﹛
How Knee Joint Cartilage Heals
﹛
Injured tissue heals faster when you give it what it needs~nutrients, proper compression and decompression
﹛
1. Chitin from the Extract of Cuttlebone Induces Acute Inflammation and Enhances MMP1 Expression
2. birds (turtles)that peck at cuttlebone lay higher quality eggs at a faster rate than those birds that don't.
3. the chemical structure of chitosan is similar with various glycosaminoglycans (GAGs), which play important roles in chondrocyte morphology modulation, differentiation, and function.
﹛
﹛
How Knee Joint Cartilage Heals
Joint Health / Pain / By DD Kelsey
UPDATED: December 2019
※The way you see the problem, is the problem.§ 每 Stephen Covey
Knee pain is a problem but it*s not THE problem. Not technically. Knee pain is a symptom but it*s a symptom that interferes with a lot of things in a person*s life so it sure feels like a pretty big problem.
When I was in PT school, I skipped most of the orthopedics lectures. Probably not the greatest thing to do but my attitude was that the lecturer, an orthopedic surgeon, was simply restating what was already in our textbook. I needed to work to get through school so I had better things to do with my time than being bored to just this side of madness several days a week.
I managed a ※B§ in the class but really knew nothing about how joints healed. We were taught, and this is still the case in many schools today, that cartilage had no capacity to heal. In fact, one of my professors said that you get one batch of cartilage and when you lose it, too bad.
That*s it.
So, if you had injured the joint cartilage or were dealing with osteoarthritis, well, too bad. The best you could do was to try to strengthen the muscles around the joint and hope that by doing so, your joint would be better protected.
I remember sitting in class wondering why; why wouldn*t cartilage heal? So I asked. My professor just looked at me like I was a complete idiot. Finally, he said, ※There*s no blood supply and no metabolism. Tissue cannot heal without metabolism. Cartilage is biologically inert.§
Inert means ※lifeless§. A chair is inert. A frying pan is inert. But cartilage? How could that be?
How could a biologic tissue be inert? It just didn*t make any sense.
So, I had to ask. ※If cartilage is biologically inert, how does it live in the body?§
Silence. For a while. ※It*s not biologically active. Now, let*s move on.§
I actually paid for that education too.
The Truth About Cartilage
I learned many years later that I was correct and my professor was wrong. Cartilage is not biologically inert.[source] Its metabolism though is very slow, sluggish. It has no blood supply, no nerve supply, and no lymph supply which makes healing difficult but not impossible.
I gave a lecture in May 2011 at the American Association of Orthopaedic Medicine conference on Regenerative Medicine in Las Vegas. My topic was the ※Mechanobiology of Exercise§ or said another way, the study of how mechanical forces generated within or imposed upon living tissues affect physiology and structure of that tissue.
I was speaking to a variety of professionals 每 physicians, chiropractors, osteopaths, podiatrists, scientists 每 about 200 people or so.
After I finished the talk, a number of people came up to me and said that they had never heard of most of the material I presented and were excited to learn that exercise had such potential to help injured tissues heal. While I had created many of the treatment techniques, much of the underlying science dates back a number of years.
We know, for example, that exercise can alter the form of the body. Bodybuilders have a certain look, sprinters look differently than marathon runners, swimmers have differently shaped bodies than gymnasts. So, the question isn*t whether exercise can alter your body. The question is how does exercise alter parts of your body that are injured and specifically cartilage.
Most everyone agrees that injured bone heals best with the application of force; controlled of course. If you break your lower leg, for example, optimal healing always includes graduated weight-bearing. If you place too much load on the fracture too quickly, you*ll impair the healing. If you don*t place enough load on the fracture, you*ll also impair the healing.
I invented nothing new here in terms of how the body heals. The science supporting the idea that cartilage has healing capability is extensive and beyond the scope of a single blog post but basically it heals in a similar way that bone heals (which makes complete sense when you consider that cartilage is the predecessor of bone) 每 controlled compression and decompression of the joint via controlled loading or weight-bearing.
Joint cartilage is ※mechanosensitive§ 每 it responds to motion and load to both maintain its health and improve it. The cells responsible for helping injured cartilage heal only ※speak§ to each other once force is applied. This is in contrast to, for example, the skin where blood is plentiful and cellular communication almost instant. The key for cartilage to heal, once injured, is finding the proper amount of motion and load.
#..human cartilage responds to physiologic loading in a way similar to that exhibited by muscle and bone, and that previously established positive symptomatic effects of exercise in patients with OA may occur in parallel or even be caused by improved cartilage properties.§
But, the wild card is how well does it heal; what*s the quality of the tissue? In some cases, the damaged cartilage will repair itself with tissue that is not the same; closer to a scar like tissue. In other cases, the cartilage may heal with higher quality tissue. In any event, some healing is better than not having it happen at all.
Turns out, some researchers from Australia studied the knees of 86 healthy subjects and initially found 19 cartilage defects where roughly one-half of the cartilage had been lost. Two years later, the subjects had MRIs to inspect the joints. Of those 19 locations, more than half improved, less than a third worsened, and the rest remained the same.[source]
Even more incredible was that in five areas where bone had been exposed (where the cartilage had been lost) four of the five showed evidence of new cartilage during the re-examination and one was at full thickness.
Evidence
The images below are from a case study. A woman in her 40*s came to see me with a diagnosis of ※knee pain§. She first saw her family doctor who prescribed an anti-inflammatory and to stop her bench step aerobics classes. She followed his instructions, went back to see him after four weeks and was told she could resume her regular routine.
So, she goes back to step aerobics and about two weeks later, her knee swells to the size of grapefruit. She then goes to see an orthopedist who sends her to me after having performed a knee arthroscopy.
We guided her through our cartilage conditioning/strengthening program. About three months later, she had no swelling, could bear above bodyweight force on her leg and felt great.
About a year later, she returned to my office with a new diagnosis. She had taken a hiking trip in the mountains in Mexico, got her leg caught between some rocks, twisted it and tore her meniscus in the same knee.
The image on the left is from her first surgery. The arrow is pointing to a large defect in her femoral condyle. In technical terms, that size of defect is called a ※mid-thickness§ injury and is thought to never heal. The image on the right is the same knee about a year later during surgery for the meniscus tear. The arrow is pointing to the original area of the defect that now has filled in about 90%.
Cartilage-healing
And below is a video of the first and second surgery. Pay special attention to the change in the firmness of the cartilage in the second video.
Video Player
00:00
02:05
Why Strengthening Muscle Is So Difficult With An Injured Joint
For those of you reading this who have had, or currently have, knee pain from a joint cartilage problem, you will probably recognize the following scenario.
Because the vast majority of providers still believe there is no hope for injured cartilage, all that is left to fix is either mechanics (how you move, how flexible you are, how well ※aligned§ your body parts are) or muscle strength.
And here*s what happens, most of the time, when you perform strengthening exercises in the presence of a joint cartilage problem as presented to me by one of my students several years ago.
※So, how*s it going with Mr. Smith?§ I asked.
※Well, okay I guess,§ replied the student.
※Okay? What are you doing with him?§ I asked.
※Oh, quad strengthening of course. Mostly trying to get his VMO to work, § she replied (VMO is the vastus medialis oblique 每 one of the quadriceps muscles).
※I see. And how many reps are you using?§
※3 sets of 10 reps,§ she replied with confidence.
※Okay and how much fatigue would you say Mr. Smith has in his muscles from the 3 sets of 10 reps?§ I asked.
※Uh#what do you mean?§ she replied.
※I mean, how tired does his leg get from the exercise,§ I replied.
