¡¡
1.GPR120 is one of the receptors of omega-3 fatty acids.
2. GPR120 is expressed in macrophages,Kupffer cells, hypothalamus and is induced in obesity.
3.GPR120 is differentially expressed along the length of mammalian intestine.
3.Omega-3 FAs Decrease M1 Proinflammatory Gene and Increase M2 Anti-inflammatory Gene Expression in Adipose Tissue.
¡¡
¡¡
GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects
Da YoungOh14SaswataTalukdar14Eun JuBae1TakeshiImamura2HidetakaMorinaga1WuQiangFan1PingpingLi1Wendell J.Lu1Steven M.Watkins3Jerrold M.Olefsky1
1
Department of Medicine, Division of Endocrinology and Metabolism, University of California, San Diego, La Jolla, CA 92093, USA
2
Division of Pharmacology, Shiga University of Medical Science, Tsukinowa, Seta, Otsu-city, Shiga, 520-2192 Japan
3
Tethys Bioscience, 3410 Industrial Boulevard, Suite 103, West Sacramento, CA 95691, USA
Summary
Omega-3 fatty acids (¦Ø-3 FAs), DHA and EPA, exert anti-inflammatory effects, but the mechanisms are poorly understood. Here, we show that the G protein-coupled receptor 120 (GPR120) functions as an ¦Ø-3 FA receptor/sensor. Stimulation of GPR120 with ¦Ø-3 FAs or a chemical agonist causes broad anti-inflammatory effects in monocytic RAW 264.7 cells and in primary intraperitoneal macrophages. All of these effects are abrogated by GPR120 knockdown. Since chronic macrophage-mediated tissue inflammation is a key mechanism for insulin resistance in obesity, we fed obese WT and GPR120 knockout mice a high-fat diet with or without ¦Ø-3 FA supplementation. The ¦Ø-3 FA treatment inhibited inflammation and enhanced systemic insulin sensitivity in WT mice, but was without effect in GPR120 knockout mice. In conclusion, GPR120 is a functional ¦Ø-3 FA receptor/sensor and mediates potent insulin sensitizing and antidiabetic effects in vivo by repressing macrophage-induced tissue inflammation.
► GPR120 is expressed in macrophages and Kupffer cells and is induced in obesity ► GPR120 functions as an omega 3 fatty acid (¦Ø-3 FA) receptor/sensor ► ¦Ø-3 FAs exert broad anti-inflammatory effects through GPR120 in macrophages ► GP120 mediates insulin sensitization by ¦Ø-3 FAs in obese mice
Ligand-Stimulated GPR120 Exerts Anti-inflammatory Effects It has been previously reported that GPR120 signals via a G¦Áq/11-coupled pathway and can respond to long chain FAs (Hirasawa et al., 2005). To pursue the biology of GPR120, a tool compound was needed, and, some years ago, Glaxo published GW9508 as a GPR40 selective agonist. However, this compound was not specific and also stimulated GPR120 (Briscoe et al., 2006). Since macrophages and adipocytes do not express GPR40 (this was confirmed by repeated q-PCR and RT-PCR measures, Figure S1A), GW9508 is a functional GPR120 specific compound in these cell types. Using this approach, we found that GW9508 treatment broadly and markedly repressed the ability of the TLR4 ligand LPS to stimulate inflammatory responses in RAW 264.7 cells (Figure 1D and E). Thus, GW9508 inhibited LPS stimulated phosphorylation of IKK¦Â and JNK, prevented I¦ÊB degradation, and inhibited TNF-¦Á and IL-6 secretion. All of these effects of GW9508 were completely abrogated by siRNA mediated knockdown of GPR120 (Figures 1D and 1E and Figure S1F).
