运动期间的脂肪代谢:新观点
Highlights:
1. epinephrine and norepinephrine are released during resistance exercises in a "fight or flight" response.
2. Growth hormone is released after resistance exercise.
3. epinephrine and norepinephrine stimulate mobilization of body fat while insulin inhibits mobolization of body fat.
4. high fat diets are harmful for endurance exercise.
5. epinephrine blocks glucose from entry into the cells, but
FAT METABOLISM DURING EXERCISE: NEW CONCEPTS
Edward F. Coyle, Ph.D.
KEY POINTS
1. People store large amounts of body fat in the form of triglycerides within fat (adipose) tissue as well as within muscle fibers (intramuscular triglycerides).When compared to carbohydrate stored as muscle glycogen, these fat stores are mobilized and oxidized at relatively slow rates during exercise.
2. As exercise progresses from low to moderate intensity, e.g., 25-65% VO2max, the rate of fatty acid mobilization from adipose tissue into blood plasma declines, whereas the rate of total fat oxidation increases due to a relatively large use of intramuscular triglycerides. Intramuscular triglycerides also account for the characteristic increase in fat oxidation as a result of habitual endurance-training programs.
3. Dietary carbohydrate intake has a large influence on fat mobilization and oxidation during exercise; when dietary carbohydrate produces sufficient carbohydrate reserves in the body, carbohydrate becomes the preferred fuel during exercise. This is especially important during intense exercise because only carbohydrate(not fat) can be mobilized and oxidized rapidly enough to meet the energy requirements for intense muscular contractions.
INTRODUCTION
The two main sources of energy during muscular exercise are fat (triglyceride) and carbohydrate (glycogen and glucose) stored within the body, and there has been much research and practical experience over the past 30 y demonstrating the importance of muscle and liver glycogen for reducing fatigue and improving athletic performance. For example, it is well known that diets containing predominantly carbohydrate are necessary to maintain glycogen stores at high levels during bouts of intense exercise and that such diets are apparently optimal for promoting training-induced improvements in performance (Simonsen et al., 1991). The primary reason that glycogen reserves are essential is that athletes can only slowly convert their body fat stores into energy during exercise. Therefore, when muscle glycogen and blood glucose concentrations are low, the intensity of exercise must be reduced to a level that can be supported by the body's limited ability to convert body fat into energy. With endurance training, athletes can markedly increase the rate at which body fat can be oxidized, thus allowing them to exercise longer before becoming exhausted due to glycogen depletion. Of course, exercise training also increases an individual's ability to exercise more intensely, so trained athletes must continue to derive most of their energy from carbohydrate during intense training and competition because their increased ability to oxidize fat cannot meet their increased energy demands.
What limits the rate at which people can convert their body fat into energy during exercise? Recent research using new techniques has begun to shed light on this question, and the emerging picture will be discussed in this article. Although we do not yet have a complete understanding of fat metabolism during exercise, there is now enough information available to cast serious doubts on many of the recent advertising claims for special diets and nutritional supplements that stress more fat, and less carbohydrate.
BODY FAT STORES
ADIPOSE TISSUE
Fat is stored in the body in the form of triglyceride, which is comprised of three fatty acids attached to a molecule of glycerol. The fatty acids consist of chains of carbon atoms with hydrogen atoms attached. There is more stored energy (9 kcal) in a gram of fat than in an equal weight of carbohydrate (4 kcal/g). Typically, about 50,000 to 60,000 kcal of energy are stored as triglycerides in the entire mass of all of the adipocytes throughout the body. Obviously, there will be more energy stored in an obese person and less in an individual who has little body fat (Figure 1). Approximately 100 kcal of energy are expended per mile of walking, so most people have sufficient stores of triglyceride energy to walk 500-1,000 miles. Because this large amount of energy is stored in a relatively small mass of triglycerides, they provide a marvelous way for people to carry fuel as they move from place to place. In contrast, if all of this energy were stored as carbohydrate in glycogen, water molecules, which are very heavy, would be bound to the glycogen molecules, resulting in a total energystore weight of more than 100 pounds. Undoubtedly, the storage of fuel as triglyceride has served nomadic human beings very well in the course of evolution when food was scarce.
INTRAMUSCULAR TRIGLYCERIDE
Triglyceride is also stored in droplets directly within the muscle fibers (intramuscular triglyceride), placing this fuel in close proximity to the site of oxidation in the muscle mitochondria. Intramuscular triglyceride accounts for 2,000-3,000 kcal of stored energy, making it a larger source of potential energy than muscle glycogen, which can contribute only about 1,500 kcal. Unfortunately, because it is technically difficult to measure intramuscular triglyceride from muscle biopsy samples, relatively little is known about the rate at which intramuscular triglyceride can be oxidized during exercise or how this energy store changes in response to acute and chronic training. It is clear, however, that intramuscular triglyceride can provide energy for intense exercise at less than one-third the rate attributed to muscle glycogen. Therefore, during strenuous training or competition energy from intramuscular triglyceride should be considered as supplementary to that supplied by muscle glycogen.
