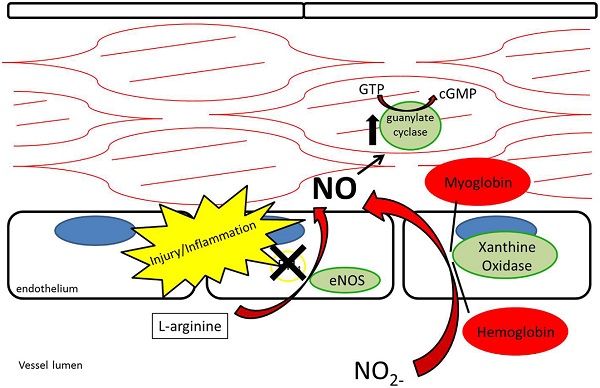
While nitrate and nitrite were long thought of as stable end-products of NO metabolism, recent evidence supports nitrate and nitrite as potential sources of NO under appropriate conditions (12, 26– 29) (Figure 4). As opposed to the NOS enzymes, which require oxygen as a substrate for NO generation, nitrite-generated production of NO has been shown to occur more readily under acidic and hypoxic conditions (30– 32, 113). Nitrate/nitrite reduction has been shown to occur via deoxygenated hemoglobin, myoglobin, enzymatic, and non-enzymatic means (33– 37). A class of molybdenum-containing enzymes, including xanthine oxidoreductase (XOR), aldehyde oxidase (AOX), and sulfite oxidase (SUOX), have been identified as enzymes that may facilitate the reduction of nitrate and nitrite to NO at the molybdenum-containing site (38). We and others have shown that XOR in particular is present within the vessel wall and tissue and contributes to NO production in intimal hyperplasia, pulmonary hypertension, and ischemia-reperfusion (12, 26, 39).
Figure 4. Nitrite reduction by xanthine oxidoreductase, myoglobin, hemoglobin, and protonation results in nitric oxide production, especially under conditions of hypoxia and acidemia.
While L-arginine is a significant contributor to plasma nitrite production through the L-arginine/NOS/NO/nitrite pathway, plasma nitrite levels are also dependent on oral consumption of nitrate and nitrite (40). The Mediterranean diet, which has been associated with a lower risk of atherosclerosis and coronary artery disease, adds credence to the importance of oral nitrate/nitrite-derived NO in vascular biology (41, 42). The Mediterranean diet, known for its high content of nitrate-rich leafy green vegetables, has also been found to lower the blood pressure of healthy volunteers (40, 43). The nitrate/nitrite/NO pathway through oral ingestion is thought to rely on a symbiotic relationship with natural oral flora. Nitrate is concentrated within the salivary glands and salivary bacteria reduce nitrate to nitrite in the oral cavity (44). Once nitrite reaches the stomach, it is reduced to NO by protonation due to the stomach’s low pH (45). NO then may act locally by enhancing mucosal blood flow to the stomach (45– 47). Nitrite is also absorbed in the stomach where it enters the blood stream (48). Due to its relative stability, nitrite then has the ability to circulate to other areas in the body and undergo reduction to NO under acidic and hypoxic conditions (33). Acting in this way, circulating nitrite has been described as a “storage pool” for NO within the body (27).
Historically, there has been concern that oral nitrate/nitrite consumption may increase the risk of some cancers, including esophageal, stomach, and colon cancer. Some epidemiological studies have suggested that high oral intake of nitrate/nitrite correlates with increased risk of gastrointestinal malignancy, though accuracy in calculating dietary exposure is difficult (49). Nitrosylation of secondary amines via nitrite occurs readily under acidic conditions, such as in the stomach, resulting in N-nitrosamines. Around 300 N-nitrosamines have been identified as carcinogenic (50). The National Toxicology Program (51) of the US Department of Health and Human Services found no evidence of carcinogenic activity in mice and rats after 2 years of exposure to oral sodium nitrite. The International Agency for Research on Cancer (52), a division of the World Health Organization, evaluated the evidence concerning dietary consumption of nitrate/nitrite and carcinogenicity in their monographs. The IARC concluded that inadequate evidence exists in humans and experimental animals for the carcinogenicity of nitrate in food and drinking water and limited evidence exists to suggest carcinogenicity of nitrite in food and drinking water (52). The IARC did, however, recognize that sufficient evidence exists in experimental animals to suggest the carcinogenicity of nitrite in combination with amines or amides and that nitrite in food is correlated with stomach cancer (52). Ongoing research will help elucidate the specific conditions in which N-nitrosamines may be carcinogenic in humans.
Multiple investigations have demonstrated that the nitrate/nitrite/NO pathway has vasoactive properties in the systemic and pulmonary circulations. Infusion of nitrite into the forearm brachial artery increased local blood flow and decreased blood pressure at rest and during exercise in humans (33). The infusion correlated with an increase in erythrocyte iron-nitrosylated hemoglobin, suggesting that hemoglobin may play a role in transporting NO through the bloodstream. Dietary supplementation has the potential to achieve similar results systemically. Larsen et al. (43) used a 3-day dietary supplementation of nitrate (0.1 mmol/kg body weight) in healthy volunteers and showed an increase in plasma nitrate (178 ± 51 vs. 26 ± 11 μM) and nitrite (219 ± 105 vs. 138 ± 38 μM). After 3 days, the volunteers also had a decrease in diastolic and mean blood pressure by 3.7 mmHg and 3.2 mmHg, respectively. In a similar study using a Japanese diet high in nitrate, Sobko et al. (53) demonstrated an increase in both salivary and plasma levels of nitrate and nitrite. These volunteers had an average 4.5 mmHg drop in diastolic blood pressure after 10 days. Dietary nitrate may also effect the pulmonary circulation. In mice exposed to hypoxia to induce pulmonary hypertension, dietary nitrate reduced vascular remodeling and right ventricular hypertrophy through pulmonary vasodilation (26). Inhaled nitrite is an alternative delivery method that has the potential to induce pulmonary vasodilation while minimizing systemic effects. Nebulized sodium nitrite reduced hypoxia-induced pulmonary hypertension in lambs by 65% with no drop in systemic blood pressure (54).
Nitric Oxide and the Vessel Response to Injury
Nitric oxide has been shown to serve many vasoprotective properties that occur after vessel injury, including reduction of platelet deposition, decrease in leukocyte adhesion, inhibition of smooth muscle cell proliferation and migration, and induction of vasodilation (55). One of the initial responses to endothelial disruption is platelet activation and plug formation. NO and NOS expression are associated with decreased platelet adhesion at the vessel wall (56, 57). NO has been shown to be a potent inhibitor of platelet adhesion, reducing thrombosis within the vessel lumen (58, 59). NO mediates platelet adhesion though upregulation of platelet-soluble guanylate cyclase production of cyclic GMP. Nitrate and nitrite-supplemented diets increase bleeding times in mice, and there is an inverse relationship between blood nitrate/nitrite levels and platelet function (60). After platelet deposition, neutrophils and macrophages begin to infiltrate the vessel wall. NO inhibits leukocyte adhesion and the subsequent vessel inflammatory response after injury (61, 62). Once the inflammatory response sets in, smooth muscle cells infiltrate the medial layer and begin proliferating. The resulting thickened medial layer narrows the lumen and stiffens the vessel wall. NO acts to reduce the smooth muscle cell response in multiple ways. NO was first recognized as the substance responsible for calcium-dependent relaxation of the vascular smooth muscle cells (63). NO upregulates soluble guanylyl cyclase within cells and leads to increased cyclic GMP. Cyclic GMP then interacts with protein kinases to lower cytoplasmic calcium, which results in vasodilation (64). Also, it has been shown in culture that NO reversibly arrests the cell cycle of vascular smooth muscle cells (65). NO inhibits smooth muscle proliferation within the vessel wall via a p21 dependent mechanism (66– 68). Overall, NO reduces smooth muscle cell migration and proliferation, which can lead to atherosclerosis and neointimal hyperplasia (69).
Nitric Oxide and Atherosclerosis
Atherosclerosis resulting in coronary artery disease and stroke are the leading causes of death in the developed world (70). Atherosclerotic plaques are formed when the endothelial layer is damaged and cholesterol accumulates within the vessel wall. Macrophages are recruited to the site of injury, form foam cells, and release cytokines leading to an inflammatory response (71). Smooth muscle cells then migrate and proliferate within the vessel wall, eventually leading to an organized plaque (72). Repeated vessel wall injury causes thrombosis and narrowing of the lumen, which leads to ischemia of the tissue bed supplied by the vessels.
While atherosclerosis is a multifactorial process, dysregulation of the arginine/NOS balance contributes to the development of atherosclerotic disease (73). For instance, iNOS inhibition in the apolipoprotein E knockout mouse model for atherosclerosis accelerates the progression of atherosclerotic disease in these mice (74). Restoring the balance of NO production at multiple points along the pathway reduces formation of atherosclerotic plaques. L-Arginine supplementation has been shown to improve vasodilation in cholesterol-fed rabbits and monkeys and reduce the progression of atherosclerosis (75– 77). Also, exogenous expression of iNOS in the arteries reduces the injury response and atherosclerotic development (78). Furthermore, supplemental oral nitrite has also been shown to be beneficial in reducing vessel inflammation and endothelial dysfunction in mice treated with a high cholesterol diet (79).
NOS enzyme dysregulation results not only in reduced NO availability, but also increased superoxide anions and arginase activity, both of which are detrimental to maintaining healthy vasculature (80, 81). Oxidized low-density lipoproteins (OxLDL) caused by the interaction between LDL and superoxide anions correlate with atherosclerotic disease (82). OxLDL is taken up by macrophages, which forms foam cells on the vessel wall (73). OxLDLs also have been shown to induce apoptosis of endothelial cells and impair endothelium-dependent arterial relaxation within atherosclerotic vessels (83– 85). On the contrary, NO has been shown to inhibit apoptosis in endothelial progenitor cells caused by oxidized low-density lipid proteins (86).
