Psoriasis Studies~Inside out disease
Features of psoriasis~ proliferation,inflammation, scales,itching
cell types in psoriasis lesion~Th17 predominant, Th1
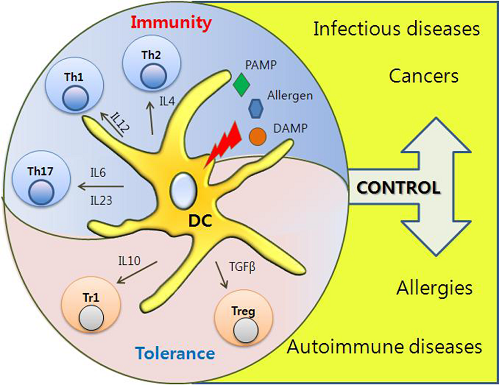
树突状细胞 Dendritic Cells ~cellular metabolism shapes the functional properties of DCs.
Dendritic cells,Macrophages, Cytokine-signaling in Psoriasis
Mast cells~ are main source of IL-17 in psoriasis lesions. mast cells, but not T cells or macrophages, were the predominant cell type producing IL-17 in psoriasis lesions.
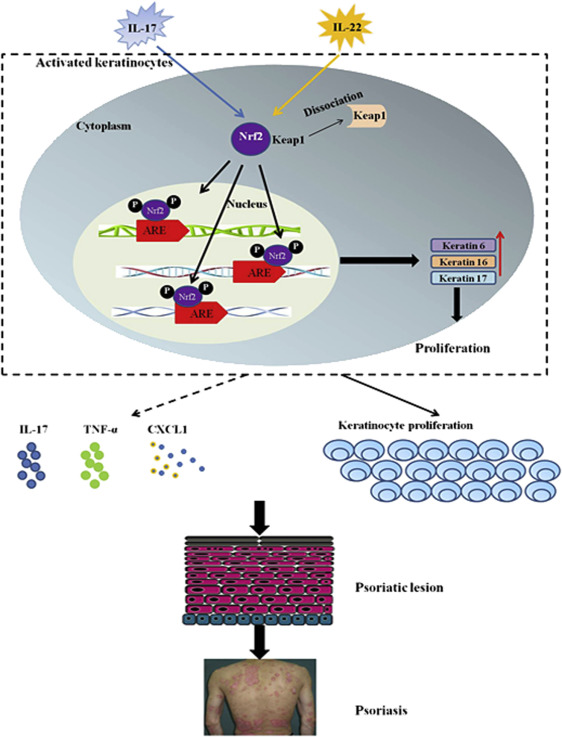
Nrf2 was overexpressed and activated in the epidermis of psoriatic lesions. K6, K16, K17 Hallmarks of psoriasis
Proliferation pathway~ DC, IL17, IL22 -Nrf2-K6, K16, K17Sequences of immune response
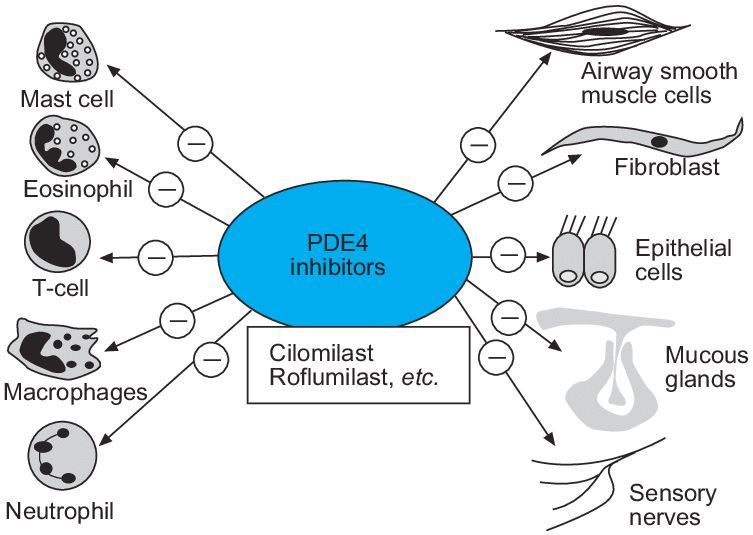
roles of phosphodiesterase type 4 (PDE-4) and its inhibitor
Bruton's tyrosine kinase inhibitor suppresses imiquimod-induced psoriasis-like inflammation. Bruton's tyrosine kinase (BTK) has been reported to execute important signaling functions in innate immune cells such as dendritic cells (DCs) and gamma delta T cells.
Role of TGF-betta in kiratinocyte profliferaiton
activated immune cells undergo anaerobic glycosis
metabolism and proinflammatory/antiinflammatory cytokine production
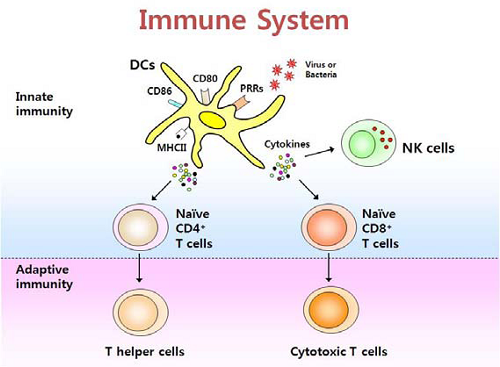
activation of dendritic cells via TLRs~Activation of DCs via TLRs promotes significant upregulation of aerobic glycolysis, which regulates the immune function of both human and mouse DCs; Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization
PI3K/ART/mTOR pathways
herbal ingredients for treating psoriasis~ danshensu, luoteolin,
Natural treatments: UVR
Autoantigens~
LPS/TLRs signaling pathway~TLR4
Effect of fatty acids on TLRs signaling pathway
Roles of IgE, IgE receptors on mast cells and B cells
sources of IL-23~ mature dendritic cells
sources of IL-17~ Th17, CD8+ effector T cells
PRO-inflammotory cytokines: IL-1,IL-6,IL-23/17, TNF-alpha
IL-17A stimulated keratinocytes activated PI3K/AKT/mTOR signaling and inhibited autophagy by simultaneously inhibiting autophagosome formation and enhancing autophagic flux.
