癌症干细胞中铁代谢的异常
Dysregulation of iron metabolism in cancer stem cells ...
Department of Biomedical Sciences for Health, University of Milan, Via
Mangiagalli 31, 20133 Milano, Italy
强调
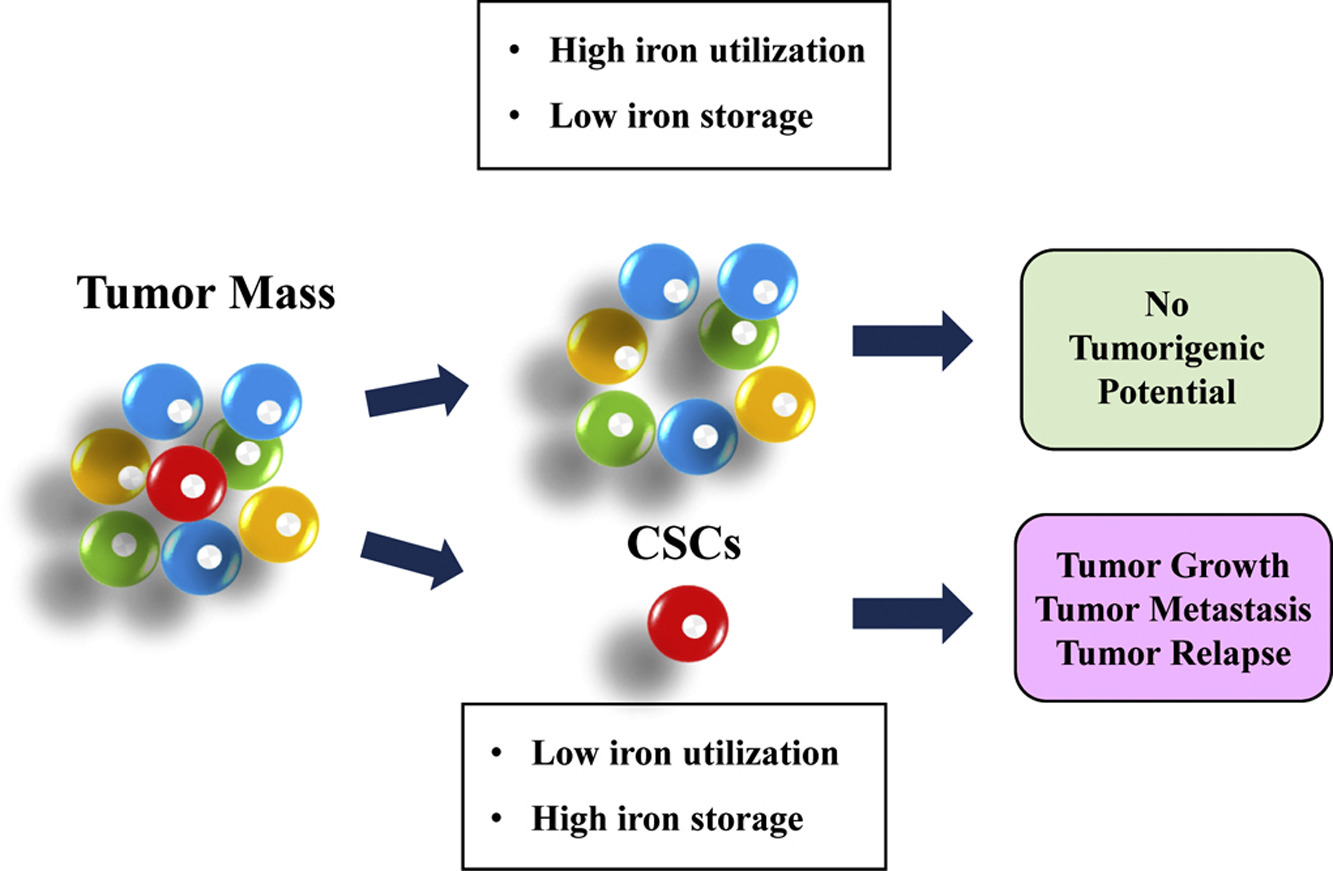
• 癌症干细胞(CSCS)是肿瘤内具有干细胞样特征的细胞。
• 人们认为CSCs可以驱动肿瘤的生长,转移和化学耐药性。
• 针对CSC的疗法的发展为改善癌症治疗抱有希望。
• 铁稳态的失调代表癌症干细胞的共同特征。
• 将铁用于目标CSC需要更好地了解CSC中的铁代谢。
抽象
癌症干细胞(CSC)是具有干细胞样特性的肿瘤细胞的独特亚群。重要的是,CSC可以在当前的标准疗法中存活下来,从而导致转移性疾病和肿瘤复发。在这里,我们描述了CSCs中铁稳态的变化,通常以高细胞内铁含量为特征。重要的是,铁代谢异常与癌症患者更快的肿瘤生长和不良预后相关。
与癌症对铁的依赖性相一致,我们还讨论了铁依赖性机制作为可药物化途径,因为已经考虑将铁螯合剂用于肿瘤治疗,并且目前作为抗肿瘤药可能会影响铁及其促进氧化的能力而提出和研究的新分子强调对癌症具有治疗价值。

Dysregulation of iron metabolism in cancer stem cells ...