Nitric Oxide and Peripheral Arterial Disease
Nitric oxide is an important regulator of the tissue response to peripheral arterial disease and lower extremity ischemia, specifically enhancing arteriogenesis, angiogenesis, and progenitor cell migration (4, 87, 88). Arteriogenesis is a recognized phenomenon that involves the enlargement of pre-existing collaterals as a result of increased sheer stress, often in response to stenotic or occluded primary vessels. Angiogenesis, on the other hand, is induced by vascular endothelial growth factor and occurs in response to tissue ischemia (89). As a result, new capillaries are formed (90). Endothelial NOS knockout mice show impaired arteriogenesis, angiogenesis, and pericyte recruitment after femoral artery ligation. All three processes are reversed in this model by intramuscular injection of adenovirus encoding eNOS, suggesting that NO is an important mediator of these processes during lower extremity ischemia (91).
In addition to eNOS-generated NO, the nitrite/NO pathway is functional in the peripheral vasculature. Intraperitoneal (IP) nitrite injections have been shown to improve tissue perfusion through increased collateral vessel development in the murine femoral ligation model of acute limb ischemia (92). IP delivered nitrite also improved angiogenesis and cutaneous flow in rat ischemic myocutaneous flaps, reducing tissue death via a nitrite/NO pathway (93). Nitrite therapy, delivered even in a delayed fashion, augments arteriogenesis in the mouse hindlimb ischemia model (94). Additionally, dietary nitrate supplementation increased capillary and bone-marrow derived progenitor cell density in ischemic hind-limbs, a process that was inhibited with antiseptic mouthwash (95). Antiseptic mouthwash reduces the concentration of the oral bacteria responsible for nitrate reduction to nitrite, thus disrupting the nitrate-nitrite-NO pathway (96). In a small study of healthy volunteers, antiseptic mouthwash increased systolic and diastolic blood pressure by 2–3.5 mm Hg during a 7 day course (97).
Nitric Oxide and Neointimal Hyperplasia
Neointimal hyperplasia is an exaggerated inflammatory healing response after vascular injury. Of particular interest is neointimal hyperplasia after balloon angioplasty and vascular stent deployment, since this may limit therapeutic success. After vessel injury, platelets adhere to the vessel wall denuded of endothelium and generate a cascade of events leading to leukocyte chemotaxis, extracellular matrix modification, endothelial cell apoptosis, and vascular smooth muscle cell migration and proliferation (55). NO has been shown to limit neointimal hyperplasia through multiple levels. Similar to atherosclerosis, NO modulates neointimal hyperplasia through inhibition of platelet aggregation, decreased leukocyte chemotaxis, and reduced vascular smooth muscle cell proliferation while stimulating that of endothelial cells (57– 59, 62, 65, 67, 68, 98, 99). The effects of NO may be limited by L-arginine shunting away from eNOS to arginase under pathological conditions. Arginase metabolism of L-arginine leads to the production of polyamines utilized in cell proliferation, and the expression of arginase I is increased in the proliferation of rat aortic smooth muscle cells (100). It has been demonstrated that arginase I activity is increased within the vessel wall after carotid balloon injury in rats, and that inhibition of arginase decreases neointimal hyperplasia in that model (13). Furthermore, Alef et al. (5) demonstrated that nitrite-supplemented drinking water acts to reduce intimal hyperplasia in the rat carotid injury model, and that this NO is generated through XOR.
Nitric Oxide and Pulmonary Arterial Hypertension
Pulmonary hypertension is a vascular disease characterized by hypoxia, pulmonary vasoconstriction, increased vascular resistance, vessel remodeling, thrombosis, and right ventricular strain (7, 101). Multiple etiologies likely contribute to the development of pulmonary hypertension, but all involve increased vascular resistance as a prominent factor. NO, an important regulator of pulmonary vascular resistance, acts as a vasorelaxing agent within the pulmonary arterial system as well as a protective agent against smooth muscle cell proliferation within the vascular wall (102, 103). It has been proposed that NO may act as a “hypoxic buffer” that leads to vasodilation under hypoxic conditions, such as occurs in pulmonary hypertension (104, 105). This theory proposes that increased nitrite reduction to NO helps to counterbalance the hypoxic pulmonary vasoconstriction by generating a vasodilatory signal. Inhaled nitrite is being utilized in pulmonary hypertension as a direct means of delivering NO to the pulmonary vasculature (106). Also, dietary nitrite in mice increases pulmonary dilation, inhibits vascular remodeling, and decreases right ventricular hypertrophy. This effect was reduced in eNOS knockout mice and after allopurinol treatment (26). In a rat model of pulmonary hypertension, it has been shown that inhaled nitrite reverses the effect of hypoxia-induced pulmonary hypertension through creation of NO via XOR (103).
Investigation into the L-arginine/nitrite/NO pathway in pulmonary hypertension has led to conflicting results as far as the importance of this system. Variation in eNOS expression has been observed in human tissue studies, despite consistently elevated eNOS in animal studies (107– 109). Inducible NOS has also been shown to be increased in some studies (110). The upregulation of the NOSs may be a compensatory response to upregulated arginase activity. Like other vascular disorders, arginase activity has been shown to be increased in pulmonary hypertension (111). Increased arginase may have a dual role of decreasing L-arginine metabolism to NO as well as polyamine-induced increases in smooth muscle cell proliferation within the vessel walls (7).
Summary
Nitric oxide is an important regulator of vascular function. An imbalance in NO production in relation to ROSs, RNSs, and other inflammatory mediators is associated with many forms of vascular dysfunction, including atherosclerosis, peripheral arterial disease, neointimal hyperplasia, and pulmonary hypertension. The recently discovered nitrate/nitrite/NO pathway is an alternative means of delivering NO to areas of deficiency. In order to harness this pathway as a therapeutic, efficient delivery to the affected tissues must be accomplished. Because of its relatively stable nature and the recognition that nitrate, nitrite, hemoglobin, and myoglobin within the blood act as a ‘storage pool’ of NO, a variety of potential delivery options to areas of vascular dysfunction exist, including dietary supplementation, inhalation, and direct intravenous infusion.Frontiers | Therapeutic Potential of the Nitrite-Generated NO Pathway in Vascular Dysfunction | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2013.00174/full
What is Tetrahydrobiopterin (BH4) and how can I make more of it?
Dr. Aaron Gardner, BSc, MRes, PhD
This post contains sponsored links. When you buy through one of these links, we may earn an affiliate commission. All sponsored links are marked with red font. To learn more, click here.
Contents [Hide]
Bh4 supporting supplements
BH4 biosynthesis and activity
BH4 as a co-factor
BH4 helps make nitric oxide
BH4 helps make serotonin
BH4 plays a role in producing dopamine
BH4 recovery
BH4 and NOS uncoupling
BH2 is converted back to BH4
BH4 Deficiency and Elevated BH2
Extreme cases of BH4 deficiency
BH4 and the One Carbon (Folate) Cycle
DHFR sits at the beginning of the folate cycle, MTHFR at the end
DHFR can replace QDPR activity
Folate and BH4 Supplementation Link
Closing Thoughts
Tetrahydrobiopterin (BH4, sometimes THB) is a vital cofactor for numerous enzymes in the body, including those involved in the formation of nitric oxide (NO), and the key neurotransmitters dopamine, serotonin and epinephrine.1 BH4 plays a critical role in both heart and cognitive health.
With this important role, many people take supplements that support BH4 levels, and it is frequently discussed together with the one carbon or folate cycle.
Bh4 supporting supplements
A lot of our readers are looking for the nutrients that support Bh4 production, so I list them here at the outset.
The following supplements support BH4 levels:
Methyl folate (View on Amazon) (MTHF supplementation can increase BH4 levels by preventing its oxidation into BH2 and by supplementing BH4 activity with NOS enzymes further preventing its degradation, but see this post before taking an MTHF supplement)23
Vitamin C4 (View on Amazon)
Curcumin5
Before we get into the health impacts of BH4 deficiency and supplementation, and its interactions with the one carbon (folate) cycle, let’s look in more detail how BH4 is formed and how it acts.
BH4 biosynthesis and activity
BH4 is formed from the abundant, simple molecule GTP in a three-step process, each step catalyzed by a different enzyme; GTP cyclohydrolase I (encoded for by the gene GCH1), pyruvoyltetrahydropterin synthase (PTS) and sepiapterin reductase (SPR).6 Pathological mutations in any of these genes can lead to BH4 deficiency, it is not yet clear if there are polymorphisms that result in milder reductions in function.
Pathway diagram showing the three enzymes involved in converting GTP into BH4.
BH4 as a co-factor
BH4 helps make nitric oxide
As mentioned above, BH4 is an important cofactor for several enzymes including nitric oxide synthase (NOS)1-3 which are responsible for converting arginine into nitric oxide (“NO”).
NO is vital in regulating blood pressure in the body.7
For more on optimizing NO production for peak sexual performance, see this episode of the podcast with Dr. Amy B. Killen, MD.
BH4 helps make serotonin
BH4 is also the cofactor for tryptophan hydroxylase (TPH), which converts tryptophan into the neurotransmitter serotonin. You’ve very likely heard of serotonin, it plays an important role in regulating muscle movement and mood.8
BH4 plays a role in producing dopamine
Additionally, BH4 is the cofactor used by phenylalanine hydroxylase in the conversion of phenylalanine into tyrosine, and its subsequent conversion by tyrosine hydroxylase (TH) into DOPA, the dopamine precursor.9 Dopamine is an important neurotransmitter in its own right, but it can also be converted into norepinephrine and epinephrine, the enzymes which control these reactions both use BH4 as a cofactor.10
BH4 is a key cofactor for several important enzymes involved in neurotransmitter formation and blood pressure regulation.