Vitamin c~glycolysis,inflammation~ NF-kB,Nrf2,TNFa, EGFR? natural antihistamine
Inhibition of glycolysis (GAPDH) that mature dentrictic/M1 macrophages/effector T cells rely on by vitamin C
Vitamin C blocks TNF-alpha-induced NF-kappaB activation
Vitamin C blocks TNF-alpha-induced NF-kappaB activation and ICAM-1 expression in human neuroblastoma cells
Vitamin C blocks TNFα-induced NF k B activation and ICAM-1 expression in human neuroblastoma cells
Vitamin c~activation of AMPK, while AMPK inhibits mTOR
vitamin C inhibited the TNF-α-induced activation of not only the mitogen-activated protein kinase (MAPKs), but also nuclear factor-kappa B (NF-κB) signaling.
Inhibition/down-regulate PI3K/ART-mTOR signaling BY EPA, DHA (PTEN), EGCG,
AA is a competitive inhibitor of adenylate cyclase~anti-proliferation?
Natural kinase inhibitors:
Polyphenol Analogues~phenolic acids (tannic, gallic, and ellagic acid.), flavonoids, anthraquinones, coumarins, and lignans. EGCG- inhibits basophils to release histamine
gallic acid~ reduced IL-6 gene expression,
Glycyrrhizic acid~ as HMGB1 inhibitor (TLR2, TLR4, RAGE), mast cell stabilizer, reducing B cell to produce IgE, suppress IL-4, restore immune balance of Th1/Th2.
GA inhibits TLR2 activation
HMGB1: The Central Cytokine for All Lymphoid Cells
Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells
Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis
Glycyrrhizin GL inhibited the activation of the NF‑κB and MAPK/ERK signaling pathways induced by HMGB1 and decreased the expression of monocyte chemoattractant protein‑1 (MCP‑1) and myeloid cell leukemia 1 (Mcl‑1).Flavonoid Analogues
Berberine/huangliansu/danshensu~Berberine/danshensu inhibits the expression of TNFalpha, MCP-1, and IL-6 in AcLDL-stimulated macrophages through PPARgamma pathway
Lactic acid~ inhibits glycolysis (chelator of nickle)~LA significantly suppressed LPS-induced cytokine production and NF-κB transcriptional activity in mouse bone marrow-derived mast cells and cytokine production in peritoneal mast cells. Mostly, lactate is recognized as a molecule capable of suppressing immune responses, through inhibition of T cells, Mϕs, and dendritic cells.
Lactic Acid Reduces LPS-Induced TNF-α and IL-6 mRNA Levels Through Decreasing IKBα Phosphorylation
Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-κB Activation via GPR81-Mediated Signaling
Magnessium~ hypomagnesemia could induce a release of histamine from dermal mast cells. A characteristic allergy-like crisis occurs spontaneously in Mg-deficient rats, the first visible symptom being a peripheral vasodilatation of the ears. A low Mg medium (0.2 mM Mg) induced much more histamine release from mast cells. Magnesium is a natural calcium antagonist. Calcium is essential for Th17 activation. We localized Th17 cells predominantly to the dermis of psoriasis skin lesions, confirmed that IL-17 mRNA increased with disease activity, and demonstrated that IL-17 mRNA expression normalized with cyclosporine (a calcium dependant calcineurin inhibitor) therapy.
Lauric acid ameliorates lipopolysaccharide (LPS)-induced liver inflammation by mediating the TLR4/MyD88 pathway in Sprague Dawley (SD) rats. CONTROVERSY studies also found lauric acid stimulate TLR4
Iowa university~GML inhibits TLR2 signaling
Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages
Palmitate and insulin synergistically induce IL-6 expression in human monocytes
linseed oil/vitamin c for psoriasis~ VC inhibits GAPDH, oxVC/DHA inhibits NFkB, mTOR
Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1
HIF1α (-VEGF) expression is required for the differentiation of Th17 cells
Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1
ALA (alpha-linolenic acid) intake not only significantly reduced tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) concentrations. ALA significantly inhibited the secretion of proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) and increased anti-inflammatory cytokine. ALA significantly inhibited the phosphorylation of IκBα and NF-κB (p65) activation in ALI. ALA showed anti-inflammatory effects in mice with LPS-induced ALI. treatment with a high concentration of ALA markedly increased the production of IL-10. ALA suppressed the activation of NF-κB in LPS-induced ALI.
ALA on mitochondrial respiration~FAO (1) The overall beta-oxidation in total mitochondria was in the order C18:3, n-3 greater than C18:2, n-6 greater than C18:1, n-9, independent of the amount of albumin in the medium.
LKB1 restrains dendritic cell function~LKB1 orchestrates dendritic cell metabolic quiescence and anti-tumor immunity. LKB1 is potentiated by AMPK (which is potentiated by vitamin c)
the capacity of DHA to activate the AMPK signalling and negatively regulate the HIF-1α functions.
Roles of HIFa~ HIF1α, a transcription factor that controls the cellular response to hypoxia, activates the glycolytic pathway and, as such, promotes inflammation 47. HIF1α expression is required for the differentiation of Th17 cells 45, a T cell subset expanded in many autoimmune and inflammatory diseases. HIF1α‐inhibitor echinomycin reduced Th1 and Th17 responses, and attenuated a mouse model of acute graft‐versus‐host disease
Natural antihistamine~vitamin c, glycyrrhzin, quercetin, EGCG, magnesium?
EGCG inhibits mTOR signaling pathway~ Epigallocatechin-3-gallate (EGCG) is an ATP-competitive inhibitor of PI3K and mTOR with Ki values around 300 nM. EGCG inhibits cell proliferation and AKT phosphorylation at Ser473 in MDA-MB-231and A549 cells. Molecular docking studies show that EGCG binds well to the PI3K kinase domain active site.