※Oh, well, I don*t know really. I guess, well, I guess I didn*t ask,§ she replied.
※Go check it out and come back and let me know what happens,§ I said.
Off goes the student to work with Mr. Smith. A few minutes later, she returns.
※What happened?§ I asked.
※Well, he didn*t have any fatigue really at 3 sets of 10,§ she replied.
※And what did you do?§ I asked.
※I increased the weight but then he couldn*t do more than about 3 or 4 reps and his knee hurt,§ she replied.
※So what did you do then?§ I asked.
※I lowered the weight and asked him to go until he fatigued. And he did about 50 reps and still wasn*t all that tired so I stopped,§ she said.
※Right. Okay, so now what?§ I asked.
※I have no idea really. I was hoping you could help me,§ she replied.
And that*s how it goes. A lot of people wasting a lot of time trying to strengthen muscles that cannot be strengthened because the effort overloads the joint.
Sound familiar at all?
What To Do
I go into considerable detail about the strategy and tactics for knee joint cartilage in my book ※The 90 Day Knee Arthritis Remedy§. Covering the entire book in a single post is not possible but here are some of the basics.
Determine how much force your joint can comfortably withstand and respect it. I have worked with clients who could tolerate 30% of their body weight on their injured leg yet refused to stop running. For example, someone who weighs 150 lbs, may be able to withstand only 45 lbs of their body weight on the painful leg while the other leg can withstand more than 150 lbs. Running produces forces well over bodyweight loads.
Injured tissue heals faster when you give it what it needs. Otherwise, it will just fight you the whole time until eventually you*re forced to stop the activity and then are faced with a much more difficult problem to solve. I like to use the Variable Incline Plane (VIP and I have no affiliation with any companies) because I can find the threshold at which you can comfortably load your leg. This information is invaluable as a training metric and for comparison over time.
Move your joint lightly, easily, and intermittently. Injured joints like movement, generally, and dislike being still. For an injured knee, I suggest things like a furniture slider, or even a paper plate to place your foot on and slide the foot forward back while in a sitting position. You can do this for 5-10 minutes a few times a day and most people find it quite helpful.
sliders
Find a repetitive motion you can do without pain and do it thirty minutes a day. I like a VIP because it*s easy to find a comfortable load level to perform squats. Other options include cycling, walking in a pool, some elliptical machines. Remember that you have to gradually increase the load or pressure in order to strengthen the joint surface. The biggest mistake people make is keeping the load levels the same for long periods of time or increasing the loads too quickly.
Eventually, you must strengthen your leg muscles 每 all of them. But to get to that point, you have to address the weakness of the cartilage.
Leg strength is built from the inside-out.
In the meantime, the old standby that almost everyone uses, quad sets, is a good thing to add but not because it helps strengthen your quadriceps (which it doesn*t). You*ll get more out of the exercise if you tighten the quadriceps muscle with your knee nearly straight while, at the same time, you tighten the hamstrings. The ※setting§ of the muscles increases the thickness of the synovial fluid which in turn helps improve the nutrient exchange within the joint and serves as an extra ※cushion§ when you place weight on your leg.1)
Add supplements. I suggest Chondroitin Sulfate and Glucosamine. I recommend Osteo Bi-Flex or Triple Flex brands (these supplements are not regulated by the FDA so quality control is a common issue and these brands have been tested and found to contain what they advertise). I also suggest Boswellia which has been shown to help reduce joint-related aches and pains.
Consider Platelet Rich Plasma Injections (PRP). I had a large medial meniscus tear in my right knee a few years ago. Having been through surgery for a tear in the other knee back in 1994, I didn*t want to go through surgery again. Preserving as much of the meniscus as you can greatly helps long term knee health. I chose to undergo a PRP injection (twice) in the right knee. I also followed strict weight-bearing restrictions following the injection and adhered to my rehab program. It worked. My meniscus healed and today the only thing about my right knee that isn*t ※normal§ is I have a very slight loss of passive knee flexion. But otherwise, it feels great and my function is very good.
Expect setbacks. Cartilage is finicky. Too much pressure, too fast and a few days later your joint will ache or swell or hurt. Not enough pressure or if the progression is too slow, and you*ll not improve much. The result is a cyclical process of improvement, then maybe a setback or two, then some improvement and then perhaps no improvement. If you have a load level test from a device like the VIP, this helps you manage the setbacks. You might think you*re worse than you really are. Numbers help.
Be patient. Healing an injured or weak joint takes time. Sometimes, a long time. Like several months or even two or three years. You have to keep working at it; keep showing up. It*s simple but not easy to do.
Maintain your improvements. Once you*ve re-established your load capacity, you have to train or exercise consistently with joint-friendly exercises to keep it there. It*s a lifestyle; a commitment. If you don*t do this, you*ll gradually decline and eventually, the problem will come back. Your body makes you earn your health.
Thanks for reading.
﹛
How Knee Joint Cartilage Heals > The Kelsey Report
https://www.dougkelsey.com/knee-cartilage/﹛
﹛
Knee Cartilage Repair: How One Patient Proved His Doctors ...
https://www.huffpost.com/entry/knee-cartilage-repair_b_876946
Aug 16, 2011 ﹞ The cartilage in my knee joints made an ugly noise, like someone rolling over a bag full of damp potato chips. A few minutes later, he delivered his verdict: "Your knees will never get better.". My condition (variously diagnosed as patellofemoral pain syndrome or chondromalacia) could worsen into osteoarthritis in as few as several years, he said.
﹛Precautions You Must Take Before Using Crystals For Joint ...
https://shop.atperrys.com/blogs/healing-crystals-blog/astonishing-ways-how-crystals...
When used for joint pain and inflammation, aquamarine stones give quick relief. The soothing energy and gentle vibes of the stone will empower and make you strong. Aquamarine is a healing stone that can penetrate your blocked chakras via the throat chakra. It is ideal for chronic joint pain.
The meniscal healing process - PubMed Central (PMC)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3666493
Jun 17, 2012 ﹞ The synovial holds the synovium-derived stem cells (SDSCs). They are a promising source of stem cells for the cartilage and meniscal repair, giving their higher condrogenic and osteogenic power than the MSC derived from the bone marrow, adipocytes or periosteum (18,19,28,54每57).﹛
﹛
﹛
Muscles Ligaments Tendons J. 2012 Jan-Mar; 2(1): 10每18.
The meniscal healing process
Pilar Mart赤nez de Albornoz1 and Francisco Forriol2
Author information Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Summary
Meniscus is a difficult structure to repair and replace. An injured or degenerative meniscus promotes osteoarthritic joint changes that should be avoided. Research focused on promoting healing or replacement must cover three different working lines: biology, mechanics and surgical technique. Biology research line looks for specific factors able to develop a collagen tissue in a matrix with cells that joins the edges of the lesion and also looks for factors able to keep the elasticity and able to regenerate the damaged meniscus fibres. On the other side, scaffolds need the adequate viscoelasticity to allow the penetration of vessels and cells to avoid reabsorption.
Keywords: meniscus, growth factors, collagen, cartilage, angiogenesis
Go to:
Introduction
The mechanisms of meniscal repair follow two patterns (1,2). The extrinsic pathway, which usually takes place in lesions of the vascular area where there is a net of capillaries, which supplied undifferentiated mesenchymal cells with nutrients to induce healing (Figs 1, ,2).2). The intrinsic pathway is based on the self-repair capacity of the meniscal fibrocartilage and the synovial fluid (Fig. 3). The more central is the location of the meniscus injury, the lower the intrinsic responsiveness. In these cases, other factors are needed to provide a biological response (3) and synovial fluid surrounding is not an element that promotes the repair. Therefore, the intrinsic pathway is rejected and becomes essential to know the specific features of meniscal cells.