BH4 recovery
When used as a cofactor in these reactions, BH4 is reduced into a molecule called BH2, or dihydrobiopterin.11
BH4 and NOS uncoupling
BH2 is an interesting product as it can also function as a cofactor for the various NOS enzymes. However, whereas NOS coupled with BH4 converts arginine into NO, NOS coupled with BH2 promotes the formation of superoxides and hydrogen peroxide, two highly toxic compounds, which are associated with several of the symptoms associated with BH4 deficiency.12
BH2 is converted back to BH4
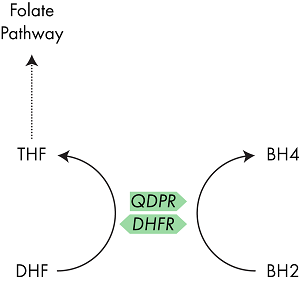
To avoid production of superoxide, BH2 in the body is rapidly converted back into BH4 through the action of the enzyme dihydropteridine reductase (QPDR).13 Another enzyme, dihydrofolate reductase (DHFR), which plays a role in the one carbon (folate) cycle, has also demonstrated the ability to convert BH2 to BH4 and I discuss the interaction between QPDR and DHFR below.14
QDPR is the enzyme which typically converts BH2 back into BH4, however the enzyme DHFR has also shown a similar activity.
BH4 Deficiency and Elevated BH2
Extreme cases of BH4 deficiency
Often diagnosed by high levels of phenylalanine (an amino acid which is processed by enzymes using BH4 into tyrosine and eventually dopamine) in the blood, BH4 deficiency is recognized as a severe metabolic disorder which is typically detected early in life due to the occurrence of serious health issues.15 This accumulation leads to impaired intellectual development and loss of muscle control. Furthermore, the lack of BH4 impairs neurotransmitter formation and the production of NO by NOS, which can lead to neurological and cardiovascular issues which John has discussed previously.16
BH4 and the One Carbon (Folate) Cycle
Right now I bet you’re asking ‘how does this link in with the folate cycle?
Well, the answer is quite straightforward, but understanding its relevance to you is a bit more tricky. QDPR is the enzyme which is typically responsible for the recovery of BH4 from its reduced form BH2.17 However, studies have also shown that the enzyme DHFR is also able perform this recovery activity. DHFR, like MTHFR, is an enzyme in the one carbon pathway, hence the similarity in their names. However, their action is rather different.18
DHFR sits at the beginning of the folate cycle, MTHFR at the end
Whereas MTHFR is responsible for converting 5,10-methylenetetrahydrofolate (MeTHF) to 5-methlytetrahydrofolate (MTHF); DHFR converts DHF into THF.19 THF, a precursor of MeTHF, is often thought as the start point of the one carbon cycle as it is where dietary folate enters the system, and so DHFR activity is key in maintaining cycle activity.
However, dietary folate is not the only source of DHF; MeTHF can be used in a reaction to directly form nucleic acids, the building blocks of DNA, rather than being used by MTHFR. When used to make DNA, MeTHF is converted into DHF, and so DHFR also plays a recovery role. Understanding how everything links together is quite complex so the pathway diagram below, summarizing everything discussed above, should prove useful.
DHFR can replace QDPR activity
Now we know what the ‘normal’ function of DHFR is, lets have a look at its ‘alternative’ activity. As mentioned above, several studies have shown that DHFR can recycle BH4 from BH2. This study by Xu et al 20 investigates its role in detail, trying to understand how QDPR and DHFR interact. Using mice completely lacking the QDPR gene, the authors discovered that DHFR was able to step in and replace its activity. However, there are a couple of caveats that go with the study. Firstly, the affinity of DHFR for BH2 is much lower than for DHF, so the authors propose that the recycling activity of DHFR only kicks in when levels of BH2 are high.
Secondly, the authors also state that the affinity of mouse DHFR for BH2 is much higher than that of the human form. So, whilst DHFR may be able to step in to assist BH2 to BH4 processing its importance might be much lower.
Finally, the authors show that high levels of BH2 also lead to a reduction in the availability of THF. The logical explanation for this would be that DHF > THF activity is reduced as DHFR is converting BH2 > BH4. However, this doesn’t seem to be the case. The authors were unable to exactly pinpoint how these were linked but hypothesized that excess BH2 may impact on the activity of other enzymes such as MTHFR.
Folate and BH4 Supplementation Link
The above data provides a link between the two pathways, but is there any evidence that dietary alteration or nutritional supplementation can have a positive effect? If BH2 levels are high then THF levels appear to be reduced.20 However, BH2 levels only reach these severely high levels in the complete absence of QDPR, which will not apply to any readers here. Supplementation with BH4 may increase the levels of BH2 but there is no evidence that this has a negative health effect, or a positive effect on folate activity.
Let’s look at it the other way. Low levels of BH4 are associated with several health issues. Can supplementation with MTHF (the preferred supplementary form) increase BH4 levels?
Interestingly, it does seem possible, although the effect is not processed through QDPR or DHFR. Rather, studies have shown that MTHF supplementation can increase BH4 levels by preventing its oxidation into BH2 and by supplementing BH4 activity with NOS enzymes further preventing its degradation.2122 Therefore, MTHF supplementation alone, or in conjunction with BH4, may prove beneficial to those with low levels of BH4.
Closing Thoughts
As always it is important to emphasize that research into these interactions and activities is fairly new.
Understanding how various enzymes and pathways interact is a complex study and so whilst beneficial effects may be observed in some, they may not be observed in others. Direct BH4 supplementation is the most obvious target in those who are BH4 deficient, however some forum posters report minor adverse effects. In these cases indirect supplementation with MTHF may prove more beneficial in some cases.BH4 biosynthesis and activity
BH4 is formed from the abundant, simple molecule GTP in a three-step process, each step catalyzed by a different enzyme; GTP cyclohydrolase I (encoded for by the gene GCH1), pyruvoyltetrahydropterin synthase (PTS) and sepiapterin reductase (SPR).6 Pathological mutations in any of these genes can lead to BH4 deficiency, it is not yet clear if there are polymorphisms that result in milder reductions in function.What is Tetrahydrobiopterin (BH4) and how can I make more of it? - Gene Food
https://www.mygenefood.com/blog/tetrahydrobiopterin-bh4-can-make/
Buy Tetrahydrobiopterin (BH4) And Increase It Naturally ...
https://mybiohack.com/blog/bh4-tetrahydrobiopterin...
BH4 is a naturally occurring essential cofactor of the three aromatic amino acid hydroxlase enzymes used in the degradation of amino acid phenylalanine and in the biosynthesis of the neurotransmitters serotonin, melatonin, dopamine, norepinephrine, epinephrine, and is a cofactor for the production of nitric oxide by nitric oxide synthases.
What is Tetrahydrobiopterin (BH4) and how can I make more ...
https://www.mygenefood.com/blog/tetrahydrobiopterin-bh4-can-make
Mar 09, 2020 · Tetrahydrobiopterin (BH4, sometimes THB) is a vital cofactor for numerous enzymes in the body, including those involved in the formation of nitric oxide (NO), and the key neurotransmitters dopamine, serotonin and epinephrine. 1 BH4 plays a critical role in both heart and cognitive health.
Tetrahydrobiopterin deficiency - Genetics Home Reference - NIH
https://ghr.nlm.nih.gov/condition/tetrahydrobiopterin-deficiency
Oct 29, 2019 · Tetrahydrobiopterin deficiency is a rare disorder characterized by a shortage (deficiency) of a molecule called tetrahydrobiopterin or BH4. This condition alters the levels of several substances in the body, including phenylalanine. Phenylalanine is a building block of proteins (an amino acid) that is obtained through the diet.It is found in foods that contain protein and in some artificial ...
Intestinal microbiota as a tetrahydrobiopterin(BH4) exogenous ...
https://www.nature.com/articles/srep39854
Jan 12, 2017 · Tetrahydrobiopterin (BH4) is an important regulatory enzyme cofactor for the catecholamine, indoleamine and nitric oxide synthase biochemical pathways. A reduced or absent BH4 endogenous pool results in severe clinical manifestations such as phenylketonuria, movement disorders, systemic and pulmonary hypertension.
Cited by: 7
Publish Year: 2017
Author: Jaques Belik, Yulia Shifrin, Erland Arning, Teodoro Bottiglieri, Jingyi Pan, Michelle C. Daigneault,...
Author: Jaques Belik
Mitochondrial Arginase II Modulates Nitric-Oxide Synthesis through Nonfreely Exchangeable l-Arginine Pools in Human Endothelial Cells
Gökce Topal, Annie Brunet, Laurence Walch, Jean-Luc Boucher and Monique David-Dufilho
Journal of Pharmacology and Experimental Therapeutics September 2006,
Abstract
Reduced synthesis of nitric oxide (NO) contributes to the endothelial dysfunction and may be related to limited availability of l-arginine, the common substrate of constitutive nitric-oxide synthase (NOS) and cytosolic arginase I and mitochondrial arginase II.To determine whether arginases modulate the endothelial NO synthesis, we investigated the effects of the competitive arginase inhibitor Nω-hydroxy-nor-l-arginine (Nor-NOHA) on the activity of NOS, arginases, and l-arginine transporter and on NO release at surface of human umbilical vein endothelial cells (HUVECs).