https://www.sciencedirect.com/science/article/pii/S0891584918312590
The pattern of iron metabolism in CSCs appears to be different from the one
represented in the current model of iron homeostasis in cancer cells, which is
characterized by low iron availability, as the iron provided by increased uptake
and decreased export is used to sustain the high requirement of growing tumor
cells .
Cited by: 5
Publish Year: 2019
Dysregulation of iron metabolism in cancer stem cells
Highlights
•
Cancer stem cells (CSCS) are cells within a tumor with stem cell like features.
•
CSCs are thought to drive tumor growth, metastasis, and chemoresistance.
•
Development of therapies targeted at CSCs holds hope for improved cancer
treatment.
•
Dysregulation of iron homeostasis represents a common feature of cancer stem
cells.
•
Exploiting iron to target CSCs requires better knowledge of iron metabolism in
CSCs.
Abstract
Cancer stem cells (CSCs) are a distinct subpopulation of tumor cells endowed
with stem-like properties. Importantly, CSCs can survive current standard
therapies, resulting in metastatic disease and tumor recurrence. Here we
describe the alterations of iron homeostasis occurring in CSCs, which in general
are characterized by high intracellular iron content. Importantly, abnormalities
of iron metabolism correlate with faster tumor growth and adverse prognosis in
cancer patients.
In line with the dependence of cancer on iron, we also discuss iron-dependent
mechanisms as druggable pathways, as iron chelators have been considered for
tumor therapy and new molecules currently proposed and studied as antineoplastic
drugs may impinge on iron and its capacity to promote oxidative stress to have
therapeutic value in cancer.
胆管癌干细胞样细胞中铁代谢失调。
Dysregulation of Iron Metabolism in Cholangiocarcinoma Stem-like Cells
摘要
胆管癌(CCA)是由胆管上皮细胞的恶性转化引起的毁灭性肝肿瘤。癌症干细胞(CSC)是具有干细胞样特性的肿瘤细胞的子集,在肿瘤的发生,复发和转移中起作用。在适当条件下,CSC形成3D球体(SPH),其保留了茎样肿瘤引发特征。
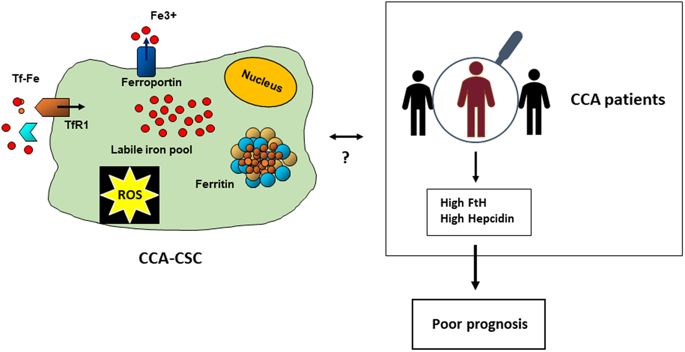
在这里,我们发现铁蛋白的不同表达表明与单层生长的肿瘤细胞相比,CCA-SPH中铁含量增加,氧化应激和CSC标志物表达更高。
暴露于铁螯合剂去铁胺(DFO)可降低SPH形成效率以及CSC标记和茎样基因的表达,而铁则具有相反的作用。 CCA样品(n = 104)中的微阵列谱显示肿瘤相对于周围肝脏的H铁蛋白,铁调素和铁转运蛋白表达降低,而转铁蛋白受体上调。此外,我们发现铁蛋白(ferrin)和铁调素(hepcidin)这两种铁代谢的主要蛋白表达水平升高的CCA患者的预后趋于恶化。
这些发现代表了铁在干细胞区室中作为一种参与CCA生长的新型代谢因子的作用的第一个证据,可能对更好的治疗方法有影响。
1 意大利罗萨诺人道临床与研究中心,自体免疫性肝病中心。
2 意大利佛罗伦斯市佛罗伦斯大学的临床试验。
3.米兰大学生物医学科学系,米兰,意大利。
4 丹麦哥本哈根哥本哈根卫生与医学科学大学生物技术研究与创新中心。
5 美国德克萨斯州坦普尔市德州A&M卫生科学中心医学部Scott & White消化疾病研究中心
6 意大利罗马萨皮恩扎大学内科和医学专业。
