
¡¡
EGFR/ErbB receptor Tyrosine Kinase Superfamily and Ligand-stimulated EGFR endocytosis
¡¡
EGFR/ErbB receptors is Tyrosine Kinase Superfamily.belong to ERK superfamily.
There are 8 ligands:TNF-al;pha, EGF, transforming growth factor-alpha (TGFA), heparin-binding EGF-like growth factor (HBEGF), betacellulin (BTC), amphiregulin (AREG), epiregulin (EREG), and epigen (EPGN).
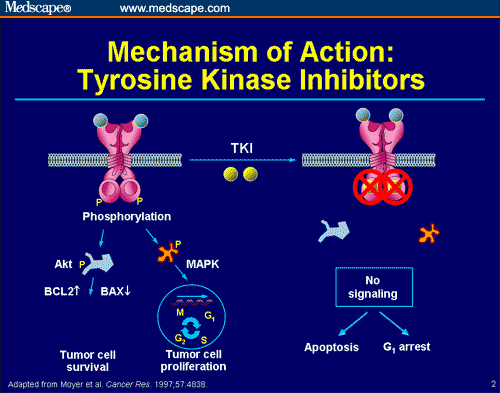
ligand-stimulated phospholation and endocytosis, binding to DNA leads to enhanced uncontrolled proliferation
Receptor Tyrosine kinase inhibitors:vitamin C,EGCG,curcumin,Dehydroascorbic acid (DHA) directly inhibits I¦ÊB¦Á kinase ¦Â (IKK¦Â) and IKK¦Á enzymatic activity
vitamin c block TNF-alpha signaling, thus
curcumin is a TKI
¡¡
¡¡

APMG Lung Molecular Pathways
http://www.apmggroup.net/innovation/molecular_testing/Lung_Pathways/lung.html
¡¡

¡¡

¡¡
¡¡
¡¡
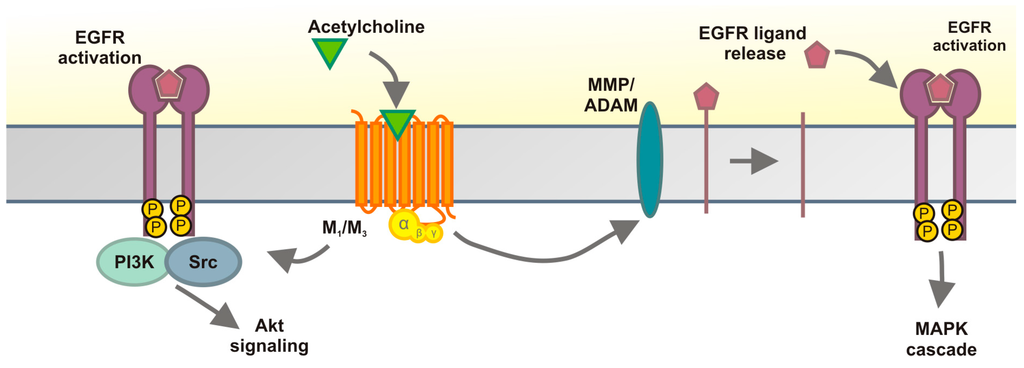
IJMS | Free Full-Text | Epidermal Growth Factor Receptor Transactivation Is
Required for Mitogen-Activated Protein Kinase Activation by Muscarinic
Acetylcholine Receptors in HaCaT Keratinocytes | HTML
https://www.mdpi.com/1422-0067/15/11/21433/htm
¡¡

¡¡

¡¡

¡¡
¡¡
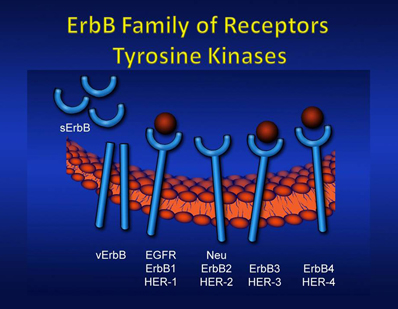
The ErbB family of proteins contains four receptor tyrosine kinases,
structurally related to the epidermal growth factor receptor (EGFR), its first
discovered member. In humans, the family includes Her1 (EGFR, ErbB1), Her2 (Neu,
ErbB2), Her3 (ErbB3), and Her4 (ErbB4). May 13 2019
ErbB - Wikipedia
en.wikipedia.org/wiki/ErbB
¡¡
Given that ligand binding is essential for the rapid internalization of
epidermal growth factor receptor (EGFR), the events induced by ligand binding
probably contribute to the regulation of EGFR internalization. These events
include receptor dimerization, activation of intrinsic tyrosine kinase activity
and autophosphorylation.
