¡¡
Does Cholesterol Play a Role in the Bacterial Selectivity of Antimicrobial Peptides?
¡¡
1.Helicobacter pylori's cholesterol uptake impacts resistance to docosahexaenoic acid
2. Cholesterol Enhances Helicobacter pylori Resistance to Antibiotics and LL-37▿
3.Interaction of Helicobacter pylori Cell Membrane with Non-Esterified Cholesterol and Other Steroids
4.Does Cholesterol Play a Role in the Bacterial Selectivity of Antimicrobial Peptides?
¡¡
Helicobacter pylori performs the unique action of assimilating exogenous non-esterified cholesterol into its cell membrane. This bacterium aggressively incorporates non-esterified cholesterol into the membrane, induces its glucosylation, and uses both non-esterified cholesterol and glucosylated cholesterols as membrane lipid compositions. The reason for this assimilation of non-esterified cholesterol into the cell membrane of H. pylori has eluded investigators for many years. Recent hypotheses posit that the sterol-uptake and sterol-glucosylation contribute to the survival of H. pylori cells in different ways. The incorporation of the non-esterified cholesterol into the cell membrane fortifies the resistance of H. pylori against the antibacterial actions of phosphatidylcholines, antibiotics, and bile salts. In parallel, the glucosylation of the non-esterified cholesterol incorporated into the cell membrane serves H. pylori in two ways. First, it helps the bacterium evade host immune responses, such as phagocytosis by macrophages and activation of antigen-specific T cells. Second, it detoxifies sterols fatal to the bacterium via a novel action of sterol glucosylation recently described in another report from our group. The reluctance of H. pylori to absorb esterified cholesterol remains unexplained. A recent study by our group has demonstrated that the phosphatidylethanolamine (PE) in the outer membrane of H. pylori serves as a steroid-binding lipid the incorporation of non-esterified cholesterol into the membrane. We have also discovered that the myristic acid (C14:0) molecule attached to the PE of this bacterium plays an important role in the selective binding of non-esterified cholesterol but not esterified cholesterol.
¡¡
Interaction of Helicobacter pylori Cell Membrane with Non-Esterified Cholesterol and Other Steroids
http://file.scirp.org/Html/11-2260054_28667.htm
¡¡
Front Immunol. 2012; 3: 195.
Does Cholesterol Play a Role in the Bacterial Selectivity of Antimicrobial Peptides?
Jeffrey R. Brender,1 Austin J. McHenry,1 and Ayyalusamy Ramamoorthy1,*
Author information Article notes Copyright and License information Disclaimer
1Biophysics and Department of Chemistry, University of Michigan, Ann Arbor, MI, USA
Antimicrobial Peptides are Promising Antibiotic Compounds
The development of novel methods to overcome the inevitable resistance that develops with common antibiotics is an important area of current research. Recent studies have shown that antimicrobial peptides (AMPs) have the potential to become excellent antibiotic compounds toward a broad-spectrum of Gram-positive and Gram-negative bacteria with less potential for bacterial resistance than conventional antibiotics (Shai, 2004). Because these compounds are highly selective toward bacteria and bacteria have difficulty in developing resistance to their effects, a large number of studies have focused on designing potent AMPs for potential pharmaceutical applications (Maloy Biopolymers; Marsh et al., 2009). One of the designed peptides, MSI-78 (also known as pexiganan), rose successfully to phase II clinical trials for treating infection in the case of diabetic foot ulcer (Gottler and Ramamoorthy, 2009).