7 意大利蒙扎米兰-比科卡大学内科和外科国际消化健康中心消化内科和自身免疫性肝病学部。pietro.invernizzi@unimib.it。
8 米兰大学生物医学科学系,米兰,意大利。gaetano.cairo@unimi.it。
Dysregulation of Iron Metabolism in Cholangiocarcinoma Stem-like Cells.
Abstract
Cholangiocarcinoma (CCA) is a devastating liver tumour arising from malignant
transformation of bile duct epithelial cells. Cancer stem cells (CSC) are a
subset of tumour cells endowed with stem-like properties, which play a role in
tumour initiation, recurrence and metastasis. In appropriate conditions, CSC
form 3D spheres (SPH), which retain stem-like tumour-initiating features. Here,
we found different expression of iron proteins indicating increased iron
content, oxidative stress and higher expression of CSC markers in CCA-SPH
compared to tumour cells growing as monolayers. Exposure to the iron chelator
desferrioxamine decreased SPH forming efficiency and the expression of CSC
markers and stem-like genes, whereas iron had an opposite effect. Microarray
profiles in CCA samples (n = 104) showed decreased H ferritin, hepcidin and
ferroportin expression in tumours respect to surrounding liver, whereas
transferrin receptor was up-regulated. Moreover, we found a trend toward poorer
outcome in CCA patients with elevated expression of ferritin and hepcidin, two
major proteins of iron metabolism. These findings, which represent the first
evidence of a role for iron in the stem cell compartment as a novel metabolic
factor involved in CCA growth, may have implications for a better therapeutic
approach.
一种有前途的癌症治疗新方法:靶向癌症干细胞中的铁代谢
A promising new approach to cancer therapy: Targeting iron metabolism in cancer stem cells
a Institut Necker-Enfants Malades (INEM), Inserm U1151-CNRS UMR 8253, France
b Université Paris Descartes-Sorbonne Paris Cité, F-75993 Paris, France
Innerm Necker-Enfants Malades(INEM),Inserm U1151-CNRS UMR 8253,法国b法国巴黎大学笛卡尔大学-75993法国巴黎,2018年5月18日接收,2018年7月24日修订,2018年7月25日接受,2018年7月30日在线可用。
铁是促进细胞增殖和生长的重要营养素。但是,铁可能有害。铁在氧化形式和还原形式之间循环的能力有助于形成自由基。过量的自由基会导致脂质过氧化,更多的活性氧和氧化应激,对DNA和其他生物分子的损害,以及(如果可能的话)肿瘤发生。铁还具有维持肿瘤微环境和转移的作用。铁的获取,外排,储存和调节途径都在癌症中受到干扰,这表明铁代谢的重编程是肿瘤细胞存活的重要方面。最近的研究揭示了铁代谢在癌症干细胞(CSC)中的作用,并表明在CSC中铁代谢的特异性靶向可能改善癌症治疗的功效。
在这篇综述中,我们首先简要总结一下我们对涉及铁的细胞内过程,饮食中铁的作用及其与癌症之间关系的理解。我们强调修饰子“铁基因”在癌症中的重要性,以及这些基因可能编码可在临床上使用的生物标志物的可能性。然后,我们提供了铁在代谢重编程,上皮-间质转化(EMT)以及CSC维持和可塑性必不可少的表观遗传标记的调控中的作用的更新。最后,我们讨论了针对CSCs中最近发现的铁调节型细胞死亡,肥大症的治疗形式的潜力。
A promising new approach to cancer therapy: Targeting iron metabolism in cancer stem cells
Author links open overlay panelMouradiEl HoutabLeïlaDos