EGCG could significantly impede expressions of HIF-1α and VEGF proteins.EGCG affects human dendritic cell differentiation and maturation~induced apoptosis in DC and Most importantly, mature DCs treated with EGCG inhibited stimulatory activity toward allogeneic T cells while secreting high amounts of IL-10. EGCG induces immunosuppressive alterations on human MODCs, both by induction of apoptosis and suppression of cell surface molecules and antigen presentation.
Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice
Epigallocatechin gallate induces apoptosis of monocytes
EGCG induced a reduction in proliferation of autoreactive T cells, production of proinflammatory cytokines, and Th1 and Th17 subpopulations, and an increase in regulatory T-cell populations.
EGCG inhibits proliferation of gastric cancer, pancreatic cancer cells
RETINOIC ACID
retinoic acid inhibiting the differentiation of dendritic cells, maturation and induction of the T-helper cell type-2 response. All-trans-retinoic acid atRA can inhibit the maturation of dendritic cells derived from cord blood mononuclear cells and that the effect can be stopped by a selective RARα antagonist, Ro 41-5253.atRA reduced the ability of dendritic cells to induce allogeneic T cells in mixed lymphocyte reaction. Similarly, Ro 41-5253 can reverse this reduction. atRA can affect dendritic cell-directed Th immune reaction and can promote Th2 response. The action may be mediated by RAR
Retinoic acid promotes the development of Arg1-expressing dendritic cells for the regulation of T-cell differentiation
Hypericin~a natural photosensitizer/vitamin c, omega-3, iron ~inhibition of HIF-1.
Conclusion: The present study demonstrated a marked increase of HIF- 1 alpha immunoreactivity in psoriatic skin.
Hypoxia-inducible factor-1α (HIF-1α) is a more important factor in psoriatic epidermal proliferation. Study on HIF-1α Gene Translation in Psoriatic Epidermis with the Topical Treatment of Capsaicin Ointment
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3263732/
Pathomechanism的角化病
表皮由基底层、棘层、颗粒层和角质层组成。在银屑病皮损中,角质形成细胞从基底层到棘层的转运时间由正常表皮层的约13天缩短至48小时。也有报道称,在银屑病皮损中,细胞周期从正常皮损中的311小时缩短到基础角质形成细胞的36小时,这表明银屑病皮损中角质形成细胞增殖明显加快。银屑病的病理机制被认为与细胞增殖加速和角质形成细胞从基底层向颗粒层快速迁移有关。角质形成细胞的增殖受到多种分子的调控,如环状抗菌肽(AMP)、蛋白激酶C、磷脂酶C和转化生长因子‐α。
Figure 1. Role of mTOR and cellular metabolism in the regulation of MHC II-dependent antigen presentation by DCs.
Metabolic pathways and upstream signaling pathways regulating these are indicated in red and black, respectively. The left and the right sides of the figure depict how MHC II-dependent antigen presentation is metabolically regulated in unactivated (immature) and TLR-stimulated (mature) DCs, respectively.Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0952791518301080
Previous studies showed that active oxidative phosphorylation in mitochondria is associated with immature or tolerogenic DCs, while increased glycolysis upon pathogen sensing can promote immunogenic DC functions.
Metabolic Control of Dendritic Cell Functions: Digesting Information - PubMed
https://pubmed.ncbi.nlm.nih.gov/31073300/
Psoriasis pathogenesis and the development of novel targeted immune therapies - PubMed
https://pubmed.ncbi.nlm.nih.gov/28887948/#&gid=article-figures&pid=figure-1-uid-0
https://onlinelibrary.wiley.com/doi/full/10.1111/1346-8138.14139
Main axis of the pathogenesis of psoriasis. Cells important in the pathogenesis of psoriasis are dendritic cells (DC), T‐helper (Th)17 (and Th1), and keratinocytes. DC activated by various stimuli excessively produce and secrete tumor necrosis factor (TNF)‐α and interleukin (IL)‐23 (including IL‐12). IL‐23 induces differentiation of naive T cells into Th17. Activated Th17 cells overproduce IL‐17 and IL‐22. TNF‐α and IL‐17 activate keratinocytes, promote epidermal hyperplasia, recruit inflammatory cells, such as neutrophils, and induce antimicrobial peptide (AMP) production. IL‐12 produced by dendritic cells also induces Th1, and Th1 produce cytokines, including interferon (IFN)‐γ. This irregular immune response continues as TNF‐α activates dendritic cells. CXCL, chemokine (C‐X‐C motif) ligand.
The close relation between IL‐17 and psoriasis was reported. High levels of IL‐17 mRNA were detected in psoriatic lesional skin, but not in non‐lesional skin.25 In keratinocytes, IL‐17 enhanced the expression of IL‐6 and IL‐8, which are known as pro‐inflammatory cytokines and exacerbate psoriasis.26 In addition, topical application of imiquimod, a Toll‐like receptor (TLR)7/8 ligand and potent immune activator, induced psoriasis‐like dermatitis in mice together with the expression of IL‐17A and IL‐17F.27 Furthermore, both CsA and anti‐TNF‐α agents decreased the levels of IL‐17A, IFN‐γ, IL‐23p19 and chemokine (C‐C motif) ligand 20 in psoriatic lesions in conjunction with improvement of psoriatic eruptions, indicating that pro‐inflammatory cytokines, including IL‐17A, are involved in the development of psoriasis.26, 28-30 These findings suggest that the IL‐17 family plays an important role in psoriasis.
Pathogenesis of psoriasis and development of treatment - Ogawa - 2018 - The Journal of Dermatology - Wiley Online Library
https://onlinelibrary.wiley.com/doi/full/10.1111/1346-8138.14139
Enhanced glucose uptake and a switch to glycolysis are key traits of M1 macrophages, whereas enhanced fatty acid oxidation and oxidative phosphorylation are the main metabolic characteristics of M2 macrophages.
Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S1550413118305138
https://www.frontiersin.org/articles/10.3389/fimmu.2014.00491/full
https://openi.nlm.nih.gov/detailedresult.php?img=PMC2582809_ar2413-1&req=4
http://link.springer.com/chapter/10.1007/978-3-319-19530-8_9
https://www.mdpi.com/1420-3049/24/11/2157
http://miriam-english.org/files/Harnessing_Infection_to_Fight_Cancer.html
http://journal.frontiersin.org/article/10.3389/fimmu.2013.00082/full
https://www.frontiersin.org/articles/10.3389/fimmu.2018.03176/full
http://www.pnas.org/content/99/1/351
https://computing.dcu.ie/~hruskin/hruskin.html
The differentiation of Th17 cells is supported by IL-6 and TGF-β, whereas IL-23, IL-1β, and IL-21 promote their expansion (2–4).
Interestingly, polymorphisms at loci encoding components of IL-23 and its receptor—IL23A, IL12B, and IL23R—have been associated with an increased risk of developing psoriasis (5–9). Psoriasis lesions contain increased amounts of IL-17 mRNA and increased numbers of Th17 cells (2, 10, 11). Similarly, increased tissue expression of IL-17 and numbers of Th17 cells are seen in rheumatoid arthritis, Crohn’s disease, autoimmune uveitis, lupus erythematosus, ankylosing spondylitis, asthma, and multiple sclerosis (12). The pathophysiologic relevance of the IL-23–IL-17 axis in autoinflammatory disease is highlighted by the clinical effectiveness of Abs targeting IL-23/IL-12 p40 and IL-17 in treating psoriasis as well as the other aforementioned diseases (13–16).Surprisingly, we observe that most IL-17+ cells in normal and psoriatic skin are mast cells, not T cells. Interestingly, mast cell numbers are decreased in psoriasis lesions after successful treatment with anthralin, psoralen plus UVA light therapy, or cyclosporine (25–27). Neutrophils also are enriched in psoriasis lesions, especially in the epidermis where they aggregate in Munro’s microabscesses (MMs) in the stratum corneum and spongiform pustules of Kogoj (SPKs) in the stratum spinosum (28).
IL-17 orchestrates innate immune responses against extracellular pathogens by inducing expression of antimicrobial peptides (AMPs) and neutrophil-tropic chemokines CXCL1, CXCL2, and IL-8 (32–35). The same AMPs and chemokines are found at extremely high levels in psoriatic epidermis (36–38). Not surprisingly, mice and humans with deficits in IL-17 production or signaling are highly susceptible to infection with extracellular bacteria and fungi (39–42).
effective antimicrobial activity by neutrophils and mast cells depends on the formation of structures called extracellular traps (ETs), termed NETs and MCETs, respectively (43–45). ETs are formed through a specialized process of cell death termed ETosis (46), where chromatin extends into fine, weblike threads to which proteins are bound. In particular, NETs can contain myeloperoxidase (MPO), proteinase 3, and AMPs such as cathelicidin (LL-37) (47). The process of NET formation (NETosis) can be triggered by extracellular bacteria and fungi or their components (48, 49). MCETs contain tryptase, LL-37, and chromatin (45), forming in response to bacteria, H2O2, or PMA (45, 48). In humans, NETs have been visualized in physiologic host responses to infections (44, 50) and have been implicated in the pathology of anti-neutrophil cytoplasmic Ab-induced vasculitis (51). Additionally, a recent study showed that lupus nephritis is associated with an inability to degrade NETs in blood (52).
We observe that mast cells and neutrophils release IL-17 into the skin though ETosis as well as conventional degranulation.
http://www.jimmunol.org/content/187/1/490
A positive feedback loop. Mast cell activation by cytokinergic IgE induces cytokine secretion by mast cells in the absence of antigen. The cytokines stimulate mast cell survival and class switching to IgE in B cells. Continued production of IgE and cytokines occurs in the absence of antigen.
Some 10 years ago it emerged that at sufficiently high concentrations certain monoclonal mouse IgEs exert previously unsuspected effects on mast cells. Thus they can both promote survival and induce activation of mast cells without the requirement for antigens. This was a wake up call that appears to have been missed (or dismissed) by the majority...
... DNCB activates immunity to release IgE from the plasma B cells, similar to the immune defense against parasites but it is associated with over-activating immunity. IgE production results in the degranulation of activating mast cells to release histamine which causes itching and edema of the skin [38]. STB, AOM and the mixture of STB and AOM alleviated the clinical AD symptoms to similar levels as the Normal-Con mice by reducing serum IgE concentrations and mast cells in the skin tissues. ...
. complex of RAG1 and RAG2 can itself initiate the process of receptor revision, since all the other machinery for repairing DNA breaks in cells is constitutive (Nemazee, 2006). RAG genes are normally silenced once a func- tional Ig is expressed in B cells, but may be re-expressed in the presence of IL-6 (Hillion et al., 2007a,b). IL-6 secretion is com- monly observed when mast cells are stimulated by cytokinergic IgE; when this occurs in the target organs of allergy, it may lead to the local expression of polyspecific/cytokinergic IgE. ...
View in full-text
How IgE mediates an allergic reaction via interaction with its two receptors. (Left) Interactions of membrane-bound IgE (mIgE, blue) with CD23 (tangerine) on B-cells regulates soluble IgE (sIgE) production. (Right) Cross-linking of IgE bound to FcεRI (scarlet) on mast cells or basophils by allergens (brown) triggers the release of mediators, causing allergy.
IgE is the antibody isotype found at the lowest concentration in the circulation. However IgE can undeniably play an important role in mediating allergic reactions; best exemplified by the clinical benefits of anti-IgE monoclonal antibody (omalizumab) therapy for some allergic diseases.
Mast cell sensitization is initiated by exposure to an allergen. In this case, a pollen grain allergen crosses the epithelial barrier and activates the production of IgE antibodies from B cells. The IgE bind surface high affinity receptors (FcRI) on the surface of mast cells and arm them for subsequent exposure.
... Crosslinking of IgE bound to mast cells by allergens, in turn, triggers the release of mediators, such as histamine and leukotrienes that are responsible for arteriolar dilation, increased vascular permeability, itching, rhinorrhea, mucus secretion, and smooth muscle contraction in the lung. The mediators and cytokines released during the early phase of an immune response to an inciting allergen trigger a further cellular inflammatory response over the next 4-8 h (late-phase inflammatory response) resulting in recurrent symptoms (usually nasal congestion) that often persist [40][41][42]. A lot of medical treatment modalities used as a treatment of AR, such as antihistamines, steroids, montelukast (Singulair), and immunotherapy.