An external file that holds a picture, illustration, etc.
Object name is mltj_1-2012_pag_10-18f1.jpg
Figure 1.
Radial cut of sheep meniscus (Polarized light microscopy).
An external file that holds a picture, illustration, etc.
Object name is mltj_1-2012_pag_10-18f2a.jpg
An external file that holds a picture, illustration, etc.
Object name is mltj_1-2012_pag_10-18f2b.jpgAn external file that holds a picture, illustration, etc.
Object name is mltj_1-2012_pag_10-18f2c.jpg
Open in a separate window
Figure 2 (a,b,c).
Sheep meniscus vascularization, A) 2 weeks old lamb; B) 1 month-old lamb; C) 5 months-old sheep.
An external file that holds a picture, illustration, etc.
Object name is mltj_1-2012_pag_10-18f3a.jpg
Open in a separate window
An external file that holds a picture, illustration, etc.
Object name is mltj_1-2012_pag_10-18f3b.jpg
Open in a separate window
An external file that holds a picture, illustration, etc.
Object name is mltj_1-2012_pag_10-18f3c.jpg
Figure 3 (a,b,c).
Pores in the avascular area of the meniscus, scanning microscopy, A) human meniscus (x3000); B) sheep meniscus (x1500); C) maccaca rhesus meniscus (x3.000).
The role of angiogenesis
Angiogenesis or new vessel formation from preexisting capillaries is essential for repairing the damaged tissues. There are signs that promote the expression of angiogenic growth factors such as metabolic parameters, inflamatory processes and genetic mutations. Inducers of angiogenesis are proteins such as fibroblastic growth factor (FGF), tissular growth factor (TGF), hepatocyte growth factor (HGF), tumoral necrosis factor (TNF), IL-8 and angiopeptin.
Angiogenesis is essential for tissue repair. From a molecular point of view, this process is based on a balance of stimulation and inhibition of different molecules. King and Vallee (4) placed angiogenin (vessel inducing protein) on a meniscal hole in 75 rabbits. They found revascularization of the defect in 52% of the animals compared with the 9% of the controls.
King (5), in 1936, studied the meniscus as a living tissue, integrated in the knee joint; in his classic work he noted that lesions in the menisci healed in the vascular area while did not in the white area. This work was later supported in different animal studies (1,6每8). Vascularization and repair were associated.
However it needs to be clarified that a meniscal injury has the potential to heal easily when is located in the vascular area, but just the vascular supply itself does not repair the meniscal defect. Cabaud et al. (9) and Heatley et al. (10) supported in dogs, monkeys and rabbits that lesions located in the periphery of the meniscus healed, due to the blood supply from the synovial.
From the initial studies of DeHaven et al. (3) who described the open meniscal suture, clinical experience has supported the good results of suturing peripheral meniscal lesions. Doubts rise when we find injuries further than the meniscal wall and disagreement in literature.
Ghadially et al. (11) performed an interesting animal trial with different species (rabbits, dogs, pigs and sheep). They produced a bucket handle lesion on the meniscus, away from the wall, and did not find any evidence of histological healing in neither of the specimens. The meniscal suture did not change the results and the bucket handle tear in the white area of the meniscus in the three different animal species not appear any healing sign at 6 months of follow up. In another group of animals, they performed ※T§ shape injuries that reached the meniscal periphery. Healing signs were found at 6 months of follow up.
Veth et al. (12,13) performed in rabbits, longitudinal lesions in the middle third and wedge lesions that reached the meniscal wall. Healing was significantly better in the wedge lesions of the meniscal wall where they found a fibrocartilage tissue similar to a control meniscus and even with higher cell count. From the 35 wedge lesions, 16 showed permanent signs of healing. In the group of the longitudinal lesions, 5 of 35 menisci showed evidence of healing. Therefore, location of the lesion is a relevant factor on the forecast of meniscal healing.
In an experimental study with sheep, Guisasola et al. (2) presented 2 cases of a longitudinal sutured lesion with healing signs. They found a new cellular infiltrate that came from the surface of the meniscus and from the channels of the suture in the gap.
Kobayashi et al. (14) developed a pilot study and showed that the power of meniscal healing was more increased in the periphery than in the avascular area. The stem cells that stimulate growth, support and tissue healing are located in the periphery of the meniscus. Other authors attribute this phenomenon to the presence of cytokines in the matrix (15 每 17).
The role of mechanical factors
The menisci are subjected to compression throughout the arc of motion of the knee. Rotation increases the stresses on the meniscus. Internal rotation of the knee increases twice the pressure on the lateral meniscus compared to neutral or external rotation. An injured meniscus is displaced and fragmented. In addition, the stress on the meniscus is also influenced by the decrease of synovial fluid and the tissue nutrition (18,19). Meniscal healing only happened in a 10% of all the meniscal ruptures reviewed by arthroscopy (20).
Kawai et al. (21) studied the healing process in dogs after performing a lesion in the vascular area of the meniscus. Subsequently to the meniscal suture, they did not apply any immobilization. A synovial pannus was confirmed in both sides of the lesions at 2 weeks. At 12 weeks, the maximum strength of the repaired tissue reached the 80% of the strength of a control meniscus. They concluded that immobilization and unloading were not relevant factors for meniscal healing in the vascular area. Years later this idea was also confirmed by Guisasola et al. (2). Conversely Zhongnan et al. (8) found better results in menisci of rabbits with immobilization. Dowdy et al. (22) pointed that the formation of collagen decreased from the 4th week of immobilization, and was even lower on the 10th week. From the 8th week of immobilization the loss of collagen was irrecoverable.
A good fixation seems to be more important than the immobilization (2,22,23). Port et al. (24) emphasize on the stability of the construct. They no find any statistical differences neither mechanical nor histological, between a group treated with two vertical sutures, another with fibrin clot plus suture, and a third one with fibrin clot plus bone marrow stem cells. The study was performed in goats. Values for tensile strength were less than 40% of the controls. Histological samples showed partial restoration with areas of giant cells and macrophages.
Molecular healing: growth factors and meniscal repair
Unlike the osseous tissue and despite immobilization, meniscus does not stimulate the cascade of the healing process. Causes of impairment would be the synovial fluid, mechanical stress, movement, loading. In our previous studies we found a vascular proliferation that did not result into a meniscal tissue formation (2).
Becker et al. (25) found an increased expression of vascular endothelial growth factor (VEGF) in menisci with lacerations on both areas, the peripheral and the free portion. The highest levels were on the 7th day after the injury and then decreased. VEGF levels were twice higher in defects located in the central area than in the periphery. This was attributable to a synergistic action of the cells of the central portion and the expression of VEGF. Endogenous anti-angiogenic factors such as troponin-1, angiostatin and endostatin are expressed to provide the lack of vessels in cartilage and in the avascular zone of the mensicus (4,6,25). The study of Becker et al. (25) showed a high expression of VEGF and mRNA in endothelial cells and fibro-chondrocytes of the vascular and avascular areas of the meniscal tissue. Despite the high concentrations of VEGF, lesions of the avascular portion did not heal successfully.
The growth factors released by the cells at the injury place together with the inflammatory infiltration of the scar tissue stimulate the meniscal cells to proliferation, migration, differentiation and matrix synthesis. The direct application of recombinant human proteins can stimulate meniscal repair but its application is limited by its short biological life and the need for repeated high doses of the growth factor.
