五倍子没食子酸 a Universal Antioxidant and Anti-cancer
gallic acid and quercetin are effective inhibitors of LDH that exert strong antiproliferative activity in breast cancer cells
propyl gallate induces apoptosis in Hela Cells via activating of caspases
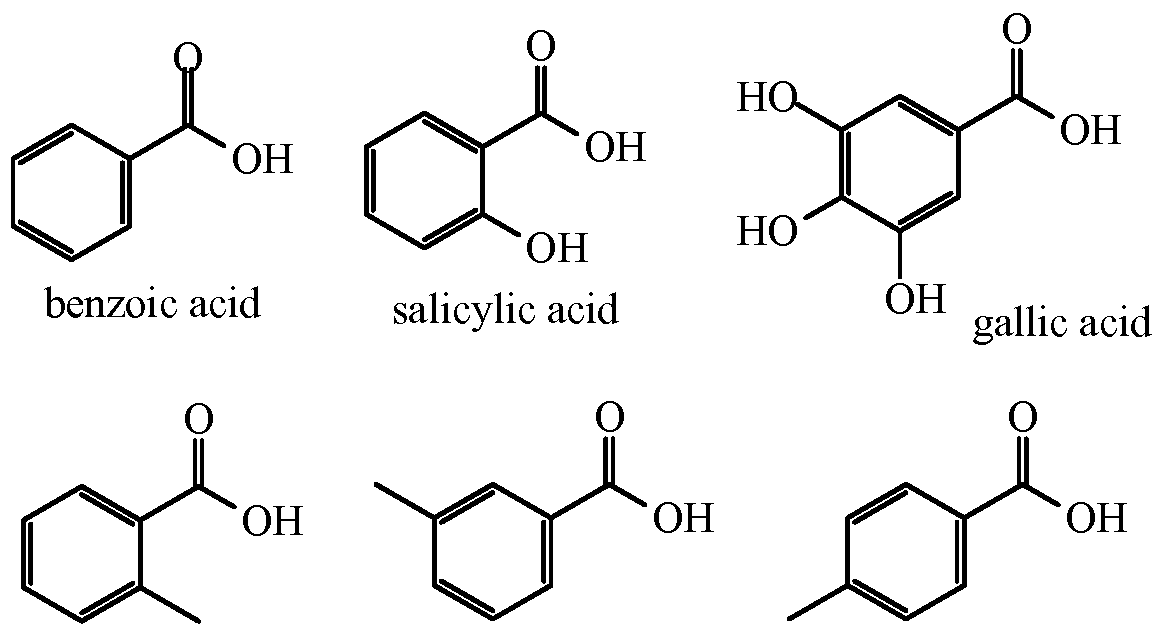
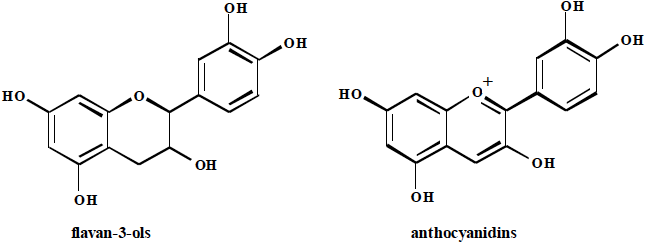
Pharmacognosy 2 | Digitális Tankönyvtár
https://www.tankonyvtar.hu/hu/tartalom/tamop412A/2011-0016_08_pharmacognosy_2/ch14.html
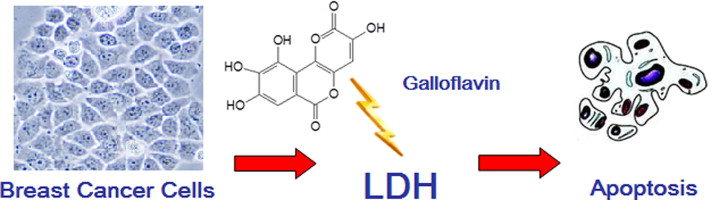
Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0928098712003235
Computational Chemistry Laboratory, Department of Chemistry, University of Delhi, Delhi-110007, India
Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications - RSC Advances (RSC Publishing) DOI:10.1039/C5RA01911G
https://pubs.rsc.org/en/content/articlehtml/2015/ra/c5ra01911g
Nanoscale Metal–Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy
Immunotherapy has become a promising cancer therapy, but only works for a subset of cancer patients. Immunogenic photodynamic therapy (PDT) can prime cancer immunotherapy to increase the response rates, but its efficacy is severely limited by tumor hypoxia. Here we report a nanoscale metal–organic framework, Fe-TBP, as a novel nanophotosensitizer to overcome tumor hypoxia and sensitize effective PDT, priming non-inflamed tumors for cancer immunotherapy. Fe-TBP was built from iron-oxo clusters and porphyrin ligands and sensitized PDT under both normoxic and hypoxic conditions. Fe-TBP mediated PDT significantly improved the efficacy of anti-programmed death-ligand 1 (α-PD-L1) treatment and elicited abscopal effects in a mouse model of colorectal cancer, resulting in >90% regression of tumors. Mechanistic studies revealed that Fe-TBP mediated PDT induced significant tumor infiltration of cytotoxic T cells.
Nanoscale Metal–Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy | Journal of the American Chemical Society
https://pubs.acs.org/doi/10.1021/jacs.8b01072
Copper-Gallic Acid Nanoscale Metal–Organic Framework for Combined Drug Delivery and Photodynamic Therapy
In this paper, we report the synthesis of bioactive copper-gallic acid nanoscale metal–organic framework for the codelivery of anticancer agent (gallic acid) and photosensitizer (methylene blue) to cancer cells. A supramolecular coordination complex of copper-bioactive frameworks (bio MOFs) were employed as the carrier of two anticancer agents. The first one is the natural phenolic acid (gallic acid), which forms a part of the framework structure (building block). The other one is the photosensitizer methylene blue, loaded as a guest molecule within the amphiphilic pores of the framework. In vitro cytotoxicity and in vivo tumor regression assays revealed enhanced cytotoxicity of dual drug nanoframework when compared with the equivalent dosages of free drugs in the presence of light.