SantosabAhmedHamaïabMaryamMehrpourab a Institut Necker-Enfants Malades (INEM),
Inserm U1151-CNRS UMR 8253, France b Université Paris Descartes-Sorbonne Paris
Cité, F-75993 Paris, France Received 18 May 2018, Revised 24 July 2018, Accepted
25 July 2018, Available online 30 July 2018. Iron is an essential nutrient that
facilitates cell proliferation and growth. Iron can be detrimental, however. The
ability of iron to cycle between oxidized and reduced forms contributes to the
formation of free radicals. An excess of free radicals leads to lipid
peroxidation, more reactive oxygen species and oxidative stress, damage to DNA
and other biomolecules, and, if potentially, tumorigenesis. Iron also has a role
in the maintenance of the tumor microenvironment and in metastasis. Pathways of
iron acquisition, efflux, storage, and regulation are all perturbed in cancer,
suggesting that reprogramming of iron metabolism is a central aspect of tumor
cell survival. Recent studies have shed light on the role of iron metabolism in
cancer stem cells (CSC) and suggest that specific targeting of iron metabolism
in CSCs may improve the efficacy of cancer therapy. In this review, we first
summarize briefly our current understanding of the intracellular processes
involving iron, the effect of dietary iron, and its relation to cancer. We
emphasize the importance of modifier “iron genes” in cancer and the possibility
that these genes may encode biomarkers that may be used clinically. We then
provide an update on the role of iron in metabolic reprogramming, the
epithelial-mesenchymal transition, and the regulation of epigenetic marks
essential for CSC maintenance and plasticity. Finally, we discuss the potential
of targeting a recently discovered form of iron-regulated cell death,
ferroptosis, in CSCs for treatment of cancer.
A promising new approach to cancer therapy: Targeting iron metabolism in
cancer stem cells - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S1044579X18300518
Frontiers | Iron Metabolism in Liver Cancer Stem Cells ...
https://www.frontiersin.org/articles/10.3389/fonc.2019.00149
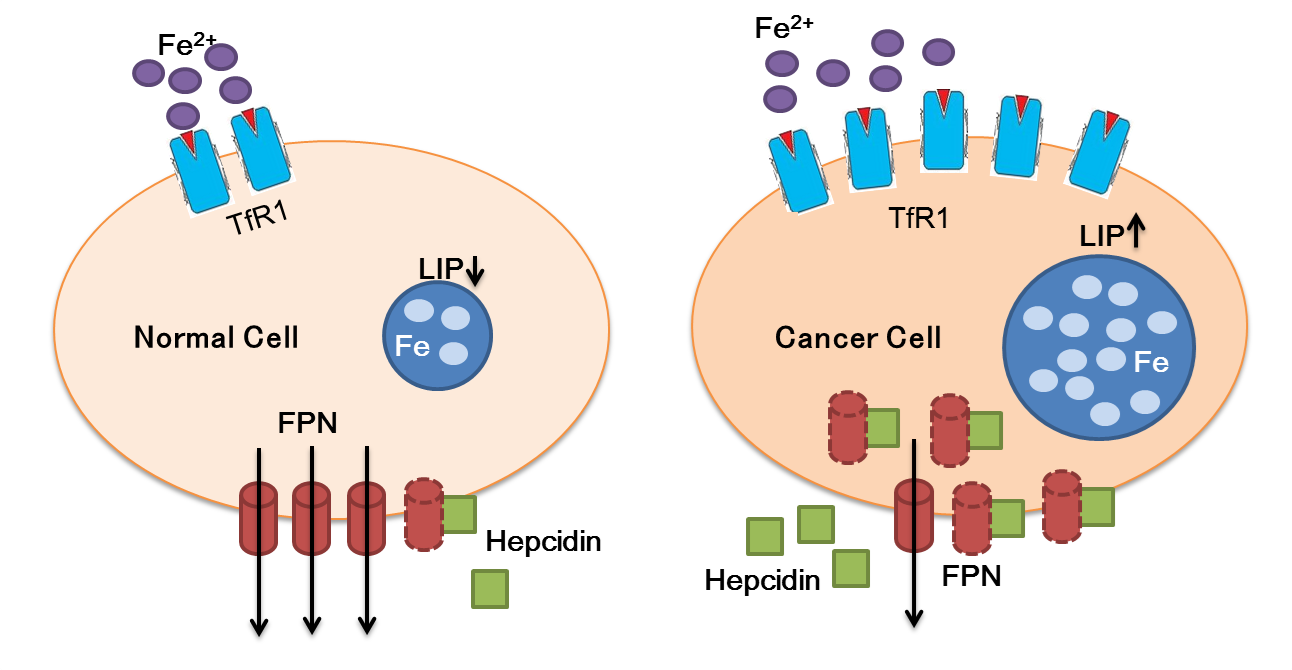
Mar 19, 2019 · In general, given the high iron needs of tumor cells to sustain
cell proliferation, the alterations of iron trafficking in cancer cells lead to
iron acquisition. To this purpose, cancer cells usually increase iron uptake,
for example by up-regulating TfR1, decrease iron release by inhibiting Fpn, or
both.