Control of epidermal growth factor receptor endocytosis by ...
www.embopress.org/doi/full/10.1038/sj.embor.7400491
Was this helpful?
Internalization and intracellular sorting of the EGF ...
https://jcs.biologists.org/content/122/19/3433
Oct 01, 2009 ¡¤ The epidermal growth factor receptor (EGFR; also known as ErbB1)
is so far the best characterized of the four ErbB-family members (EGFR, ErbB2,
ErbB3 and ErbB4). EGFR binds to EGF with high affinity in a specific and
saturable manner (for a review, see Carpenter and Cohen, 1979). This binding
elicits the internalization and intracellular sorting of receptor-ligand
complexes and activates ¡
Cited by: 151
Publish Year: 2009
Author: Inger Helene Madshus, Espen Stang
EGF receptor trafficking: consequences for signaling and ...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3884125
Ligand-stimulated EGFR endocytosis: a positive and negative regulator of
signaling. However, internalization of activated EGFR also enables specific
signaling pathways from intracellular sites, and endocytic trafficking of EGFRs
is required for optimal activation of a subset of signal transducers [2].
Cited by: 399
Publish Year: 2014
Author:
¡¡
PLoS One. 2013;8(3):e58148. doi: 10.1371/journal.pone.0058148. Epub 2013 Mar
5.
Internalization mechanisms of the epidermal growth factor receptor after
activation with different ligands.
Henriksen L1, Grandal MV, Knudsen SL, van Deurs B, Grøvdal LM.
Author information
1
Department of Cellular and Molecular Medicine, University of Copenhagen,
Copenhagen, Denmark.
Abstract
The epidermal growth factor receptor (EGFR) regulates normal growth and
differentiation, but dysregulation of the receptor or one of the EGFR ligands is
involved in the pathogenesis of many cancers. There are eight ligands for EGFR,
however most of the research into trafficking of the receptor after ligand
activation focuses on the effect of epidermal growth factor (EGF) and
transforming growth factor-¦Á (TGF-¦Á). For a long time it was believed that
clathrin-mediated endocytosis was the major pathway for internalization of the
receptor, but recent work suggests that different pathways exist. Here we show
that clathrin ablation completely inhibits internalization of EGF- and
TGF-¦Á-stimulated receptor, however the inhibition of receptor internalization in
cells treated with heparin-binding EGF-like growth factor (HB-EGF) or
betacellulin (BTC) was only partial. In contrast, clathrin knockdown fully
inhibits EGFR degradation after all ligands tested. Furthermore, inhibition of
dynamin function blocked EGFR internalization after stimulation with all
ligands. Knocking out a number of clathrin-independent dynamin-dependent
pathways of internalization had no effect on the ligand-induced endocytosis of
the EGFR. We suggest that EGF and TGF-¦Á lead to EGFR endocytosis mainly via the
clathrin-mediated pathway. Furthermore, we suggest that HB-EGF and BTC also lead
to EGFR endocytosis via a clathrin-mediated pathway, but can additionally use an
unidentified internalization pathway or better recruit the small amount of
clathrin remaining after clathrin knockdown.
Internalization mechanisms of the epidermal growth factor receptor after
activation with different ligands. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/23472148
¡¡
Ligand-activated epidermal growth factor receptor (EGFR)
signaling governs endocytic trafficking of unliganded receptor monomers by
non-canonical phosphorylation
Tomohiro Tanaka‡, Yue Zhou‡,¡ì, Tatsuhiko Ozawa¶, Ryuya Okizono‡,
Ayako Banba‡, Tomohiro Yamamura‡, Eiji Oga‡, Atsushi Muraguchi¶ and Hiroaki
Sakurai‡1
- Author Affiliations
From the Departments of ‡Cancer Cell Biology and
¶Immunology, Graduate School of Medicine and Pharmaceutical Sciences, University
of Toyama, Toyama 930-0194, Japan and
the ¡ìMOE Key Laboratory for Standardization of Chinese Medicines and the
Shanghai Key Laboratory of Compound Chinese Medicines, Institute of Chinese
Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai
201203, China
Abstract
The canonical description of transmembrane receptor function is initial binding
of ligand, followed by initiation of intracellular signaling and then
internalization en route to degradation or recycling to the cell surface. It is
known that low concentrations of extracellular ligand lead to a higher
proportion of receptor that is recycled and that non-canonical mechanisms of
receptor activation, including phosphorylation by the kinase p38, can induce
internalization and recycling. However, no connections have been made between
these pathways; i.e. it has yet to be established what happens to unbound
receptors following stimulation with ligand. Here we demonstrate that a
minimal level of activation of epidermal growth factor receptor (EGFR) tyrosine
kinase by low levels of ligand is sufficient to fully activate downstream
mitogen-activated protein kinase (MAPK) pathways, with most of the remaining
unbound EGFR molecules being efficiently phosphorylated at intracellular
serine/threonine residues by activated mitogen-activated protein kinase(MAPK).