Copper-Gallic Acid Nanoscale Metal–Organic Framework for Combined Drug Delivery and Photodynamic Therapy | ACS Applied Bio Materials
https://pubs.acs.org/doi/10.1021/acsabm.9b00116
US PATENT
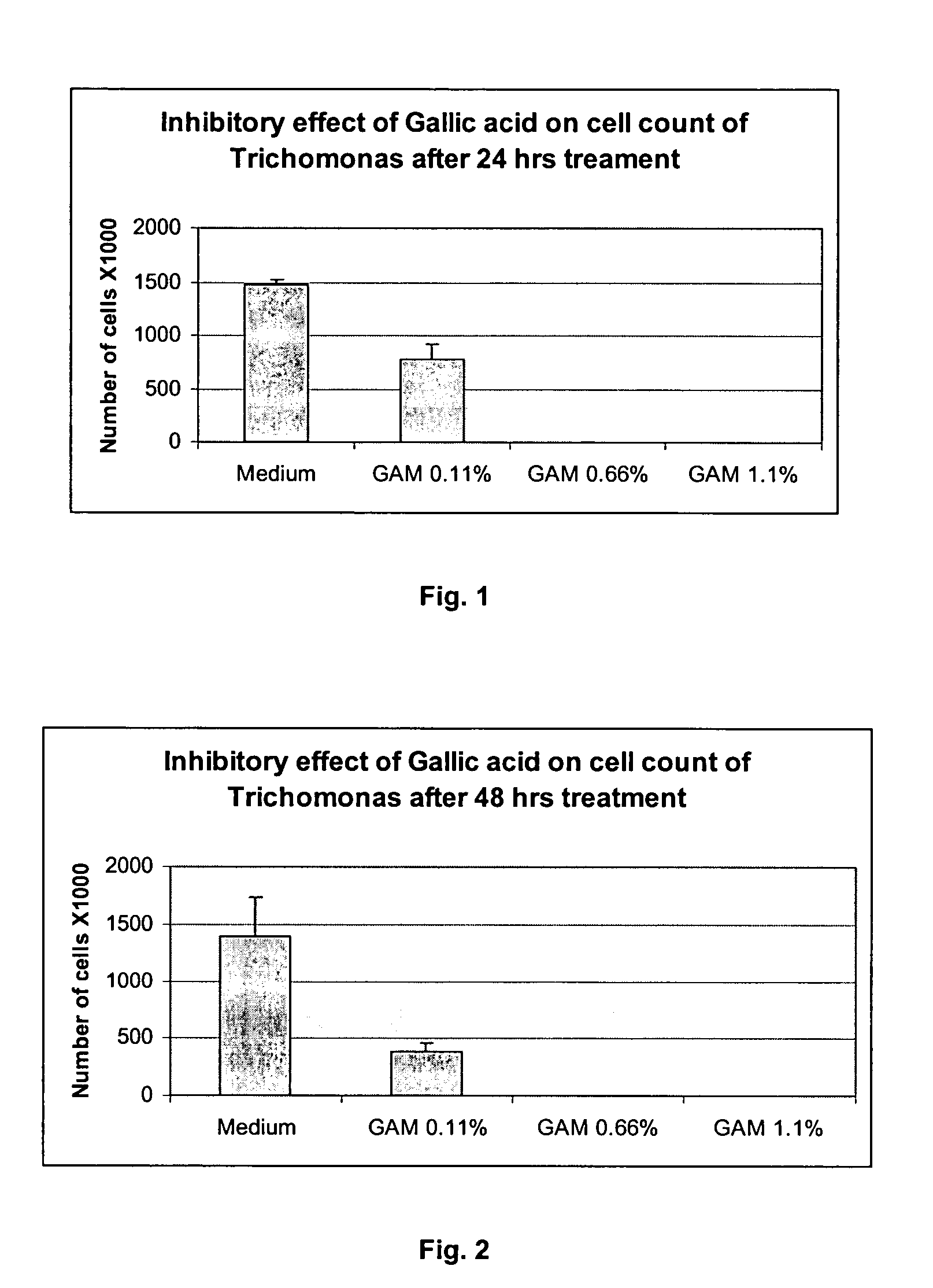
Method for preventing and/or treating vaginal and vulval infections
Abstract
The invention provides a method for treating and/or preventing a wide scope of vaginal and vulval infections, such as those caused by bacteria, parasites or yeasts, by administering gallic acid to a subject in need of treatment. A composition containing gallic acid for treating vaginal infections is also disclosed. It has been found that gallic acid is capable of selectively inhibiting the growth of Trichomonas vaginalis, a parasite that causes trichomonas vaginitis; Gardnerella vaginalis, a bacterium that causes bacterial vaginosis; and Candida albicans, a yeast that causes candidiasis and vulvitis; while not inhibiting the growth of Lactobacillus acidophilus, the dominant bacteria in a healthy vaginal ecosystem. Gallic acid is safe and cost-effective, and can be used alone or incorporated into different vaginal health products to treat and/or prevent vaginal infections.
US20060217443A1 - Method for preventing and/or treating vaginal and vulval infections - Google Patents
https://patents.google.com/patent/US20060217443
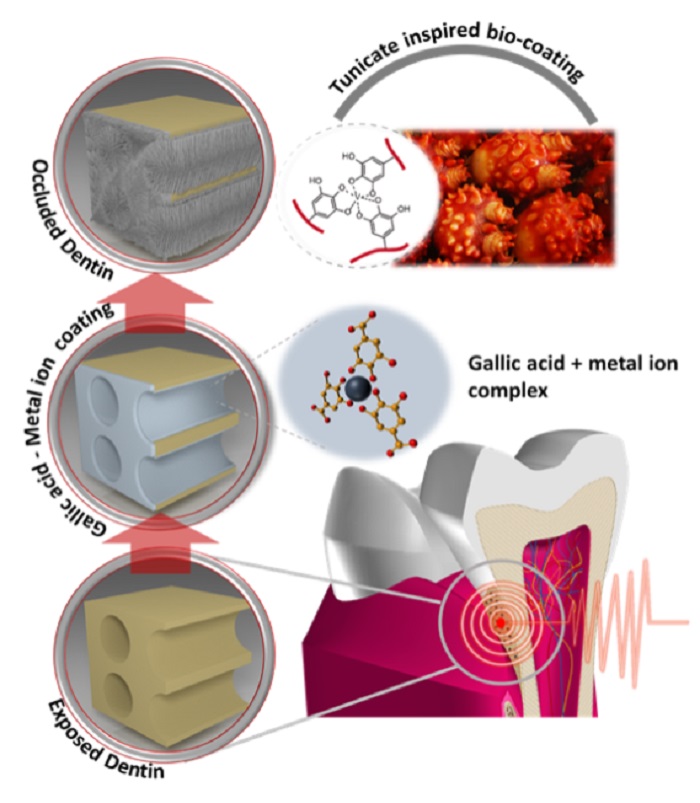
Sea squirts emerge as good dental solution
Feb 19, 2016
When your teeth smart, you can now rely on sea squirts to help ease the pain.
The Ministry of Oceans and Fisheries has found a solution in the gallic acid secreted from the marine invertebrate as a way to cure toothaches, a first-of-its-kind in the world.
Tongyeongsi_20141124_Article_04.jpg
The gallic acid from sea squirts can be effective against toothaches.
In the water, with a strong current and full of algae, sea squirts can survive even when their tissue has been cut or wounded. This is because of the gallic acid they secrete, a chemical compound that helps them heal cuts and wounds in just a couple of hours.
Taking advantage of this same principle, a recent study found that combining gallic acid from the sea squirt with metal ions could provide a good cure to toothaches. Results found that a coating could be administered to the painful teeth and that the pain would be eased in just a few minutes.
This new treatment applies a coating over the dentin that causes the pain, blocking any nerve stimulus. It also boosts teeth health by combining with the calcium and the saliva and then producing a bony component that helps to bring damaged teeth back to health.
Dentists currently rely on two treatments: toothpastes with potassium ion and a method whereby they apply a tooth-colored resin over the lower section of the teeth so that it can’t be exposed to external stimuli. However, these two methods have their own downsides. The potassium-ion toothpaste can ease the pain only temporarily, while the resin coating is likely to be rubbed off when eating or brushing your teeth.
Prof. Hwang Dong-su from Pohang University of Science and Technology, who led the research team, said, “Gallic acid can be found easily, not only in sea squirts, but also in tree bark and herbs, too. This new cure can be a real money-saver in terms of medical expenses and it can be safe to swallow, as well.”
By Sohn JiAe
Korea.net Staff Writer
Photos: Jeon Han, the Ministry of Oceans and Fisheries
jiae5853@korea.kr
When an exposed dentin causes pain, a new treatment uses gallic acid to coat the exposed surface, relieving the toothache.