Author: Stefania Recalcati, Margherita Correnti, Elena Gammella, Chiara Raggi,
Pietro Invernizzi, Gaetano Ca...
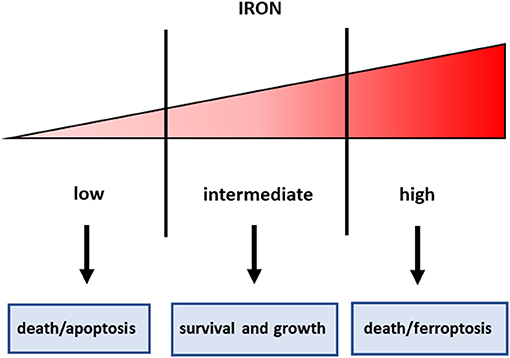
Figure 1. Iron threshold concept. Certain iron levels are required for cell survival and homeostasis, but iron concentrations too low lead to apoptotic cell death, whereas excess iron equally triggers cell death.
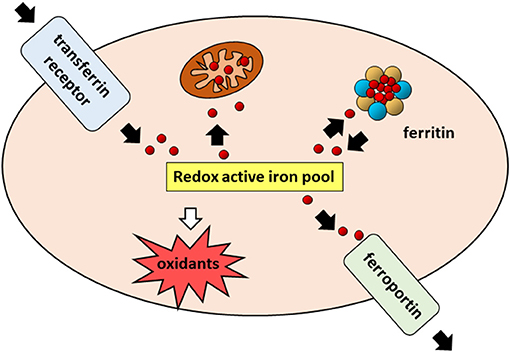
FIGURE 2
www.frontiersin.org
Figure 2. Cellular iron pathways in a nutshell. Transferrin bound iron,
internalized through endocytosis of the transferrin receptor (TfR1), enters a
pool of redox-active iron whose concentration is kept under control by
mechanisms ensuring that the iron which is not used for biochemical processes,
particularly in mitochondria, is either safely stored in cytoplasmic ferritin or
exported by ferroportin.
Publish Year: 2019'
The mechanism of dysregulated iron homeostasis in cancer ...
https://molnut621.wixsite.com/molecularnutrition/single-post/2016/09/08/The-mechanism...