Spindler et al. (26) showed that the avascular meniscal cells did not react to the presence of PDGF while Tumia and Johnstone (27) found that the IGF-1 stimulated the fibrochondrocytes of the avascular area. The activity of fibrochondrocytes is stimulated by the IGF-1 isolated or combined with 10% of fetal calf serum (FCS) by increasing the metabolism of thymidine and the DNA formation more in the inner zone than in the outer meniscus.
It has been shown (19) that synovial cells treated with hyaluronic acid (0.1 mg/mL) and Hylan® (Synvisc) (0.1 mg/mL) increased the expression of TGF-汕1 and VEGF. However, the Hylan® decreased the connective tissue growth factor (CTGF) (0.66 times) and the VEGF (0.78 times) compared with the hyaluronic acid. Fortier et al. (28) proved the protective effects on synoviocytes of the tetracycline followed by the minocycline and compared with the doxycycline. They are all interesting products for the osteoarthritis management.
Therefore a growth factor carrier must meet the following conditions (29): (i) to release growth factors in time and with the adequate dosage. (ii) The presence of a substrate to stimulate the recruitment and cell adhesion, promoting chemotaxis in a space that allows cell migration and angiogenesis. (iii) To be biodegradable without causing immune, inflammatory or toxic reactions that would inhibit the repair process.
There have been reported four types of carriers for the growth factors: inorganic materials, synthetic polymers, natural polymers or ※composites§ and compounds made of the materials cited above. Among the most commonly used materials there are the type I collagen, hyaluronic acid gels, and polylactic and polyglycolic acid. The type I collagen is a carrier interesting for its fibrillar structure and for being the most abundant protein the extracellular matrix of bone and meniscus.
Other materials as acellular hydrogels have been implanted instead of a complete meniscus in rabbit and sheep (30,31). Degradable porous sponges have been developed (32,33). An attempt has been made to study natural materials as SIS (Small Intestinal Submucosa) or collagen and tissular implants (12,13,34每37). Many of these studies use animals and showed certain degree of chondral protection. Not all of them achieved a meniscal-like structure and its final function as protector of the cartilage (38). Baker et al. (15) have worked in a nanofibrous matrix of polycaprolactone (PCL) (E-caprolactone) combined with meniscal cells or mesenchymal cells. These group used cells derived from meniscal resection with good results; appropriate for their potential for autologous therapies without immune response and an appropriate phenotype.
The development of injectable carriers with growth factors is according to Seeherman et al. (39), one of the currently working lines.
Many of the synthetic polymers originate from the family of the polyesters that are degraded by gradual hydrolysis. The polymers are made from polyglycolic acid, polylactic and other copolymers. These polymers by changing its composition can adjust their mechanical properties and degradation time to simulate the repair tissue. The polymer matrix is made to allow the penetration of vascular structures to stimulate the cells to grow, develop and differentiate. Matrix can be supplemented with growth factors to induce the cellular development. Complex polymers are designed with different layers, to release different growth factors and improve the incorporation of the matrix and cell proliferation (35).
Most of the studies about meniscal repair confirm that the longitudinal lesions of the avascular area, the most common, are not able to heal properly and the meniscus do not reach its standard biomechanical conditions. There is evidence in experimental studies (2,40) that the meniscal strength after 2每3 months of the lesion does not reach the 30% of strength of a healthy meniscal tissue.
As we show, peripheral lesions located in the vascular area of the meniscus have better forecast. However results in literature are not homogeneous. The rate of success and failure is uncertain (41). It is difficult to assess giving the variety of lesions and the absence of registration. Any meniscal repair technique has to connect the edges of the lesion to protect it from the stresses of the joint. The meniscal repair process must prioritize the same principles as for other organic tissue repair: immobilization, an adequate fixation, rest, and delivery of healing substances.
To extend the indications of repairing in the avascular area, new techniques have been developed bringing blood supply from the capsule to the avascular area or directly repairing the injury.
Uysal et al. (42) analyzed 38 menisci from cadavers and surgical patients, aged below 40 years. There were three groups: non injured, with traumatic lesions and degenerative lesions. They studied the apoptotic cells in each group. The apoptotic cell count was statistically higher in the pathological groups than the health samples.
They did not find any differences between the pathological samples. Meniscal explants from the vascular and avascular areas showed an intrinsic healing response similar in vitro (43). This suggested that the meniscal repair process could be improved in vivo under appropriate intraarticular conditions.
External stimuli:
Haemarthrosis and fibrin clot
Arnoczky et al. (7) knowing the relevance of the hematoma in the initial healing phase of any injury applied a clot of fibrin in a meniscal defect in the avascular area of 12 dogs. The results showed a healed meniscal tissue with fibrocartilage. The origin of the repair cells was unknown giving the lack of vessels in the area of the defect. The clot provides a scaffold rich in platelet derived growth factors (PDGF) and fibronectine to enhance chemotaxis and mitogenic stimuli to the healing cells.
The fibrin clot seems to have the ideal features to guide the intrinsic meniscal response to heal, as a scaffold and as a source of stimulating factors. Spindle meniscal cells responded to chemotactic and mitogenic factors (43每46). Meniscal cells are able to multiply and develop an extracellular matrix in vitro when they are exposed to mitogenic and chemotactic factors existing in the hematoma (43每46). The origin of the repair cells is uncertain, but the early presence of fibroblasts in the meniscal injury suggests an important role of the superficial meniscal cells and the synovial cells as a source of stem cells in the joint. The control group did not show macroscopic healing, but in some cases there was a thin layer of tissue that filled the gap, probably due to a residual haemarthrosis of the surgery or to a mild proliferative response of the meniscal tissue. Webber et al. (44,46) looked on meniscal surgery with optimism. In vitro, fibrochondrocytes proliferated and synthesized extracellular matrix without blood supply whenever, they were surrounded by the appropriate media. For these authors the fibrin clot has the features to guide the intrinsic meniscal response, as a scaffold and as an input of growth factors to promote cell replication.
Van Trommel et al. (47) treated five complete radial external meniscal lesions, in the avascular popliteal portion, with a fibrin clot. Three years later, the arthroscopy control and the MRI testified that the lesions had healed. In the clinical experience, meniscal repair associated to the reconstruction of the ACL establishes a propitious environment. The haemarthrosis is full of growth factors that promote the meniscal repair (47). In an in vitro study, fibrochondrocytes exposed to the growth factors of a clot showed an increased proliferation rate and synthesis of a cartilage matrix (26,46每49).
Fibrin glue
The problem of the application of the fibrin clot is its poor adhesive property. It cannot stimulate healing if it doesn*t remain fixed and stable. Fibrin glue is a combination of coagulation factors (fibrinogen, thrombin, CaCl2 and Factor XIII) with aprotinin. The adhesive properties of the fibrin glue are superior to the clot but it lacks the biological properties. The fibrin glue is able to keep overlapping the edges of the injury without stimulating the repair process, therefore is only indicated in stable and small lesions (50). It has also been shown that the association of the fibrin glue and bone marrow stem cells promoted the meniscal healing, as well as the combination of fibrin glue and VEGF (51). To overcome the application problems of the fibrin clot, the preparation technique has been improved, increasing its consistency and the fibrin content. The fibrin glue can be used combined with a suture or be fragmented and applied with a syringe (52,53).
Synovial graft
The synovial holds the synovium-derived stem cells (SDSCs). They are a promising source of stem cells for the cartilage and meniscal repair, giving their higher condrogenic and osteogenic power than the MSC derived from the bone marrow, adipocytes or periosteum (18,19,28,54每57). Under adequate stimulation conditions they are able to migrate to the cartilage defect and produce a chondrogenic differentiation (58). They are also stable cells in vitro cultures from the step #3 to #10. These properties make them useful for transplantation to the avascular area of the meniscus and synovial cells play an important role in repopulation of the synovium grafts. They have a high differentiation rate and serve as a rich source of stem cells (54).