In unstimulated cells, Nor-NOHA dose-dependently reduced the arginase activity with maximal inhibition at 20 μM. When HUVECs were stimulated by thrombin without extracellular l-arginine, Nor-NOHA dose-dependently increased the NOS activity and the NO release with maximal effects at 20 μM. Extracellular l-arginine also dose-dependently increased NO release and arginase activity. When HUVECs were stimulated by thrombin in the presence of 100 μM l-arginine, NOS activity and NO release were similar in untreated and Nor-NOHA-treated cells. However, despite activation of l-arginine uptake, the inhibition of arginase activity by Nor-NOHA was still significant. The depletion of freely exchangeable l-arginine pools with extracellular l-lysine did not prevent Nor-NOHA from increasing the NO release. This indicates the presence of pools, which are accessible to NOS and arginase, but not exchangeable. Interestingly, the mitochondrial arginase II was constitutively expressed, whereas the cytosolic arginase I was barely detectable in HUVECs. These data suggest that endothelial NO synthesis depends on the activity of arginase II in mitochondria and l-arginine carriers in cell membrane.
Mitochondrial Arginase II Modulates Nitric-Oxide Synthesis through Nonfreely Exchangeable l-Arginine Pools in Human Endothelial Cells | Journal of Pharmacology and Experimental Therapeutics
http://jpet.aspetjournals.org/content/318/3/1368
A Novel Arginase Inhibitor Derived from Scutellavia indica Restored Endothelial Function in ApoE-Null Mice Fed a High-Cholesterol Diet
Hye Mi Hwang, Jeong Hyung Lee, Byung Sun Min, Byeong Hwa Jeon, Kwang Lae Hoe, Young Myeong Kim and Sungwoo Ryoo
Journal of Pharmacology and Experimental Therapeutics October 2015,
Figure1
Abstract
Elevated endothelial arginase activity decreases nitric oxide (NO) production by competing with the substrate l-arginine, previously reported, and reciprocally regulating endothelial nitric oxide synthase (eNOS) activity. Thus, arginase inhibitors may help treat vascular diseases associated with endothelial dysfunction. A screening of metabolites from medicinal plants revealed that (2S)-5,2′,5′-trihydroxy-7,8-dimethoxy flavanone (TDF) was a noncompetitive inhibitor of arginase.We investigated whether TDF reciprocally regulated endothelial NO production and its possible mechanism. TDF noncompetitively inhibited arginase I and II activity in a dose-dependent manner. TDF incubation decreased arginase activity and increased NO production in human umbilical vein endothelial cells and isolated mouse aortic vessels and reduced reactive oxygen species (ROS) generation in the endothelium of the latter. These TDF-mediated effects were associated with increased eNOS phosphorylation and dimerization but not with changes in protein content.
Endothelium-dependent vasorelaxant responses to acetylcholine (Ach) were significantly increased in TDF-incubated aortic rings and attenuated by incubation with soluble guanylyl cyclase inhibitor. Phenylephrine-induced vasoconstrictor responses were markedly attenuated in TDF-treated vessels from wild-type mice. In atherogenic-prone ApoE−/− mice, TDF attenuated the high-cholesterol diet (HCD)-induced increase in arginase activity, which was accompanied by restoration of NO production and reduction of ROS generation. TDF incubation induced eNOS dimerization and phosphorylation at Ser1177. In addition, TDF improved Ach-dependent vasorelaxation responses and attenuated U46619-dependent contractile responses but did not change sodium nitroprusside–induced vasorelaxation or N-NAME-induced vasoconstriction. The findings suggest that TDF may help treat cardiovascular diseases by reducing pathophysiology derived from HCD-mediated endothelial dysfunction.
A Novel Arginase Inhibitor Derived from Scutellavia indica Restored Endothelial Function in ApoE-Null Mice Fed a High-Cholesterol Diet | Journal of Pharmacology and Experimental Therapeutics
http://jpet.aspetjournals.org/content/355/1/57
L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity
Roger Geiger
Jan C. Rieckmann
Tobias Wolf
Nicola Zamboni
Federica Sallusto
Antonio Lanzavecchia 7
Open AccessPublished:October 13, 2016DOI:https://doi.org/10.1016/j.cell.2016.09.031
Highlights
• Dataset on dynamic metabolome/proteome profiles of activated human naive T cells
• Intracellular L-arginine levels regulate several metabolic pathways in T cells
• T cells with increased L-arginine display enhanced survival and anti-tumor activity
• LiP-MS identified proteins that are structurally modified by high L-arginine levels
Summary
Metabolic activity is intimately linked to T cell fate and function. Using high-resolution mass spectrometry, we generated dynamic metabolome and proteome profiles of human primary naive T cells following activation.We discovered critical changes in the arginine metabolism that led to a drop in intracellular L-arginine concentration. Elevating L-arginine levels induced global metabolic changes including a shift from glycolysis to oxidative phosphorylation in activated T cells and promoted the generation of central memory-like cells endowed with higher survival capacity and, in a mouse model, anti-tumor activity.
Proteome-wide probing of structural alterations, validated by the analysis of knockout T cell clones, identified three transcriptional regulators (BAZ1B, PSIP1, and TSN) that sensed L-arginine levels and promoted T cell survival. Thus, intracellular L-arginine concentrations directly impact the metabolic fitness and survival capacity of T cells that are crucial for anti-tumor responses.
L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity: Cell
https://www.cell.com/cell/fulltext/S0092-8674(16)31313-7#%20
Arginine Metabolism in Myeloid Cells Shapes Innate and Adaptive Immunity
imagePaulo C. Rodriguez1, imageAugusto C. Ochoa2,3 and imageAmir A. Al-Khami2,4*
1Augusta University, Georgia Cancer Center, Augusta, GA, USA
2Stanley S. Scott Cancer Center, Louisiana State University Health Sciences Center, New Orleans, LA, USA
3Department of Pediatrics, Louisiana State University Health Sciences Center, New Orleans, LA, USA
4Department of Genetics, Louisiana State University Health Sciences Center, New Orleans, LA, USA
Arginine metabolism has been a key catabolic and anabolic process throughout the evolution of the immune response. Accruing evidence indicates that arginine-catabolizing enzymes, mainly nitric oxide synthases and arginases, are closely integrated with the control of immune response under physiological and pathological conditions. Myeloid cells are major players that exploit the regulators of arginine metabolism to mediate diverse, although often opposing, immunological and functional consequences. In this article, we focus on the importance of arginine catabolism by myeloid cells in regulating innate and adaptive immunity. Revisiting this matter could result in novel therapeutic approaches by which the immunoregulatory nodes instructed by arginine metabolism can be targeted.Frontiers | Arginine Metabolism in Myeloid Cells Shapes Innate and Adaptive Immunity | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2017.00093/full
Infection Immunology, 1996 Dec.
Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells.
B P Mahon, M S Ryan, F Griffin, and K H Mills
Department of Biology, St. Patrick's College, Maynooth, County Kildare, Ireland.
ABSTRACT
Using a murine respiratory infection model, we have demonstrated previously that infection with Bordetella pertussis or immunization with a whole-cell pertussis vaccine induced antigen-specific Th1 cells, which conferred a high level of protection against aerosol challenge. In contrast, immunization with an acellular vaccine, consisting of the B. pertussis components detoxified pertussis toxin, filamentous hemagglutinin, and pertactin adsorbed to alum, generated Th2 cells and was associated with delayed bacterial clearance following challenge. In this study, we demonstrated that addition of interleukin-12 (IL-12) either in vitro or in vivo enhanced type 1 T-cell cytokine responses induced with an acellular vaccine. Furthermore, the rate of bacterial clearance in mice coinjected with IL-12 and the acellular vaccine was similar to that observed following immunization with a potent whole-cell vaccine. Analysis of IL-12 secretion by murine macrophages suggested that this cytokine is produced in vivo following B. pertussis infection or immunization with the whole-cell vaccine. IL-12 was detected in the supernatants of lung, splenic, and peritoneal macrophages infected with live B. pertussis or stimulated with heat-killed whole B. pertussis or B. pertussis lipopolysaccharide. In contrast, IL-12 could not be detected following stimulation of macrophages with the bacterial antigens filamentous hemagglutinin, detoxified pertussis toxin, and pertactin, the components of acellular vaccines. Our findings suggest that induction of endogenous IL-12 may contribute to the high efficacy of pertussis whole-cell vaccines and also demonstrate that it is possible to attain these high levels of protection with a less reactogenic acellular vaccine incorporating IL-12 as an adjuvant.
白细胞介素-12是由巨噬细胞产生,以应对活的或杀死的百日咳博德菌,并通过促进Th1细胞的诱导,提高无细胞百日咳疫苗的疗效。
B P Mahon, M S Ryan, F Griffin, K H Mills
爱尔兰基尔代尔郡梅努斯圣帕特里克学院生物系。
摘要
利用小鼠呼吸道感染模型,我们先前证明了百日咳博德菌感染或全细胞百日咳疫苗免疫可诱导抗原特异性Th1细胞,从而提供了对气溶胶挑战的高水平保护。相比之下,接种由百日咳毒素解毒成分、丝状血凝素和透明质酸吸附在明矾上组成的非细胞疫苗,可产生Th2细胞,并与挑战后的细菌清除延迟有关。在这项研究中,我们证明了在体外或体内添加白细胞介素-12 (IL-12)可增强非细胞疫苗诱导的1型T细胞因子反应。此外,联合注射IL-12和无细胞疫苗的小鼠的细菌清除率与接种高效全细胞疫苗后观察到的相似。小鼠巨噬细胞分泌IL-12的分析表明,该细胞因子是在百日咳感染或免疫全细胞疫苗后体内产生的。在感染活百日咳的肺、脾和腹腔巨噬细胞的上清液中检测到IL-12,或经热致死的全百日咳或百日咳脂多糖刺激后检测到IL-12。相反,用细菌抗原丝状血凝素、百日咳解毒毒素和非细胞疫苗的成分pern刺激巨噬细胞后,不能检测到IL-12。我们的研究结果表明,内源性IL-12的诱导可能有助于提高百日咳全细胞疫苗的有效性,同时也表明,使用含有IL-12作为佐剂的低反应性无细胞疫苗可以达到这些高水平的保护。Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC174522/
IL-12 Produced by Dendritic Cells Augments CD8+ T cell ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2716729
Dec 15, 2008 · Interleukin-12 (IL-12) is produced by mature DC in response to infection by various intracellular pathogens ( 14, 22 ). Due to the potent effects that IL-12 has on T cell activation, it is largely viewed as an important bridge between the innate and adaptive immune systems ( 23 – 29 ).