This non-canonical, p38-mediated phosphorylation of the C-tail of EGFR, near
Ser-1015, induces the clathrin-mediated endocytosis of the unliganded EGFR
monomers, which occurs slightly later than the canonical endocytosis of
ligand-bound EGFR dimers via tyrosine autophosphorylation. EGFR endocytosed
via the non-canonical pathway is largely recycled back to the plasma membrane as
functional receptors, whereas p38-independent populations are mainly sorted for
lysosomal degradation. Moreover, ligand concentrations balance these endocytic
trafficking pathways. These results demonstrate that ligand-activated EGFR
signaling controls unliganded receptors through feedback phosphorylation,
identifying a dual-mode regulation of the endocytic trafficking dynamics of
EGFR.
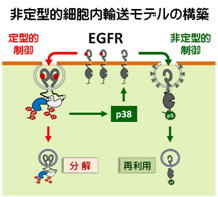
Ligand-activated epidermal growth factor receptor (EGFR) signaling governs
endocytic trafficking of unliganded receptor monomers by non-canonical
phosphorylation
http://www.jbc.org/content/293/7/2288.short
¡¡
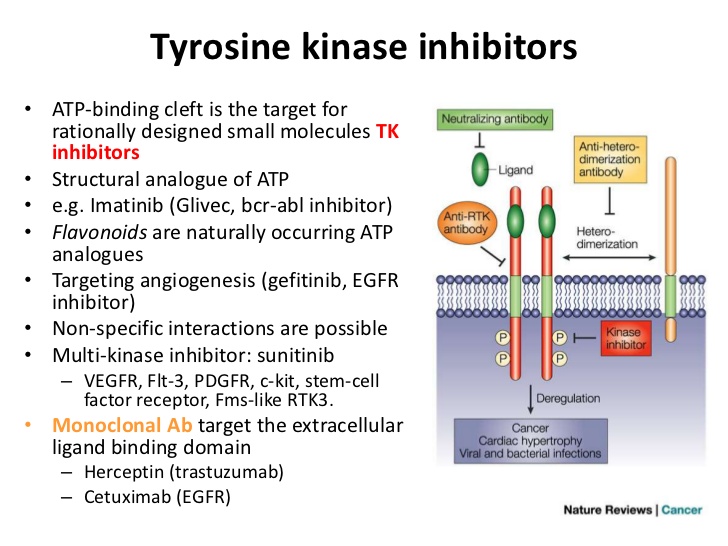
Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, EGCG.

f2-ijms-9-6-1034: Schematic representation of the erbB family and the IGF/IGF-1R
system. (A) The family of erbB receptors includes four members: EGFR (erbB1),
HER2 (neu/erbB2), HER3 (erbB3), and HER4 (erbB4). All members have an
extracellular ligand binding region (cysteine rich domain), a single
membrane-spanning region, and a cytoplasmic tyrosine-kinase-containing domain.
Ligand, such as TGF-¦Á etc, binding to erbB receptors induces the formation of
receptor homo- and heterodimers and the activation of the intrinsic kinase
domain, thus resulting in phosphorylation on specific tyrosine residues within
the cytoplasmic tail. These phosphorylated residues serve as docking sites for a
range of proteins, the recruitment of which leads to the activation of
intracellular signaling pathways, including the Ras/MAPK and PI3K/Akt pathways.