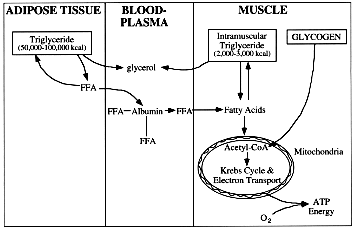
FIGURE 1. Scheme of the storage and mobilization of the stored triglyceride. Triglyceride from adipose tissue can be broken down to glycerol and free fatty acids (FFA), and FFA can be mobilized by binding to plasma albumin for transportation in the circulation to skeletal muscle and other tissues. Intramuscular triglyceride can also be broken down to glycerol and fatty acids, which enter the mitochondria for oxidation during exercise.
In addition to energy supplied by intramuscular triglycerides, it should be noted that plasma triglycerides are another source of energy for muscle. In the fasted state, there is a small amount of triglyceride produced by the liver that is bound to very-low-density lipoproteins in plasma. Although muscle can break down this plasma triglyceride to some extent during exercise, its contribution to energy is very small (Kiens et al., 1993).
MOBILIZATION AND OXIDATION OF FAT DURING EXERCISE
MOBILIZATION OF FREE FATTY ACIDS (FFA) FROM ADIPOSE TISSUE
The large stores of triglyceride within adipose tissue are mobilized at relatively slow rates during exercise. In this process, exercise stimulates an enzyme, hormone sensitive lipase, to dissolve the lipid or triglyceride molecule into three molecules of unbound or free fatty acids (FFA) and one glycerol molecule (Figure 1) ; this process of breaking down triglycerides is known as lipolysis. The glycerol released from this reaction is water soluble and diffuses freely into the blood. Its rate of appearance in the blood provides a direct measure of the amount of triglyceride hydrolyzed in the body. The primary factor thought to be responsible for the stimulation of adipose tissue lipolysis during exercise is the increasing plasma concentration of epinephrine, which activates betareceptors in adipocytes (Arner et al. , 1990); additional hormonal factors probably also play a role.
The fate of the three FFA molecules released from adipose tissue during lipolysis is complex (Figure 1). These fatty acids are not water soluble and thus require a protein carrier to allow them to be transported through cells and within the blood stream. At rest, about 70% of the FFA released during lipolysis are reattached to glycerol molecules to form new triglycerides within the adipocytes. However, during low-intensity exercise, this process is attenuated at the same time as the overall rate of lipolysis increases; as a result, the rate of appearance of FFA in the plasma increases by up to five fold (Klein et al., 1994; Romijn et al., 1993; Wolfe et al., 1990). Once they enter the plasma, the FFA molecules are loosely bound to albumin, a plasma protein, and transported in the circulation. Some of the fatty acids are eventually released from albumin and bound to intramuscular proteins, which in turn transport the FFA to the mitochondria for oxidation (Turcotte et al., 1991).
FIGURE 2. Contribution of the four major fuel substrates to energy expenditure after 30 min. of exercise at 25%, 65% and 85% of maximal oxygen uptake in fasted subjects. Reproduced with permission from Romijn et al. (1993).
Recent studies of endurance-trained men who had fasted overnight found that the rate of appearance of FFA in plasma declines as the intensity of exercise progressively increases from low (25% VO2max, comparable to a walking pace) to moderate (65% VO2max, comparable to the greatest running pace that can be sustained for 2-4 h) to high (85% VO2max, the greatest pace that can be sustained for 30-60 min) (Figure 2). The contributions of carbohydrate, i.e. muscle glycogen and blood glucose, and of fat, i.e., plasma FFA from adipose tissue plus intramuscular triglyceride, to total energy expenditure during exercise at these various intensities are shown in Figure 2. It should be noted that although the contribution of plasma FFA to the fuel supply declines as exercise intensity increases from 25% to 65% VO2max, total fat oxidation increases. Furthermore, although the use of plasma FFA for energy is reduced as intensity increases from 25% to 65% VO2max, we can't discount the possibility that at an intermediate intensity, e.g., 45% VO2max, plasma FFA might contribute more energy than at 25% VO2max.
INTRAMUSCULAR TRIGLYCERIDE OXIDATION DURING EXERCISE
It has been recognized for quite some time that intramuscular triglyceride must be important for fat oxidation during exercise of certain intensities (Essen et al., 1977), especially in dogs (Issekutz & Paul , 1968). During low-intensity exercise, e.g., 25% VO2max, it is assumed that plasma FFA are almost the exclusive fat source as a fuel because of the very close matching between the rate of fat oxidation and the rate at which FFA molecules disappear from the blood. However, during exercise at higher intensities, total fat oxidation in endurance-trained people is far in excess of the rate of plasma FFA disappearance, thus indicating that additional fat oxidation must be derived from a pool of intramuscular triglyceride. This point is illustrated in Figure 2 and 3. Intramuscular triglyceride oxidation was calculated to be very low during exercise at 25% VO2max, but during exercise at 65% VO2max, intramuscular triglyceride accounts for approximately one-half of the total fat oxidation. Intramuscular triglyceride oxidation was calculated to be somewhat reduced during exercise at 85% VO2max. These observations are preliminary, and more research is needed to fully elucidate the influence of exercise intensity, diet, and training status on intramuscular triglyceride oxidation.