Sep 08, 2016 · The mechanism of dysregulated iron homeostasis in cancer cells
and the roles of Egr1 and ATF3 in regulation of cancer growth and iron
metabolism September 8, 2016 Medicia Kartawijaya
Author: Medicia Kartawijaya
哺乳动物的铁代谢及其受铁调节蛋白的控制☆
Mammalian iron metabolism and its control by iron regulatory proteins☆
抽象
铁调节蛋白1和2(IRP1和IRP2)维持细胞铁稳态。
IRP与位于编码铁的摄取,储存,利用和输出的蛋白质的mRNA非翻译区中的铁响应元件(IREs)结合。在过去的十年中,在理解IRP如何通过铁依赖性和铁依赖性机制以及小鼠IRP2缺乏的病理后果方面取得了重大进展。对涉及多种细胞途径的新型IREs的鉴定表明,IRP-IRE网络延伸至铁稳态以外的过程。对IRP调节的机械理解可能会为铁代谢紊乱的基础产生重要的见解。本文是《金属细胞生物学》特刊的一部分。
强调
►IRP1和IRP2是哺乳动物细胞铁稳态的主要调节剂。 ►IRP与位于铁吸收,储存,利用和输出相关的mRNA非翻译区中的铁反应元件(IRE)结合。
►IRP受铁,活性氧和氮物质的翻译后调控。 ►新型IREs的发现揭示了IRP-IRE网络已扩展到细胞铁稳态之上。
►小鼠IRP缺乏会破坏铁体内平衡,并导致血液学,神经退行性疾病和代谢性疾病。
Mammalian iron metabolism and its control by iron regulatory proteins☆
Abstract
Cellular iron homeostasis is maintained by iron regulatory proteins 1 and 2
(IRP1 and IRP2). IRPs bind to iron-responsive elements (IREs) located in the
untranslated regions of mRNAs encoding protein involved in iron uptake, storage,
utilization and export. Over the past decade, significant progress has been made
in understanding how IRPs are regulated by iron-dependent and iron-independent
mechanisms and the pathological consequences of IRP2 deficiency in mice. The
identification of novel IREs involved in diverse cellular pathways has revealed
that the IRP–IRE network extends to processes other than iron homeostasis. A
mechanistic understanding of IRP regulation will likely yield important insights
into the basis of disorders of iron metabolism. This article is part of a
Special Issue entitled: Cell Biology of Metals.
Highlights
► IRP1 and IRP2 are the principal regulators of mammalian cellular iron
homeostasis. ► IRPs bind to iron-responsive elements (IREs) located in the
untranslated regions of mRNAs involved in iron uptake, storage, utilization and
export. ► IRPs are post-translationally regulated by iron and reactive oxygen
and nitrogen species. ► The identification of novel IREs reveals the presence of
an expanded IRP–IRE network beyond cellular iron homeostasis. ► IRP deficiency
in mice disrupts iron homeostasis and leads to hematological, neurodegenerative
and metabolic disorders.
Mammalian iron metabolism and its control by iron regulatory proteins -
ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0167488912001267
食管鳞状细胞癌中转铁蛋白受体CD71的过表达及其致瘤特性
Overexpression of transferrin receptor CD71 and its
tumorigenic properties in esophageal squamous cell carcinoma
肯尼思·赖
香港大学
食管鳞状细胞癌(ESCC)是亚洲流行地区的主要食管癌类型。在本研究中,我们调查了转铁蛋白受体CD71在ESCC中的临床意义和作用。
CD71在细胞铁摄入中具有生理作用,并且与各种类型的肿瘤的癌变有关。在我们的队列中,使用定量聚合酶链反应在61.5%的患者中检测到CD71转录超过2倍的上调。免疫组织化学分析还显示,石蜡包埋的肿瘤中CD71的膜和细胞质均很强。用增殖标志物Ki-67染色平行肿瘤切片显示Ki-67染色的模式与CD71表达有关。临床病理数据分析表明,CD71过表达可作为晚期T4期的指标(p
=
0.0307)。这些数据表明CD71与ESCC之间有很强的联系。随后使用短干扰RNA(siRNA)抑制CD71表达的体外试验证实了ESCC中CD71的致癌特性。在CD71抑制的细胞中观察到细胞生长抑制和S周期细胞停滞。潜在的机制涉及MEK
/ ERK途径的激活。总而言之,本研究提供了证据,显示了CD71在ESCC中的致癌特性与临床相关性,并建议靶向CD71作为ESCC的治疗策略。
转铁蛋白是一种血清糖蛋白,负责在血液中转运铁,并与细胞表面的转铁蛋白受体结合,从而导致铁内在化。许多研究报道,由于铁的需求,转铁蛋白受体在包括膀胱癌,脑癌,乳腺癌,肺癌和淋巴瘤在内的恶性癌细胞中被上调[89]
[90] [91]。转铁蛋白缀合的药物递送系统的发展使药物可以通过受体介导的内吞作用渗透细胞[92]。 ...