The synovial cells have an unknown function in the meniscal repair. In a study performed by this team showed that these cells were sensible to the stimuli of different growth factors. They modified the gene expression and stimulated the fibroblast derived growth factors. These factors had an important effect in expressing the type II collagen that simulates the meniscal healing and the MMP expression that are also involved in remodeling.
During the repair process, the cell lineage of the first 6 weeks is mostly fibroblastic. The origin of these cells is twofold: from the meniscal fibro-chondrocytes (44每46) and from the synovial cells. They reached the defect from an invagination of the meniscal surface, upper and lower surfaces, and from the channels performed by the suture. Collagen fibers were scarce and disorganized.
We believe that the use of substances to induce cell proliferation is a field of high interest in the meniscal tissue repair. The placement of a synovial flap over the sutured lesion serves as a stimulus for blood supply and for synovial stem cells. Kobuna et al. (57) have show conclusive results in dogs. By microangiography they demonstrated that vessels located on the femoral surface and the inner part of the meniscus reached the sutured area. At 6 weeks the lesions showed healing with a fibrovascular tissue. Cisa et al. (59) published good results in rabbits after using synovial flaps. Healing of the superficial layers is a consequence of the arrival of cellular elements from the synovial fluid and the fibro-chondrocytes. Healing of the deeper area is a consequence of the synovial autograft. However, the trigger factor of the healing process remains unknown.
Synovial cells have shown an over expression of agrecans, MMP-2 and bone morphogenetic protein-7 (BMP-7), when they were stimulated with fibroblastic growth factor (FGF), tissular growth factor (TGF), insulin growth factor (IGF) and BMP-7. We did not find an over expression of collagens or any effect when they were cultured with hyaluronic acid and a chondroprotective (60每62).
Following this idea, in longitudinal lesions in the medial meniscus of dogs, Shirakura et al. (63) analyzed the effectiveness of a free synovial autograft, a free muscular autograft on a Dacron® mesh and a control group of just sutured meniscus. Eleven of the 35 synovial autografts healed in 12 weeks with a fibrous tissue. They found new capillaries from the periphery without reaching the lesion. In none of the other groups the repair was achieved.
Jitsuiki et al. (64) advanced in the study of healing using synovial flaps. They tried to differentiate two concepts in the process of meniscal healing: the effect of vascularization and the effect of synovial tissue and synovial fluid. Meniscal lesions in rabbits healed by covering them with the synovial tissue. Fibro-chondrocytes had an intrinsic ability to repair, but they did not migrate and proliferate (44每46). Therefore, the reparative cells that appeared on the surface of the meniscus had to derive from cellular elements of the synovial fluid. These findings indicated that the healing process in the avascular zone of the meniscus was primarily due to synovial autograft together with the diffusion of synovial fluid and local factors.
The synovial autograft would simplify the surgical technique, avoiding the cutting of a flap, surgically more challenging. In vitro, the synovial graft showed a higher cell proliferation and collagen neoformation (65). On the 4th week the group of the synovial graft showed obvious signs of healing while in the control group there was an increase of cellular components on both sides of the lesion without filling. The cells of the fibrin clot covered the scaffold and the defect, but there was not collagen neoformation.
Periosteum
Parallel to the implementations o synovial grafts, the periosteum is a potential source of stem cells. From the periosteum arise chondrocytes and fibrochondrocytes that are involved in the meniscal repair process. In an animal study in dogs, the meniscal lesion of the avascular area was repaired with free periosteum graft and fibrin clot. After 16 weeks, the new tissue in the old defect was similar to the adjacent (52). The periosteum has been elected as a donor tissue in musculoskeletal bioengineering as a source of chondrogenic factors (58). The chondrogenesis of the periosteum has two different phases: a cellular proliferation followed by a cellular differentiation and the deposition of a matrix rich in proteoglycans (66). In vitro cartilage tissue can be obtained from periosteum and TGF-汕1 stimulation (58).
PRP and MSC
Platelet-rich plasma (PRP) is a source of growth factors that induce healing response by stimulating the angiogenesis, chemotaxis, collagen matrix synthesis and cell proliferation (26). The role of the PRP in meniscal healing in literature is conflicting. Ishida et al. (67) reported good results in rabbits both in vitro and in vivo. In vitro, PRP stimulated the meniscal cell proliferation, the extracellular matrix synthesis, and the expression of fibrocartilage-related mRNA. In vivo, PRP together with Gelatin hydrogel showed better repair tissue in the meniscal defects than the control groups. These good results contrast with the study of Zellner et al. (68). They performed circular meniscal punch defects in the avascular zone of rabbit menisci. They were left empty of filled with different substances. Neither bone marrow nor PRP loaded in matrices induced improvement in meniscal healing.
Mesenchymal stem cells (MSC) are pluripotent cells that, thanks to their developmental plasticity are able to differentiate into specific therapeutic cell types (69). Studies have shown the production of abundant extracellular matrix around the cells in the avascular area and restoring a meniscal like tissue (17,68每71). The association of growth factors and MSC within scaffold implants demonstrated to increase proteoglycans and collagen synthesis (72). The combination of MSC and suturing, using or not fibrin glue, seems to be the most effective treatment (73).
It has been deeply studied the autologous chondrocyte implantation previously cultured in vitro, for cartilage defects (74,75). Peretti et al. (76,77) used the same idea and technology for meniscal defects in the avascular area, in rabbits. A meniscal allogenic scaffold with cultures cells is the most promising pattern to get meniscal repair (77). The same team (76) performed a longitudinal lesion in the avascular area of the menisci of 16 pigs. They divided the sample in 4 groups. In one of the groups they placed isolated chondrocytes over devitalized allogenic meniscal slices and maintained the structure with two sutures. The other three groups were managed as controls. One had an allogenic matrix without cells, another just a suture of the lesion without any external substances, and the last one was left untreated. The first group was the only one that showed meniscal repair signs. Dutton et al. (17) proved in pigs that the implant of MSC in meniscal lesions improved macroscopic and histological structure of the lesion but not their mechanical properties.
Cultured cells require a suitable medium, a biomaterial to growth and develop. Generally they consist in porous materials, with holes of 100每200 米m of diameter and a three dimensional arrangement. These materials have the advantages of being easily adaptable in the defect, and injectable, avoiding more aggressive insertion techniques (78,79). In a future, it could be of interest to obtain a viscous or liquid material that solidifies at body temperature, or to change other environmental conditions, as the pH.
Future outlines in meniscal repair
An important feature managing growth factors is their placement on suitable carrier materials or biomaterials. It is not easy to find the appropriate material for the meniscus. Bio-degradable porous substances are recommended to keep the concentration of growth factors in the target place (39). It is difficult to obtain a combination of a certain material that is able to retain the growth factor and which simultaneously is degraded to allow revascularization. Degradation rate has to be compatible with formation rate. The mechanical integrity of the repair process cannot commit the elimination process of the material. These characteristics must be commensurate with the type of treated tissue.
To study the possibilities of gene transfer in meniscal allografts, Martinek et al. (80) made a meniscal replacement using a meniscal allograft previously treated ex vivo with a retrovirus. They analyzed the expression of the gene marker: lacZ. In the superficial layers of the meniscus remained the gene expression. In the deeper layers of the meniscus they found transduced cells at the junction of the meniscus with the transplanted synovial.