Composition of Membranes is Key to Amp Selectivity
Bacteria have difficulty in developing resistance to AMPs because the toxicity of AMP is mostly mediated by a non-specific process rather than by an interaction with a specific protein target. Most AMPs lyse bacteria by directly interacting with the lipid bilayer of the bacterial cell membrane and disrupting the lipid bilayer structure (Oren and Shai, 1998; Epand and Vogel, 1999; Shai, 2002; Bechinger, 2011). The development of more potent and selective AMPs requires that the molecular basis of activity and selectivity be understood. Substantial progress has been made in recent years in this area, particularly using cutting-edge solid-state NMR spectroscopy to provide insights into the mechanisms of membrane disruption by AMPs (Bechinger, 1999; Durr et al., 2006; Bhattacharjya and Ramamoorthy, 2009; Ramamoorthy, 2009; Nguyen et al., 2011). For example, the high-resolution 3D structure, membrane orientation, and mechanism of membrane disruption are reported for several important peptides including LL-37 (Wildman et al., 2003; Porcelli et al., 2008), MSI-78 (Hallock et al., 2003), MSI-594 (Ramamoorthy et al., 2006; Bhunia et al., 2009), and pardaxin (Hallock et al., 2002; Porcelli et al., 2004; Bhunia et al., 2010). Biophysical studies have also revealed the role of anionic lipids, (Thennarasu et al., 2010) cholesterol, and lipopolysaccharides (Bhunia et al., 2009, 2010; Domadia et al., 2010) in Gram-negative bacteria on the antimicrobial activities of these AMPs. In addition, substantial progress has been in understanding the molecular determinants of AMP activity. For example, recent studies have shown the ability to form oligomeric aggregates in the cell membrane enhances the potency of an AMP (Toke et al., 2004; Tremouilhac et al., 2006; Marquette et al., 2008; Ramamoorthy et al., 2008; Strandberg et al., 2008). Studies have also shown that the presence of d-amino acids (Mangoni et al., 2006) and disulfide bridges (Dhople et al., 2006) can enhance resistance against proteolytic degradation without affecting the antimicrobial activity.
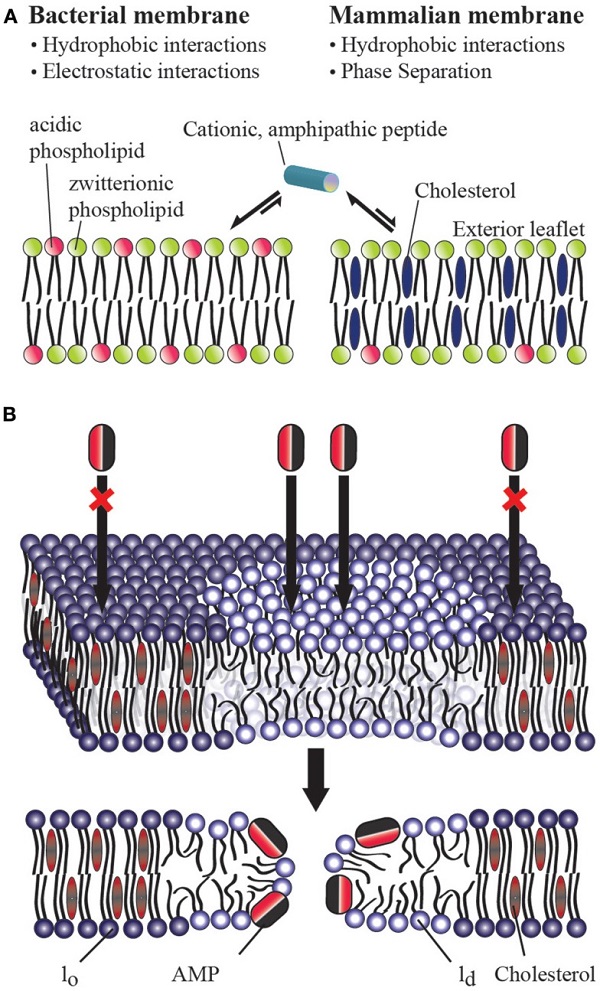
From these studies, a picture of how AMPs preferentially target bacteria has begun to emerge. The selectivity of AMPs therefore largely lies in their ability to distinguish between prokaryotic and eukaryotic membranes (Glukhov et al., 2005; Epand et al., 2006b). Biophysical studies have shown the importance of two factors in the membrane selectivity of an AMP (Figure (Figure1A):1A): (a) the electrostatic interaction between a cationic AMP and the acidic bacterial membrane which is composed of about ∼25% anionic lipids (POPS, POPG, and/or cardiolipin; Glukhov et al., 2005; van Meer et al., 2008; Epand et al., 2010) and (b) the presence of a large amount of cholesterol in a eukaryotic cell membrane which inhibits membrane disruption by