WHOLE-BODY FAT OXIDATION DURING EXERCISE OF INCREASING INTENSITY
There is much interest in the effect of exercise intensity on fat oxidation and the sources of that fat. It is often assumed that the intensity of exercise must be kept low to burn fat optimally. However, from Figures 2 and 3 it can be seen that the rate of total fat oxidation was higher at 65% than at 25% VO2max -110 cal · kg-1 · min-1 vs. 70 cal · kg-1 · min-1. At 25% VO2max, almost all of the energy expenditure during exercise was derived from fat, but fat oxidation at 65% VO2max accounted for only 50% of the energy expenditure. However, because the total rate of energy expenditure was so much greater (2.6-fold) at 65% VO2max, the absolute rate of fat oxidation was greater, i.e., it was 50% of a much larger value (Figure 3). Therefore, expressing energy derived from fat simply as a percentage of energy expenditure without consideration of the rate of total energy expenditure is misleading. Likewise, the reduction in the rate of appearance of plasma FFA with increasing intensity of exercise does not prove that exercising at a low intensity is the best way to reduce fat stored in adipose tissue.
Figure 3. Expanded views of teh sources of fat for oxidation during exercise at 25% (walking pace), 65% (moderate running) and 85% (intense running) of maximal oxygen uptake in fasted subjects.
Both the rate of energy expenditure and the duration of exercise are critical in determining fat loss. Another consideration is the effect that exercise has on energy expenditure during the recovery periods between exercise sessions. Reductions in body fat stores as a result of long-term exercise training depend primarily on the total daily energy expenditure and not simply the actual fuel oxidized during exercise (Ballor et al., 1990).
FAT SUPPLEMENTATION DURING EXERCISE
INGESTION OF LONG-CHAIN TRIGLYCERIDES
It is not possible to ingest FFA because they are too acidic and because they need a protein carrier for intestinal absorption. Thus, the only practical way of significantly raising fat in the blood is by ingesting triglycerides. Normal long-chain dietary triglycerides enter the blood 3-4 h after ingestion and are bound to chylomicrons, which are lipoprotein carriers in the plasma. The rate of breakdown of triglycerides bound to plasma chylomicrons and the rate of uptake of those triglycerides by muscles during exercise are relatively low, and these chylomicron-associated triglycerides are used primarily to replenish intramuscular triglycerides during recovery from exercise (Mackie et al., 1980; Oscai et al., 1990). Therefore, although not proven, it is unlikely that ingestion of long-chain triglycerides has much potential to provide significant fuel for muscle during exercise (Terjung et al., 1983) .
INGESTION OF MEDIUM-CHAIN TRIGLYCERIDES
Unlike long-chain triglycerides, ingested medium-chain triglycerides (MCT) are directly absorbed into the blood and liver and are rapidly broken down to fatty acids and glycerol. They therefore provide a theoretical means of rapidly elevating plasma FFA. Another theoretical advantage of MCT is that they appear to be readily transported through cells and into the mitochondria for oxidation. Recent studies have shown that a large percentage of ingested MCT is oxidized and that the oxidation increases more rapidly when the MCT is consumed along with carbohydrate (Jeukendrup et al., 1995). However, most individuals cannot consume more than 30 g of ingested MCT without experiencing severe gastrointestinal discomfort and diarrhea. Accordingly, MCT ingestion can only contribute 3-6% of the total energy expended during exercise (Jeukendrup et al., 1995). Furthermore, when MCT is consumed with a carbohydrate feeding, the carbohydrate-stimulated insulin secretion partially inhibits the mobilization of the body's own fat stores, resulting in large reductions in fat oxidation compared to exercise when fasted.
INTRAVENOUS LIPID INFUSIONS THAT RAISE PLASMA FFA CONCENTRATIONS
A technique used in research studies to raise plasma FFA is to intravenously infuse a triglyceride emulsion, e.g., Intralipid°, followed by heparin, which causes the release of a lipolytic enzyme, lipoprotein lipase, from its storage site in adipose tissues and muscle into the blood, where the enzyme splits triglyceride into glycerol and FFA (Vukovich et al., 1993). Infusion rates must be carefully controlled because an excessive elevation of FFA in the blood is harmful. There are some conditions during exercise in which the concentration of FFA in plasma is less than optimal so that there may be some theoretical benefit of artificially raising the plasma FFA concentration. For example, plasma FFA mobilization and concentration are low during intense exercise (discussed above) as well as during exercise following carbohydrate ingestion (discussed below). Under these conditions, the elevation of FFA via intravenous infusion of triglyceride and heparin slightly reduces the rate of muscle glycogen utilization (Costill et al., 1977; Vukovich et al., 1993). However, this effect is relatively small, and any benefit to performance has yet to be demonstrated.