Sea squirts emerge as good dental solution : Korea.net : The official website of the Republic of Korea
http://www.korea.net/NewsFocus/Sci-Tech/view?articleId=132794
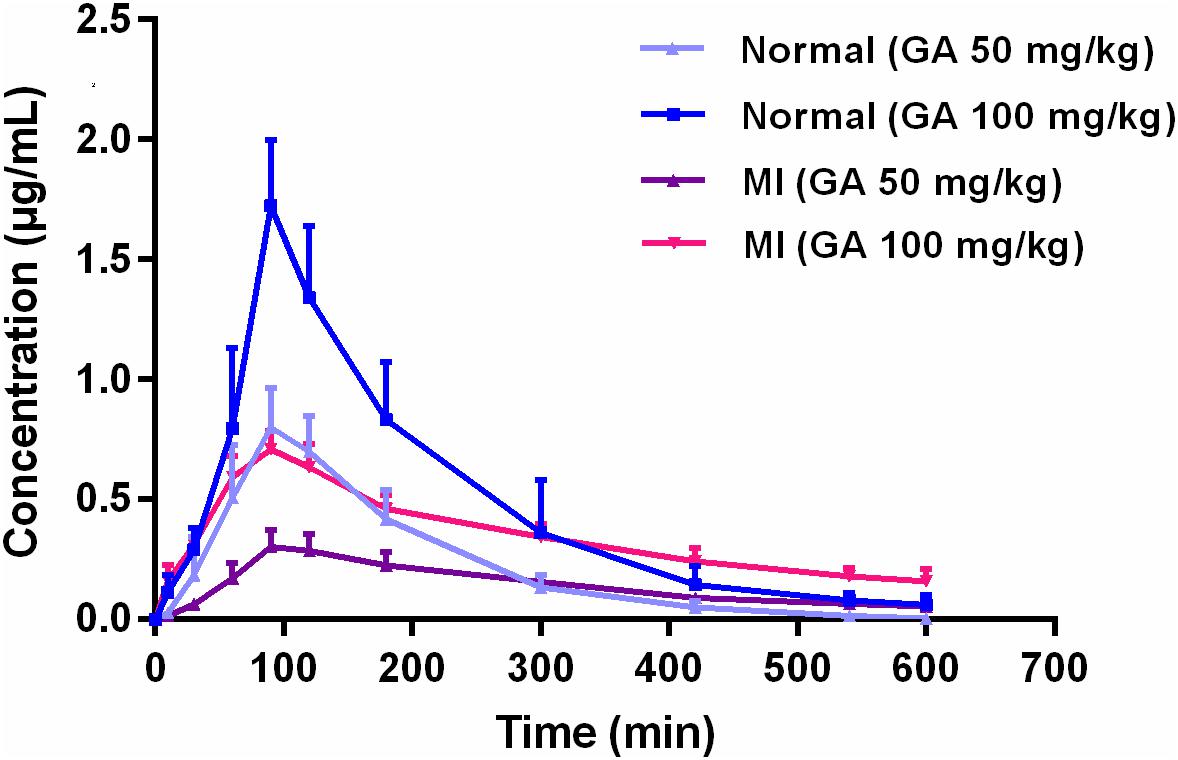
Comparative Pharmacokinetics of Gallic Acid After Oral Administration of Gallic Acid Monohydrate in Normal and Isoproterenol-Induced Myocardial Infarcted Rats
Zhe Yu1†, Fan Song2†, Yu-Chen Jin2,3,4†, Wei-Min Zhang2,3†, Ya Zhang2, En-Jun Liu2, Dan Zhou2,5, Lin-Lin Bi2, Qian Yang2, Hua Li2*, Bang-Le Zhang4* and Si-Wang Wang2*
1Department of Pharmaceutical Analysis, School of Pharmacy, Fourth Military Medical University, Xi’an, China
2Department of Natural Medicine, School of Pharmacy, Fourth Military Medical University, Xi’an, China
3Cadet Brigade, Fourth Military Medical University, Xi’an, China
4Department of Pharmaceutics, School of Pharmacy, Fourth Military Medical University, Xi’an, China
5Department of Pharmacy, Ninth Hospital of Xi’an, Xi’an, China
Gallic acid (GA) is a polyphenolic natural product widely distributed in food, beverage, and traditional Chinese herbs with beneficial effects on the cardiovascular system. In this research, a comparative study was conducted to investigate the possible difference of pharmacokinetic process in normal and isoproterenol-induced myocardial infarcted rats after oral administration of GA monohydrate with the dose of 50 and 100 mg/kg, respectively. Quantification of GA in rat plasma was achieved by using a simple and rapid high-performance liquid chromatographic method. The results revealed that pharmacokinetics of GA were greatly different between normal and pathological state. GA exhibited slower absorption into the bloodstream, and yielded 1.7-fold (50 mg/kg GA) and 1.3-fold (100 mg/kg GA) less values of area under concentration–time curve as well as 2.5-fold lower of maximum blood concentration (Cmax) in MI rats than those in normal rats. In addition, significant prolonged T1/2 and MRT as well as decreased CL were also registered in MI rats. Our findings suggest that myocardial infarction could alter the pharmacokinetic process of GA, and thus the potential pharmacokinetic differences of herbal preparations (or dietary nutrition) containing GA between normal and pathological conditions should be brought to the forefront seriously in clinical practice.
Frontiers | Comparative Pharmacokinetics of Gallic Acid After Oral Administration of Gallic Acid Monohydrate in Normal and Isoproterenol-Induced Myocardial Infarcted Rats | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2018.00328/full
Pharmacognosy 2 | Digitális Tankönyvtár
https://www.tankonyvtar.hu/hu/tartalom/tamop412A/2011-0016_08_pharmacognosy_2/ch14.html
Hydrophilic Graphene Preparation from Gallic Acid Modified Graphene Oxide in Magnesium Self-Propagating High Temperature Synthesis Process | Scientific Reports
https://www.nature.com/articles/srep35184
Free radicals produced by the oxidation of gallic acid: An electron paramagnetic resonance studyBackground
Gallic acid (3,4,5-trihydroxybenzoic acid), found in a variety of plants, is extensively used in tanning, ink dyes, as well as in the manufacturing of paper [1]. In addition, the gallate moiety is a key component of many foods and drinks, e.g. there are two gallate moieties in the important polyphenol, (-)-epi-gallocatechin-3-gallate (EGCG); this and related polyphenols are responsible for the antioxidant, anticarcinogenic, and antiviral properties of some of the most widely consumed beverages in the world, such as green tea [2,3]. The three aromatic phenoxyl groups of gallic acid are prone to oxidation with the formation of hydrogen peroxide, quinones, and semiquinones [4]. We have observed the formation of two distinct semiquinones formed upon the oxidation of gallic acid. Here we have investigated the nature of these two different radicals.
1Free Radical and Radiation Biology, Med Labs B180, The University of Iowa, Iowa City, IA 52242-1181, USA
2Department of Applied Radiation and Isotopes, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand
corresponding authorCorresponding author.
Background
Gallic acid (3,4,5-trihydroxybenzoic acid) is found in a wide variety of plants; it is extensively used in tanning, ink dyes, as well as in the manufacturing of paper. The gallate moiety is a key component of many functional phytochemicals. In this work electron paramagnetic spectroscopy (EPR) was used to detect the free radicals generated by the air-oxidation of gallic acid.
Results
We found that gallic acid produces two different radicals as a function of pH. In the pH range between 7-10, the spectrum of the gallate free radical is a doublet of triplets (aH = 1.00 G, aH = 0.23 G, aH = 0.28 G). This is consistent with three hydrogens providing hyperfine splitting. However, in a more alkaline environment, pH >10, the hyperfine splitting pattern transforms into a 1:2:1 pattern (aH (2) = 1.07 G). Using D2O as a solvent, we demonstrate that the third hydrogen (i.e. aH = 0.28 G) at lower pH is a slowly exchanging hydron, participating in hydrogen bonding with two oxygens in ortho position on the gallate ring. The pKa of this proton has been determined to be 10.
Conclusions
This simple and novel approach permitted the understanding of the prototropic equilibrium of the semiquinone radicals generated by gallic acid, a ubiquitous compound, allowing new insights into its oxidation and subsequent reactions.