Quercetin is a naturally occurring polyphenol flavonoid which is rich in antioxidants. It has anti-allergic functions that are known for inhibiting histamine production and pro-inflammatory mediators. Quercetin can regulate the Th1/ Th2 stability, and decrease the antigen-specific IgE antibody releasing by B cells. Quercetin has a main role in anti-inflammatory and immunomodulatory function which makes it proper for the management of different diseases. Allergic diseases are a big concern and have high health care costs. In addition, the use of current therapies such as ß2-agonists and corticosteroids has been limited for long term use due to their numerous side effects. Since the effect of quercetin on allergic diseases has been widely studied, in the current article, we review the effect of quercetin on allergic diseases, such as allergic asthma, allergic rhinitis (AR), and atopic dermatitis (AD).
Upon activation, naïve CD4+ T cells differentiate into a number of specialized T helper (Th) cell subsets. Th2 cells are central players in immunity to helminths and are implicated in mediating the inflammatory pathology associated with allergies. https://pubmed.ncbi.nlm.nih.gov/31611881/
Figure 1. Allergy response pathway. As part of an allergic response, allergens are presented to naïve T cells, which activates Th2 cells in the presence of IL-4. Th2 cells then release cytokines including IL-3, IL-4 and IL-13. This in turn activates the release of IgE-producing antibodies as well as activating eosinophils and mast cells. Figure adapted from Fujita H, Meyer N, Akdis M et al (2012).
Memory Th2 cell responses are recognised as a significant contributor of exacerbated allergic diseases. Th2 cells comprise a core part of the adaptive immune system. It has been proposed that minimal bacterial exposure early in life shifts the balance of the Th1/Th2 ratio towards a dominant Th2 immune response. Th2 cells secrete cytokines including IL-4, IL-5, IL-6 and IL-13. This in turn activates IgE antibody production, as well as mast cells and eosinophils (Figure 1).
https://www.esgct.eu/Blog/Treating-allergy-with-a-single-vaccine.aspx
Th2 Cell - an overview | ScienceDirect Topics
https://www.sciencedirect.com/topics/medicine-and-dentistry/th2-cell
Cytokines such as IL-4 and IL-5 released by Th2 cells stimulate, respectively, B-cell switching to the production of IgE antibody and activation of eosinophils. The coordinate actions of these effector mechanisms result in heightened immunity against, for example, helminthic parasites, which can be coated with IgE and destroyed by the toxic granular contents of eosinophils.
LKB1 signaling in dendritic cells limits their T cell-activating potential. LKB1 is phosphorylated in DCs in the tumor microenvironment, while it is depleted by LPS or E. coli favoring Treg expansion. LKB1 limits the ability of DCs to induce T cell priming by repressing a variety of activating pathways. These effects lead to LKB1-deficient DCs to promote dysregulated T cell effector activity, with predominant increase in thymus-derived regulatory T cell priming but also increased priming of pro-immunogenic effector Th17, Th1 and CD8+ T cells. Mechanistically, upon loss of LKB1, DCs enhance their expression of MHC molecules, co-stimulatory molecules (e.g., CD86, OX40L), cytokines (e.g., IL-6, IL-2) and migration receptors (e.g., CCR7) — all of which contribute to enhanced T cell priming. The predominant activation of regulatory T cells and Th17 cells upon LKB1 deletion in DCs contributes to tumor growth
Overall, LKB1 emerges as a fundamental regulator of the core DC function to control T cell responses and maintain their immunological quiescence, at least partially via limiting DC migration, co-stimulatory molecule (CD86 and OX40L) and cytokine (IL-6) expression (Fig. 1).9–11 LKB1 loss in DCs results in their uncontrolled stimulation of T cells, foremost of Tregs by cDC2s in the thymus and periphery as well as peripheral Th17 cells. Prevention of mTOR signaling in DCs, likely in concert with limiting glycolytic metabolism,2–4 appears to account for aspects of LKB1-mediated regulation of T cell immunity by DCs, such as Treg homeostasis.
LKB1 restrains dendritic cell function
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6796839/
http://www.jimmunol.org/content/198/6/2223
Nrf2 Promotes Keratinocyte Proliferation in Psoriasis through Up-Regulation of Keratin 6, Keratin 16, and Keratin 17 - Journal of Investigative Dermatology
https://www.jidonline.org/article/S0022-202X(17)31561-0/fulltext
Jounard of Investigation Dermatology. 2008 May;
Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells
1Laboratory for Investigative Dermatology, The Rockefeller University, New York, New York 10021, USA.
The importance of T helper 17 (Th17) cells in inflammation and autoimmunity is now being appreciated. We analyzed psoriasis skin lesions and peripheral blood for the presence of IL-17-producing T cells. We localized Th17 cells predominantly to the dermis of psoriasis skin lesions, confirmed that IL-17 mRNA increased with disease activity, and demonstrated that IL-17 mRNA expression normalized with cyclosporine therapy. IL-22 mRNA expression mirrored IL-17 and both were downregulated in parallel with keratin 16. Th17 cells are a discrete population, separate from Th1 cells (which are also in psoriasis lesions), and Th2 cells. Our findings suggest that psoriasis is a mixed Th1 and Th17 inflammatory environment. Th17 cells may be proximal regulators of psoriatic skin inflammation, and warrant further attention as therapeutic targets.寻常型银屑病病变包含离散的Th1和Th17 T细胞群
T helper 17 (Th17)细胞在炎症和自身免疫中的重要性正在得到重视。我们分析了银屑病皮肤损伤和外周血中是否存在il -17产生T细胞。我们将Th17细胞主要定位于银屑病皮损的真皮,证实IL-17 mRNA随疾病活动而增加,并证实在环孢素(cyclosporine)治疗后IL-17 mRNA表达正常化。IL-22 mRNA表达与IL-17一致,且与角蛋白16平行下调。Th17细胞是一个离散的群体,与Th1细胞(也存在于银屑病病变中)和Th2细胞分离。我们的发现提示银屑病是Th1和Th17混合的炎症环境。Th17细胞可能是银屑病皮肤炎症的近端调节因子,值得进一步关注作为治疗靶点。Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells - PubMed
https://pubmed.ncbi.nlm.nih.gov/18200064/
Phosphodiesterase-4 (PDE4) inhibitors have the potential to suppress inflammatory cells and structural cells in chronic obstructive pulmonary disease patients, giving a broad spectrum anti-inflammatory profile.