Cited by: 113
Publish Year: 2008
Author: Curtis J. Henry, David A. Ornelles, Latoya M. Mitchell, Kristina L. Brzoza-Lewis, Elizabeth M. Hiltb...
High-level IL-12 production by human dendritic cells ...
https://academic.oup.com/intimm/article/10/11/1593/814922
Nov 01, 1998 · IL-12 is a key cytokine in the development of Th1 responses. IL-12 production by antigen-presenting cells (APC) can be induced by the interaction between CD40 on the APC and CD40 ligand (CD40L) expressed on T cells after activation. Our previous study indicated that in dendritic cells (DC), the only APC that can activate naive T (h)...
Cited by: 481
Publish Year: 1998
Author: Alies Snijders, Pawel Kalinski, Catharien M. U. Hilkens, Martien L. Kapsenberg
IL-12 production by human monocyte-derived dendritic cells ...
https://www.ncbi.nlm.nih.gov/pubmed/16000948
IL-12p70 is synthesized and secreted in response to inflammatory cytokines, bacterial/viral components, and CD40 ligation. This study investigated the production of IL-12 by DCs at the single-cell level using a sensitive intracellular cytokine flow cytometry-based assay system.
Cited by: 18
Publish Year: 2005
Author: Jan Muller-Berghaus, Walter C. Olson, Rachel A. Moulton, William T. Knapp, Dirk Schadendorf, Walter ...
Interleukin-12 is produced by dendritic cells and mediates ...
https://www.ncbi.nlm.nih.gov/pubmed/8605935
Established sources of IL-12 are stimulated macrophages, neutrophils and B cells. As dendritic cells (DC) process antigen in the periphery and then migrate to lymphoid organs to sensitize T cells and induce cell mediated immunity, we reasoned that DC should constitute a critical source of IL-12.
Cited by: 916
Publish Year: 1996
Author: Christine Heufler, Franz Koch, Ursula Stanzl, Gerda Topar, M
Oxford Academic
International Immunology
High-level IL-12 production by human dendritic cells requires two signals.
A Snijders, P Kalinski, C M Hilkens, M L Kapsenberg
Abstract
IL-12 is a key cytokine in the development of Th1 responses.IL-12 production by antigen-presenting cells (APC) can be induced by the interaction between CD40 on the APC and CD40 ligand (CD40L) expressed on T cells after activation.
Our previous study indicated that in dendritic cells (DC), the only APC that can activate naive T(h) cells efficiently, the mere CD40 engagement is insufficient to induce IL-12 production.
The aim of the present study was to dissect the conditions for efficient IL-12 production by DC further. Using populations of naive and memory Th cells, recombinant CD40L, neutralizing and blocking antibodies, and by determining IFN-gamma production and CD40L expression levels, we here show that T cell-induced IL-12 production by DC results from the action of two signals, mediated by CD40L and IFN-gamma, and that the inability of naive T(h) cells to induce IL-12 production resides in their inability to produce IFN-(gamma).
Other factors than CD40L and IFN-gamma can provide the required signals for IL-12 production by DC, as either factor could be replaced by lipopolysaccharide (LPS). The two-signal requirement proved unique for the production of IL-12, since either CD40 engagement or LPS was sufficient for the efficient production of tumor necrosis factor-alpha, IL-8 and the p40 subunit of IL-12, and may be considered as a safety mechanism for optimal control of potentially harmful T(h)1 responses.
High-level IL-12 production by human dendritic cells requires two signals. | International Immunology | Oxford Academic
https://academic.oup.com/intimm/article/10/11/1593/814922
J Immunol. Author manuscript; available in PMC 2009 Dec 15.
IL-12 Produced by Dendritic Cells Augments CD8+ T cell Activation through the Production of the Chemokines CCL1 and CCL171
Curtis J. Henry, David A. Ornelles, Latoya M. Mitchell, Kristina L. Brzoza-Lewis, and Elizabeth M. Hiltbold
Author information Copyright and License information Disclaimer
Department of Microbiology and Immunology, Wake Forest University School of Medicine, Winston-Salem, NC 27157
2Address correspondence to: Elizabeth M. Hiltbold, Ph.D., 5109 Gray Building, Department of Microbiology and Immunology, Wake Forest University School of Medicine, Winston-Salem, NC 27157
Abstract
Interleukin-12 (IL-12) family members are an important link between innate and adaptive immunity. IL-12 drives Th1 responses by augmenting IFN-γ production, which is key for clearance of intracellular pathogens. Interleukin-23 (IL-23) promotes the development of IL-17 producing CD4+ T cells that participate in the control of extracellular pathogens and the induction of autoimmunity. However, recent studies have shown that these cytokines can modulate lymphocyte migration and cellular interactions. Therefore, we sought to determine the individual roles of IL-12 and IL-23 in naïve CD8+ T cell activation by addressing their ability to influence interferon gamma (IFN-γ) production and cellular interaction dynamics during priming by Listeria monocytogenes (Lm)-infected dendritic cells (DC). We found that IL-12 was the major cytokine influencing the level of IFN-γ production by CD8+ T cells while IL-23 had little effect on this response. In addition, we observed that IL-12 promoted longer duration conjugation events between CD8+ T cells and DC. This enhanced cognate interaction time correlated increased production of the chemokines CCL1 and CCL17 by wild-type but not IL-12 deficient DC. Neutralization of both chemokines resulted in reduced interaction time and IFN-γ production demonstrating their importance in priming naïve CD8+ T cells. Our study demonstrates a novel mechanism through which IL-12 augments naïve CD8+ T cell activation by facilitating chemokine production thus promoting more stable cognate interactions during priming.
Keywords: T cells, Dendritic Cells, Cytokines, Chemokines, Cell ActivationIL-12 Produced by Dendritic Cells Augments CD8+ T cell Activation through the Production of the Chemokines CCL1 and CCL17
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2716729/
J Immunother. 2005 Jul-Aug;28(4):306-13.
IL-12 production by human monocyte-derived dendritic cells: looking at the single cell.
Muller-Berghaus J1, Olson WC, Moulton RA, Knapp WT, Schadendorf D, Storkus WJ.
Author information
1 Department of Surgery, University of Pittsburgh, Pennsylvania, USA. jan.m-b@web.de
Abstract
Dendritic cells (DCs) are under investigation as immunotherapeutic agents in the treatment of cancer and infectious diseases. One of the important factors in skewing the immune response toward clinically beneficial TH1-type immunity is interleukin-12p70. IL-12p70 is synthesized and secreted in response to inflammatory cytokines, bacterial/viral components, and CD40 ligation.This study investigated the production of IL-12 by DCs at the single-cell level using a sensitive intracellular cytokine flow cytometry-based assay system. The authors observed that immature DCs could be stimulated with several compounds to produce IL-12, but that IL-12 production was a feature of a minority of activated DCs. IL-12+ DCs were characterized as being partially matured (ie, absent or low CD83 expression, with variable expression of CD1a and CD64). Interestingly, activated DCs lacked expression of the CD16 and CD64 Fc gammaR, which may have important implications for exogenous antigen-loading strategies.
PMID: 16000948 DOI: 10.1097/01.cji.0000163594.74533.10IL-12 production by human monocyte-derived dendritic cells: looking at the single cell. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/16000948
Eur J Immunol. 1996 Mar;26(3):659-68.
Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells.
Heufler C1, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G.
Author information
1
Department of Dermatology, University of Innsbruck, Australia.
Abstract
Interleukin-12 (IL-12), a 70-kDa heterodimeric cytokine composed of covalently linked p35 and p40 chains, is to date the most critical factor for skewing the immune response towards a T helper 1 (Th1) of cytokine profile [high interferon-gamma (IFN-gamma), low IL-4].Established sources of IL-12 are stimulated macrophages, neutrophils and B cells. As dendritic cells (DC) process antigen in the periphery and then migrate to lymphoid organs to sensitize T cells and induce cell mediated immunity, we reasoned that DC should constitute a critical source of IL-12.
The criteria used to detect IL-12 in DC were the demonstration of p40 and p35 mRNA (semiquantitative polymerase chain reaction, northern blotting, and in situ hybridization) as well as IL-12 protein (p70 enzyme-linked immunosorbent assay, p70 antigen capture followed by IFN-gamma bioassay, free p40 chain radioimmunoassay or immunoprecipitation).
We found that conventional stimuli such as Staphylococcus aureus induced production of IL-12 by murine as well as human DC in amounts comparable to spleen cells, peritoneal macrophages or peripheral mononuclear cells. DC exhibited, however, features that had not been seen with other antigen-presenting cells: they produced bioactive IL-12 upon antigen-specific interaction with T cells without any other stimuli; in an allogeneic mixed leukocyte reaction model, neutralizing anti-IL-12 antibodies showed that DC-derived IL-12 was critical for optimal proliferation and IFN-gamma production by activated Th1 blasts; and finally, the priming of resting, naive allogeneic T cells by DC, followed by restimulation of primed T blasts by DC, skewed the response to Th1 without the need for any exogenous cytokines or stimuli such as microorganisms. This skewing to Th1 cytokine production, which depended on DC-derived IL-12, but did not require anti-IL-4, exogenous IL-12, or microbes, might be a major function of DC.