Kenneth K Y Lai
he University of Hong Kong
Esophageal squamous cell carcinoma (ESCC) is the predominant type of esophageal
cancer in endemic Asian regions. In the present study, we investigated the
clinical implication and role of transferrin receptor CD71 in ESCC. CD71 has a
physiological role in cellular iron intake and is implicated in the
carcinogenesis of various types of tumors. In our cohort, more than a 2-fold
upregulation of the CD71 transcript was detected in 61.5% of patients using
quantitative polymerase chain reaction. Immunohistochemical analysis also showed
strong membranous and cytoplasmic localization of CD71 in paraffin-embedded
tumors. Staining parallel tumor sections with the proliferative marker Ki-67
revealed that the pattern of Ki-67 staining was associated with CD71 expression.
Analysis of clinicopathological data indicated that CD71 overexpression can be
used as an indicator for advanced T4 stage (p=0.0307). These data suggested a
strong link between CD71 and ESCC. Subsequent in vitro assays using short
interfering RNA (siRNA) to suppress CD71 expression confirmed the tumorigenic
properties of CD71 in ESCC; cell growth inhibition and cell cycle arrest at S
phase were observed in CD71-suppressed cells. The underlying mechanism involved
activation of the MEK/ERK pathway. In summary, the present study provides
evidence showing the tumorigenic properties of CD71 in ESCC with clinical
correlations and suggests targeting CD71 as a strategy for the treatment of
ESCC.
Overexpression of transferrin receptor CD71 and its tumorigenic properties in
esophageal squamous cell carcinoma | Request PDF
https://www.researchgate.net/publication/259770754_Overexpression_of_transferrin_receptor_CD71_and_its_tumorigenic_properties_in_esophageal_squamous_cell_carcinoma
铁,炎症和癌细胞侵袭
Iron, inflammation and invasion of cancer cells
慢性炎症与肿瘤细胞从良性肿瘤发展为扩散性癌症的转移有关。通过在肿瘤生长部位相互作用的各种介质促进的血管生成促进了这种转移进程。血管生成引起两个主要变化,这些变化由癌细胞的糖基化和新抗原呈递改变而辅助。血管生成促进的病理变化包括炎症增强和组织基质降解,从其起源部位释放出肿瘤细胞。降解的肿瘤细胞释放由于糖基化改变而产生的新抗原。向T细胞呈递的新抗原加剧了转移和炎症。铁可调节炎症感染和某些感染中的炎症。根据离散报告,我们提出铁,炎症,血管生成和肿瘤生长之间的联系。更好地了解这种联系可能有助于我们制定癌症免疫疗法的新策略。
关键词:铁代谢,癌症,铁调素,靶向治疗
Iron, inflammation and invasion of cancer cells
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4632882
Jul 01, 2015 · Iron excess is associated with tumorigenesis because it is the
source of mutagenic hydroxyl radical formation that interferes with DNA repair
and affects the signal transduction in cancer cells, acting as a nutrient for
proliferating tumors . The other groups of compounds used for iron-targeted
anti-tumor therapy are the iron chelators.
Cited by: 11
Publish Year: 2015
Author: Eva Fischer-Fodor, Natalia Miklasova, Ioana Berindan-Neagoe, Bhaskar
Saha
Iron, inflammation and invasion of cancer cells
Chronic inflammation is associated with the metastasis of tumor cells evolving
from a benign tumor to disseminating cancer. Such a metastatic progression is
fostered by the angiogenesis propelled by various mediators interacting at the
site of tumor growth. Angiogenesis causes two major changes that are assisted by
altered glycosylation and neo-antigen presentation by the cancer cells. The
angiogenesis-promoted pathological changes include enhanced inflammation and
degradation of tissue matrices releasing tumor cells from the site of its
origin. The degraded tumor cells release the neo-antigens resulting from altered
glycosylation. Presentation of neo-antigens to T cells escalates metastasis and
inflammation. Inflammasome activation and inflammation in several infections are
regulated by iron. Based on the discrete reports, we propose a link between
iron, inflammation, angiogenesis and tumor growth. Knowing the link better may
help us formulate a novel strategy for cancer immunotherapy.