(B) The IGF/IGF-1R system is composed of ligands (IGF-1 and IGF-2), receptor
(IGF-1R), and ligand binding proteins (IGFBPs). IGF-1 and IGF-2 are found in the
circulation complexed to IGFBPs, which serve to regulate the bioavailability of
these ligands in the tissues. The IGF-1R contains two ¦Á (cysteine rich domain)
and two ¦Â (tyrosine kinase domain) subunits which are joined by disulfide
bridges to form a heterotetrameric receptor complex. The IGF/IGF-1R interaction
results in phosphorylation of tyrosine residues in the tyrosine kinase domain.
After autophosphorylation, the receptor kinase phosphorylates intracellular
proteins, which enable activation of the PI3K/Akt and Ras/MAPK signaling
pathways. A detailed description of the downstream signaling pathway is provided
in ¡°Figure 3¡±.
Abstract
Tea is one of the most popular beverages consumed worldwide. Epidemiologic
studies show an inverse relationship between consumption of tea, especially
green tea, and development of cancers. Numerous in vivo and in vitro studies
indicate strong chemopreventive effects for green tea and its constituents
against cancers of various organs. (-)-Epigallocatechin-3-gallate (EGCG), the
major catechin in green tea, appears to be the most biologically active
constituent in tea with respect to inhibiting cell proliferation and inducing
apoptosis in cancer cells. Recent studies indicate that the receptor tyrosine
kinases (RTKs) are one of the critical targets of EGCG to inhibit cancer cell
growth. EGCG inhibits the activation of EGFR (erbB1), HER2 (neu/erbB2) and also
HER3 (neu/erbB3), which belong to subclass I of the RTK superfamily, in various
types of human cancer cells. The activation of IGF-1 and VEGF receptors, the
other members of RTK family, is also inhibited by EGCG. In addition, EGCG alters
membrane lipid organization and thus inhibits the dimerization and activation of
EGFR. Therefore, EGCG inhibits the Ras/MAPK and PI3K/Akt signaling pathways,
which are RTK-related cell signaling pathways, as well as the activation of AP-1
and NF-kappaB, thereby modulating the expression of target genes which are
associated with induction of apoptosis and cell cycle arrest in cancer cells.
These findings are significant because abnormalities in the expression and
function of RTKs and their downstream effectors play a critical role in the
development of several types of human malignancies. In this paper we review
evidence indicating that EGCG exerts anticancer effects, at least in part,
through inhibition of activation of the specific RTKs and conclude that
targeting RTKs and related signaling pathway by tea catechins might be a
promising strategy for the prevention of human cancers.
Bottom Line: In addition, EGCG alters membrane lipid organization and thus
inhibits the dimerization and activation of EGFR.These findings are significant
because abnormalities in the expression and function of RTKs and their
downstream effectors play a critical role in the development of several types of
human malignancies.In this paper we review evidence indicating that EGCG exerts
anticancer effects, at least in part, through inhibition of activation of the
specific RTKs and conclude that targeting RTKs and related signaling pathway by
tea catechins might be a promising strategy for the prevention of human cancers.
Schematic representation of the erbB family and the IGF | Open-i
https://openi.nlm.nih.gov/detailedresult?img=PMC2658783_ijms-9-6-1034-f2&req=4
¡¡
European Journal of Medicinal Chemistry
Volume 181, 1 November 2019, 111512
European Journal of Medicinal Chemistry
Review article
Curcumin as tyrosine kinase inhibitor in cancer treatment
Author links open overlay
panelA.GolonkoaH.LewandowskabR.ŚwisłockacU.T.Jasi¨½skaaW.PriebedW.Lewandowskia
a
Institute of Agricultural and Food Biotechnology, Rakowiecka 36, 02-532, Warsaw,
Poland
b
Institute of Nuclear Chemistry and Technology, Centre for Radiobiology and
Biological Dosimetry, Dorodna 16, 03-195, Warsaw, Poland
c
Bialystok University of Technology, Faculty of Civil Engineering and
Environmental Engineering, Department of Chemistry, Biology and Biotechnology,
Wiejska 45E, 15-351, Bialystok, Poland
d
Department of Experimental Therapeutics, University of Texas MD, Anderson Cancer
Center, Houston, TX, USA

Highlights
•
Our article summarizes knowledge about the mechanisms of curcumin activity
directed to signaling pathways of tyrosine kinases.
•
We have attempted to explain how the antitumor activity can be enhanced by
modifications in the structure of the molecule.
•
We have discussed the possibility of using curcumin in anti-cancer therapy and
problems with its bioavailability.