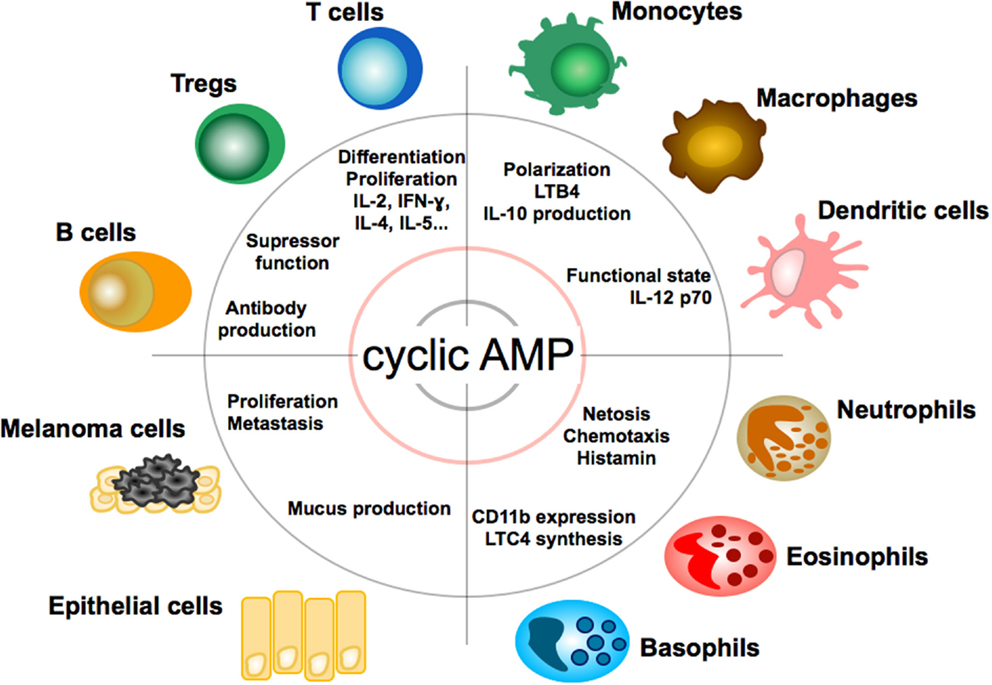
The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases
Department of Dermatology, University Medical Center Mainz, Johannes Gutenberg-University Mainz, Mainz, Germany
Dendritic Cells
As professional antigen-presenting cells of the immune system, DCs are equipped with a unique capability to induce and regulate adaptive immune responses. In DC, cyclic AMP suppresses the release of pro-inflammatory mediators (TNF-α, IL-17, IFN-γ) (61) and promotes the release of anti-inflammatory mediators, such as IL-10 (62). As a functional consequence, cAMP concentrations in DC regulate T cell immunity (63). Pharmacological inhibition of cyclic nucleotide PDE4, which is highly expressed in DC, for example, suppresses the DC Th1-polarizing capacity (64, 65) and commands secretion of IL-6 and TGF-beta and subsequent induction of Th17 differentiation (66). It, thus, appears that cAMP levels differentially regulate cytokine production by DC as a response to changes in the microenvironment. Apart from spatio-temporal fine-tuning of DC activities, cAMP activities in DC depend on the stage of DC maturation: prostaglandin E2 (PGE2), a key inducer of cAMP, exerts a stimulatory function for immature DCs in peripheral tissues (67) but inhibitory function for mature DCs in lymph nodes (68).Frontiers | The cAMP Pathway as Therapeutic Target in Autoimmune and Inflammatory Diseases | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2016.00123/full
Figure 3. Calcium (Ca2+)–calcineurin–nuclear factor of an activated T cell (NFAT) signaling pathway as a novel therapeutic target. Orai1 is a plasma membrane protein with four transmembrane segments. Stromal interaction molecule 1 (STIM1) is a single-pass transmembrane protein located in the endoplasmic reticulum (ER). The increase in intracellular Ca2+ concentration by Ca2+ entry through Ca2+ release-activated Ca2+ (CRAC)/Orai1 induces the phosphatase calcineurin. As a result, the activated calcineurin dephosphorylates several serine residues of the NFAT. The NFAT then translocates to the nucleus where it binds to DNA and regulates target gene expression. Some ions or small molecules, including La3+, SKF96365, and 2-ABP, are able to inhibit the CRAC channel. Again, already-commercialized cyclosporine A and tacrolimus inhibit calcineurin. Since dysregulated Ca2+ signaling is involved in the pathogenesis of autoimmune diseases, intervention of Ca2+ signaling in store-operated Ca2+ entry (SOCE) through the Orai1–STIM1 pathway may be a promising approach to control autoimmune diseases.