¡¡
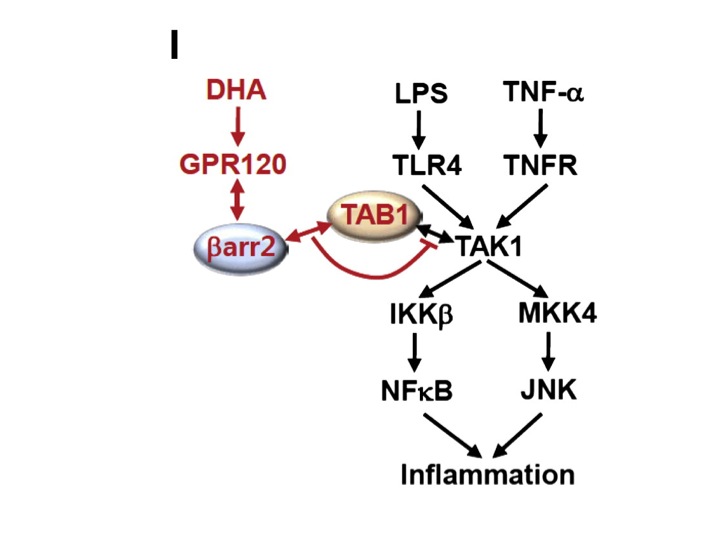
Schematic diagram of the ¦Â-arrestin2 and GPR120-mediated anti-inflammatory mechanism. Red colored letters and arrows indicate the DHA-mediated anti-inflammatory effect, and black colored letters and arrows indicate the LPS- and TNF-¦Á-induced inflammatory pathway.
¡¡Omega-3 FAs Reduce Inflammatory Macrophages in Adipose Tissue
The activation of GPR120 by ¦Ø-3 FAs, as well as its expression in adipocytes and macrophages, led us to study whether DHA, a representative ¦Ø-3 FA, can modulate inflammation through GPR120 in these cells. To examine this, we pretreated RAW 264.7 cells and 3T3-L1 adipocytes with GW9508 or DHA for 1 hr, followed by LPS (TLR4), TNF-¦Á, TLR2, or TLR3 stimulation, respectively. We found that GW9508 and, more importantly, DHA, strongly inhibited LPS-induced phosphorylation of JNK and IKK¦Â, I¦ÊB degradation, cytokine secretion and inflammatory gene expression level in RAW 264.7 cells (Figures 2B¨C2D) as well as TNF-¦Á, TLR2 and TLR3-induced JNK and IKK¦Â phosphorylation in 3T3-L1 adipocytes (Figure S2B) or RAW 264.7 cells (Figure S2C). All of the effects of GW9508 and DHA were completely prevented by GPR120 knockdown, demonstrating that these anti-inflammatory effects were specifically exerted through GPR120 (Figure 1, Figure 2, Figure S1, and Figure S2). Similar results were seen in primary wild-type (WT) intraperitoneal macrophages (IPMacs) and GPR120 knockout (KO) IPMacs (Figures 2E and 2F). These data argue that GPR120 is an ¦Ø-3 FA receptor or sensor, and provide a molecular mechanism for the anti-inflammatory effects of this class of FAs.
Omega-3 FAs Decrease M1 Proinflammatory Gene and Increase M2 Anti-inflammatory Gene Expression in Adipose Tissue
As shown in Figure 7A, expression of M1 inflammatory genes such as IL-6, TNF-¦Á, MCP-1, IL-1¦Â, iNOS, and CD11c was increased by HFD compared to chow diet in both genotypes, and was reduced in the ¦Ø-3 FA treated WT mice, but not in the GPR120 KO mice. Even on chow diet, expression of several inflammatory genes was higher in GPR120 KOs compared to WT, consistent with the insulin resistance observed in the chow-fed KO mice. Expression of the M2 anti-inflammatory genes, arginase 1, IL-10, MGL1, Ym-1, Clec7a, and MMR was increased by ¦Ø-3 FAs in WT, but not in the GPR120 KO adipose tissue (Figure 7B). These results are consistent with Figure 6 and demonstrate that the dietary change from HFD to ¦Ø-3 FA supplemented HFD resulted in an overall decreased proinflammatory profile in adipose tissue from WT, but not in GPR120 KO mice. These changes in gene expression were predominantly manifested in the SVF, except for MCP-1 and IL-6, which are known to be readily expressed in adipocytes (Figure S7). Qualitatively similar results were seen in the liver (Figures S6D and S6E).¡¡
Discussion
In this report we show that GPR120 functions as an ¦Ø-3 FA receptor/sensor in proinflammatory macrophages and mature adipocytes. By signaling through GPR120, DHA and EPA (the major natural ¦Ø-3 FA constituents of fish oil), mediate potent anti-inflammatory effects to inhibit both TLR and TNF-¦Á inflammatory signaling pathways. The mechanism of GPR120-mediated anti-inflammation involves inhibition of TAK1 through a ¦Â-arrestin2/TAB1 dependent effect. Since chronic tissue inflammation is an important mechanism causing insulin resistance (Xu et al., 2003, Shoelson et al., 2007, Schenk et al., 2008), the anti-inflammatory actions of ¦Ø-3 FAs exert potent insulin sensitizing effects. The in vivo anti-inflammatory and insulin sensitizing effects of ¦Ø-3 FAs are dependent on expression of GPR120, as demonstrated in studies of obese GPR120 KO animals and WT littermates. Thus, GPR120 is highly expressed in proinflammatory macrophages and functions as an ¦Ø-3 FA receptor, mediating the anti-inflammatory effects of this class of FAs to inhibit both the TLR2/3/4 and the TNF-¦Á response pathways and cause systemic insulin sensitization.Dietary DHA is rapidly esterified into chylomicrons during the process of gastrointestinal absorption, and is also packaged into VLDL triglycerides by the liver. DHA can also be esterified into phospholipids and cholesterol esters associated with circulating lipopoproteins and only a small proportion (∼5%) of