rigidifying the lipid bilayer structure (Benachir et al., 1997; Matsuzaki, 1999; Glukhov et al., 2005; Epand et al., 2006a; Verly et al., 2008). These factors controlling the membrane selectivity of AMPs can also be exploited for other pharmaceutical targets. For example, several AMPs have been shown to have anticancer activities; this property has been attributed to the presence of anionic lipids in the outer leaflet of the cancer cell plasma membrane (Hoskin and Ramamoorthy, 2008). Similarly, most AMPs also kill fungi, protozoa, and even enveloped viruses, which all show a lipid distribution different than a normal eukaryotic cell (Oren and Shai, 1998; Epand and Vogel, 1999; Shai, 2002; Bechinger, 2011; Nguyen et al., 2011; Pius et al., 2012). Despite this progress in understanding the molecular determinants of AMP activity, there are still unresolved questions, particularly with regards to the preferential targeting of bacterial membranes. While the role of anionic lipids in membrane targeting of AMPs is well established, the role of cholesterol is still not clear. Accordingly, this opinion article focuses on the distinct roles of cholesterol in homogenous versus heterogeneous lipid bilayers.
Figure 1
(A) Role of cholesterol on the bacterial selectivity of antimicrobial peptides. Lipid bilayers mimicking bacterial (A) and eukaryotic (B) cell membranes are commonly used in in vitro studies on AMPs. In eukaryotic cell membranes, the outer leaflet consists primarily of zwitterionic phosphatidylcholine lipids (such as POPC), and cholesterol (∼25%) while the inner leaflet contains anionic lipids (such as POPS). Bacterial cell membranes typically lack cholesterol and contain ∼25% acidic lipids (like POPG and cardiolipin), and ∼55% phosphatidylethanolamine (POPE). AMPs have been shown to directly interact with the lipid bilayer of bacterial cell membranes and lyse the cell by disrupting the membrane via one of the several proposed mechanisms including barrel-stave, toroidal-pore, and detergent-type disturbances. The presence of cholesterol in the eukaryotic cell membrane enhances the rigidity of lipid bilayers to inhibit the membrane disruption activities of antimicrobial peptides. The electrostatic interaction between a cationic antimicrobial peptide and the anionic lipids (POPS) present in the outer leaflet of bacterial membranes plays a vital role in bacterial selectivity and the absence of cholesterol makes the membrane disruption by an AMP easier. In the case of Gram-negative bacteria, the presence of anionic lipopolysaccharides attracts cationic AMPs. (B) Mechanism of action of an antimicrobial peptide in a raft-containing membrane. In a heterogeneous mixture of lipids, the presence of cholesterol in the raft domain (lo) resists the permeation of an antimicrobial peptide while the disordered (ld) lipid domain is easily disrupted by an antimicrobial peptide.