ENDURANCE TRAINING INCREASES FAT OXIDATION BUT NOT FFA MOBILIZATION INTO PLASMA DURING EXERCISE
SOURCE OF THE INCREASE IN FAT OXIDATION
As discussed in a recent issue of Sports Science Exchange (Terjung, 1995), one of the most functional adaptations to endurance training is an increase in the size and number of muscle mitochondria to greatly enhance aerobic metabolism , i.e., the ability of muscles to use oxygen to metabolize fat and carbohydrate for energy. During exercise at a given absolute submaximal power output, endurance-trained people experience less muscular fatigue, less disturbance of energy balance, and less reliance on muscle glycogen as a fuel than do untrained individuals. The reduction in glycogen use is accompanied by an increase in fat oxidation, and there are two reports of research that investigated the source of the additional fat breakdown by measuring the contribution ofintramuscular
Figure 4. Substrates providing energy during exercise at a given absolute intensity (64% of pre-training VO2max). Measurements were made when subjects were untrained (Before Training) and Trained (After Training) for endurance for 12 wk. After Training, oxidation of carbohydrate plasma FFA was reduced, whereas estimated intramuscular triglyceride use was increased. Statistical significant differences between before and after training treaments are indicated by *. Redrawn from Martin et al. (1993) with permission.
triglyceride and plasma FFA during exercise at 64% pretraining VO2max, before and after 12 wk of strenuous running and cycling (Hurley et al., 1986; Martin et al., 1993). The results of these studies are displayed in Figure 4. The reduction in muscle glycogen oxidation as a result of endurance training was directly associated with an increase in oxidation of triglycerides derived from within muscle, but not from plasma. The factors accounting for the increased intramuscular triglyceride use are not clear. Theoretically, previously reported increases in intramuscular triglyceride concentration after training (Morgan et al., 1969) could have been involved, but such an increase did not appear to take place in the two studies in question. Surprisingly, the rate of disappearance of plasma FFA was actually reduced following training. This suggests that mobilization and oxidation of fatty acids derived from adipose tissue during moderate intensity exercise does not change much as a result of endurance training. As described below, this result is consistent with those of cross-sectional studies comparing untrained and endurance trained people during low intensity exercise. Therefore, it appears that intramuscular triglyceride is the primary source of the fat that is oxidized at a greater rate as an adaptation to endurance training and that it is the oxidation of this intramuscular fat that is associated with a reduction in muscle glycogen utilization and with improved endurance performance.
We have recently compared the rates of plasma FFA mobilization and whole body lipolysis in untrained compared to endurance-trained men (Klein et al., 1994). During this experiment, both groups walked on a treadmill for 4 h at a brisk pace that elicited a VO2 of 20 mL · kg-1 · min-1. This elicited about 28% VO2max in the trained subjects compared to 43% VO2max in the untrained. As expected, total oxidation of body fat was about one-third greater in the trained than in the untrained subjects. Interestingly, at this low intensity of exercise, during which little intramuscular triglyceride use was expected, it appeared that the rate of plasma FFA disappearance very closely matched the rate of total fat oxidation in the trained subjects. This suggests that the endurance-trained individuals were able to oxidize fatty acids from adipose tissue at the same rate at which they were mobilized. In contrast, in the untrained subjects, even though the rates of whole body lipolysis and plasma FFA mobilization were identical to those in the trained subjects, the rate of fat oxidation was lower than in the trained subjects. Although the rate of disappearance of plasma FFA was similar in the two groups, trained subjects appeared capable of oxidizing a greater percentage of the FFA leaving the circulation. This indicates that untrained subjects have greater ability to mobilize than to oxidize FFA , and therefore a sizable portion of the mobilized FFA is reincorporated into triglyceride in some tissues. The major adaptation allowing trained subjects to oxidize more fat while walking seems to be an increase in the capacity of the muscles to oxidize FFA and not an increase in the mobilization of FFA from adipose tissue into plasma.
DIETARY CARBOHYDRATE INFLUENCES FAT OXIDATION DURING EXERCISE
EATING CARBOHYDRATE DURING THE HOURS BEFORE EXERCISE
Fat oxidation during exercise is very sensitive to the interval between eating carbohydrate and the onset of exercise and to the duration of the exercise. This is due in part to the elevation in plasma insulin in response to the carbohydrate meal and the resultant inhibition of lipolysis in adipose tissues, thus reducing the mobilization of FFA into the plasma. This effect is evident for at least 4 h after eating 140 g of carbohydrate that has a high glycemic index (Montain et al., 1991). Under these conditions, the carbohydrate meal reduces both total fat oxidation and plasma FFA concentration during the first 50 min of moderate-intensity exercise. However, this suppression of fat oxidation is reversed as the duration of exercise is increased; after 100 min of exercise, the rate of fat oxidation is similar, whether or not carbohydrate was eaten before exercise. It appears that the body relies heavily on carbohydrate and less on fat when people have eaten carbohydrate during the previous few hours, and therefore carbohydrate is preferred when it is available. It is likely that insulin plays a role in regulating the mixture of carbohydrate and fat oxidized during exercise.