Abstract
Curcumin is a natural substance known for ages, exhibiting a multidirectional
effect in cancer prevention and adjuvant cancer therapies. The great advantage
of using nutraceuticals of vegetable origin in comparison to popular cytostatic
drugs is the minimized side effect and reduced toxicity. The targets in
oncological therapy are, among others, tyrosine kinases, important mediators of
signaling pathways whose impaired expression is observed in many types of
cancer. Unfortunately, the hydrophobic nature of the curcumin molecule often
limits its bioavailability, which is why many studies focus on the chemical
modification of this compound. Current research is aimed at modifying structures
that improve the pharmacokinetic parameters of curcumin, e.g. the formation of
nanoparticles, complexes with metals or the synthesis of curcumin derivatives
with functional substituents that allow tumor targeting. The article is a review
and analysis of current literature on the properties of curcumin and its
derivatives in the treatment of cancers directed to signaling pathways of
tyrosine kinases and confronts the problem of low assimilation of curcumin with
potential therapeutic effects.
Curcumin as tyrosine kinase inhibitor in cancer treatment - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0223523419306361
¡¡

¡¡


¡¡
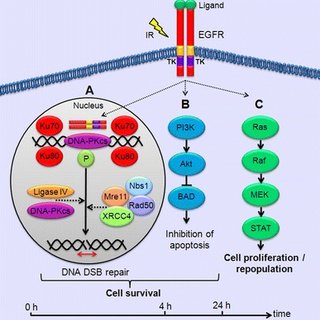
NORMAL MOLECULAR PATHWAYS AND ASSOCIATED MUTATIONS
The RAS-RAF-MEK-ERK signaling pathway (MAPK pathway) is a classical
intracellular pathway that plays a crucial role in the homeostasis of normal
cell turnover, cellular proliferation, differentiation, survival, and apoptosis.
When activated aberrantly, this signaling pathway can induce tumorigenesis and
has been associated with various malignancies.
MAPK Signaling Pathway
edge
EGFR
The Epidermal Growth Factor Receptor (EGFR), also known as HER1 and ERBB1, is an
important trans-membrane tyrosine kinase receptor involved in the initiation of
the MAPK pathway. Activating mutations in EGFR can lead to aberrant cellular
proliferation, inhibition of apoptosis, angiogenesis, increased cell survival,
and gene transcription.
Some patients with tumors carrying EGFR mutations may respond to selective
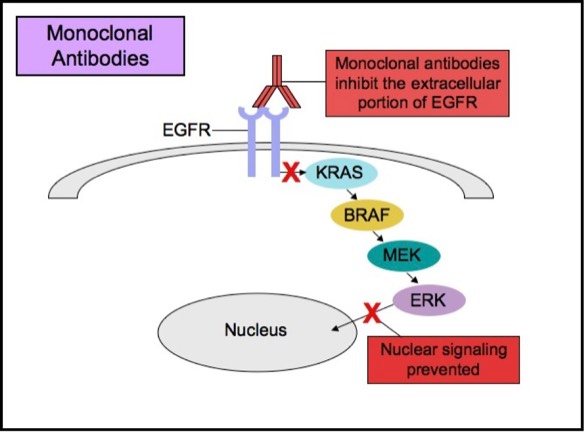
anti-EGFR therapies. EGFR has an extracellular ligand-binding region and a
cytoplasmic tyrosine kinase-containing domain. Currently, there are two major
types of anti-EGFR drug therapies. The first drug type (cetuximab, panitumumab)
is a monoclonal antibody that inhibits EGFR by binding to an extracellular
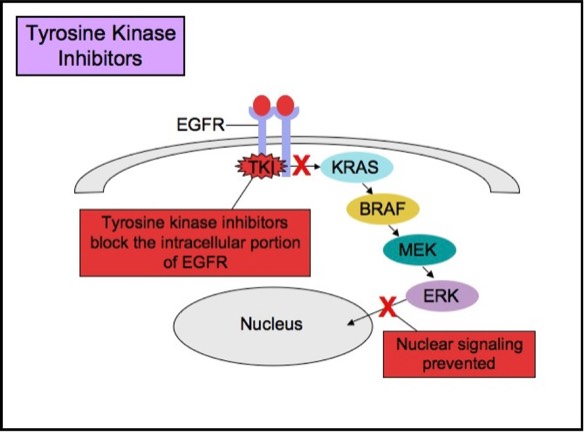
portion of the EGFR protein. The second type (gefitinib, erlotinib) is an
intracellular tyrosine kinase inhibitor that inhibits EGFR by crossing the cell
membrane and blocking the receptor¡¯s active site.