我们发现,传统的刺激,如金黄色葡萄球菌诱导小鼠和人类DC产生IL-12的数量可与脾细胞、腹膜巨噬细胞或外周单核细胞相媲美。然而,DC表现出其他抗原呈递细胞未见的特征:它们在没有任何其他刺激的情况下,通过与T细胞的抗原特异性相互作用产生具有生物活性的IL-12;在异基因混合白细胞反应模型中,中和抗IL-12抗体显示,dc来源的IL-12对活化Th1细胞的最佳增殖和ifn -产生至关重要;最后,DC启动静止的同种异体T细胞,然后DC再刺激启动的T细胞,在不需要任何外源性细胞因子或刺激(如微生物)的情况下,使对Th1的反应发生倾斜。这种偏向于Th1细胞因子的产生依赖于DC衍生的IL-12,但不需要抗il -4、外源性IL-12或微生物,这可能是DC的一个主要功能。
PMID: 8605935 DOI: 10.1002/eji.1830260323Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/8605935
IL-12 Production by Human Monocyte-Derived Dendritic Cells
Dendritic cells (DCs) are under investigation as immunotherapeutic agents in the treatment of cancer and infectious diseases. One of the important factors in skewing the immune response toward clinically beneficial T(H)1-type immunity is interleukin-12p70.
IL-12p70 is synthesized and secreted in response to inflammatory cytokines, bacterial/viral components, and CD40 ligation.
This study investigated the production of IL-12 by DCs at the single-cell level using a sensitive intracellular cytokine flow cytometry-based assay system. The authors observed that immature DCs could be stimulated with several compounds to produce IL-12, but that IL-12 production was a feature of a minority of activated DCs.
IL-12(+) DCs were characterized as being partially matured (ie, absent or low CD83 expression, with variable expression of CD1a and CD64). Interestingly, activated DCs lacked expression of the CD16 and CD64 Fc gamma R, which may have important implications for exogenous antigen-loading strategies.
IL-12 Production by Human Monocyte-Derived Dendritic Cells | Request PDF
https://www.researchgate.net/publication/247597019_IL-12_Production_by_Human_Monocyte-Derived_Dendritic_Cells
ACS Applied Materials & Interfaces 10(6) · January 2018
Gold Nanoparticle-Based Photoluminescent Nanoswitch Controlled by Host-Guest Recognition and Enzymatic Hydrolysis for Arginase Activity Assay福建医科大学
The development of simple yet powerful methods for monitoring enzyme activity is of great significance. Herein, a facile, convenient, cost-effective, and continuous fluorescent method for the detection of arginase and its inhibitor has been reported based on a host-guest interaction- and enzymatic hydrolysis-controlled luminescent nanoswitch.The fluorescence intensity of 6-aza-2-thiothymine-stabilized gold nanoparticle (ATT-AuNP) is enhanced by L-arginine, owing to the formation of supramolecular host-guest assembly between the guanidine group of L-arginine and ATT molecules capped on AuNP surface.
However, hydrolysis of L-arginine, catalyzed by arginase, leads to a decrease of fluorescence intensity of L-arginine/ATT-AuNPs hybrids. Upon incorporation of the arginase inhibitor L-norvaline, the fluorescence of the ATT-AuNP-based detecting system is restored. The linear range of arginase activity determination is from 0.0625 to 1.15 U/mL and the limit of detection is 0.056 U/mL. The half-maximal inhibition value IC50 of L-norvaline is determined to be 5.6 mM. The practicability of this luminescent nanoswitch is validated by assaying arginase activity in rat liver and monitoring the response of rat liver arginase to pharmacological agent. Compared to the existing fluorescent method of arginase activity assay, the approach demonstrated here does not involve any complicated technical manipulation, thereby greatly simplifying the detection steps. We propose that this AuNP-based luminescent nanoswitch would find wide applications in the field of life sciences and medicine.
(PDF) Gold Nanoparticle-Based Photoluminescent Nanoswitch Controlled by Host-Guest Recognition and Enzymatic Hydrolysis for Arginase Activity Assay
https://www.researchgate.net/publication/322728337_Gold_Nanoparticle-Based_Photoluminescent_Nanoswitch_Controlled_by_Host-Guest_Recognition_and_Enzymatic_Hydrolysis_for_Arginase_Activity_Assay
Insights Into Nitric Oxide Modulated Quorum Sensing Pathways
Ilana Heckler and Elizabeth M. Boon*†
Department of Chemistry, Institute of Chemical Biology & Drug Discovery, Stony Brook University, Stony Brook, NY, United States
The emerging threat of drug resistant bacteria has prompted the investigation into bacterial signaling pathways responsible for pathogenesis. One such mechanism by which bacteria regulate their physiology during infection of a host is through a process known as quorum sensing (QS). Bacteria use QS to regulate community-wide gene expression in response to changes in population density. In order to sense these changes in population density, bacteria produce, secrete and detect small molecules called autoinducers. The most common signals detected by Gram-negative and Gram-positive bacteria are acylated homoserine lactones and autoinducing peptides (AIPs), respectively. However, increasing evidence has supported a role for the small molecule nitric oxide (NO) in influencing QS-mediated group behaviors like bioluminescence, biofilm production, and virulence. In this review, we discuss three bacteria that have an established role for NO in influencing bacterial physiology through QS circuits. In two Vibrio species, NO has been shown to affect QS pathways upon coordination of hemoprotein sensors. Further, NO has been demonstrated to serve a protective role against staphylococcal pneumonia through S-nitrosylation of a QS regulator of virulence.
Introduction
Quorum sensing (QS) refers to the process by which bacteria regulate gene expression in response to small molecules called autoinducers. Autoinducers, typically acylated homoserine lactone molecules in Gram-negative bacteria or oligopeptides in Gram-positive bacteria, are synthesized inside the cell and freely diffuse across the membrane. As cell density rises, the concentration of autoinducers in the surrounding environment also increases. When a threshold level of autoinducers is reached, autoinducers bind to their respective receptors and initiate a signal transduction cascade downstream, ultimately resulting in the repression or expression of specific genes. QS has been shown to mediate several bacterial processes including bioluminescence, sporulation, competence, antibiotic production, biofilm formation, and virulence factor production (Miller and Bassler, 2001).
Bacterial receptors of autoinducers can be membrane bound hybrid histidine kinase proteins (Ng and Bassler, 2009). Hybrid histidine kinases contain a receiver domain, in addition to an input domain and kinase core. Upon binding ATP, the receptor will autophosphorylate on a conserved histidine residue in the kinase core. Phosphate is then transferred from the histidine residue to an aspartate residue on the same polypeptide. Hybrid histidine kinases rely on an intermediate histidine phosphotransferase (HPT) protein to then transfer the phosphate from the receiver domain of the kinase, to the receiver domain of a response regulator. The most common autoinducers sensed by Gram-negative bacteria are acylated homoserine lactones, or an interfuranosyl borate diester interspecies signal named autoinducer-2 (Papenfort and Bassler, 2016). In Gram-positive bacteria, autoinducing peptides (AIPs) which are synthesized by ribosomes and secreted extracellularly by specific transporters, bind to receptor kinases at high cell density to initiate QS pathways.
Nitric oxide (NO) is a diatomic membrane-permeable gas molecule that has been implicated in a wide range of physiological processes in both eukaryotes and bacteria. Many of the interesting biological properties of NO can be attributed to its unique physical properties. Due to its size, neutral charge, and hydrophobic nature, NO is able to freely diffuse into the cytosol, where it may undergo a variety of reactions including protein S-nitrosylation and metal coordination. While at high (micromolar) concentrations, NO is cytotoxic, at low concentrations, NO has been shown to be a signaling molecule in both bacteria and eukaryotes (Murphy, 1999; Arora et al., 2015).
In mammals, NO is synthesized by nitric oxide synthases (NOS) from L-arginine, oxygen and NADPH. The first report of bacterial NOS activity came from studies of the Nocardia species (Chen and Rosazza, 1994). Although it does not appear that most bacteria synthesize NO enzymatically, bacteria can produce NO as a by-product of denitrification, through the anaerobic reduction of nitrite by nitrite reductase. NO is then subsequently reduced to nitrous oxide by NO reductase. Indeed, the expression of nitrite reductase and NO reductase in Pseudomonas aeruginosa biofilms were found to be QS-dependent, suggesting that QS is responsible for the maintenance of NO levels in this organism (Hentzer et al., 2005).
Bacteria could also respond to NO that is produced by a eukaryotic host during symbiotic or pathogenic engagement. The ability to respond to NO to mediate QS related behaviors may provide microbes with an evolutionary advantage when encountering NO during infection of a host. In addition to a role for NO as a QS signal, there may also be a connection between QS and the regulation of intracellular levels of NO.
No and Innate Immunity
In response to a bacterial infection, the mammalian host produces high levels of NO through the upregulation of (iNOS). Nitrosative stress is an important component of the hosts innate immunity as it curbs microbial growth through the disruption of the fundamental physiological process including respiration, metabolism, and DNA replication (Murphy, 1999). However, bacteria have evolved to cope with nitrosative stress in order to circumvent host defenses during infection. In this section, we will discuss the discovery of a NO sensitive QS circuit in Staphylococcus aureus that may provide the bacterium with an evolutionary advantage when encountering high levels of NO during infection of a human host.