As it has been shown, the meniscus is a difficult structure to repair and replace. An injured or degenerative meniscus promotes osteoarthritic joint changes that should be avoided. Research focused on promoting healing or replacement must cover three different working lines: biology, mechanics and technique.
Biology research line looks for specific factors able to develop a collagen tissue in a matrix with cells that joins the edges of the lesion. Over time, the scar tissue in the defect would increase its stiffness as similar as a healthy meniscus. The biology line, also looks for factors able to keep the elasticity and able to regenerate the damaged meniscus fibres.
Mechanics research line looks for creating scaffolds with the adequate features to allow the penetration of vessels and cells. Other important features are viscoelasticity, an excess of fragility to avoid reabsorption, and an excess of toughness to avoid the damage of cartilage.
Finally, the technical research line looks for managing meniscal injuries by arthroscopy avoiding more aggressive techniques in the joint. Currently, arthroscopically therapies are focused on meniscal transplantation, scaffold implantation and placing healing substances in the damaged meniscus.
The development of these three research lines presents a challenge to the orthopaedic surgeons and scientists to decrease the number of degenerative processes in the joint for the coming years.﹛
The meniscal healing process
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3666493/﹛
Nutritional Properties of Cuttlebone for Pets
Cuttlefish bone, or cuttlebone, is the inner shell of the cuttlefish, a kind of invertebrate mollusk. Cuttlefish are the only cephalopods that have this kind of shell, as squids have a very different inner structure that resembles a long and very thin cartilage known as squid pen.
This shell is made of aragonite, and it is very appreciated in animal care thanks to its high calcium and mineral salt content. Cuttlebone has a slender and elegant shape that resembles a refined ship design, so it's very easy to recognize.
Keep reading this AnimalWised article and discover all about the nutritional properties of cuttlebone for pets, from birds to reptiles.
What nutrients does cuttlebone have?
Cuttlebone is made of aragonite, that is, crystallized calcium carbonate. This mineral has a beautiful lattice shape, which explains its buoyancy and absorbent power.
Apart from this huge amount of calcium carbonate, cuttlefish shell includes different essential trace elements - withvarying composition percentages - in its composition. Among the nutrients of cuttlebone you can also find calcium phosphate, It also contains calcium phosphate, sodium, magnesium, phosphorus and other mineral salts.
Why is cuttlefish bone good for birds?
Cuttlefish bone is often found in bird cages for birds to nibble on. Do you know why?
The reason why bird enthusiasts have cuttlebone inside their bird's cage is due to the large amount of natural calcium carbonate that makes up the shell. When a bird nibbles on it, its calcium intake is boosted. This is very important, as the diet of birds is naturally deficient in calcium.
Thanks to cuttlebone's highly absorbible natural calcium, their bones get stronger and regenerate. Birds also use cuttlebone to sharpen and wear down their beak, which stops the beak from growing excessively. Moreover, birds that peck at cuttlebone lay higher quality eggs at a faster rate than those birds that don't.
The trace elements also have important properties, and when birds molt their feathers the new ones will be healthier, stronger and more beautiful.
Why is cuttlebone good for turtles and tortoises?
The cuttlebone is a magnificent dietary supplement for turtles and tortoises, especially when they are growing. The composition of turtles' shell and bones requires an adequate supply of calcium, which the cuttlebone can provide.
In the wild, these reptiles naturally absorb calcium by consuming the shells of snails that they come across. Just like with birds, cuttlebone allows turtles and tortoises to sharpen their teeth and control their growth at the same time. The mineral salts provided by the cuttlebone are also good for their organism, so it's understandable why its multiple properties are so beneficial.
Cuttlebone can be placed inside the terrarium for a pet tortoise to peck at. For tortoises and turtles that do not live in terrariums, you can grate the cuttlebone onto its usual food.﹛
﹛
Nutritional Properties of Cuttlebone for Pets - AGROWASTE HX
http://agrowastehx.com/news/nutritional-properties-of-cuttlebone-for-pets/526.html﹛
﹛
Nutritional value of cuttlefish
Cuttlefish is a good source of nutrition when eaten in moderation. A 3-ounce serving of this mollusk provides you with 134 calories, 1 gram of carbohydrate, 28 grams of protein, and 1 gram of fat. They contain unsaturated fats, especially omega 每 fatty acids like eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both of which play a major role in reducing the risks of heart problems by lowering blood pressure, lowering triglyceride levels, preventing blood clots and blockage of arteries. Cuttlefish is a great source of many vitamins. It consists of many of the health benefitting vitamins like vitamin A, vitamin C, niacin, riboflavin, vitamin B6, folate and vitamin B12. It also provides you with significant amounts of essential minerals like calcium, magnesium, iron, phosphorus, potassium, sodium, zinc, copper, manganese and selenium. As mentioned earlier, cuttlefish is likely to have contaminant such as mercury and cadmium and hence it is recommended to limit their consumption to twice a week (5 ounce servings).
Cuttlebone and its uses
Cuttlebone is the hard and porous internal structure found in the cuttlefish, which is made of aragonite. It helps in controlling the buoyancy of cuttlefish in water. This structure is unique to cuttlefish, and is one of the features that differentiate them from other mollusks such as squid and octopus. The cuttlebone of each species of cuttlefish varies in size, shape, texture and pattern of ridges. It finds use in jewellery making as they can be used as casting moulds. The cuttlebone is also give to parakeets and other such birds as a dietary source of calcium. Since it has a grainy texture similar to pumice, it helps in wearing down the overgrown beaks of these birds.
Cuttlebone is considered to possess many medicinal properties and has been used in traditional Chinese medicine for treating many health problems including asthma, bleeding and perforation caused by ulcer and gastric and duodenal ulcer. Dentists use it to put an end to bleeding after a tooth is pulled out. Cuttlebone contains Calcium carbonate that can help in neutralizing stomach acid, reducing pepsin activity, changing the pH of the contents of your intestine and in promoting the healing of ulcers. Studies conducted on animals reveal that cuttlebone may help in repairing bone defects.﹛
Health benefits of cuttlefish | Value Food
http://www.valuefood.info/2023/health-benefits-of-cuttlefish/﹛
Chitin and Chitosan for Regenerative Medicine pp 61-82 | Cite as
Chitosan-Based Scaffolds for Cartilage Regeneration
Authors
Authors and affiliations
Xuezhou LiJianxun DingEmail authorXiuli ZhuangFei ChangJincheng WangEmail authorXuesi Chen
Abstract
Intra-joint trauma often accompanies cartilage damage, as one of the main reasons of osteoarthritis, which often induce severe pain and limited joint function in the final stage. Because of the poor regenerative capacity, cartilage repair has been on the top list of regenerative medicine from decades ago. Recently, the researches of cartilage regeneration are mainly focused on the development of novel scaffolds, which can provide spatial frame and logistic template for stem cells, other progenitor cells, or chondrocytes to proliferate or differentiate into cartilage-like tissues. Among the dazzling scaffolds, chitosan-based systems, including physical hydrogels, chemically cross-linked hydrogels, or porous scaffolds, show great potential in cartilage tissue regeneration. Chitosan possesses superior characteristics, such as biocompatibility, biodegradability, bioabsorbability, low immunogenicity, and intrinsic antibacterial nature, for potential applications in tissue engineering. Specially, the chemical structure of chitosan is similar with various glycosaminoglycans (GAGs), which play important roles in chondrocyte morphology modulation, differentiation, and function. In addition, appropriate mechanical properties and porosity, excellent cell adhesion, and even control release of functional growth factors are achieved in chitosan-based scaffolds. In this chapter, the advancements of different types of chitosan-based scaffolds for cartilage regeneration are systemically summarized, and the future directions are predicted.