total plasma DHA is found in the FFA pool. Through the action of lipoprotein lipase bound to the luminal surface of endothelial cells, ¦Ø-3 FAs are cleaved from circulating triglycerides where they can act as ligands or be taken up by peripheral tissues (Polozova and Salem Jr., 2007). Recent studies have also indicated that metabolic products derived from ¦Ø-3 FAs, such as 17S-hydroxy-DHA, resolvins, and protectins may play a role in the long term resolution of inflammation and this might attenuate insulin resistance in the context of obesity (Gonz¨¢lez-P¨¦riz et al., 2009). If this proves to be correct, then this could provide an additional mechanism for long term ¦Ø-3 FA-induced anti-inflammatory, insulin sensitizing effects. However, in the current studies, we found that these ¦Ø-3 FA derivatives were unable to stimulate GPR120 activation in our reporter cell assay (data not shown), indicating that GPR120 functions as a receptor for ¦Ø-3 FAs and not their biochemical products. Resolution of inflammation versus anti-inflammatory actions are distinct processes, and it is certainly possible that the products derived from ¦Ø-3 FA metabolism work on the former but not the latter.
In summary, we have found that GPR120 functions as an ¦Ø-3 FA receptor/sensor and mediates robust and broad anti-inflammatory effects, particularly in macrophages. After ligand stimulation, GPR120 couples to ¦Â-arrestin2 which is followed by receptor endocytosis and inhibition of TAB1-mediated activation of TAK1, providing a mechanism for inhibition of both the TLR and TNF-¦Á proinflammatory signaling pathways. Since chronic tissue inflammation is linked to insulin resistance in obesity, we used GPR120 KO mice to demonstrate that ¦Ø-3 FAs cause GPR120-mediated anti-inflammatory and insulin sensitizing effects in vivo. Overall, these results strongly argue that anti-inflammatory effects can ameliorate insulin resistance in obesity. Taken together, GPR120 emerges as an important control point in the integration of anti-inflammatory and insulin sensitizing responses, which may prove useful in the future development of new therapeutic approaches for the treatment of insulin resistant diseases.GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0092867410008883¡¡
Potential roles of GPR120 and its agonists in the management of diabetes
Dan Zhang, Po Sing Leung
School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
Abstract: Free fatty acids (FFAs) serve not only as nutrients that provide energy but also as extracellular signaling molecules that manipulate intracellular physiological events through FFA receptors (FFARs) such as FFAR4. FFAR4 is also known as G-protein coupled receptor 120 (GPR120). The main role of GPR120 is to elicit FFA regulation on metabolism homeostasis. GPR120 agonism correlates with prevention of the occurrence and development of metabolic disorders such as obesity and diabetes. GPR120 activation directly or indirectly inhibits inflammation, modulates hormone secretion from the gastrointestinal tract and pancreas, and regulates lipid and/or glucose metabolism in adipose, liver, and muscle tissues, which may help prevent obesity and diabetes. This review summarizes recent advances in physiological roles of GPR120 in preventing insulin resistance and protecting pancreatic islet function, and examines how resident GPR120 in the pancreas may be involved in modulating pancreatic islet function.¡¡
Distribution
Expression of GPR120 differs between species (Table 1). In the rat, the highest expression of GPR120 was found in the colon, whereas in mouse and human, the highest GPR120 expression is found in the lung.11,13 Beyond the lung, GPR120 is expressed mainly in tissues and cells related to energy metabolism, such as the gastrointestinal tract, tongue, and adipose tissue. Various macrophages also express high levels of GPR120.GPR120 expression is also identified in macrophages, such as mouse bone marrow-derived macrophages and intraperitoneal macrophages.3 In liver, GPR120 expression is limited to resident macrophages (Kupffer cells [KCs]) and its expression can be increased significantly in macrophages of adipose and liver by HFD-feeding in mice.3,9 Table 1 summarizes the tissue distribution of GPR120 in various species.