This reduction in fat oxidation and increase in carbohydrate oxidation is not usually detrimental if all of the increase in carbohydrate oxidation is derived from glucose in the blood from the meal, thus having little influence on muscle glycogen use. Therefore, at present, there is little basis for recommending that people refrain from eating carbohydrate before exercise because such a meal will simply shift energy metabolism to less of a reliance on oxidation of plasma FFA and more on blood glucose oxidation, with lesser effects on muscle glycogen and intramuscular triglyceride utilization.
Plasma FFA mobilization is remarkably sensitive to even small increases in plasma insulin (Jensen et al., 1989), and it seems that lipolysis is influenced for a long time after eating carbohydrate (Montain et al., 1991). Diets that are lower in carbohydrate or that contain carbohydrates that cause less insulin secretion, probably still elicit enough of an insulin response to reduce plasma FFA mobilization. Therefore, any commercially available product or diet that claims to increase FFA mobilization and oxidation would have to almost totally eliminate the insulin response to the carbohydrate in their product, which seems unlikely. At the very least, the developers of these products must demonstrate that FFA mobilization is increased by their diets and is somehow beneficial. As discussed above, increased FFA mobilization would certainly not seem to be of any value for untrained people because their mobilization of FFA normally exceeds the ability of the muscles to oxidize FFA.
ELIMINATING CARBOHYDRATE FROM THE DIET OF ENDURANCE-TRAINED PEOPLE
Recognizing that even small amounts of dietary carbohydrate might influence fat metabolism, a study was performed by Phinney et al. (1983) during which they fed endurance-trained men a high-fat diet containing almost no carbohydrate, i.e., less than 20 g/d for 4 wk. This diet reduced the concentration of muscle glycogen by one-half, and it markedly increased fat oxidation during exercise at moderate intensities of 62-64% VO2max. However, the diet did not increase the length of time that exercise could be maintained, despite the fact that fat oxidation was increased. Furthermore, these subjects were not capable of exercising at higher intensities. Even with this extreme diet, it seems clear that fat oxidation cannot be increased sufficiently to fully replace muscle glycogen as a source of energy for intense exercise. Furthermore, high fat intake is a risk factor for cardiovascular and other diseases.
SUMMARY
People store large amounts of body fat in the form of triglyceride within adipose tissue as well as within muscle fibers. These stores must be mobilized into FFA and transported to muscle mitochondria for oxidation during exercise. Fatty acids from adipose tissue are mobilized into plasma and carried by albumin to muscle for oxidation. As exercise intensity increases from low (25% VO2max) to moderate (65% VO2max) to high (85% VO2max), plasma FFA mobilization declines. However, total fat oxidation increases when intensity increases from 25% to 65% VO2max, due to oxidation of intramuscular triglycerides, which provide about one-half of the fat for oxidation. Endurance training characteristically increases fat oxidation during moderate intensity exercise by accelerating the oxidation of intramuscular triglyceride without increasing the mobilization or oxidation of plasma FFA. Similarly, during low-intensity exercise with little intramuscular triglyceride oxidation, the increased fat oxidation of trained people does not appear to be caused by increased mobilization of FFA into plasma, but rather by a greater rate of oxidation of the FFA removed from the blood during exercise. Therefore, it seems that untrained people have greater abilities to mobilize FFA than they do to oxidize it when they exercise in the fasted state. Carbohydrate ingestion during the hours before exercise, even in relatively small amounts, reduces fat oxidation during exercise largely through the action of insulin. Fat supplementation and special diets have limited ability to increase fat oxidation in people, especially during sport competitions. Therefore, fat from body stores and/or dietary supplementation cannot adequately replace muscle glycogen and blood glucose as fuels for intense exercise.SSE #59: Fat Metabolism During Exercise: New Concepts
https://www.gssiweb.org/en-ca/article/sse-59-fat-metabolism-during-exercise-new-concepts
Yes! You do Burn Fat During Resistance Exercise
Lawrence Herrera and Len Kravitz, Ph.D.
Article reviewed:
Ormsbee, M. J., Thyfault, J. P., Johnson, E. A., Kraus, R. M., Choi, M. D., and Hickner, R. C. (2007). Fat metabolism and acute resistance exercise in trained men. Journal of Applied Physiology, 102, 1767-1772.