Nitrosylation of a QS Regulator Inhibits Virulence of S. aureus
In addition to hemoprotein coordination, NO may exert its influence over bacterial QS pathways by protein S-nitrosylation (Kovacs and Lindermayr, 2013). NO modification of cystine residues is an important post translation modification governing protein function that has been observed in mammals, bacteria and plants. Free radical NO can react directly with thiyl radicals or may first react with an oxidant such as superoxide, oxygen or redox metals to form S-nitrosylating agents like peroxynitrite (Kovacs and Lindermayr, 2013). Not surprisingly, considering the inhibitory effect of NO on microbial survival, targets of S-nitrosylation within the genome of the commensal bacterium, S. aureus, include proteins involved in carbohydrate and lipid metabolism, tRNA and cell wall biosynthesis, DNA replication and repair, and amino acid metabolism (Urbano et al., 2018). Interestingly however, a small subset of S-nitrosylated proteins in S. aureus are responsible for antibiotic resistance and virulence. AgrA, a major transcriptional activator of virulence genes and a key component of staphylococcal QS, was found to be a target of S-nitrosylation at three separate cysteine residues C6, C123, and C199 (Urbano et al., 2018). In S. aureus, when cell density is high, AgrA is activated through phosphotransfer from the autophosphorylated AgrC receptor (Figure 3). When phosphosphylated, AgrA subsequently binds to several promotors responsible for the expression of virulence factors, including agrPIII, leading to positive autoregulation of the agr operon. Upon addition of a NO donor, the transcription of several AgrA targets was inhibited in a concentration dependent matter. Further, a NO insensitive mutant, in which C199 was replaced with serine residue, exhibited resistance to NO (Urbano et al., 2018). These findings suggest that NO inhibits QS-mediated virulence in S. aureus through the S-nitrosylation of AgrA cysteine residues, particularly C199. The authors also hypothesize that considering the conservation of cysteine residues across the LytTR family of regulators, NO might affect similar processes in other bacterial species through cysteine modification.Frontiers | Insights Into Nitric Oxide Modulated Quorum Sensing Pathways | Microbiology
https://www.frontiersin.org/articles/10.3389/fmicb.2019.02174/full
Differential Regulation of Nitric Oxide Synthase-2 and Arginase-1 by Type 1/Type 2 Cytokines In Vivo: Granulomatous Pathology Is Shaped by the Pattern of l-Arginine Metabolism
Matthias Hesse, Manuel Modolell, Anne C. La Flamme, Marco Schito, José Manuel Fuentes, Allen W. Cheever, Edward J. Pearce and Thomas A. Wynn
J Immunol December 1, 2001, 167 (11) 6533-6544; DOI:*Schistosomiasis Immunology and Pathology Unit and
†Max Planck Institut für Immunbiologie, Freiburg, Germany;
‡Department of Microbiology/Immunology, Cornell University, Ithaca, NY 14853;
§Immunobiology Section, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892;
¶Department of Biochemistry, University of Extremadura, Caceres, Spain; and
∥Biomedical Research Institute, Rockville, MD 20852
Abstract
Type 2 cytokines regulate fibrotic liver pathology in mice infected with Schistosoma mansoni. Switching the immune response to a type 1-dominant reaction has proven highly effective at reducing the pathologic response. Activation of NOS-2 is critical, because type 1-deviated/NO synthase 2 (NOS-2)-deficient mice completely fail to control their response. Here, we demonstrate the differential regulation of NOS-2 and arginase type 1 (Arg-1) by type 1/type 2 cytokines in vivo and for the first time show a critical role for arginase in the pathogenesis of schistosomiasis. Using cytokine-deficient mice and two granuloma models, we show that induction of Arg-1 is type 2 cytokine dependent.Schistosome eggs induce Arg-1, while Mycobacterium avium-infected mice develop a dominant NOS-2 response. IFN-γ suppresses Arg-1 activity, because type 1 polarized IL-4/IL-10-deficient, IL-4/IL-13-deficient, and egg/IL-12-sensitized animals fail to up-regulate Arg-1 following egg exposure. Notably, granuloma size decreases in these type-1-deviated/Arg-1-unresponsive mice, suggesting an important regulatory role for Arg-1 in schistosome egg-induced pathology. To test this hypothesis, we administered difluoromethylornithine to block ornithine-aminodecarboxylase, which uses the product of arginine metabolism, l-ornithine, to generate polyamines. Strikingly, granuloma size and hepatic fibrosis increased in the ornithine-aminodecarboxylase-inhibited mice. Furthermore, we show that type 2 cytokine-stimulated macrophages produce proline under strict arginase control. Together, these data reveal an important regulatory role for the arginase biosynthetic pathway in the regulation of inflammation and demonstrate that differential activation of Arg-1/NOS-2 is a critical determinant in the pathogenesis of granuloma formation.
While the immune response generated against a pathogenic micro-organism is important to control infection, a persistent inflammatory reaction can actually do more harm than the pathogen itself. To develop new treatment strategies for chronic inflammatory diseases, it is important not only to characterize the mechanisms that initiate immune responses, but also to gain a deeper understanding of the factors that maintain these reactions. Indeed, knowledge about the downstream effector cells that regulate inflammatory responses and how their functional activity is controlled by the immune response may reveal new and/or improved approaches for disease intervention in general.
Mice infected with the helminth parasite Schistosoma mansoni develop a pathologic reaction that closely mimics the human disease. Some of the eggs laid by adult worm pairs are trapped in the liver, a process that can lead to marked inflammation, tissue eosinophilia, collagen deposition, and ultimately portal hypertension and severe hepatic fibrosis in some patients. In mice the inflammatory response is dominated by the production of type 2-associated cytokines, including IL-4, IL-5, IL-10, and IL-13. Several studies have demonstrated that these type 2 cytokines control many aspects of the egg-induced immunopathologic response (1, 2).
Previously, we showed that much of the granulomatous liver pathology is reduced in infected mice when the egg-specific response is converted from a Th2-dominant to a Th1-dominant reaction. This is accomplished by sensitizing mice to schistosome egg Ag in the presence of the Th1-inducing adjuvant, IL-12, before infection (3). Several type 1-associated cytokines were shown to be involved in the protective response (4), although recent studies suggested that NO synthase-2 (NOS-2)4 expression is particularly important (5). NOS-2-deficient mice sensitized with eggs/IL-12 not only fail to control their egg-induced inflammatory response, but actually display a marked exacerbation in the reaction despite developing a Th1-deviated response. A similar role for NOS-2 was recently described in Mycobacterium avium-infected mice (6). These findings suggest that while Th1 cytokines are critical, the downstream functional activity of NO-producing cells is equally important. Thus, NOS-2 is important not only for its antimicrobial activities (7), but also because it can serve as a potent anti-inflammatory and antifibrotic mediator.
Murine macrophages are a dominant feature of egg-induced granulomas and represent the main cellular source of inducible NO. However, these cells possess two inducible enzymes, NOS-2 and arginase, that share l-arginine as a common substrate. Although it is clear that NOS-2 plays an important role in schistosomiasis, the specific contribution of arginine metabolism to the regulation of granulomatous pathology remains unclear. In previous in vitro studies, we showed that different combinations of cytokines induce NOS-2 and the hepatic isoform of arginase (arginase type 1 (Arg-1)). The Th1-associated cytokines IFN-γ and TNF-α activate NOS-2, whereas the Th2-type cytokines IL-4, IL-10, and IL-13 induce Arg-1 (8). Of interest, the preferential activation of these enzymes is also observed during Ag presentation by macrophages and dendritic cells to corresponding CD4+ Th1 or Th2 lymphocytes (9, 10). Thus, in a manner similar to the Th1/Th2 paradigm, the cytokine-mediated activation of one enzyme is accompanied by the active suppression of the other.
In mammals, two arginase isoforms are expressed: the cytosolic Arg-1 and the mitochondrial Arg-2 (11). The isoforms catalyze the same reaction, but are encoded by different genes and differ in their tissue distribution. Arg-1 is an essential enzyme of the urea cycle and is expressed at high levels in hepatocytes. This enzyme hydrolyses l-arginine to urea and l-ornithine; therefore, its main function in the liver is the detoxification of ammonia. Although it is expressed at other sites, the exact role of Arg-1 in extrahepatic cells and tissues is not well understood. Because l-ornithine, a product of arginase activity, is a necessary metabolite for the production of polyamines and prolines, which control cell proliferation and collagen production, respectively, arginase activity appears to be critically linked with cell growth and connective tissue production. Notably, both of these activities are key parameters in the pathogenesis of inflammatory responses.