Keywords
Cartilage regeneration Chitosan Chondrocytes Hydrogel Porous scaffolds Stem cells Three-dimensional﹛
Chitosan-Based Scaffolds for Cartilage Regeneration | SpringerLink
https://link.springer.com/chapter/10.1007/978-81-322-2511-9_3﹛
﹛
Chitosan: A promising polymer for cartilage repair and viscosupplementation
March 2017Bio-medical materials and engineering 28(s1):S209-S215
DOI: 10.3233/BME-171643University of Carlifornia, San Franscico
﹛
Osteoarthritis (OA) is a painful, degenerative and inflammatory disease that affects the entire synovial joints. Nowadays, no cure exists, and the pharmacological treatments are limited to symptoms alleviation. There is a need for a new efficient and safe treatment. Viscosupplementation is a process that aims to restore the normal rheological properties of synovial fluid. For the past years, hyaluronic acid was usually used but this molecule has some limitations including the short residency time in joint cavity.﹛
Recently, in vitro studies have suggested that chitosan could promote the expression of cartilage matrix components and reduce inflammatory and catabolic mediator's production by chondrocytes. In vivo, chitosan prevented cartilage degradation and synovial membrane inflammation in OA induced rabbit model. Several studies have also shown that chitosan could induce chondrogenic differentiation of mesenchymal stem cells. Therefore, chitosan is an interesting polymer to design scaffold and hydrogel for cartilage lesion repair, cells transplantation, sustained drug release and viscosupplementation.
Chitosan: A promising polymer for cartilage repair and viscosupplementation | Request PDF
https://www.researchgate.net/publication/315909818_Chitosan_A_promising_polymer_for_cartilage_repair_and_viscosupplementation﹛
﹛
Chitin and chitosan sources and reported uses.
﹛
﹛
﹛
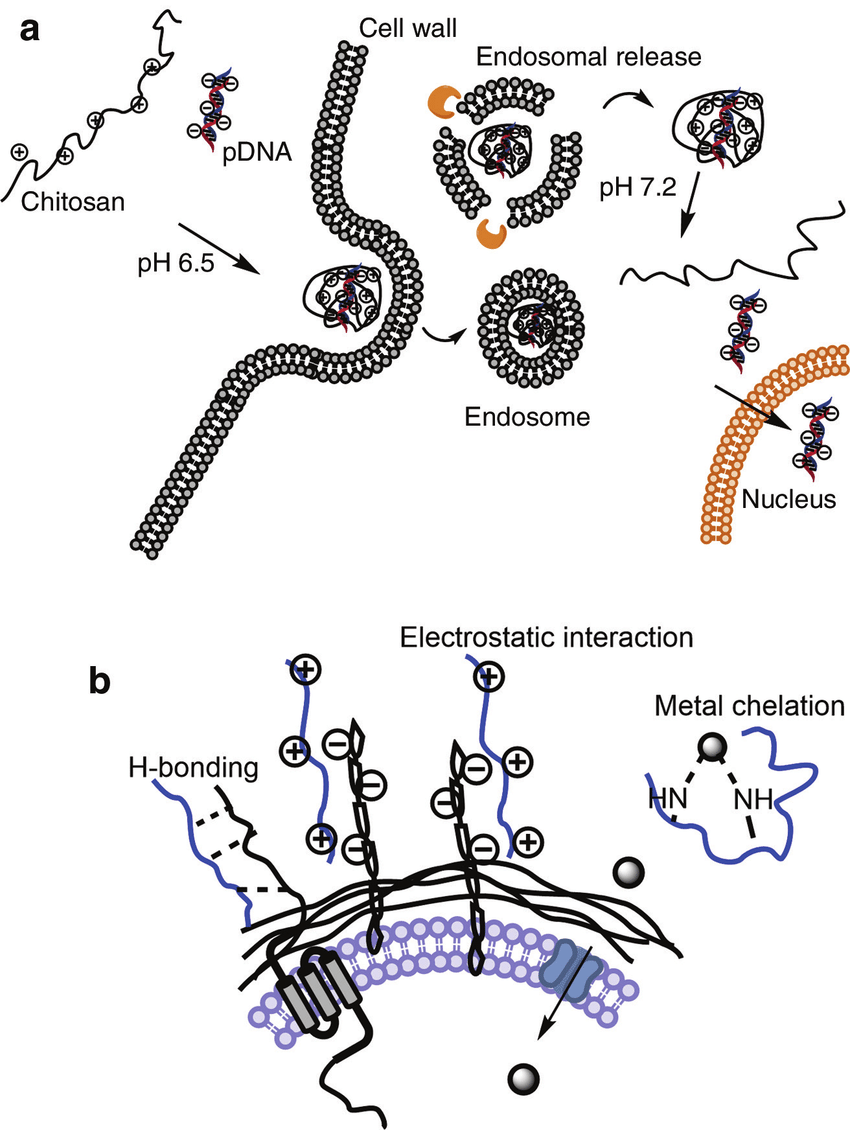
(a) Gene transfection model with chitosan as vector. (b) Antimicrobial mechanisms via H-bonding, electrostatic interaction and essential metal chelation.
﹛
﹛
https://www.researchgate.net/figure/Chitin-and-chitosan-sources-and-reported-uses_fig1_320845033
﹛
﹛
﹛
﹛
﹛
﹛
Chitin And Chitosan | Meron Biopolymers
https://www.meronbiopolymers.com/chitin-chitosan
CHITIN & CHITOSAN. Chitin is the second most abundant natural biopolymer in the world, behind only cellulose. It is also the most abundant naturally occurring polysaccharide that contains amino sugars. This abundance, combined with the specific chemistry of chitin and its derivative chitosan, make for the array of potential applications.
What is Chitin and Chitosan - Sinudom Agriculture Products ...
https://www.chitosanthai.com/en/what-is-chitin-and-chitosan
Apr 23, 2018 ﹞ What is Chitin and Chitosan Chitin/Chitosan is a substance that can be found from nature, especially in crustaceans like crab, prawn, and insects. Chitin/chitosan is a substance needed by cells of living things in addition to the five main food groups needed by the body, namely carbohydrate, protein, fat, vitamins, and minerals.
Chitin and chitosan: Commercial and biomedical ...
https://www.versiondaily.com/chitin-and-chitosan-commercial-and-biomedical-applications
Mar 27, 2017 ﹞ Chitin and chitosan are biological polymers with promising commercial and biomedical applications. Researchers have long explored the properties and derivative functions of these two polymers. Because of their expansive use, chitin and chitosan are poised to become one of the most important natural resources in the future.﹛
﹛
Biomol Ther (Seoul). 2013 May 30; 21(3): 246每250.
Chitin from the Extract of Cuttlebone Induces Acute Inflammation and Enhances MMP1 Expression
Ki Man Lee,1 Hong Shim,1 Geum Seon Lee,1 Il Ho Park,1 Ok Sang Lee,2 Sung Cil Lim,2,* and Tae Jin Kang1,*
1Institute of Chronic Diseases and College of Pharmacy, Sahmyook University, Seoul 139-742
2Department of Pharmacy, College of Pharmacy, Chungbuk National University, Cheongju 361-763, Republic of Korea
Abstract
We previously reported that the extract from cuttlebone (CB) has wound healing effect in burned lesion of rat.﹛
In present study, the main component of CB extract was analyzed and its wound healing activity was evaluated by using in vitro acute inflammation model.