Free radicals produced by the oxidation of gallic acid: An electron paramagnetic resonance study
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2924338/
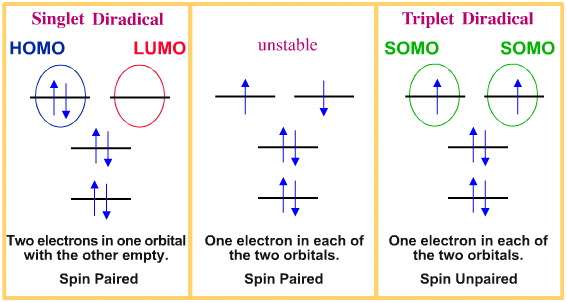
Diradical Chemistry | Chemogenesis
https://www.meta-synthesis.com/webbook/16_diradical/diradical.html
Mechanism of Photosensitized Generation of Singlet Oxygen ...
https://pubs.acs.org/doi/10.1021/jp026189d
Mechanism of Photosensitized Generation of Singlet Oxygen during Oxygen Quenching of Triplet States and the General Dependence of the Rate Constants and Efficiencies of O 2 (1 Σ g +), O 2 (1 Δ g), and O 2 (3 Σ g-) Formation on Sensitizer Triplet State Energy and Oxidation Potential
Cited by: 52
Publish Year: 2003
Author: Claude Schweitzer, Zahra Mehrdad, Astrid Noll, and Er
Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol–gel process
Photocatalytic degradation of phenol and benzoic acid in aqueous solution was studied using zinc oxide (ZnO) powder synthesized by sol–gel process. Synthesized catalyst was characterized by X-ray diffraction and transmission electron microscopy. The Brunauer–Emmett–Teller surface area, pHpzc, and the band gap of the catalyst samples were also measured. The influence of various key parameters such as amount of photocatalyst, initial solution pH, and the initial concentration of phenol and benzoic acid was investigated.
The photocatalytic activity of ZnO is almost similar to that of Titania, i.e., ZnO is found to be as reactive as TiO2 under concentrated sunlight (as the band gap energy of ZnO is same as that of TiO2, i.e., 3.2 eV). But in some cases, the photocatalytic activity of ZnO is considered to be less than that of TiO2, due to photocorrosion tendency of ZnO. In aqueous solution, ZnO shows photocorrosion tendency with the illumination of UV light [7], [8]
However, the photocatalytic activity of ZnO is limited to irradiation wavelengths in the UV region because ZnO semiconductor has a wide band gap of about 3.2 eV and can only absorb UV light with wavelengths below 387 nm [9]. It is particularly interesting to synthesize ZnO by the sol–gel process because sol–gel chemistry is an efficient tool for controlling morphology and reactivity of solids. In recent decades, it has permitted the development of new highly dispersed materials, presenting both good homogeneity and purity [10], [11], [12]. The efficiency of photocatalytic processes has been shown to depend on several different characteristics of the semiconductor particles, such as their surface properties, the position of their band gap potentials, and the mobility and recombination rate of the charge carriers generated by UV light absorption. Moreover, a relevant role is also played by the chemical and adsorption properties of the degradation substrate, depending also on experimental conditions, such as pH and the substrate to photocatalyst concentration ratio [13].
In this work, ZnO powder was synthesized by sol–gel method using zinc acetate dihydrate as a precursor and characterized by using techniques such as nitrogen adsorption isotherms, X-ray diffraction (XRD), scanning electron microscopy (SEM), and UV–Vis spectroscopy. Photocatalytic activity of catalyst was evaluated by measuring the photodegradation of phenol and benzoic acid. The effect of different operating conditions, such as catalyst loading, initial pH, and pollutant concentrations, was also studied.4. Conclusions
The sol–gel process used in this work allowed producing a crystalline pure ZnO powder after a calcinations step. This calcined ZnO powder was very active for the degradation of phenol and benzoic acid under UV irradiation.
The photocatalytic degradation of various toxic organic compounds has been proposed as a viable process to detoxify aquatic solutions.
The photocatalytic process was influenced by several operating variables such as pH of the solution, catalyst loading and initial phenol and benzoic acid concentrations. In this work, it was established that the use of a pH = 2.5 for the medium, a ZnO concentration = 1.5 g L−1 and initial phenol concentration = 0.20 g L−1 allowed the degradation of about 60% of phenol after 120 min.
These first results being interesting, the degradation of phenol and benzoic acid by-products, such as catechol and hydroquinone, are actually studied on ZnO photocatalyst synthesized by the sol–gel process. In parallel, the sensitization of ZnO under visible light is developed by doping with alkaline elements such as Li, Na, and K.
Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol–gel process - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S1110016813000367
DEGRADATION OF SALICYLIC ACID BY UV, UV/ H2O2,
UV/O3, PHOTOFENTON PROCESSES
The present study was used to probe the treatment of simulated wastewater containing salicylic acid by UV, UV/
H2O2, UV/O3, photofenton processes. Experiments were conducted in a batch photoreactor to examine the effects of
operating variables like pH, ratio of H2O2/COD, and different combinations of oxidizing agents with UV, and their
degradation rate is compared. A pseudo-first order kinetic model was adopted to represent the photo-oxidative
degradation of salicylic acid. The degradation rate of salicylic acid obeys the following sequence:
photoperoxone(O3/UV/ H2O2) > photofenton(UV/Fe2+/ H2O2)>photoperoxidation(UV/ H2O2) > photolysis(UV).
Keywords: Salicylic acid, photoperoxone, photoperoxidation, photofenton, photolysis.
© 2011 RASĀYAN. All rights reserved
Microsoft Word - 24_Vol.4_3_, 2011, RJC-821, 640-647.doc
http://rasayanjournal.co.in/vol-4/issue-3/24.pdf
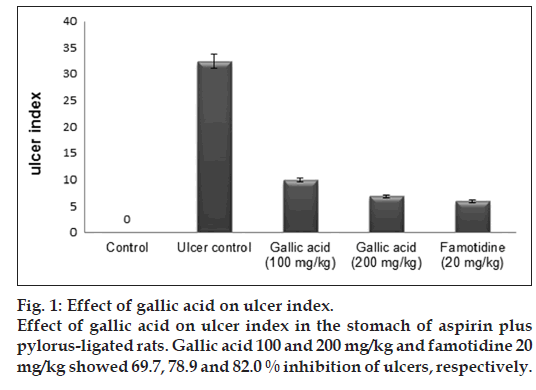
Antiulcerogenic effect of gallic acid in rats and its effect on oxidant and antioxidant parameters in stomach tissue
S. Sen, K. Asokkumar*, M. Umamaheswari, A. T. Sivashanmugam and V. Subhadradevi
Department of Pharmacology, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore-641 044, India
Abstract
In the present study, we investigate the antiulcerogenic effect of gallic acid against aspirin plus pyrolus ligation-induced gastric ulcer in rats. Rats were treated with gallic acid (100 and 200 mg/kg) and famotidine (20 mg/kg) for 1 week, followed by induction of gastric ulcer using the aspirin plus pyrolus ligation model. At the end of 4 h after ligation, the rats were sacrificed and ulcer index, gastric juice volume, pH and other biochemical parameter of gastric juice were evaluated. Stomachs of rats were evaluated biochemically to determine oxidant and antioxidant parameters. Pretreatment with gallic acid significantly decreased ulcer index, gastric juice volume, free and total acidity, total protein, DNA content and increased pH and carbohydrates concentration. Gallic acid at a dose of 100 and 200 mg/kg exerted 69.7 and 78.9% ulcer inhibition, respectively. The levels of superoxide dismutase, catalase, reduced glutathione, glutathione reductase, glutathione peroxidise, glucose-6-phosphate dehydrogenase were increased while reduction in myeloperoxidase and lipid peroxidation were observed in the stomach tissues of the drug treated rats. The histopathological studies further confirmed the antiulcer activity of gallic acid. We conclude that the gallic acid possesses antiulcer effect and that these occur by a mechanism that involves attenuation of offensive factors, improvement of mucosal defensive with activation of antioxidant parameters and inhibition of some toxic oxidant parameters.