We hypothesized that type 1/type 2 cytokines would regulate the dominance of NOS-2 Arg-1 vs expression in vivo, which, in turn, would regulate the character and magnitude of inflammatory reactions. To test this hypothesis, we examined l-arginine metabolism in two different models of granulomatous disease. Initially, we investigated whether the expression of Arg-1 and NOS-2 was regulated in a manner similar to that described in in vitro studies. Specifically, we examined in several cytokine-deficient mouse strains whether arginase was activated following exposure to schistosome eggs, a potent Th2-inducing stimulus. In separate studies, mice treated with schistosome eggs and IL-12 or Mycobacterium avium-infected animals were used to examine the pattern of Arg-1/NOS-2 expression during type 1-dominated inflammatory responses. Finally, additional in vitro and in vivo studies were designed to determine more directly whether granulomatous pathology is influenced by the activation of arginase.Differential Regulation of Nitric Oxide Synthase-2 and Arginase-1 by Type 1/Type 2 Cytokines In Vivo: Granulomatous Pathology Is Shaped by the Pattern of l-Arginine Metabolism | The Journal of Immunology
https://www.jimmunol.org/content/167/11/6533




.png)

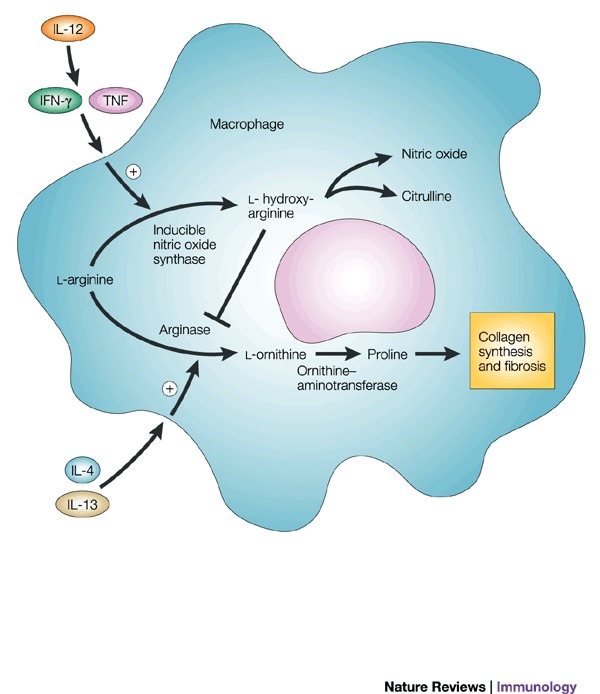

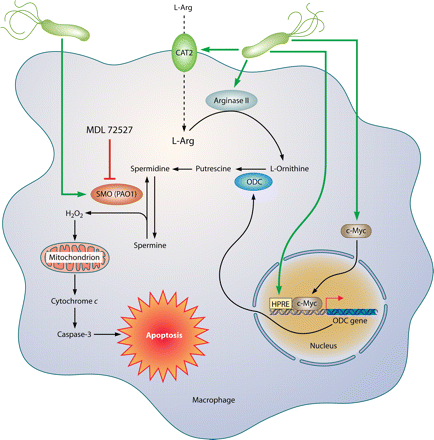


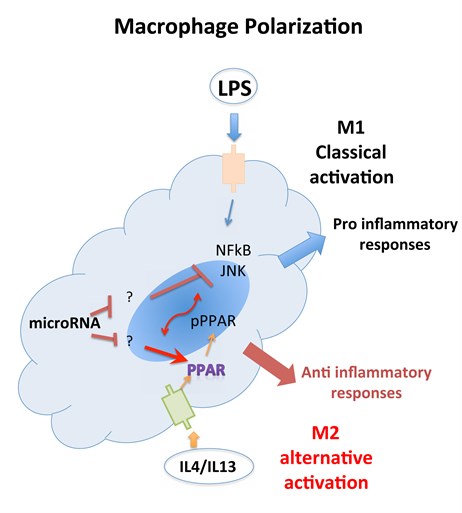
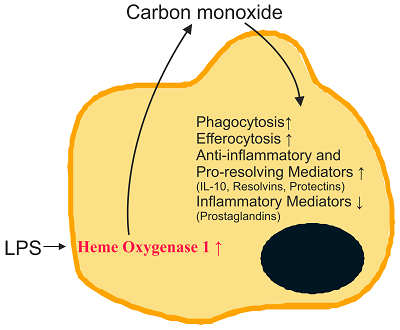
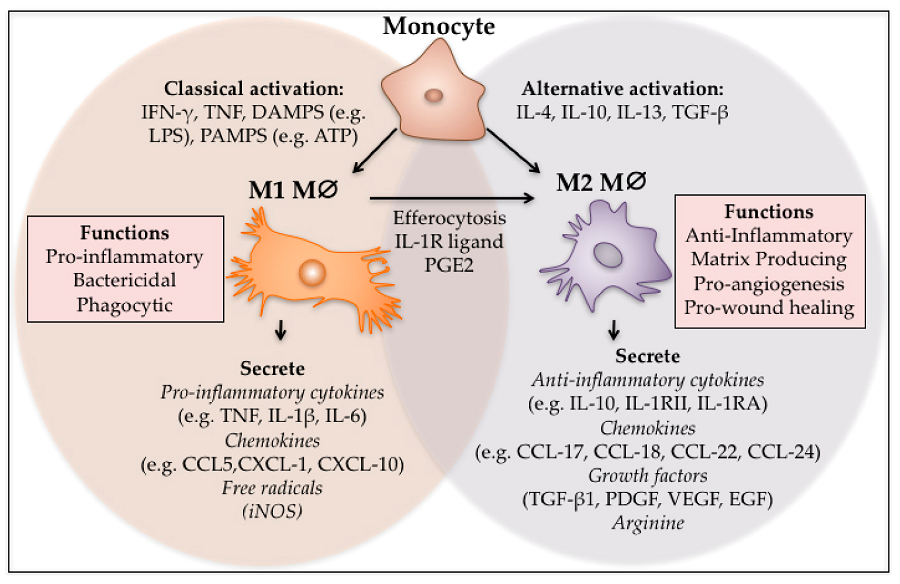
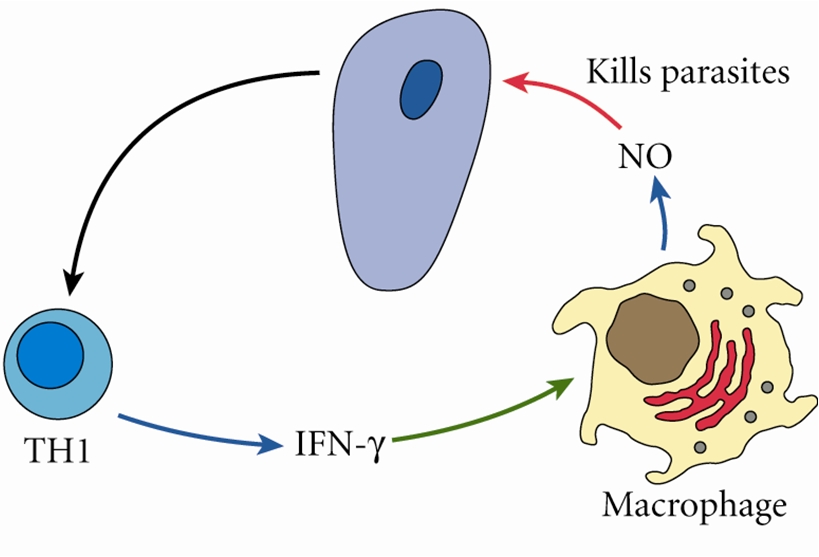
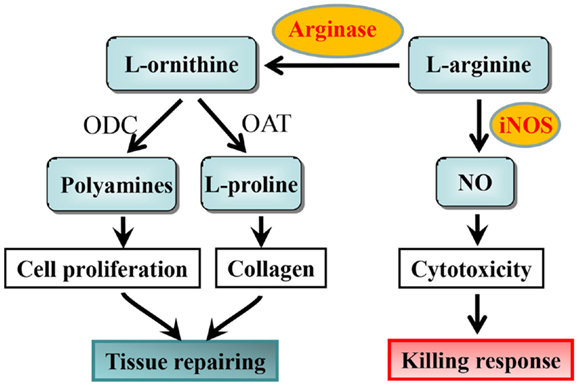

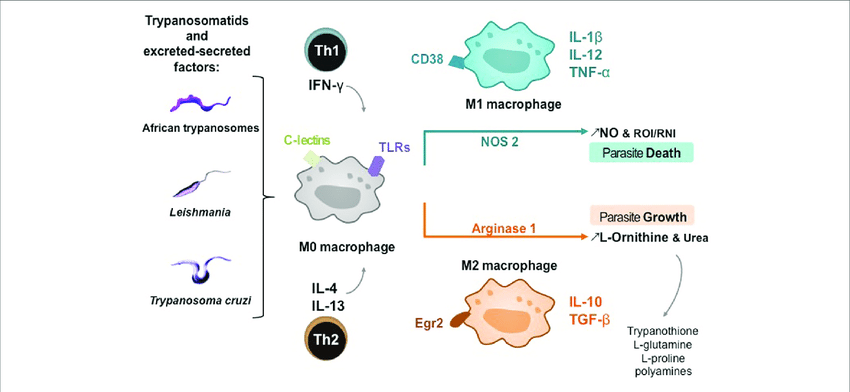
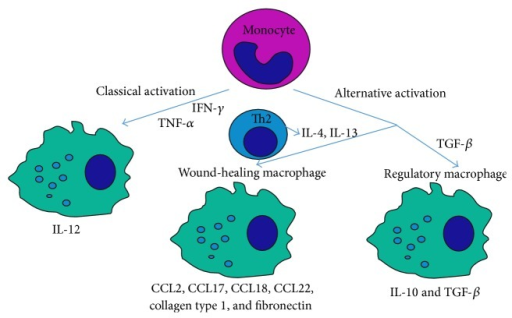
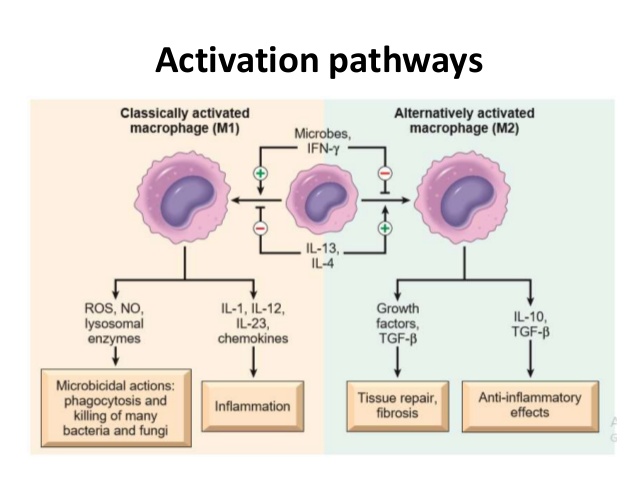
.jpg)
.jpg)