Calcineurin (CaN) is a calcium and calmodulin dependent serine/threonine protein phosphatase (also known as protein phosphatase 3, and calcium-dependent serine-threonine phosphatase).[2] It activates the T cells of the immune system and can be blocked by drugs. Calcineurin activates nuclear factor of activated T cell cytoplasmic (NFATc), a transcription factor, by dephosphorylating it. The activated NFATc is then translocated into the nucleus, where it upregulates the expression of interleukin 2 (IL-2), which, in turn, stimulates the growth and differentiation of the T cell response. Calcineurin is the target of a class of drugs called calcineurin inhibitors, which include ciclosporin, voclosporin, pimecrolimus and tacrolimus.Mechanism of action
When an antigen-presenting cell interacts with a T cell receptor on T cells, there is an increase in the cytoplasmic level of calcium, which activates calcineurin by binding a regulatory subunit and activating calmodulin binding.[3] Calcineurin induces transcription factors (NFATs) that are important in the transcription of IL-2 genes. IL-2 activates T-helper lymphocytes and induces the production of other cytokines. In this way, it governs the action of cytotoxic lymphocytes. The amount of IL-2 being produced by the T-helper cells is believed to influence the extent of the immune response significantly.钙调神经磷酸酶(CaN)是一种钙和钙调蛋白依赖性丝氨酸/苏氨酸蛋白磷酸酶(也称为蛋白磷酸酶3,和钙依赖性丝氨酸-苏氨酸磷酸酶)。它可以激活免疫系统的T细胞,可以被药物阻止。钙调神经磷酸酶通过去磷酸化激活T细胞胞质核因子(NFATc),一种转录因子。激活的NFATc随后被转移到细胞核中,上调白细胞介素2 (IL-2)的表达,进而刺激T细胞的生长和分化反应。钙调神经磷酸酶是一类称为钙调神经磷酸酶抑制剂的药物的靶点,这类药物包括环孢素,voclosporin, pimecrolimus和他克莫司。
作用机制
当抗原呈递细胞与T细胞上的T细胞受体相互作用时,细胞质中的钙水平会升高,钙调磷酸酶通过与调节亚基结合激活钙调蛋白结合。[3]钙调神经磷酸酶诱导转录因子(nfat),在IL-2基因的转录中起重要作用。IL-2激活t辅助淋巴细胞并诱导其他细胞因子的产生。通过这种方式,它控制着细胞毒性淋巴细胞的活动。t辅助细胞产生的IL-2的数量被认为会显著影响免疫应答的程度。Calcineurin - Wikipedia
https://en.wikipedia.org/wiki/Calcineurin[Magnesium: a natural calcium antagonist]
[Magnesium: a natural calcium antagonist] - PubMed
https://pubmed.ncbi.nlm.nih.gov/8926707/The dangers of magnesium deficiency | Dr. Thomas E. Levy ...
https://www.peakenergy.com/articles/nh20140407/The...
Apr 07, 2014 · Probably the single most important property of magnesium in the body is its ability to act as a natural biological antagonist to calcium. As most adults have excess calciumthroughout their bodies, it is this reciprocal relationship between magnesium and calcium that makes most people in need of regular magnesium supplementation.
Frontier Immunology, 10 March 2020 |
The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases
Calcium (Ca2+) is an essential signaling molecule that controls a wide range of biological functions. In the immune system, calcium signals play a central role in a variety of cellular functions such as proliferation, differentiation, apoptosis, and numerous gene transcriptions. During an immune response, the engagement of T-cell and B-cell antigen receptors induces a decrease in the intracellular Ca2+ store and then activates store-operated Ca2+ entry (SOCE) to raise the intracellular Ca2+ concentration, which is mediated by the Ca2+ release-activated Ca2+ (CRAC) channels. Recently, identification of the two critical regulators of the CRAC channel, stromal interaction molecule (STIM) and Orai1, has broadened our understanding of the regulatory mechanisms of Ca2+ signaling in lymphocytes. Repetitive or prolonged increase in intracellular Ca2+ is required for the calcineurin-mediated dephosphorylation of the nuclear factor of an activated T cell (NFAT). Recent data indicate that Ca2+-calcineurin-NFAT1 to 4 pathways are dysregulated in autoimmune diseases. Therefore, calcineurin inhibitors, cyclosporine and tacrolimus, have been used for the treatment of such autoimmune diseases as systemic lupus erythematosus and rheumatoid arthritis. Here, we review the role of the Ca2+-calcineurin–NFAT signaling pathway in health and diseases, focusing on the STIM and Orai1, and discuss the deregulated calcium-mediated calcineurin-NFAT pathway in autoimmune diseases.Psoriasis
Psoriasis is a chronic inflammatory skin disease characterized by various sized thick scaly erythematous plaques (108). The histopathology of psoriatic plaques shows epidermal proliferation and inflammation of the dermis (109). Both innate and adaptive immune cells, including keratinocytes and T cells, participate in the initiation and perpetuation of psoriasis (58, 110). Psoriasis is a well-established T cell-mediated skin disease (110, 111). In particular, various cytokines induce the activation of immune cells, particular Th1 and Th17 cells (111), and the functional imbalance of Th1 or Th17 over Tregs is considered a key pathway for the progression of psoriasis (111). For example, psoriatic skin lesions show a strong IFN-γ signature and have an abundance of IFN-γ (+) Th1 cells (112). An imbalance between Tregs and effector T (Teff) cells is observed in the peripheral blood of psoriasis patients (113). Moreover, the Tregs of psoriasis patients are functionally deficient in suppressing Teff cells (114). Recently, the association between IL-9 and the Th17 pathway has been reported in psoriasis. Expressions of IL-9 and IL-9R are markedly increased in psoriatic skin lesions (115), and IL-9 stimulates the production of IL-17A by CD4+ T cells isolated from patients with psoriasis (116).
It is well-established that calcineurin inhibitors suppress T cell activation and the differentiation of naive T cells to memory T cells (117). In particular, calcineurin inhibitors downregulate the expression of STAT1, IFN-γ, and several IFN-γ-downstream genes, repressing the generation of Th1 cells (118). Moreover, the expressions of IL-17, IL-22, and IL-17-inducible genes, including DEFB-2, LCN2, IL-1β, S100A12, and CCL20, are markedly suppressed by calcineurin inhibitors (119). Given the importance of Th1 and Th17 cells in psoriasis pathogenesis, the inhibition of the calcineurin–NFAT pathway seems to be therapeutically relevant to psoriasis. Interestingly, the actions of calcineurin inhibitors are not limited to T cells. NFAT1 expression was first described by Northrop et al. (120) in mice skin, and then calcineurin expression was subsequently reported in the human epidermis (121). Calcineurin inhibitors reduce antigen presentation by Langerhans' cells and suppress neutrophil chemotaxis through the inhibition of psoriatic monocytes (122). Epidermal IL-1 and IL-8 expressions in psoriatic skin can be blocked by the calcineurin inhibitor cyclosporine (123). Indeed, cyclosporine and tacrolimus, both calcineurin inhibitors, have been widely used in psoriasis treatment with high efficacy (124).