Introduction
Am I burning fat while doing resistance exercise? This is a question that clients ask personal trainers and fitness professionals regularly. Resistance training is an essential component of any weight management program due to it's chief role in maintaining and/or increasing lean body mass (muscle). Muscle contributes significantly to resting metabolic rate, which is the energy expended to maintain all bodily functions at rest. And a guiding principle of weight management is the attainment and maintenance of a 'negative' energy balance (i.e., burning more calories than storing) over extended periods of time. However, what physiological function does weight training actually provide to fat metabolism during and immediately following an exercise session? Surprisingly, this investigation led by Ornmsbee and colleagues (2007) is the first study to examine the specific effects of resistance exercise on adipose tissue fat metabolism. This research team also examined the extent the body uses fat as a fuel during and after a resistance training session.
Fat Metabolism 101: The Principle Physiological Functions
Fat is stored in the body in the form of triglycerides. Triglycerides are made up of three fatty acid molecules held together by a molecule of glycerol. The mobilization of fat refers to the initial process of releasing fat from storage sites (adipocytes) in adipose tissue. Lipolysis follows, which is the progression of reactions that biologically 'disassemble' the triglyceride into three fatty acids and glycerol, which are released into the blood. The metabolism of fat describes the complete biological breakdown or oxidation (which means loss of electrons) of fatty acids into energy that can be used by the cells of the body.
At the start of exercise the adrenal medulla (in the kidneys) secretes epinephrine and norepinephrine, which are part of the body's 'fight or flight' autonomic response to physical stress (such as exercise). Epinephrine and norepinephrine are major stimulatory hormones of hormone sensitive lipase (HSL). When HSL is stimulated, it acts to break apart the triglyceride in the manner defined above called lipolysis. HSL actions can be inhibited by insulin. Therefore, during exercise the rate of lipolysis is largely regulated by the balance between the stimulating effect of epinephrine and norepinephrine and the inhibitory effect of insulin.
The Study
Subjects
The subjects of this study were 8 physically active males in their mid-twenties who gave their written consent to participate before beginning the investigation. The volunteers answered a health history and physical activity questionnaire which showed that they had been participating in resistance exercise more than 3 days a week for the last 2 years. The researchers chose this specific population of exercisers because there is evidence that the lipolytic response to catecholamines (combined name for epinephrine and norepinehrine) may be compromised somewhat in overweight/obese populations (Bennard, Imbeautl and Doucet, 2005). Subjects were also free from any existing acute or chronic illness or from any known metabolic, cardiovascular or pulmonary disease. None were taking any medications or supplements and all subjects were nonsmokers.
Procedures
The subjects had three separate visits to the exercise physiology laboratories. During the first visit, baseline information including height, weight, body composition and 10 repetition maximum (10-RM) for all weight training exercises was collected. During the second and third visits, the participants were randomly assigned to either a resistance training day or a nonexercise control day. It should be noted that the participants abstained from vigorous activity, alcohol and caffeine 48 hours prior to each scheduled testing day. Also, at least 7 days passed between the two experimental testing days.
Body Composition and 10-RM
The subjects were weighed on an electronic scale and height was determined with a standard stadiometer (measurement device with movable horizontal board which comes in contact with head). Seven skinfold measurement sites (chest, midaxillary, tricep, subscapular, abdominal, supraillium, and thigh) were measured and used to calculate body density and estimate body fat percentage. The subjects 10-RM was assessed for the following exercises: chest press, lateral pull down, shoulder press, leg press, leg extension and leg curl.
Microdialysis and Resistance Exercise
During and immediately after each testing trial the subjects had microdialysis probes inserted into abdominal adipose tissue to measure lipolysis. Microdialysis is a technique used to determine the chemical components of the fluid in tissues. A tiny sterilized probe is inserted into the fat tissue. The tube is made of a semi-permeable membrane which allows specific molecules to pass. In this study the researchers measured glycerol, as it is an index of lipolysis.
The substrate (i.e., fat and carbohydrate) energy expenditure before, during and after the resistance training and control trials was measured with indirect calorimetry. With this laboratory technique each subject wears a mouth piece (attached to gas analyzers) for the collection and measurement of oxygen and carbon dioxide, the gases that are exchanged during respiration (oxygen being consumed while carbon dioxide is expired). Since fat and carbohydrates liberate energy when they are utilized by the cells, the energy expenditure can be measured (indirectly) and the specific contributions of fat and carbohydrate can be determined.
Subjects were instructed to fast 10-12 hours before reporting to the lab the day of testing as different foods might inhibit or accelerate certain steps of metabolism. Once at the lab the subjects were inserted with the microdialysis probe in subcutaneous fat tissue and underwent resting indirect calorimetry. The subjects were randomly assigned to either do a resistance training workout or no exercise (control) on their 2 experimental trials. On the resistance training day the volunteers performed 3 sets of 10 reps using a load of 85-100% of the subjects 10-RM on the chest press, lateral pull down, leg press, shoulder press, leg extension, and leg curl. Rest periods were kept to 90 seconds or less between all sets and exercises. Every step of the testing protocol was the same for the control day, except the subjects did not participate in the resistance exercise; they were kept resting in a supine position during that time. Immediately following the exercise session or the controlled rest period the subject underwent indirect calorimetry for 45 minutes. Microdialysis was continued for 5 hours post the exercise or control phase.
