内皮细胞研究 Endothelial Cells Studies
effects of insulin/glucose/nitric oxide/ROS on EC
studies in vitro demonstrated a nitric oxide-dependent vasodilator effect of insulin on arterioles from skeletal muscle
Insulin also enhanced the expression of the potent vasoconstrictor endothelin-1 by endothelial cells in vitro (28) and in vivo (29).
Interaction between insulin and nitric oxide
uptake of glucose: The endothelial cell uptake of glucose is rapid, is glucose transporter (GLUT)-4 independent (GLUT-1 appears to be the predominant glucose transporter) and occurs in proportion to the plasma glucose concentration (40). The endothelial cell uptake of glucose is rapid, is glucose transporter (GLUT)-4 independent (GLUT-1 appears to be the predominant glucose transporter) and occurs in proportion to the plasma glucose concentration (40). acute increases in circulating glucose concentrations to provoke endothelial dysfunction. In clinical studies excess FFA levels secondary to lipid infusion (8) as well as an acute high fat meal can cause endothelial dysfunction. A high fat meal can also acutely increase plasma endotoxin concentrations (44), again supporting the endothelium as an early sensor and responder to factors that provoke insulin resistance. Thus, excess nutrient supply (of either carbohydrate or fat) adversely affects the endothelial cell.
endothelial progenitor cells
microvascular retinal endothelial cells do not decrease glucose uptake when exposed to high extracellular glucose concentrations, whereas brain- and heart-derived endothelial cells do [14]
metformin on EC: First-line therapy of type 2 diabetes is metformin, which is very effective in lowering blood glucose and could be nephroprotective for patients with DKD [74]. Beyond its anti-hyperglycemic properties, studies have shown beneficial effects of metformin therapy on endothelial cells. In endothelial cells, metformin decreases mitochondrial oxidative stress, increases NO bioavailability and reduces endothelial senescence and apoptosis [75]. These effects are probably mediated by reduced oxidative stress, as well as activation of the AMPK pathway leading to decreased mTOR signaling [75]. Clinical studies have provided evidence that metformin improves endothelium-dependent vasodilation in vivo [75].
FDA-approved drugs inhibit the polyol pathway(glucose to sorbitol to fructose): anti-oxidants, like α-lipoic acid, vitamin E, and vitamin C
effects of AGEs: via RAGE, deposition, in situ glycation
The Endothelial Cell: an “Early Responder” in the ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594570
The endothelial cell uptake of glucose is rapid, is glucose transporter (GLUT)-4 independent (GLUT-1 appears to be the predominant glucose transporter) and occurs in proportion to the plasma glucose concentration (40).
Cited by: 61
Publish Year: 2013
Author: Eugene J. Barrett, Eugene J. Barrett, Zhenqi Liu
Toxins (Basel). 2019 Oct;
Endothelial Toxicity of High Glucose and its by-Products in Diabetic Kidney Disease
Laetitia Dou1,* and Noémie Jourde-Chiche1,2,†
1Aix Marseille University, INSERM, INRA, C2VN, Faculté de Pharmacie, 27 Bd Jean Moulin, 13005 Marseille, France;
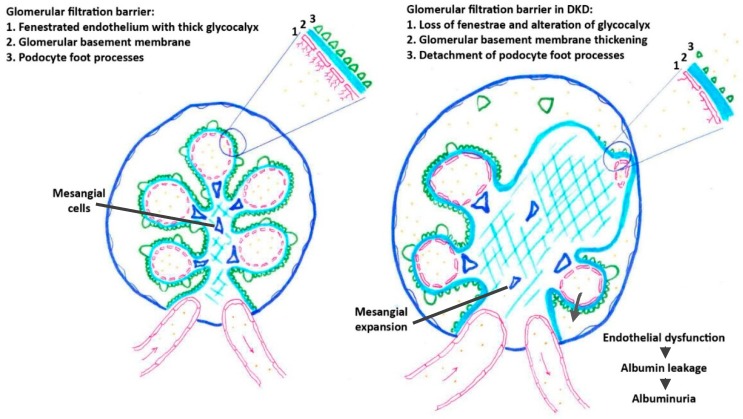
Alterations of renal endothelial cells play a crucial role in the initiation and progression of diabetic kidney disease. High glucose per se, as well as glucose by-products, induce endothelial dysfunction in both large vessels and the microvasculature.Toxic glucose by-products include advanced glycation end products (AGEs), a group of modified proteins and/or lipids that become glycated after exposure to sugars, and glucose metabolites produced via the polyol pathway. These glucose-related endothelio-toxins notably induce an alteration of the glomerular filtration barrier by increasing the permeability of glomerular endothelial cells, altering endothelial glycocalyx, and finally, inducing endothelial cell apoptosis.