Cholesterol is Believed to Play a Role in Bacterial Selectivity of AMPs
One of the major differences between bacterial and eukaryotic cell membranes is the presence of a large amount of cholesterol in eukaryotic cell membranes and a complete absence in bacterial cell membranes (Figure (Figure1A).1A). Cholesterol has been shown to protect human erythrocytes from attack by magainin 2 (Matsuzaki et al., 1995b). Similar studies on model membranes have demonstrated that the presence of cholesterol reduces AMP binding and suppresses the disruption of lipid bilayer structure by AMPS (Feigin et al., 1995; Matsuzaki et al., 1995a; Tytler et al., 1995; Raghuraman and Chattopadhyay, 2004; Glukhov et al., 2005; Verly et al., 2008; Wu et al., 2010). Solid-state NMR studies have provided high-resolution insights into the role of cholesterol against the function of several AMPs (Benachir et al., 1997; Wildman et al., 2003; Ramamoorthy et al., 2010). Cholesterol is known to increase membrane cohesion and mechanical stiffness (Evans and Waugh, 1977; Henriksen et al., 2006) which may resist the membrane bending required for many AMPs to function (Allende et al., 2005). This interaction reduces the tilt of the paradaxin helix relative to the bilayer normal, which in turn reduces the stability of the paradaxin pore (Ramamoorthy et al., 2010). However, for most AMPs a noticeable inhibitory effect of cholesterol is only noticeable after the formation of liquid ordered lipid phase at high concentrations of cholesterol (∼20%; McHenry et al., submitted) which suggests it may be due to an indirect effect due to a modulation of membrane properties rather than a direct interaction (Feigin et al., 1995). Despite these advances, the actual reason for the reduced affinity of many AMPs for cholesterol containing membranes is not fully understood. As noted above, this is traditionally been interpreted as a consequence of the increased acyl chain order in the liquid ordered phase of cholesterol containing membranes. In this context, it is interesting to compare cholesterol's effects on AMPs which do not clearly prefer the disordered liquid crystalline lipid phase or ordered gel phase. Surprisingly, cholesterol still strongly inhibits these peptides, which suggests an additional factor, such as dehydration of the headgroup region (M¡¯Baye et al., 2008) is partially responsible for cholesterol's effect.
Cholesterol Loses Its Effectiveness in Inhibiting AMPs When Incorporated into Raft-Like Domains
While biophysical studies have shown the ability of cholesterol to suppress the action of an AMP against a homogeneous lipid bilayer, recent studies have revealed that cholesterol does not have this same effect in heterogeneous lipid systems (Pokorny and Almeida, 2005; Pokorny et al., 2006). Though few studies have looked at membrane disruption by AMPs in heterogeneous systems with phase separation [particularly in liquid ordered (lo) liquid-disordered (ld) domain coexistences often referred to as ¡°lipid rafts¡±], two studies by the Almeida group demonstrated the permeabilizing activity of ¦Ä-lysin in raft-like palmitoyl-2-oleoylphosphatidylcholine/cholesterol/sphingomyelin (POPC/Chol/SM) mixtures (Pokorny and Almeida, 2005; Pokorny et al., 2006). These studies revealed that membrane permeabilization by ¦Ä-lysin occurs exclusively in the ld phase in membranes with ld − lo phase segregation and that the localization of ¦Ä-lysin to the ld phase results in greater membrane disruption than would be expected in the absence of phase segregation. Our own group recently demonstrated that this important effect occurs among a diverse set of AMPs (MSI-78, MSI-594, MSI-843, and MSI-367) encompassing several membrane disruptive mechanisms (McHenry et al., submitted). These combined results indicate that the phase separation naturally occurring in eukaryotic membranes is likely to nullify the effect of cholesterol against membrane disruption by AMPs. This surprising result suggests either cholesterol is not as important in determining the selectivity of AMPs toward bacterial membranes as once supposed, or unknown additional factors mitigate this effect in eukaryotic cells.
The mechanism of action of an AMP in a heterogeneous lipid system is depicted in Figure Figure1B.1B. These results suggest that raft formation localizes the concentration of cholesterol in the cell membrane in such a way that non-raft domains of the cell membrane can be easily disrupted by AMPs and toxins. It is also possible that the phase behavior of the membrane and the physicochemical properties of the boundaries connecting the ordered and disordered domains play important roles in the membrane disruption process by AMPs. For instance, paradaxin has been shown to segregate a homogeneous membrane into cholesterol rich and cholesterol poor domains (Epand et al., 2006a). While the AMPs that have been investigated so far function by the non-specifically mechanically disrupting the membranes (carpet, detergent-type, or toroidal-pore formation) mechanism (Figure (Figure1A),1A), it is unclear how AMPs resembling more traditional ion channels (barrel-stave mechanism) would interact with heterogeneous lipid systems (Figure (Figure1B).1B). Therefore, it is important to further investigate the interaction of a variety of AMPs with more heterogeneous lipid systems.