Monoclonal Anti-EGFR Therapy
edge
Tyrosine Kinase Inhibitor Therapy
edge
Studies show that patients whose lung cancers exhibit EGFR mutations may benefit
from anti-EGFR therapies. Tumor subtypes most likely to respond to anti-EGFR
therapies include adenocarcinoma, non-mucinous bronchioloalveolar carcinoma
(BAC), and adenosquamous carcinoma. EGFR mutations are rare in other histologic
types of lung cancer (small cell carcinoma, squamous cell carcinoma, and large
cell carcinoma). Thus, anti-EGFR therapies are most commonly employed for
treatment of adenocarcinoma. The two most common EGFR mutations, comprising
about 90% of all EGFR mutations in lung adenocarcinomas, are on exon 19 and 21
(L858R). Patients with these mutations are likely to respond to anti-EGFR
therapies. There are also less common mutations (exon 20 insertion), including
those that actually cause poor response to anti-EGFR therapies, but these
mutations are relatively uncommon.
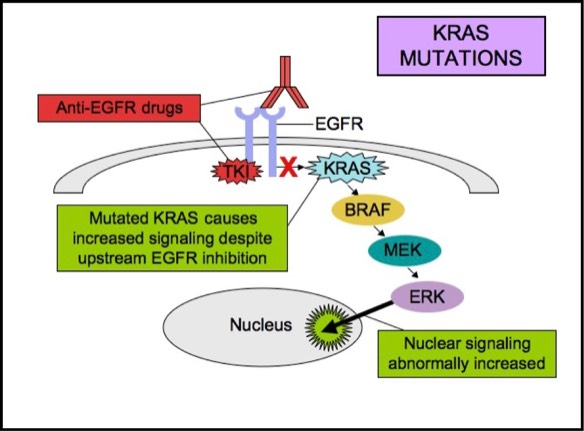
KRAS
KRAS is a small G-protein that is important for EGFR signaling. KRAS mutations
activate downstream signaling cascade despite the upstream EGFR regulation.
Anti-EGFR therapies are ineffective in tumors with such activating KRAS
mutations, because the KRAS mutations trigger the EGFR-signaling cascade at a
point downstream of the therapy¡¯s target. Therefore, both monoclonal antibodies
and tyrosine kinase inhibitors may fail to respond in the presence of KRAS
mutations.
KRAS mutations are seen in about 15-20% of non-small cell lung cancers and
30-50% of lung adenocarcinomas. EGFR and KRAS mutations are essentially mutually
exclusive in lung adenocarcinomas. The presence of both EGFR and KRAS mutations
in lung adenocarcinomas is rare, but if they do occur together, EGFR inhibitors
are less likely to be effective. KRAS mutation is a poor prognostic indicator.
The presence of KRAS mutation usually means failure to respond to anti-EGFR
therapy. Anti-EGFR therapies are likely to be ineffective in patients with
advanced or metastatic lung adenocarcinomas when a KRAS mutation is present.
In patients with lung adenocarcinoma, non-mucinous BAC, or adenosquamous
carcinoma, EGFR mutation analysis by polymerase chain reaction (PCR) is
recommended to detect those patients who may benefit from anti-EGFR therapy.
Laboratories often offer EGFR mutation PCR test, with reflex to KRAS (if EGFR is
negative), since the two mutations are mutually exclusive.
KRAS Mutations
edge
ALK
Non-small cell lung cancers have also been linked to rearrangements of the gene
encoding anaplastic lymphoma kinase (ALK). The most common ALK rearrangement
found in non-small cell lung cancers is the fusion between echinoderm
microtubule-associated protein-like 4 (EML4) and ALK gene on chromosome 2p23.
EML4-ALK non-small cell lung cancer is a unique subset of non-small cell lung
cancer that is most commonly seen in adenocarcinomas of never or light smokers,
whose tumors lack EGFR and KRAS mutations. Patients with ALK rearrangements tend
to be younger than most patients with non-small cell lung cancer. These patients
usually do not benefit from EGFR-specific therapy, but may benefit from specific
ALK inhibitors. Crizotinib is an oral ALK tyrosine kinase inhibitor approved for
advanced or metastatic ALK-positive non-small cell lung cancer (i.e patients who
harbor the ALK gene rearrangement). FISH is the current standard method for
determining the presence of the EML4-ALK gene translocation. EML4-ALK
translocations, KRAS mutations, and EGFR mutations in lung cancer are almost
always mutually exclusive.