Antiulcerogenic effect of gallic acid in rats and its effect on oxidant and antioxidant parameters in stomach tissue
http://www.ijpsonline.com/articles/antiulcerogenic-effect-of-gallic-acid-in-rats-and-its-effect-on-oxidant-and-antioxidant-parameters-in-stomach-tissue.html
Gallic Acid Alleviates Hypertriglyceridemia and Fat Accumulation via Modulating Glycolysis and Lipolysis Pathways in Perirenal Adipose Tissues of Rats Fed a High-Fructose Diet
by Da-Wei Huang 1, Wen-Chang Chang 2, Heng-Jui Yang 3, James Swi-Bea Wu 2 and Szu-Chuan Shen 3,*
1
Department of Food and Beverage Management, China University of Science and Technology, No. 245, Sec. 3, Academia Road, Taipei 11581, Taiwan
2
Graduate Institute of Food Science and Technology, National Taiwan University, P.O. Box 23-14, Taipei 10672, Taiwan
3
Department of Human Development and Family Studies, National Taiwan Normal University, No. 162, Sec. 1, Heping East Road, Taipei 10610, Taiwan
This study investigated the ameliorative effect of gallic acid (GA) on hypertriglyceridemia and fat accumulation in perirenal adipose tissues of high-fructose diet (HFD)-induced diabetic rats. The previous results showed that orally administered GA (30 mg/kg body weight) for four weeks significantly reduced the levels of plasma glucose and triglyceride (TG) in HFD rats. GA also markedly decreased the perirenal adipose tissues weight of HFD rats in present study (p < 0.05). Western blot assay indicated that GA restored expression of insulin signaling-related proteins, such as insulin receptor (IR), protein kinase C-zeta (PKC-ζ), and glucose transporter-4 (GLUT4) in the perirenal adipose tissues of HFD rats. Moreover, GA enhanced expression of glycolysis-related proteins, such as phosphofructokinase (PFK) and pyruvate kinase (PK), and increased the expression of lipolysis-related proteins, such as adipose triglyceride lipase (ATGL), which is involved in lipolysis in the perirenal adipose tissues of HFD rats. This study revealed that GA may alleviate hypertriglyceridemia and fat accumulation through enhancing glycolysis and lipolysis pathways in perirenal adipose tissues of HFD rats. These findings also suggest the potential of GA in preventing the progression of diabetes mellitus (DM) complications
IJMS | Free Full-Text | Gallic Acid Alleviates Hypertriglyceridemia and Fat Accumulation via Modulating Glycolysis and Lipolysis Pathways in Perirenal Adipose Tissues of Rats Fed a High-Fructose Diet
https://www.mdpi.com/1422-0067/19/1/254
Pharmacological effects of gallic acid in health and diseases: A mechanistic review
Department of Pharmacognosy, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
Antimicrobial activity
Structure-activity relationship studies of phenolic acids show that some parameters such as the basic chemical structure, the position, and the number of hydroxyl groups as well as their substituents on the phenolic ring, and the esterification of the carboxyl group, can affect the antimicrobial activity. Generally, hydroxycinnamic acids have higher antibacterial activity compared with hydroxybenzoic acids (5). Hydroxybenzoic acids with a lower degree of hydroxylation in phenol groups, highly methoxylated phenol groups, highly oxidized phenol groups, or ester derivatives with long alkyl chains showed higher antibacterial activities in comparison with their parent structures (5). On the other hand, hydroxybenzoic acids with more free –OH groups on the phenol ring were found more potent against the human immunodeficiency virus (HIV) and hepatitis C virus (HCV) (5-9).
From the mechanistic point of view, gallic acid can inhibit motility, adherence and biofilm formation of Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus mutans, Chromobacterium violaceum, and Listeria monocytogenes (10-12). The compound can also disrupt the integrity of the cell membrane in Gram-positive and Gram-negative bacteria and change the charge, hydrophobicity, and permeability of the membrane surface (13). Gallic acid can interfere with the membrane permeability of Campylobacter jejuni and elevate the antibiotic accumulation in the microorganism (14). Moreover, it can disintegrate the outer membrane of Gram-negative bacteria via chelation of divalent cations (15).
In addition to its effects on the bacterial cell membrane, there are some reports on the inhibitory activity of gallic acid against bacterial dihydrofolate reductase and its excitatory activity on topoisomerase IV-mediated DNA cleavage in different bacteria (16). Alkyl gallates can also penetrate the bacterial cell membrane and interfere with the electron transport chain and cellular respiration (17).
Some ester derivatives of gallic acid, i.e., octyl gallate, use the hydrophilic catechol part as a hook to bind to the polar surface of the cell membrane and enter the lipid bilayer using the hydrophobic alkyl part. Subsequently, they act as a nonionic surfactant and interfere with the selective permeability of cell membrane in fungi (17).
Gallic acid can inhibit HIV-1 integrase, HIV-1 transcriptase, HIV-1 protease dimerization (18-22), HCV attachment and penetration, HCV replication, HCV serine protease (23-26), the herpes simplex virus (HSV)-1 and HSV-2 attachment and penetration (22). It also causes disruption in Haemophilus influenza A and B particles (27).
In connection with protozoa, gallic acid can bind to the glutamate-gated chloride channels in the nervous system of Caenorhabditis elegans and initiates the hyperpolarization of the cell membranes and excitation of muscles. These events finally result in worm paralysis and death (28).
Gallic acid, alkyl gallates and chitosan-based formulations of gallic acid can potentiate the antimicrobial activity of other antibiotics, including erythromycin, gentamicin, norfloxacin, ciprofloxacin, ampicillin, penicillin, and oxacillin via synergism (29-34) (Table 1).
Gallic acid interferes with various intra-cellular inflammatory pathways that induce ulcerative colitis. The compound inhibits the expression of nuclear transcription factors, such as nuclear factor (NF)-κB and signal transducer and activator of transcription 3 (STAT3), and down-regulates their inflammatory downstream targets (50). It also reduces the expression and/or activity of pro-inflammatory cytokines and inflammatory proteins, including TNF-α, interferon-γ (INF-γ), interleukin (IL)-1β, IL-6, IL-17, IL-21, IL-23, cyclooxygenase (COX)-2, and i-NOS, and decreases the expression and infiltration of neutrophils and CD68+ macrophages into the colon (50-51).
Pharmacological effects of gallic acid in health and diseases: A mechanistic review
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6528712/
Inhibiting NF-κB Activation by Small Molecules As a ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2955987
During the last 5 years, a number of compounds have been reported to inhibit NF-κB by inhibiting acetylation. Gallic acid obtained from natural products such as gallnuts, sumac, oak bark, and green tea was recently reported to possess anti-histone acetyltransferase activity, thus showing potential to downregulate NF-κB activation .
Effects of Gallic Acid and Its Derivates on Inflammatory ...
biomedpharmajournal.org/vol11no3/effects-of-gallic-acid-and-its-derivates-on...
Inflammatory regulation was assessed from NF-kB mRNA expression with qRT-PCR and IL-6 secretion levels with ELISA. Gallic acid and its derivatives showed a decrease in the relative expression of NF-kB, significantly at dose 102,4 μg/ml. IL-6 although not statistically significant.