Future Scope
While the development of AMPs for antibiotic applications is highly important, it is essential to understand the origin of their bacterial selectivity. As mentioned above, recent studies have shown that AMPs easily disrupt the structure of heterogeneous lipid systems, and therefore cholesterol is unlikely to play a major role in reducing the toxicity or increasing the selectivity of AMPs. Since a natural eukaryotic cell membrane contains heterogeneous lipid systems and domains, cholesterol poor domains must be easily disruptable by an AMP. Further studies probing the role of cholesterol in different types of lipid bilayers with a variety of AMPs are essential to better understand the exact role of cholesterol on the toxicity and selectivity of AMPs. Such studies would aid in the design of more efficient AMPs.Does Cholesterol Play a Role in the Bacterial Selectivity of Antimicrobial Peptides?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3398343/¡¡
International Journal of Medical Microbiology
Volume 304, Issues 3¨C4, May 2014, Pages 314-320
Helicobacter pylori's cholesterol uptake impacts resistance to docosahexaenoic acid
Author links open overlay panelMartaCorreiaabcSusanaCasaldJoãoVinagreabeRaquelSerucaabCeuFigueiredoabElietteTouaticJos¨¦ C.Machadoab
a Institute of Molecular Pathology and Immunology of the University of Porto, Rua Dr. Roberto Frias, s/n, 4200-465 Porto, Portugal
b Faculdade de Medicina da Universidade do Porto, Al. Prof. Hernâni Monteiro, 4200-319 Porto, Portugal
c Institut Pasteur, Unit¨¦ de Pathogen¨¨se de Helicobacter, 25, 28 Rue du Docteur Roux, 75724 Paris Cedex 15, France
d REQUIMTE/Laborat¨®rio de Bromatologia e Hidrologia, Faculdade de Farm¨¢cia do Porto, Rua de Jorge Viterbo Ferreira, 228, 4050-313 Porto, Portugal
e Institute of Biomedical Sciences Abel Salazar of the University of Porto (ICBAS), 4099-003 Porto, Portugal
Abstract
Helicobacter pylori colonizes half of the world population and is associated with gastric cancer. We have previously demonstrated that docosahexaenoic acid (DHA), an n-3 polyunsaturated fatty acid known for its anti-inflammatory and antitumor effects, directly inhibits H. pylori growth in vitro and in mice. Nevertheless, the concentration of DHA shown to reduce H. pylori mice gastric colonization was ineffective in vitro. Related to the auxotrophy of H. pylori for cholesterol, we hypothesize that other mechanisms, in addition to DHA direct antibacterial effect, must be responsible for the reduction of the infection burden. In the present study we investigated if DHA affects also H. pylori growth, by reducing the availability of membrane cholesterol in the epithelial cell for H. pylori uptake. Levels of cholesterol in gastric epithelial cells and of cholesteryl glucosides in H. pylori were determined by thin layer chromatography and gas chromatography. The consequences of epithelial cells¡¯ cholesterol depletion on H. pylori growth were assessed in liquid cultures. We show that H. pylori uptakes cholesterol from epithelial cells. In addition, DHA lowers cholesterol levels in epithelial cells, decreases its de novo synthesis, leading to a lower synthesis of cholesteryl glucosides by H. pylori. A previous exposition of H. pylori to cholesterol influences the bacterium response to the direct inhibitory effect of DHA. Overall, our results suggest that a direct effect of DHA on H. pylori survival is modulated by its access to epithelial cell cholesterol, supporting the notion that cholesterol enhances the resistance of H. pylori. The cholesterol-dependent resistance of H. pylori to antimicrobial compounds raises new important aspects for the development of new anti-bacterial strategies.Helicobacter pylori's cholesterol uptake impacts resistance to docosahexaenoic acid - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S1438422113001914¡¡
Antimicrob Agents Chemother. 2011 Jun; 55(6): 2897¨C2904.