The glomerular endothelial dysfunction results in albuminuria.
In addition, high glucose and by-products impair the endothelial repair capacities by reducing the number and function of endothelial progenitor cells.
In this review, we summarize the mechanisms of renal endothelial toxicity of high glucose/glucose by-products, which encompass changes in synthesis of growth factors like TGF-β and VEGF, induction of oxidative stress and inflammation, and reduction of NO bioavailability. We finally present potential therapies to reduce endothelial dysfunction in diabetic kidney disease.
Pathological changes affecting glomerular filtration barrier in DKD.
Keywords: AGEs, diabetic kidney disease, endothelial dysfunction, glucose, polyols
Endothelial Toxicity of High Glucose and its by-Products ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6833015
Dev Cell. 2017 Jun 5
Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer
Prostate cancer (PCa) bone metastasis is frequently associated with bone-forming lesions, but the source of the osteoblastic lesions remains unclear. We show that the tumor-induced bone derives partly from tumor-associated endothelial cells that have undergone endothelial-to-osteoblast (EC-to-OSB) conversion. The tumor-associated osteoblasts in PCa bone metastasis specimens and patient-derived xenografts (PDXs) were found to co-express endothelial marker Tie-2. BMP4, identified in PDX-conditioned medium, promoted EC-to-OSB conversion of 2H11 endothelial cells. BMP4 overexpression in non-osteogenic C4-2b PCa cells led to ectopic bone formation under subcutaneous implantation. Tumor-induced bone was reduced in trigenic mice (Tie2cre/Osxf/f/SCID) with endothelial-specific deletion of osteoblast cell-fate determinant OSX compared with bigenic mice (Osxf/f/SCID). Thus, tumor-induced EC-to-OSB conversion is one mechanism that leads to osteoblastic bone metastasis of PCa.
Keywords: bone metastasis; endothelial-to-osteoblast conversion; osteoblast; paracrine factors; prostate cancer; proteomics.Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer - PubMed
https://pubmed.ncbi.nlm.nih.gov/28586644/
Conversion of vascular endothelial cells into multipotent stem-like cells
Mesenchymal stem cells can give rise to several cell types, but varying results depending on isolation methods and tissue source have led to controversies about their usefulness in clinical medicine. Here we show that vascular endothelial cells can transform into multipotent stem-like cells by an activin-like kinase-2 (ALK2) receptor–dependent mechanism. In lesions from individuals with fibrodysplasia ossificans progressiva (FOP), a disease in which heterotopic ossification occurs as a result of activating ALK2 mutations, or from transgenic mice expressing constitutively active ALK2, chondrocytes and osteoblasts expressed endothelial markers. Lineage tracing of heterotopic ossification in mice using a Tie2-Cre construct also suggested an endothelial origin of these cell types. Expression of constitutively active ALK2 in endothelial cells caused endothelial-to-mesenchymal transition and acquisition of a stem cell–like phenotype. Similar results were obtained by treatment of untransfected endothelial cells with the ligands transforming growth factor-β2 (TGF-β2) or bone morphogenetic protein-4 (BMP4) in an ALK2-dependent manner. These stem-like cells could be triggered to differentiate into osteoblasts, chondrocytes or adipocytes. We suggest that conversion of endothelial cells to stem-like cells may provide a new approach to tissue engineering.血管内皮细胞转化为多能干样细胞
间充质干细胞可以产生几种细胞类型,但是取决于分离方法和组织来源的不同结果导致了有关其在临床医学中的用途的争议。在这里,我们显示血管内皮细胞可以通过激活素样激酶2(ALK2)受体依赖性机制转变为多能干细胞样细胞。在具有骨化性增生性纤维增生症(FOP)的个体的病变中,由于激活ALK2突变而发生异位骨化的疾病,或表达组成性活性ALK2的转基因小鼠,软骨细胞和成骨细胞表达的内皮标记。使用Tie2-Cre构建体的小鼠异位骨化的谱系追踪也暗示了这些细胞类型的内皮起源。组成性活性ALK2在内皮细胞中的表达引起内皮向间充质的转变和干细胞样表型的获得。通过以ALK2依赖性方式用转化生长因子-β2(TGF-β2)或骨形态发生蛋白-4(BMP4)的配体处理未转染的内皮细胞,可获得类似的结果。可以触发这些干样细胞分化为成骨细胞,软骨细胞或脂肪细胞。我们建议内皮细胞向干细胞样细胞的转化可能为组织工程提供一种新方法。
Conversion of vascular endothelial cells into multipotent stem-like cells — MD Anderson Cancer Center
https://mdanderson.elsevierpure.com/en/publications/conversion-of-vascular-endothelial-cells-into-multipotent-stem-li-2