Cited by: 1
Publish Year: 2018
Author: Arleni Bustami, Popi Sopiah, R. Muharam, He
Inhibiting NF-κB Activation by Small Molecules As a Therapeutic Strategy
Cytokine Research Laboratory, Department of Experimental Therapeutics, The University of Texas M. D. Anderson Cancer Center, Houston, Texas 77030
Because nuclear factor-κB (NF-κB) is a ubiquitously expressed proinflammatory transcription factor that regulates the expression of over 500 genes involved in cellular transformation, survival, proliferation, invasion, angiogenesis, metastasis, and inflammation, the NF-κB signaling pathway has become a potential target for pharmacological intervention. A wide variety of agents can activate NF-κB through canonical and noncanonical pathways. Canonical pathway involves various steps including the phosphorylation, ubiquitnation, and degradation of the inhibitor of NF-κB (IκBα), which leads to the nuclear translocation of the p50- p65 subunits of NF-κB followed by p65 phosphorylation, acetylation and methylation, DNA binding, and gene transcription. Thus, agents that can inhibit protein kinases, protein phosphatases, proteasomes, ubiquitnation, acetylation, methylation, and DNA binding steps have been identified as NF-κB inhibitors. Here, we review the small molecules that suppress NF-κB activation and thus may have therapeutic potential.
introduction
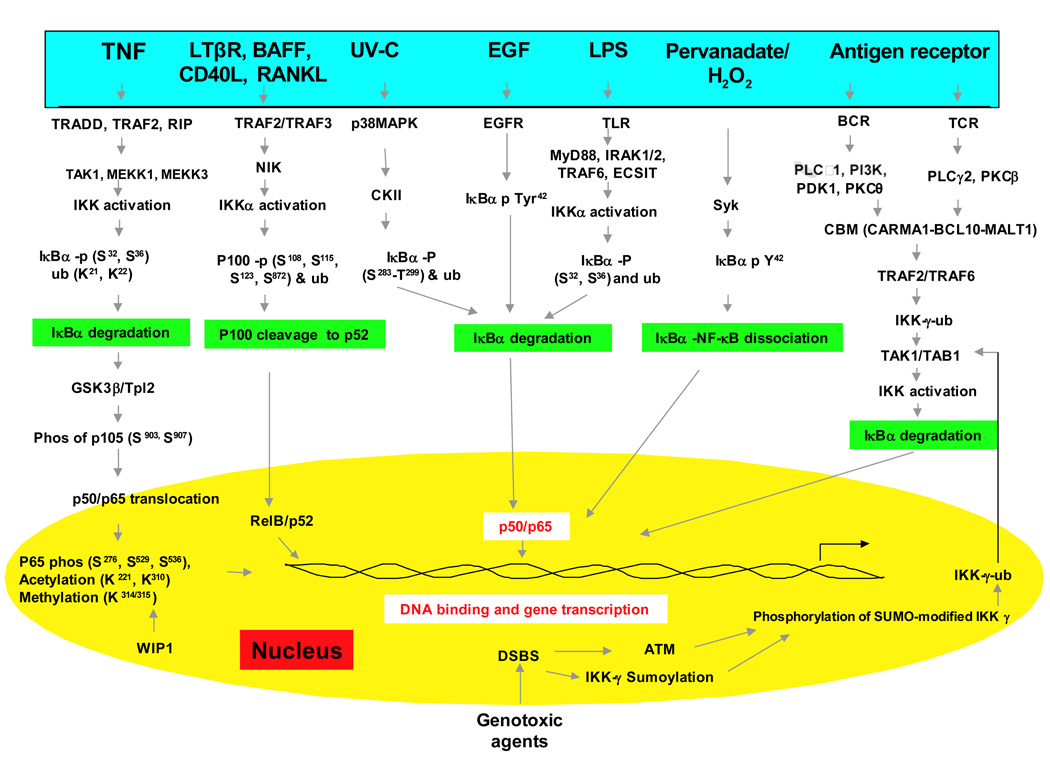
The nuclear factor-κB (NF-κB) signaling pathway plays a major role in the development, maintenance, and progression of most chronic diseases. NF-κB controls the expression of genes involved in a number of physiological responses, including immune inflammatory responses, acute-phase inflammatory responses, oxidative stress responses, cell adhesion, differentiation, and apoptosis [1]. Recent studies have suggested that NF-κB dysregulation is associated with many diseases including AIDS, atherosclerosis, asthma, arthritis, diabetes, inflammatory bowel disease, stroke, muscle wasting and viral infections. Mounting evidence indicates that NF-kB acts as a link between inflammation and cancer progression [2–10], making NF-κB essential to and a potential drug target in hematological malignancies and solid tumors [11, 12]. NF-κB was first identified in 1986 by Sen and Baltimore [5] in the nucleus bound to an enhancer element of the immunoglobulin kappa light chain gene in B cells [5, 13]. It is now known to be ubiquitous in nature present in all the cell types and is evolutionary conserved. It belongs to the family of Rel proteins that includes c-Rel, RelA (p65), RelB, NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100) all of which can form hetero- or homodimers [14–16].
NF-κB activation is tightly regulated mainly through its localization. In resting cells, NF-κB proteins are kept in the cytoplasm in association with inhibitory IkB proteins including IκBα, IκBβ, and IκBε [15] among which IκBα is the most abundant. NF-κB signaling occurs through the canonical (classical) pathway initiated by NF-κB1 (p50/p105) and a noncanonical (alternative) pathway initiated by NF-κB2 (p52/p100) (Fig 1). Before the active NF-κB is translocated into the nucleus, NF-κB1 and NF-κB2 are cleaved to the active p50 and p52 subunits, respectively. While the classical pathway depends on IKK complex consisting of IKKα, IKKβ, IKKγ and the inhibitory subunit IκBs, the alternative pathway depends on IKKα homodimers and NF-κB inducing kinase (NIK) [17–19]. During classical activation, the IKK complex specifically phosphorylates IκBs on two conserved N-terminal serine residues which target them for E2- and E3-ligase-mediated polyubiquitination and subsequent 26S proteasomal mediated degradation. This process releases and activates NF-κB which now translocates to the nucleus. The activation of alternative pathway, which is commonly associated with RelB results in regulated processing of the p100 precursor protein to p52 and subsequent translocation of p52-RelB heterodimers to the nucleus[18]. Although NF-κB activation occurs mainly through canonical and non-canonical pathways, during the past decade a number of pathways for NF-κB activation has been elucidated (Fig 1).
Keywords: Inflammation, NF-κB, small molecule inhibitors, therapeuticsInhibiting NF-κB Activation by Small Molecules As a Therapeutic Strategy
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2955987/
https://pubs.acs.org/doi/10.1021/jf303177p#
Bactericidal Action of Photoirradiated Gallic Acid via Reactive Oxygen Species Formation
It is known that gallic acid shows antimicrobial activity. In the present study, photoirradiation induced reactive oxygen species formation was investigated for augmentation of the antimicrobial activity of gallic acid. Staphylococcus aureus suspended in 4 mmol/L gallic acid was exposed to blue light of a LED at 400 nm. This treatment killed the bacteria, and a >5-log reduction of the viable counts was observed within 15 min. By contrast, neither the LED treatment alone nor the treatment with gallic acid alone showed substantial bactericidal effect. When hydroxyl radical scavengers were added to the suspension, the bactericidal effect of photoirradiated gallic acid was attenuated. Furthermore, electron spin resonance analysis demonstrated that hydroxyl radicals were generated by the photoirradiation of gallic acid. Thus, the present study suggests that the photo-oxidation can enhance the antimicrobial activity of gallic acid via hydroxyl radical formation.