Keywords: fatty acid, G protein-coupled receptors, inflammation, pancreas, obesity
[Full text] Potential roles of GPR120 and its agonists in the management of diabet | DDDT
https://www.dovepress.com/potential-roles-of-gpr120-and-its-agonists-in-the-management-of-diabet-peer-reviewed-fulltext-article-DDDT
¡¡Free Fatty Acid Receptors pp 221-231 | Cite as
Anti-Inflammatory and Insulin-Sensitizing Effects of Free Fatty Acid Receptors
Junki Miyamoto1,Mayu Kasubuchi1, Akira Nakajima1, Ikuo Kimura
1.Department of Applied Biological Science, Graduate School of AgricultureTokyo University of Agriculture and TechnologyFuchu-shiJapan
Part of the Handbook of Experimental Pharmacology book series (HEP, volume 236)
Abstract
Chronic low-grade inflammation in macrophages and adipose tissues can promote the development of obesity and type 2 diabetes. Free fatty acids (FFAs) have important roles in various tissues, acting as both essential energy sources and signaling molecules. FFA receptors (FFARs) can modulate inflammation in various types of cells and tissues; however the underlying mechanisms mediating these effects are unclear. FFARs are activated by specific FFAs; for example, GPR40 and GPR120 are activated by medium and long chain FFAs, GPR41 and GPR43 are activated by short chain FFAs, and GPR84 is activated by medium-chain FFAs. To date, a number of studies associated with the physiological functions of G protein-coupled receptors (GPCRs) have reported that these GPCRs are expressed in various tissues and involved in inflammatory and metabolic responses. Thus, the development of selective agonists or antagonists for various GPCRs may facilitate the establishment of novel therapies for the treatment of various diseases. In this review, we summarize current literature describing the potential of GPCRs as therapeutic targets for inflammatory and metabolic disorders.
Keywords
Anti-inflammation GPCRs Insulin sensitivityAnti-Inflammatory and Insulin-Sensitizing Effects of Free Fatty Acid Receptors | SpringerLink
https://link.springer.com/chapter/10.1007%2F164_2016_47¡¡
Cell Biol Int. 2019 Jul 19. doi: 10.1002/cbin.11204. [Epub ahead of print]
Lipopolysaccharide inhibits GPR120 expression in macrophages via Toll-like receptor 4 and p38 MAPK activation.
Zhao YY1, Fu H1, Liang XY1, Zhang BL1, Wei LL1, Zhu JX1, Chen MW2, Zhao YF1.
Author information
1
Institute of Basic Medical Sciences, Xi'an Medical University, Xi'an, 710021, China.
2
Shaanxi Provincial Research Center for Prevention and Treatment of Respiratory Diseases, Xi'an Medical University, Xi'an, 710021, China.
Abstract
Free fatty acid receptor G protein-coupled receptor 120 (GPR120) is highly expressed in macrophages and was reported to inhibit lipopolysaccharide (LPS)-stimulated cytokine expression. Under inflammation, macrophages exhibit striking functional changes, but changes in GPR120 expression and signaling are not known.In this study, the effects of LPS treatment on macrophage GPR120 expression and activation were investigated. The results showed that LPS inhibited GPR120 expression in mouse macrophage cell line Ana-1 cells. Moreover, LPS treatment inhibited GPR120 expression in mouse alveolar macrophages both in vitro and in vivo.