Cholesterol Enhances Helicobacter pylori Resistance to Antibiotics and LL-37▿
David J. McGee,1,* Alika E. George,1,2 Elizabeth A. Trainor,1 Katherine E. Horton,1 Ellen Hildebrandt,3 and Traci L. Testerman1
1Department of Microbiology and Immunology, Louisiana State University Health Sciences Center¡ªShreveport, Shreveport, Louisiana 71130
2College of Pharmacy, University of Louisiana at Monroe, Monroe, Louisiana 71209
3Department of Cell Biology and Biochemistry, Mailstop 6540, Texas Tech University Health Sciences Center, Lubbock, Texas 79430
ABSTRACT
The human gastric pathogen Helicobacter pylori steals host cholesterol, modifies it by glycosylation, and incorporates the glycosylated cholesterol onto its surface via a cholesterol glucosyltransferase, encoded by cgt. The impact of cholesterol on H. pylori antimicrobial resistance is unknown. H. pylori strain 26695 was cultured in Ham's F12 chemically defined medium in the presence or absence of cholesterol. The two cultures were subjected to overnight incubations with serial 2-fold dilutions of 12 antibiotics, six antifungals, and seven antimicrobial peptides (including LL-37 cathelicidin and human alpha and beta defensins). Of 25 agents tested, cholesterol-grown H. pylori cells were substantially more resistant (over 100-fold) to nine agents than were H. pylori cells grown without cholesterol. These nine agents included eight antibiotics and LL-37. H. pylori was susceptible to the antifungal drug pimaricin regardless of cholesterol presence in the culture medium. A cgt mutant retained cholesterol-dependent resistance to most antimicrobials but displayed increased susceptibility to colistin, suggesting an involvement of lipid A. Mutation of lpxE, encoding lipid A1-phosphatase, led to loss of cholesterol-dependent resistance to polymyxin B and colistin but not other antimicrobials tested. The cgt mutant was severely attenuated in gerbils, indicating that glycosylation is essential in vivo. These findings suggest that cholesterol plays a vital role in virulence and contributes to the intrinsic antibiotic resistance of H. pylori.¡¡
H. pylori grown in the presence of cholesterol is more resistant to LL-37 but not the alpha and beta defensins.
H. pylori encounters antimicrobial peptides in vivo. H. pylori induces the expression of bactericidal, cationic antimicrobial peptides in tissue culture models and in patients, including LL-37 cathelicidin, human alpha defensins (HNP-1 to HNP-3), and human beta defensins (especially human beta defensin-2 [HBD-2]) (6, 10, 15, 16, 25, 27, 37, 40, 41, 43). The beta defensins are produced by gastric epithelial cells and other cell types, while the alpha defensins are stored in azurophilic granules of neutrophils and are released following infiltration of the neutrophils into gastric tissues infected with H. pylori. LL-37 is secreted by gastric epithelial cells (10) and other cell types (26).
Under our growth conditions, H. pylori cultured in the presence of cholesterol was completely resistant to LL-37, whereas there was dose-dependent killing of H. pylori cultured in the absence of cholesterol (Fig. 6). The cgt mutant behaved similarly to wild-type H. pylori, namely, there was still similar cholesterol-dependent resistance to LL-37 with the cgt mutant compared to the wild-type strain (data not shown).At the highest concentrations tested (range, 0.5 to 10 ¦Ìg/ml), H. pylori was inherently resistant to all four human beta defensins (HBD-1 to -4) and to alpha defensins (HNP-1 and HNP-2), regardless of cholesterol presence in the growth medium (data not shown). Defensin concentrations higher than 10 ¦Ìg/ml were not tested due to the expense and concerns about physiologic relevance.
The cholesterol glucosyltransferase, Cgt, is essential for colonization of gerbils.
To determine the role of Cgt in vivo, we assessed the ability of the cgt knockout strain, compared with that of the isogenic SS1 wild-type strain, to colonize gerbils. While the wild type colonized the antrum, body, and fundus regions of the stomach, the cgt mutant was severely attenuated in all three regions (Fig. 7).In summary, the data presented here suggest that cholesterol plays a critical role in some antibiotic and LL-37 resistance of H. pylori. Cholesterol uptake by H. pylori in the gastric mucosa may contribute to the difficulties encountered in treating patients infected with this human pathogen.
Cholesterol Enhances Helicobacter pylori Resistance to Antibiotics and LL-37
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3101455/¡¡
.jpg)