Bactericidal Action of Photoirradiated Gallic Acid via Reactive Oxygen Species Formation | Journal of Agricultural and Food Chemistry
https://pubs.acs.org/doi/10.1021/jf303177p#
Sci Rep. 2018 Aug 27;8(1):12888. doi: 10.1038/s41598-018-31195-x.
A novel pathway for the photooxidation of catechin in relation to its prooxidative activity.
1
Tohoku University Graduate School of Dentistry, 4-1 Seiryo, Aoba-ku, Sendai, 980-8575, Japan.
2
Kitasato Institute for Life Sciences, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo, 108-8641, Japan.
3
Tohoku University Graduate School of Dentistry, 4-1 Seiryo, Aoba-ku, Sendai, 980-8575, Japan. niwano@m.tohoku.ac.jp.
4
Faculty of Nursing, Shumei University, 1-1 Daigaku-cho, Yachiyo, Chiba, 276-0003, Japan. niwano@m.tohoku.ac.jp.
Abstract
In the present study, we evaluated the prooxidative mode of action of photoirradiated (+)-catechin at 400 nm in relation to reactive oxygen species generation and its possible application to disinfection. Photoirradiation of (+)-catechin at a concentration of 1 mg/mL yielded not only hydrogen peroxide (H2O2) but hydroxyl radical (·OH) in a total amount of approximately 20 μM in 10 min. As a result, photoirradiated catechin killed Staphylococcus aureus, and a > 5-log reduction in viable bacteria counts was observed within 20 min. Liquid chromatography-high-resolution-electrospray ionization-mass spectrometry showed that photoirradiation decreased the (+)-catechin peak (molecular formula C15H14O6) whilst it increased two peaks of a substance with the molecular formula C15H12O6 with increasing irradiation time. Nuclear magnetic resonance analysis revealed that the two C15H12O6 peaks were allocated to intramolecular cyclization products that are enantiomers of each other. These results suggest that photoirradiation induces oxidation of (+)-catechin resulting in the reduction of oxygen to generate H2O2. This H2O2 is then homolytically cleaved to ·OH, and alongside this process, (+)-catechin is finally converted to two intramolecular cyclization products that are different from the quinone structure of the B ring, as proposed previously for the autoxidation and enzymatic oxidation of catechins.A novel pathway for the photooxidation of catechin in relation to its prooxidative activity. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/30150642
Frontiers | Antimetabolic Effects of Polyphenols in Breast ...
https://www.frontiersin.org/articles/10.3389/fnut.2018.00025/full
Apr 16, 2018 · Gallic acid appears to be a non-competitive inhibitor of the enzyme, whereas quercetin appears to be a competitive inhibitor. Because these two compounds inhibited the proliferation of MCF-7 human breast cancer cells, it was concluded that gallic acid and quercetin are effective inhibitors of UGDH that exert strong antiproliferative activity in breast cancer cells ( 85 ).
Cited by: 7
Publish Year: 2018
Author: Elisa Keating, Fátima MartelEvaluation of the anti-tumor effects of lactate dehydrogenase inhibitor galloflavin in endometrial cancer cells | Journal of Hematology & Oncology | Full Text
https://jhoonline.biomedcentral.com/articles/10.1186/s13045-014-0097-x
Jan 29, 2015 · Galloflavin (GF), which is synthesized from gallic acid, is a new lactate dehydrogenase inhibitor that inhibits both the A and B isoforms of LDH [].By serving as a competitive inhibitor with NADH for LDH, GF has been shown to disrupt aerobic glycolysis and decrease cell viability effectively across many cancer cell types, including breast, colon and liver cancers as well as Burkitt lymphoma [2-4].
Cited by: 11
Publish Year: 2015
Author: Xiaoyun Han, Xiugui Sheng, Hannah M Jones, Am
Tryptamine-Gallic Acid Hybrid Prevents Non-steroidal Anti-inflammatory Drug-induced Gastropathy
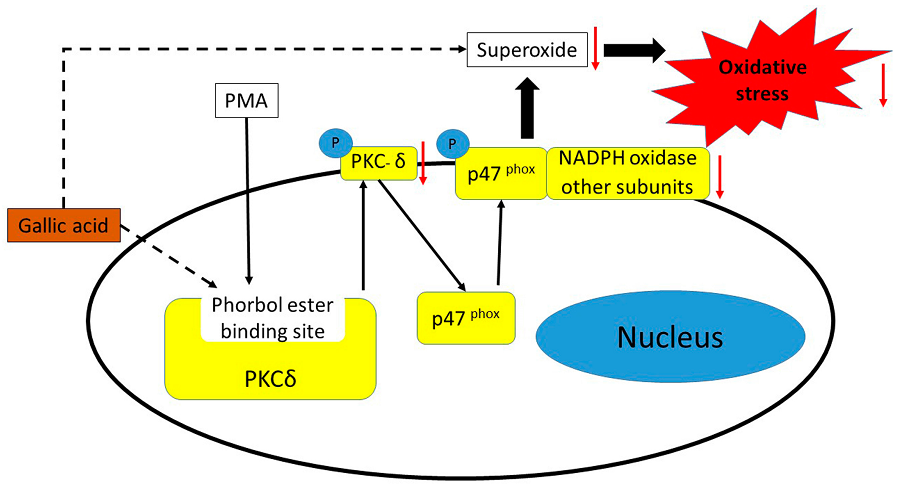
CORRECTION OF MITOCHONDRIAL DYSFUNCTION AND INHIBITION OF APOPTOSIS IN GASTRIC MUCOSAL CELLS*
Chinmay Pal‡, Samik Bindu‡, Sumanta Dey‡, Athar Alam‡, Manish Goyal‡, Mohd. Shameel Iqbal‡, Souvik Sarkar‡, Rahul Kumar‡, Kamal Krishna Halder§, Mita Chatterjee Debnath§, Susanta Adhikari¶ and Uday Bandyopadhyay‡,1
- Author Affiliations
From the ‡Division of Infectious Diseases and Immunology and
the §Nuclear Medicine Division, Indian Institute of Chemical Biology, 4 Raja S. C. Mullick Road, Jadavpur, Kolkata-700032, West Bengal, India and
the ¶Department of Chemistry, University of Calcutta, 92 A.P.C. Road, Kolkata-700009, West Bengal, India
Capsule
Background: Non-steroidal anti-inflammatory drugs (NSAIDs) induce gastropathy by promoting mitochondrial pathology, oxidative stress, and apoptosis in gastric mucosal cells.
Results: We have synthesized SEGA (3a), a tryptamine-gallic acid hybrid, which prevents NSAID-induced gastropathy by preventing mitochondrial oxidative stress, dysfunction, and apoptosis.
Conclusion: SEGA (3a) bears an immense therapeutic potential against NSAID-induced gastropathy.
Significance: This novel molecule is a significant addition in the discovery of gastroprotective drugs.
We have investigated the gastroprotective effect of SEGA (3a), a newly synthesized tryptamine-gallic acid hybrid molecule against non-steroidal anti-inflammatory drug (NSAID)-induced gastropathy with mechanistic details. SEGA (3a) prevents indomethacin (NSAID)-induced mitochondrial oxidative stress (MOS) and dysfunctions in gastric mucosal cells, which play a pathogenic role in inducing gastropathy. SEGA (3a) offers this mitoprotective effect by scavenging of mitochondrial superoxide anion (O2̇̄) and intramitochondrial free iron released as a result of MOS. SEGA (3a) in vivo blocks indomethacin-mediated MOS, as is evident from the inhibition of indomethacin-induced mitochondrial protein carbonyl formation, lipid peroxidation, and thiol depletion. SEGA (3a) corrects indomethacin-mediated mitochondrial dysfunction in vivo by restoring defective electron transport chain function, collapse of transmembrane potential, and loss of dehydrogenase activity. SEGA (3a) not only corrects mitochondrial dysfunction but also inhibits the activation of the mitochondrial pathway of apoptosis by indomethacin. SEGA (3a) inhibits indomethacin-induced down-regulation of bcl-2 and up-regulation of bax genes in gastric mucosa. SEGA (3a) also inhibits indometacin-induced activation of caspase-9 and caspase-3 in gastric mucosa. Besides the gastroprotective effect against NSAID, SEGA (3a) also expedites the healing of already damaged gastric mucosa. Radiolabeled (99mTc-labeled SEGA (3a)) tracer studies confirm that SEGA (3a) enters into mitochondria of gastric mucosal cell in vivo, and it is quite stable in serum. Thus, SEGA (3a) bears an immense potential to be a novel gastroprotective agent against NSAID-induced gastropathy.