The inhibitory effect of LPS on GPR120 expression was blocked by Toll-like receptor 4 (TLR4) inhibitor TAK242 and p38 mitogen-activated protein kinase inhibitor LY222820, but not by ERK1/2 inhibitor U0126 and c-Jun N-terminal kinase inhibitor SP600125. LPS-induced inhibition of GPR120 expression was not attenuated by GPR120 agonists TUG891 and GW9508. TUG891 inhibited the phagocytosis of alveolar macrophages, and LPS treatment counteracted the effects of TUG891 on phagocytosis. These results indicate that pretreatment with LPS inhibits GPR120 expression and activation in macrophages.
It is suggested that LPS-induced inhibition of GPR120 expression is a reaction enhancing the LPS-induced pro-inflammatory response of macrophages.
© 2019 International Federation for Cell Biology.Lipopolysaccharide inhibits GPR120 expression in macrophages via Toll-like receptor 4 and p38 MAPK activation. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/31322778¡¡
Published: 26 December 2016
Ginsenoside Rb2 enhances the anti-inflammatory effect of ¦Ø-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression
Qi Huang, Ting Wang & He-yao Wang
Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 201203, Shanghai, China
University of Chinese Academy of Sciences, 100049, Beijing, China
Abstract
Recent studies confirm that chronic low-grade inflammation is closely associated with metabolic syndromes, and anti-inflammatory therapy is a potential approach for treating cardiovascular diseases and type 2 diabetes. Accumulating evidence suggests that GPR120 activation is a feasible solution to ameliorating chronic inflammation and improving glucose metabolism. In this study we investigated whether ginsenoside Rb2 (Rb2), which exhibited regulatory activities in glucose and lipid metabolism, affected GPR120 expression in lipopolysaccharide (LPS)-activated mouse macrophage RAW264.7 cells, and examined the contribution of GPR120 activation to reducing the LPS-induced inflammatory response.LPS (100 ng/mL) activated the macrophages, resulting in dramatic increases in TNF-¦Á, IL-6, IL-1¦Â and NO production. Treatment with a ¦Ø-3 fatty acid ¦Á-linolenic acid (ALA, 50 ¦Ìmol/L) produced moderate reduction in LPS-stimulated inflammatory cytokines and NO production (TNF-¦Á and IL-6 were decreased by 46% and 42%, respectively). Pre-incubation with Rb2 (1 or 10 ¦Ìmol/L) for 12 h before ALA treatment dramatically amplified the inhibitory effects of ALA (TNF-¦Á and IL-6 were decreased by 74% and 86%, respectively). Compared to the treatment with ALA alone, pre-incubation with Rb2 resulted in a more prominent reduction in LPS-stimulated expression of iNOS and COX-2 and LPS-stimulated IKK/NF-¦ÊB phosphorylation and MAPK pathway activation. Rb2 (0.1¨C100 ¦Ìmol/L) dose- and time-dependently increased both mRNA and protein expression of GPR120 in RAW264.7 cells, but treatment with Rb2 alone did not exert anti-inflammatory effect in LPS-activated RAW264.7 cells.
In RAW264.7 cells transfected with GPR120 shRNA, the ameliorating effects of Rb2 on LPS-induced inflammation were abolished. In conclusion, Rb2 exerts anti-inflammatory effect in LPS-stimulated mouse macrophage RAW264.7 cells in vitro by increasing GPR120 expression and subsequently enhancing ¦Ø-3 fatty acid-induced GPR120 activation.
Ginsenoside Rb2 enhances the anti-inflammatory effect of ¦Ø-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression | Acta Pharmacologica Sinica
https://www.nature.com/articles/aps2016135¡¡
Prostaglandins Leukot Essent Fatty Acids. 2015 Jun; 97: 27¨C34.