Dietary Control
The subjects were instructed to record their dietary intake for 2 days prior to the first test session (control day or resistance training day). They were instructed to replicate this 2-day dietary intake for the next testing session so that diet could not affect the study results.
Results
There are some very practical and important findings from this original investigation. Energy expenditure was elevated approximately 10.5% higher for 40 minutes after the workout day as compared to the control day. This effect confirms research shown in other studies (Bennard, Imbeautl and Doucet, 2005).
Secondly, and perhaps more meaningfully, microdialysis data indicated that glycerol levels (the marker for lipolysis) were raised 78% during and 75% after the resistance training as compared with corresponding times on the control day. In addition, the indirect calorimetry data showed that fat oxidation was 105% higher after the workout day as compared to the control session. Thus fat is definitely being used above resting values as a fuel (in conjunction with carbohydraes) during and after the resistance training bout. The enhanced lipolysis during and after exercise is hypothesized to be due to the increased levels of epinephrine and norepinephrine (Ormsbee et al., 2007; Bennard, Imbeautl and Doucet, 2005). In addition, previous research (Bennard, Imbeautl and Doucet, 2005) shows that growth hormone (a powerful activator of lipolysis) has been shown to be elevated after exercise and thus also contributes greatly to this post-exercise fat oxidation.
Essential Message for Personal Trainers and Fitness Professionals
This study is the first to directly show that resistance exercise increases adipose tissue lipolysis and thus contributes to improved body composition. This boost in lipolysis is apparently due to the excitatory effect of resistance training on specific hormones (e.g., epinephrine, norepinephrine and growth hormone). As this study design was completed with trained male subjects, it is hoped that the methods and procedures will be completed with other subject populations (e.g., females, untrained persons, youth, seniors, overweight, etc.) in future research.
Additional Reference:
Bennard, P., Imbeault, P., and Doucet, E. (2005). Maximizing acute fat utilization: Effects of exercise, food, and individual characteristics. Canadian Journal of Applied Physiology, 30(4), 475-499.Burning Fat During Resistance Exercise
https://www.unm.edu/~lkravitz/Article%20folder/burnfatUNM.html
Metabolic effects of catecholamines
Adrenaline and noradrenaline are both amines derived from the catechol nucleus, and the term catecholamines is often used to cover them both (see Fig. 5.10). It will be appreciated that the catecholamines have indirect effects on metabolism which are mediated through 'physiological' changes - heart rate, blood flow, etc. They also have indirect effects mediated through changes in hormone secretion, as well as direct effects in some tissues.
7.3.3.1 Glycogenolysis
In the liver, the catecholamines stimulate glycogen breakdown (glycogenolysis) through P2 (adenylyl cyclase-linked) receptors and the 'cascade' mechanism discussed earlier (see Box 2.4). In addition, they can activate glycogenolysis through a second mechanism, via the a1 (phospholipase C-linked) receptors (see Table 5.1) and elevation of the cytoplasmic Ca2+ concentration. (This was mentioned in Box 2.4; one particular situation in which elevation of intracellular Ca2+ concentration stimulates glycogenolysis will be covered in Section 8.4.3 and Fig. 8.8.) The degradation of glycogen, via glucose 1-phosphate, leads to production of glucose, which can be released into the bloodstream. This is a major response to hypoglycaemia (a fall in the blood glucose concentration) and leads to rapid restoration of the glucose concentration provided there is adequate glycogen stored in the liver. There is much experimental evidence that the liver is supplied with sympathetic nerves and that these can activate glycogenolysis directly, although adrenaline from the adrenal medulla is also released under such conditions and will certainly play a role. In humans, it has been very difficult to show directly that the sympathetic nerves are involved, but people whose adrenals have been removed can respond fairly normally to glucose deprivation, implying that at least in that situation the sympathetic nerves to the liver can play a role.
In skeletal muscle, the catecholamines are undoubtedly important for stimulation of glycogenolysis; but they are not in themselves sufficient to activate it. The activation of skeletal muscle glycogen breakdown is intimately linked with the stimulation of muscle contraction, which, as we have seen, is brought about by the cholinergic fibres of the somatic nervous system. (The links between contraction and glycogenolysis will be fully discussed in Chapter 8; see Fig. 8.8.) Glycogenolysis seems to be 'primed' by catecholamines, perhaps released in response to anticipation of the exercise. Circulating adrenaline is likely to be more important in this respect than noradrenaline from sympathetic nerve terminals, since the main (possibly the only) sympathetic supply to muscle is to the smooth muscle of the blood vessels and is responsible for regulation of blood flow.