Keywords: iron metabolism, cancer, hepcidin, targeted therapy
Depletion of Iron From Tumor Cells Prior To Vitamin C Therapy Quells Cancer
By Bill Sardi
January 2, 2019
donate FacebookTwitterShare
It is widely known that mega-dose vitamin C transiently produces hydrogen
peroxide, an oxidant, to selectively kill cancer cells. In the late 1970s Linus
Pauling and Ewan Cameron were first to report of success utilizing intravenous
vitamin C to produce 1-year survival among 22 percent of otherwise hopeless
cancer patients (chemotherapy at the time was far less effective). Subsequent
studies concluded oral vitamin C could not possibly reach adequate blood
concentrations of vitamin C to produce hydrogen peroxide (cancer cell killing
effect) even though the data from that study ran contrary to the conclusions
drawn. [Knowledge of Health 2016]
Thereafter Steve Hickey and Hilary Roberts posited a dynamic flow theory of
ascorbate (vitamin C) therapy that addressed the fact vitamin C is rapidly
excreted and its oral absorption is drastically reduced when consumed in
mega-doses. Therefore, Hickey and Roberts posed intermittent oral administration
of vitamin C to maintain high blood levels sufficient to exert a cell killing
(cytotoxic) effect in cancer cells. [Journal Orthomolecular Medicine Vol. 20,
2005] Liposomal vitamin C further increases vitamin C delivery inside cells to
potentially effect a cure.
Curing the Incurable: ...
MD JD Thomas E Levy
Best Price: $3.00
Buy New $18.89
(as of 10:30 EDT - Details)
These revelations have led to a renaissance in the use of vitamin C for cancer.
[Knowledge of Health, 2016]
Now another breakthrough in the scientific understanding of vitamin C therapy
for cancer is being reported. Researchers in Japan report extracellular iron
(outside cancer cells) decomposes hydrogen peroxide generated by vitamin C. The
inhibition of cancer cell growth via vitamin C (hydrogen peroxide) is completely
blocked by the presence of iron. [Scientific Reports 2018] This was demonstrated
in leukemia cells (cancer of the blood) in a lab dish. Given that most of the
iron stored in the body is bound to hemoglobin in red blood cells, it is
understandable why iron plays such an important role in leukemia.
The anti-cancer effect of vitamin C is completely abolished by iron. Iron serves
as a growth factor for tumor cells.
With this new understanding, researchers employed iron-binding molecules prior
to vitamin C treatment with demonstrable anti-cancer effects. An iron-chelating
(key-lay-ting) drug, or donation of blood to reduce iron load, or a low iron
diet (in humans, avoidance of red meat) increased the cell-killing effect of
vitamin C.
A reduction of iron combined with vitamin C infusions worked synergistically
(more than additively) to the point where detection and invasion by tumor cells
was completely eliminated. Iron storage (ferritin) was dramatically reduced by
the above measures and then vitamin C-activated hydrogen peroxide killed off
cancer cells readily. Researchers conclude this approach produces a “novel
anti-leukemic effect.” [Scientific Reports 2018]
Optimal Nutrition for ...
Thomas E. Levy
Best Price: $2.29
Buy New $13.61
(as of 07:40 EDT - Details)
The idea of utilizing a natural iron chelator, such as IP6 (phytic acid or
phytate) derived from rice bran, prior to oral vitamin C therapy now needs to be
put to the test. The comparative iron chelating properties of various natural
iron chelators is presented in the chart below. It reveals IP6 as a superior
iron chelator even over EDTA which is often employed by clinicians who perform
chelation therapy.
The prospect of home cancer therapy looms as neither IP6 rice bran extract nor
vitamin C are toxic or induce acute side effects. A regimen of IP6 (up to 1600
mg is orally absorbed) followed by a vitamin C chaser is proposed.
The advantage of home vitamin C therapy is that intravenous vitamin C infusions
cannot practically or economically be administered on a daily basis at doctor’s
offices. Cancer rages unchecked in between chemotherapy or even intravenous
vitamin C infusions. Daily oral iron chelation with IP6 rice bran followed by
oral vitamin C therapy (3000 mg vitamin C as ascorbic acid four times a day), or
as an alternative, liposomal vitamin C, may prove to be a safe and novel way of
introducing self-care to cancer therapy. Be aware, home-made mixtures of
lecithin + vitamin C produce emulsified ascorbate but not the microscopic lipid
bi-layered delivery system defined as a liposome. [Whole Foods Magazine Aug.
2018]
Depletion of Iron From Tumor Cells Prior To Vitamin C Therapy - LewRockwell
https://www.lewrockwell.com/2019/01/bill-sardi/depletion-of-iron-from-tumor-cells-prior-to-vitamin-c-therapy-quells-cancer/