Published online 2015 Apr 11. doi: 10.1016/j.plefa.2015.03.002
Docosahexaenoic acid differentially affects TNF¦Á and IL-6 expression in LPS-stimulated RAW 264.7 murine macrophages
Kaori L. Honda, Stefania Lamon-Fava, Nirupa R. Matthan, Dayong Wu, and Alice H. Lichtenstein*
Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111, USA
Docosahexaenoic acid (DHA) is generally reported to have anti-inflammatory properties, however, prior work has documented differential effects on individual pro-inflammatory cytokines: reduced IL-6, but not TNF¦Á, mRNA expression in macrophages. To elucidate the mechanism, the roles of prostaglandin E2 (PGE2), cyclic AMP response element-binding protein (CREB), and NF¦ÊB were examined in RAW 264.7 macrophages. DHA did not influence CREB activity, but significantly reduced PGE2 production by 41% and NF¦ÊB activity by 32%. Exogenous PGE2 inhibited TNF¦Á mRNA expression dose dependently. Unexpectedly, inhibiting PGE2 production with NS-398 also decreased TNF¦Á mRNA expression, suggesting a concentration-dependent dual role of PGE2 in regulating TNF¦Á expression. IL-6 expression was unaffected by endogenous or exogenous PGE2. Partial block of NF¦ÊB activation (SN50; 46%, or, BAY-11-7082; 41%) lowered IL-6 to a greater extent than TNF¦Á mRNA expression. The differential effect of DHA on TNF¦Á and IL-6 mRNA expression may be mediated via reduction in NF¦ÊB activity.
Keywords: TNF¦Á, IL-6, TLR4, Macrophages, PGE2, CREB
Docosahexaenoic acid differentially affects TNF¦Á and IL-6 expression in LPS-stimulated RAW 264.7 murine macrophages
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4562472/¡¡
PLoS One. 2012; 7(1): e30571.
Unsaturated Fatty Acids Revert Diet-Induced Hypothalamic Inflammation in Obesity
Dennys E. Cintra, 1 , 3 Eduardo R. Ropelle, 2 Juliana C. Moraes, 1 Jos¨¦ R. Pauli, 2 Joseane Morari, 1 Claudio T. de Souza, 1 Renato Grimaldi, 4 Marcela Stahl, 4 Jos¨¦ B. Carvalheira, 2 Mario J. Saad, 2 and Licio A. Velloso 1 , 2 , *
1 Laboratory of Cell Signaling, University of Campinas, Campinas, Brazil,
BackgroundDefective hypothalamic activity plays an important role in the development of obesity [1], [2], [3]. A number of recent studies have shown that in both diet-induced and genetically-determined animal models of obesity, inflammation of the hypothalamus is an important mechanism leading to the anomalous control of caloric intake and energy expenditure [4], [5], [6], [7], [8], [9]. Saturated fatty acids, highly consumed in western diets, induce hypothalamic inflammation by activating signal transduction though TLR4, which leads to endoplasmic reticulum stress, in situ expression of inflammatory cytokines and eventually, apoptosis of neurons, all contributing to the development of adipostatic hormone resistance and anomalous expression of the neurotransmitters involved in the regulation of energy homeostasis [5], [6].
In experimental models, hypothalamic inflammation is an early and determining factor in the installation and progression of obesity. Pharmacological and gene-based approaches have proven efficient in restraining inflammation and correcting the obese phenotypes. However, the role of nutrients in the modulation of hypothalamic inflammation is unknown.
Methodology/Principal Findings
Here we show that, in a mouse model of diet-induced obesity, partial substitution of the fatty acid component of the diet by flax seed oil (rich in C18:3) or olive oil (rich in C18:1) corrects hypothalamic inflammation, hypothalamic and whole body insulin resistance, and body adiposity. In addition, upon icv injection in obese rats, both ¦Ø3 and ¦Ø9 pure fatty acids reduce spontaneous food intake and body mass gain. These effects are accompanied by the reversal of functional and molecular hypothalamic resistance to leptin/insulin and increased POMC and CART expressions. In addition, both, ¦Ø3 and ¦Ø9 fatty acids inhibit the AMPK/ACC pathway and increase CPT1 and SCD1 expression in the hypothalamus. Finally, acute hypothalamic injection of ¦Ø3 and ¦Ø9 fatty acids activate signal transduction through the recently identified GPR120 unsaturated fatty acid receptor.
Conclusions/Significance
Unsaturated fatty acids can act either as nutrients or directly in the hypothalamus, reverting diet-induced inflammation and reducing body adiposity. These data show that, in addition to pharmacological and genetic approaches, nutrients can also be attractive candidates for controlling hypothalamic inflammation in obesity.¡¡
Unsaturated Fatty Acids Revert Diet-Induced Hypothalamic Inflammation in Obesity
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3261210/¡¡