7.3.3.2 Lipolysis
Human fat cells have both a2- and P1-adrenergic receptors. There are also P3 ('atypical') receptors that are responsible for stimulation of lipolysis in rodent fat cells, although their role in humans is presently unclear.
The a2 receptors are linked, via inhibitory G; proteins, to adenylyl cyclase, and reduce its activity. The P1 receptors are linked to it through Gs proteins and stimulate its activity. Activation of adenylyl cyclase will increase the cellular concentration of cAMP and activate hormone-sensitive lipase (see Box 2.4), bringing about a breakdown of the triacylglycerol stores and the release of non-esterified fatty acids into the plasma.
There is usually a balance between stimulatory and inhibitory effects, and in normal sedentary daily life it is probable that regulation of hormonesensitive lipase by insulin predominates. However, in response to any kind of stress, including exercise, there is activation of the P1 receptors so that lipolysis is stimulated. Blockade of the P receptors with the P-antagonist propranolol reduces, or may completely suppress, the liberation of non-esterified fatty acids into the plasma in response to exercise (Fig. 7.7). Activation of hormone-sensitive lipase can be brought about purely by mental stress. Stimulation of lipolysis is an important feature of the response to physical stresses such as surgical operations or injury. Again, it is not certain to what extent the direct innervation of adipose tissue is involved, or whether circulating adrenaline plays the major role. But, as with glycogenolysis, people without adrenals can raise their plasma non-esterified fatty acid concentration in response to lack of glucose, so the sympathetic nerves must play a role in that situation.
Not only is the rate of lipolysis regulated by the nervous system, but also the rate of blood flow through adipose tissue. This can have indirect effects on the release of non-esterified fatty acids. In very severe stress states, typified by physical injury with major blood loss, a-adrenergic effects predominate in the blood vessels of adipose tissue and cause it to constrict. Presumably the body is trying to preserve blood for more vital organs and tissues. This reduces the ability of adipose tissue to liberate fatty acids into the plasma, since the binding
Catecholamine Effects Urine
Fig. 7.7 Propranolol (a ^-adrenergic blocker) inhibits lipolysis in response to exercise. The figure shows changes in the concentration of glycerol (released in fat mobilisation) in the interstitial fluid in adipose tissue, measured with a small probe. During exercise (0-30 min) the glycerol concentration rises, indicating lipolysis. When propranolol is introduced (via the probe) the rise is inhibited. In separate experiments, when phentolamine (an a-adrenergic blocker) was introduced, glycerol release was not affected. Based on Arner, P., Kriegholm, E., Engfeldt, P. & Bolinder, J. (1990) Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest 85: 893-898. Reproduced with permission.
Fig. 7.7 Propranolol (a ^-adrenergic blocker) inhibits lipolysis in response to exercise. The figure shows changes in the concentration of glycerol (released in fat mobilisation) in the interstitial fluid in adipose tissue, measured with a small probe. During exercise (0-30 min) the glycerol concentration rises, indicating lipolysis. When propranolol is introduced (via the probe) the rise is inhibited. In separate experiments, when phentolamine (an a-adrenergic blocker) was introduced, glycerol release was not affected. Based on Arner, P., Kriegholm, E., Engfeldt, P. & Bolinder, J. (1990) Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest 85: 893-898. Reproduced with permission.
sites on the albumin become saturated, and fatty acids may accumulate within the tissue. Thus, after moderately severe injuries or during surgical operations, the level of non-esterified fatty acids in the plasma is usually very high, but after very severe injuries the level may be relatively normal. Although there is no doubt that lipolysis is activated, the fatty acids are unable to leave the adipose tissue as rapidly as they are released from triacylglycerol (Table 7.1). The same phenomenon may come into play to some extent during strenuous exercise (see Chapter 8).
7.3.3.3 Glucose utilisation
There is consistent evidence that elevated plasma adrenaline concentrations impair glucose utilisation by skeletal muscle. One plausible mechanism is that adrenaline stimulates glycogenolysis, so there is an accumulation of glucose 6-phosphate, which will inhibit hexokinase and reduce the entry of further glucose into the cell. This might seem odd during exercise, but in that situation other mechanisms operate to stimulate glucose entry, mainly exercise-induced translocation of GLUT4 to the cell membrane. Also, during exercise glycolysis will be rapid and so any build-up of glucose 6-phosphate probably minimised. But we can see that during hypoglycaemia, inhibition of muscle glucose utilisation by adrenaline would spare glucose for use by the brain.
However, there is also evidence for P-adrenergic receptor-stimulation of glucose uptake into muscle. This seems to be mediated by the sympathetic nervous system rather than by circulating adrenaline: effects of hypothalamic.Metabolic effects of catecholamines - Adipose Tissue
https://www.alpfmedical.info/adipose-tissue/metabolic-effects-of-catecholamines.html