ROS1
ROS1 is a receptor tyrosine kinase. ROS1 fusions are encountered in
approximately 2% of non-small cell lung cancers and are identified as a
potential driver mutation in non-small cell lung cancers. ROS1
rearrangement-positive lung cancer is a distinct subset of lung cancer with
clinical characteristics similar to ALK-rearranged lung cancer. Similar to ALK
rearrangements, ROS1 fusions are more commonly seen in light or never smokers.
They are also associated with younger age and adenocarcinomas. ROS1
fusion-positive cancers are reportedly sensitive to tyrosine kinase inhibitors
that inhibit ROS1. Crizotinib has shown early evidence of clinical response in
ROS1 fusion-positive patients. FISH is the current standard method for detecting
ROS1 rearrangement. ROS1 mutations are non-overlapping with other oncogenic
mutations found in non-small cell lung cancer such as ALK, EGFR, and KRAS
mutations.
CLINICAL INDICATIONS FOR EGFR/KRAS/ALK/ROS1 TESTING
Testing should be performed on:
Patients with metastatic adenocarcinoma
Patients with unresectable tumors
Patients with recurrent tumors
Patients who cannot tolerate conventional chemotherapy
Patients with mixed lung cancers with any adenocarcinoma component in a lung
resection specimen. For patients without any adenocarcinoma component by
histology or IHC (i.e. pure SCC or small cell carcinoma) EGFR and ALK testing is
not recommended
Patients with limited specimens (i.e. core biopsy or cytology) where an
adenocarcinoma component cannot be excluded and the clinical presentation is
suspicious for adenocarcinoma (i.e. young age or absence of smoking history).
Patients with multiple separate primary lung adenocarcinomas. Each separate
primary may be tested, however, testing of different areas within the same
primary adenocarcinoma is not recommended.
Testing may be useful at the time of the initial resection specimen even in
patients who do not have advanced metastatic disease, in order to prevent
difficulties/delays in obtaining archival material for molecular testing at the
time of recurrence (sometimes resulting in patients receiving additional
procedures)
Acceptable specimens usually include:
Formalin-fixed paraffin-embedded tissue
Fresh snap-frozen tissue
Fine needle aspiration cell block
PROPOSED ALGORITHM FOR EGFR/KRAS/ALK/ROS1 TESTING
Testing for EGFR, ALK, KRAS, and ROS1 mutations may all be ordered separately at
the time of the resection. Alternatively, since all these mutations are
essentially mutually exclusive, KRAS and ROS1 testing are often ordered as
reflex, if EGFR or ALK1 is negative, respectively. I am recommending (as
available) automatic reflex to KRAS and ROS1 when EGFR and ALK1 are negative.
See algorithm below.
edge
**Although it is counter-intuitive to pursue further testing in a patient who is
EGFR- negative, determining the presence of KRAS mutation is still useful
information because patients who are EGFR-mutation negative often are still
candidates for anti-EGFR therapy as a second-line agent. Studies have shown that
anti-EGFR therapy may be useful in patients with NSCLC as second-line,
third-line, or as maintenance therapy, including those patients who are EGFR
mutation-negative.
The above information is largely based upon the National Comprehensive Cancer
Network (NCCN) recommendations and guidelines.
TEST COMMENTS
EGFR mutation analysis by PCR is available upon request. This test is intended
to detect EGFR mutation-positive patients. Pulmonary non-small cell carcinomas,
including adenocarcinomas, non-mucinous bronchioloalveolar carcinomas, and
adenosquamous carcinomas with certain EGFR mutations may respond to anti-EGFR
therapies. Alternatively, rare EGFR mutations predict failure to respond to
anti-EGFR therapies.
EML4-ALK gene translocation and ROS1 fusion analysis by FISH are also available
upon request. These tests are intended to detect patients who may respond to
anti-ALK therapy.
KRAS mutation analysis by PCR is also available upon request. This test is
useful in patients found to be negative for EGFR mutations. Patients with KRAS
mutation may also show relative insensitivity to anti-EGFR therapies that target
the RAS/RAF pathway.
TEST INTERPRETATION
PCR GENE MUTATION ANALYSIS INTERPRETATION:
EGFR Mutation Analysis:
Positive = Mutation Detected; specify genotype*
Negative = Mutation Not Detected
*When an EGFR mutation is detected, a specific genotype is reported. Although
most EGFR mutations predict a favorable response to anti-EGFR therapies, rare
mutations are associated with a lack of clinical response to anti-EGFR
therapies.