Mitochondria Mitochondrial Apoptosis Oxidative StressTryptamine-Gallic Acid Hybrid Prevents Non-steroidal Anti-inflammatory Drug-induced Gastropathy
https://www.jbc.org/content/287/5/3495
Boiled Greeen Banana Health Benefits For Treating Gastritis!
May 16, 2017
Boiled green bananas health benefits for the treatment of stomach ulcers and gastritis are simply amazing! Try this treatment out and get rid of the pain now!
Gastritis is one of the major problems that many people face worldwide. It is nothing more than irritation and inflammation of the gastric mucosa. Some of your symptoms may be heartburn and a feeling of fullness.
When it is not taken care of promptly, it can generate stomach ulcers and other types of stomach problems. If you have this problem or know someone who has it, this article will be of interest to you.
Preparation and use:
To be able to use the boiled green bananas health benefits to fight gastritis or stomach ulcers, first you should grind them. For this, all you have to do is take a green banana, peel it and chop it into slices. Let them dry in the open air for several days. Once they are completely dry, pass them through a mill to get a kind of fine powder. This powder will be the potent remedy you will use to combat gastritis.
Now, heat a cup of mineral water until it begins to boil. At this point, pour the water into a container and dilute 1 tablespoon of the ground banana. Take this infusion 3 times a day, every day.See The Boiled Green Bananas Health Benefits For Treating Gastritis!
https://thehealthyville.com/recipes/boiled-green-bananas-health-benefits/
Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future.
Significant evidences have shown that the polyphenol-rich diets have the ability to protect against diabetes. Since the last several reviews focused on the nutrition and health effects including type 2 diabetes of polyphenols in 2007-2008, a number of related original publications have appeared in this area. This review summarizes important advances related to influence of dietary polyphenols and polyphenol-rich diets on preventing and managing type 2 diabetes, as well as diabetes-mediated changes in bioactivities of dietary polyphenols. It looks like that anthocyanins or anthocyanin-rich foods intakes are related to the risk of type 2 diabetes, but there is no association for other polyphenol subclasses. It illustrated that procyanidins are more active when administered individually than when mixed with food. The benefits of dietary polyphenols for type 2 diabetes can be summarized as: protection of pancreatic β-cells against glucose toxicity, anti-inflammatory, antioxidant, inhibition of starch digestion, inhibition of α-amylases or α-glucosidases, and inhibition of advanced glycation end products formation. Moreover, type 2 diabetes also significantly influence the benefits of dietary polyphenols, although there are very limited studies have been conducted so far. However, how type 2 diabetes impact the pharmacology of dietary polyphenols are not well understood. An understanding of type 2 diabetes-mediated changes in pharmacokinetics and bioactivity of dietary polyphenols will lead to improve the benefits of these phytochemicals and clinical outcomes for type 2 diabetics.(PDF) Dietary Polyphenols and Type 2 Diabetes: Current Insights and Future.
https://www.researchgate.net/publication/263777475_Dietary_Polyphenols_and_Type_2_Diabetes_Current_Insights_and_Future
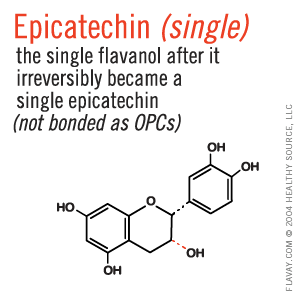
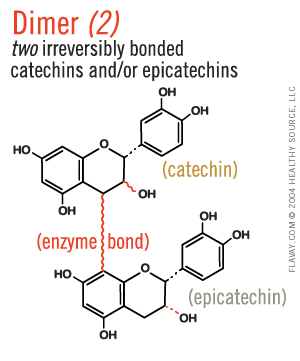
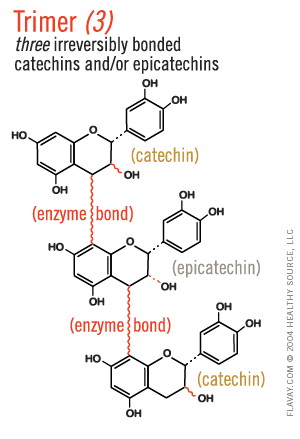
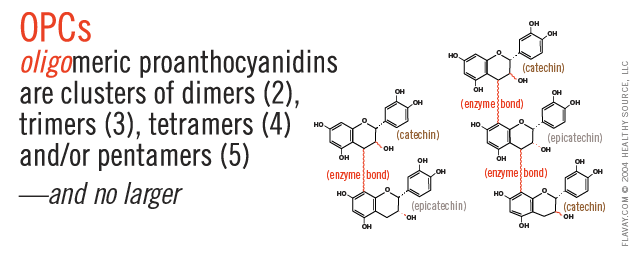
Only Small Proanthocyanidins Are Safe and Effective
Oligo means few or small.
Only the small (2-5) clusters of molecules are small enough to fit between collagen fibrils and gently protect delicate tissues. (Clusters of 6+ are too large and their affinity for proteins is too strong to be healthful.)
https://www.flavay.com/composition.html
Arch Toxicol. 2009 Sep;
The anti-apoptotic effects of caspase inhibitors on propyl gallate-treated HeLa cells in relation to reactive oxygen species and glutathione levels
Department of Physiology, Centers for Healthcare Technology Development, Institute for Medical Sciences, Chonbuk National University, Jeonju, Republic of Korea.
Propyl gallate (PG) as a synthetic antioxidant is widely used in processed food, cosmetics and medicinal preparations. Despite the assumed low toxicity of PG, it exerts a variety of effects on tissue and cell functions. In the present study, we evaluated the anti-apoptotic effects of caspase inhibitors on PG-treated human cervix adenocarcinoma HeLa cells in relation to the changes of reactive oxygen species (ROS) and glutathione (GSH) levels. PG induced apoptosis in a dose-dependent manner, as evidenced by sub-G1 cells and annexin V staining cells. Treatment with pan-caspase inhibitor, caspase-3 inhibitor, caspase-8 inhibitor or caspase-9 inhibitor significantly prevented apoptosis in PG-treated HeLa cells at 24 h. The intracellular ROS levels including O (2) (*-) were increased or decreased in PG-treated HeLa cells depending on the incubation times (1 or 24 h). PG depleted intracellular GSH content in HeLa cells at 24 h. Treatment with caspase inhibitor reduced ROS levels and significantly prevented GSH depletion in PG-treated HeLa cells at 24 h. In conclusion, PG induced apoptosis in HeLa cells. The anti-apoptotic effect of caspase inhibitor on PG-induced HeLa cell death was closely related to the reduction of ROS levels, especially mitochondrial O (2) (*-) , as well as to the inhibition of GSH depletion.The anti-apoptotic effects of caspase inhibitors on propyl gallate-treated HeLa cells in relation to reactive oxygen species and glutathione levels - PubMed
https://pubmed.ncbi.nlm.nih.gov/19434396/
.png)


.gif)

.png)