﹛
The extract of CB stimulated macrophages to increase the production of TNF-汐. The extract also enhanced the production of TGF-汕 and VEGF, which were involved in angiogenesis and fibroblast activation.
﹛
The treatment with CB extract enhanced proliferation of murine fibroblast. CB extract also induced the activation of fibroblast to increase the secretion of matrix metalloproteases 1 (MMP1).
﹛
The constituent of CB extract which has wound healing activity was identified as chitin by HPLC analysis. The mechanism that the CB extract helps to promote healing of burned lesion is associated with that chitin in CB extracts stimulated wound skins to induce acute inflammation and to promoted cell proliferation and MMP expression in fibroblast.
﹛
Our results suggest that CB or chitin can be a new candidate material for the treatment of skin wound such as ulcer and burn.
﹛
INTRODUCTION
The wound healing consists of acute inflammation, angiogenesis, and re-epithelialization and a cellular, physiologic, and biochemical reaction initiated after the stimulus of injury to tissue (Singer and Clark, 1999). When a dermal wound is filled by a clot, inflammatory cells migrate to skin lesion and secret growth factors, such as transforming growth factor-汕 (TGF-汕) and vascular endothelial growth factor (VEGF), which stimulate fibroblasts from the adjacent dermis to migrate to the wounded site and involve in angiogenesis.
MMPs (matrix metalloproteases) are enzymes pivotal to the remodeling of the extracellular matrix (ECM) in a variety of physiological processes. MMPs are also involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling (Sternlicht and Werb, 2001). Among the MMPs, MMP-1 is the main collagenase secreted by fibroblasts and degrades the native collagens initiating tissue remodeling (Birkedal-Hansen et al., 1993).
Cuttlebone (CB), which is known as cuttlefish bone, has been traditionally used as a medicine for treating sore skin. Recent studies showed antibacterial activity (Shanmugam et al., 2008) and bone healing properties (Garc赤a-Enriquez et al., 2010) of CB. We, previously, demonstrated that the extract of CB showed the wound healing activity on skin ulcer lesion in burned rat (Jang et al., 2013). However, the component showing wound healing has not been elucidated yet. In this study, CB extract were evaluated for their wound healing activity by using in vitro acute inflammation model and its component was analyzed by HPLC. In addition, MMP-1 activity and mRNA expression in cells stimulated with the extract were investigated.﹛
The extract of CB increased production of TNF-汐, TGF-汕 and VEGF by macrophages
The cytotoxicity the CB extract used in this study was evaluated in RAW 264.7 cells and fibroblasts using MTT assay and trypan blue exclusion method, and there is no effect on cell viability at the concentrations used and at even higher dose, 200 米g/ml (Fig. 2).﹛
The mechanisms underlying wound healing processes involve the acute inflammatory mediators. The new formation of blood vessels (angiogenesis) is necessary and a vital component in wound healing. The migrating fibroblasts fill the wounded site and stimulate the formation of granulation tissue (Peppa et al., 2003; Folkman, 2007). TNF-汐 has a pivotal role in the activation of vascular endothelial cell, the induction of angiogenesis, and proliferation of fibroblast. TGF-汕 and VEGF are involved in fibroblast migration and angiogenesis, respectively. Therefore we first examined the in vitro mechanism of CB extract on wound healing and accessed whether the wound healing effect of CB extract is due to the activation of macrophages to produce cytokines, TGF-汕 and VEGF. RAW 264.7 cells were treated with CB extract and the levels of TNF-汐, TGF-汕 and VEGF were measured with the cell culture supernatant. The treatment with CB extract induced the activation of macrophages and increased the production of TNF-汐, TGF-汕 and VEGF in macrophages at dose dependent manner (Fig. 3). It seems that CB extract activates macrophages to induce acute inflammation for wound healing.
﹛
MMP-1 activity and expression
MMP-1 is secreted from fibroblast during inflammation for cell migration and wound healing. We, thus, examined whether CB extract induced MMP-1 activation in fibroblast. The MMP- 1 activity was increased after treatment of the CB compared to non-treated group (Fig. 5A). We then investigated whether the increased MMP-1 activity is caused by the induction of MMP-1 mRNA. The mRNA level of MMP-1 were determined by RT-PCR and increased slightly by treatment of the extract (Fig. 5B).﹛
DISCUSSION
Our study has a new finding that may have potential therapeutic implications. During research for novel topical drug for burn injury from natural products, we demonstrated that the CB extract has wound healing effect in burned animal model (Jang et al., 2013). However, it is unknown which constituents of CB extract show the healing in burn wounds. This study showed that the constituent of CB extract showing wound healing activity is identified as chitin by HPLC analysis (Fig. 1B). In addition, we found that CB extract induced acute inflammation. Our results suggest that the increased production of TNF-汐 , TGF-汕, and VEGF in the macrophages treated with CB extract (Fig. 4), through an unknown mechanism, is associated with wound healing effect. It is possible that the enhanced healing of wounds in burned animal by CB extract is a result of its inflammatory activity and its capacity to stimulate wound healing.
The important relationship of wound healing has been found to exist between macrophages and fibroblast (Raghow, 1994). TNF-汐 induced the activation of vascular endothelial cell and proliferation of fibroblast. We found that the CB extract increased the production of TNF-汕 in a dose-dependent manner. Furthermore, our results also demonstrated that the production of VEGF and TGF-汕 also increased in macrophages treated with CB extract, suggesting that VEGF and TGF-汕 accelerate wound healing (Fig. 4). VEGF is one of the most potent angiogenesis stimulating growth factors and functions as an inducer of vascular permeability and an endothelial cell mitogen (Ferrara, 1999; Yamagishi et al., 1999; Gavard and Gutkind, 2006). Several reports showed that VEGF increase re-epithelialization in wounded sites (Michaels et al., 2005; Saaristo et al., 2006; Li et al., 2007).
Wound healing involving a number of processes is an orderly but complex phenomenon. An essential feature of wound healing is re-epithelialization which depends on two basic functions of keratinocytes, proliferation and migration (Broughton et al., 2006). Re-epithelialization process is influenced by a combination of growth factors and cytokines including VEGF and TGF-汕 (Amendt et al., 2002). The initial immune response to burn injury is largely pro-inflammatory.
MMP-1 is one of the most abundant enzymes in the MMP family and tight regulation of MMP-1 secretion and activity is important for tissue development and homeostasis (Sternlicht and Werb, 2001). In this study we investigated the role of CB in the regulation of MMP-1 expression in fibroblasts. CB induced the expression of MMP1 gene and the secretion of MMP1 protein, which may play an important role in the regulation of acute inflammatory reaction in pathologic status such as burn.
Chitin activates macrophage by interacting with cell surface receptors such as mannose receptor and toll-like receptor-2 (Lee, 2009). Chitin-activated macrophage enhances the formation of tissue in the wound and the production of endothelial growth factor, consistent with our results (Ueno et al., 2001). Moreover, chitin is known to play an essential role in hemostasis (Valentine et al., 2010).
Our results suggest that CB or chitin can be a new candidate material for the treatment of skin wound such as ulcer and burn. Identifying the relation between each step involved in wound healing and CB was pivotal for better understanding of the molecular mechanism underlying the cellular response to CB treatment during wound healing. In future studies, we will investigate the role of CB or chitin in normal functions of the connective tissue as well as in angiogenesis.
Keywords: Cuttlebone, Chitin, Fibroblast, Matrix Metalloprotease, Cytokine﹛
Chitin from the Extract of Cuttlebone Induces Acute Inflammation and Enhances MMP1 Expression
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3830125/﹛
﹛
﹛