KRAS Mutation Analysis:
Positive = Mutation Detected: Unlikely to respond to anti-EGFR therapy
Negative = Mutation Not Detected: May respond to anti-EGFR therapy
ALK Fusion Analysis:
Positive = Fusion Detected: May respond to anti-ALK therapy
Negative = Fusion Not Detected: Unlikely to respond to anti-ALK therapy
ROS1 Rearrangement Analysis:
Positive = Fusion Detected: May respond to anti-ALK therapy
Negative = Fusion Not Detected: Unlikely to respond to anti-ALK therapy
SUGGESTED READING
Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond.
Modern Pathology 21: S16-S22, 2008.
http://www.nature.com/modpathol/journal/v21/n2s/full/3801018a.html
CAP/IASLC/AMP: Molecular Testing Guideline for the Selection of Lung Cancer
Patients for EGFR and ALK Tyrosine Kinase Inhibitors. Archives of Pathology
2013, 137:828-860
CAP Lung Molecular Reporting Template
CAP Webinar on lung cancer testing can be viewed here:
CAP Resource Page on Molecular Testing in Lung Cancer
CAP Today article on lung cancer molecular testing.
CAP Patient Guide to Lung Cancer Testing.
CAP FAQs on Lung Cancer IHC Guidelines.
APMG Lung Molecular Pathways
http://www.apmggroup.net/innovation/molecular_testing/Lung_Pathways/lung.html
¡¡
¡¡
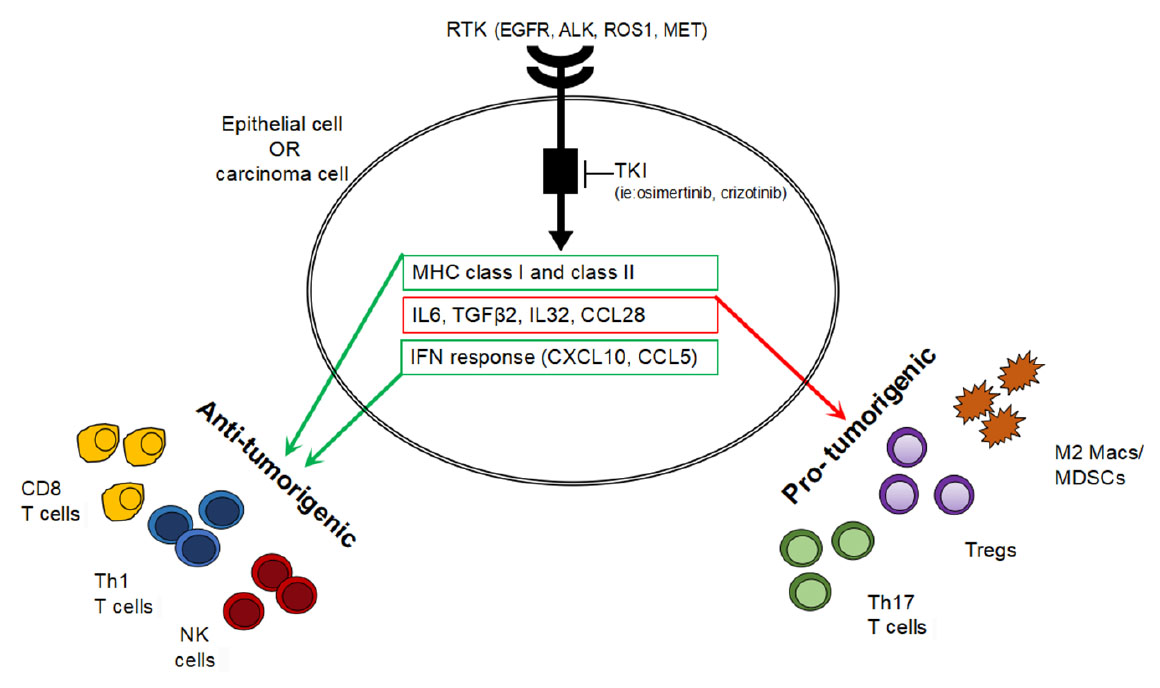
Linking tyrosine kinase inhibitor-mediated inflammation with normal
epithelial cell homeostasis and tumor therapeutic responses
https://cdrjournal.com/article/view/2662
¡¡