STIM1 and Orai1 in keratinocytes, CRAC channels, have been implicated in the proliferation and differentiation of keratinocytes. It has been demonstrated that keratinocyte differentiation is induced by the change of extracellular Ca2+ concentration (125). Increased extracellular Ca2+ concentration triggers phospholipase C-mediated intracellular Ca2+ signals, which activate SOCE. Moreover, siRNA-mediated knockdown of either STIM1 or Orai1 suppresses SOCE and almost completely abolishes the Ca2+-mediated keratinocyte differentiation and growth (125). Menon and Elias (126) reported a defective Ca2+ gradient in the keratinocytes of psoriasis patients. Keratinocytes isolated from psoriasis patients showed a decreased response after Ca2+ store depletion as well as reduced mRNA/protein expression of CRAC channels (127, 128). In line with these findings, another study reported reduced mRNA and protein expression of TRPC channels (128), and the incubation of keratinocytes isolated from psoriasis patients with the TRPC6 agonist partly restores their differentiation and proliferation defect (129). Therefore, it remains to be determined whether Ca2+ sensing and signaling pathway plays an inductive or protective role in the pathologic differentiation and proliferation of keratinocytes in psoriasis.
Taken together, the earlier reports indicate that Ca2+ sensing and signaling pathway can be an excellent target for the treatment of psoriasis. Currently, phase 1 of a clinical trial of CRAC channel inhibitor for plaque psoriasis is in progress. CRAC channel inhibitors, a new class of oral immunomodulatory drugs, potently inhibit Orai1, Th1, Th2, and Th17-derived cytokine production and T cell proliferation, which are involved in chronic inflammatory responses in psoriasis (130).Frontiers | The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2020.00195/full
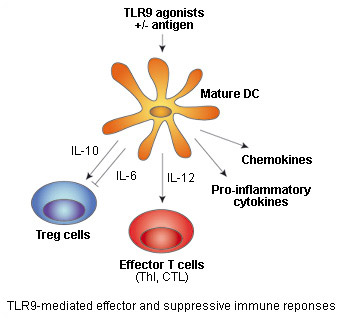
TLR9 agonists: double-edge sword for immune therapies
Toll-like receptor 9 (TLR9) senses unmethylated CpG dinucleotides, a hallmark of microbial DNA, that can be mimicked by synthetic oligonucleotides containing CpG motifs (CpG ODNs).
TLR9 stimulation by CpG DNA or CpG ODNs triggers intracellular signaling leading to the activation of macrophages, dendritic cells (DC) and B cells, and the production of cytokines, chemokines, and immunoglobulins. Subsequently, cytokines produced by DC, such as IL-12, induce the differentiation of naive T cells into T helper 1 (Th1) and cytotoxic T-cells (CTL).
CpG ODNs as vaccine adjuvants
Therefore, TLR9 agonists can elicit innate immune defenses and antigen T-cell specific responses, a property that underlines their development as vaccine adjuvants or immunotherapeutics for infectious diseases and cancer.
Studies in animal models have demonstrated that the immune defenses mounted by CpG ODNs alone or as vaccine adjuvants can protect against a variety of viral, bacterial, and parasitic diseases [1]. Promising results in the prophylactic treatment of hepatitis B have been obtained from phase III trials with a combination of a CpG ODN and hepatitis B surface antigen (Heplisav) [2].
CpG ODNs as antitumor agents
Antitumor activity of CpG ODNs has also been established in numerous mouse models. Encouraging results in the treatment of cancers have come from phase I and II clinical trials using CpG ODNs as a tumor vaccine adjuvant, monotherapy, or in combination with chemotherapy [2].
However, there have been also some disappointing results with one pharmaceutical company recently dropping its clinical program with a TLR9 agonist in non-small cell lung cancer. The interim data of two phase 3 trials of PF-3512676 (formerly called CpG 2006) showed that it failed to improve the clinical outcomes compared to chemotherapy alone [2].TLR9受体激动剂:免疫治疗的双刃剑
toll样受体9 (TLR9)感受未甲基化的CpG二核苷酸,这是微生物DNA的一个标志,可以被含有CpG基序的合成寡核苷酸(CpG ODNs)模仿。
CpG DNA或CpG ODNs刺激TLR9可触发细胞内信号,导致巨噬细胞、树突状细胞(DC)和B细胞的激活,并产生细胞因子、趋化因子和免疫球蛋白。随后,DC产生的细胞因子,如IL-12,诱导原始T细胞分化为T助手1 (Th1)和细胞毒性T细胞(CTL)。
CpG ODNs作为疫苗佐剂
因此,TLR9激动剂可以诱导先天免疫防御和抗原t细胞特异性应答,这一特性突出了TLR9作为疫苗佐剂或免疫疗法用于感染性疾病和癌症的发展。
动物模型研究表明,单独使用CpG ODNs或作为疫苗佐剂进行免疫防御,可预防多种病毒、细菌和寄生虫疾病[1]。联合CpG ODN和乙型肝炎表面抗原(Heplisav)[2]的III期临床试验在预防治疗乙型肝炎方面取得了有希望的结果。
CpG ODNs作为抗肿瘤药物
CpG ODNs的抗肿瘤活性也已在许多小鼠模型中得到证实。使用CpG ODNs作为肿瘤疫苗佐剂、单药治疗或与化疗[2]联合治疗癌症的I期和II期临床试验取得了令人鼓舞的结果。
然而,也有一些令人失望的结果,最近一家制药公司放弃了其TLR9激动剂治疗非小细胞肺癌的临床项目。两项PF-3512676(前称CpG 2006)三期临床试验的中期数据显示,与单纯化疗[2]相比,PF-3512676并不能改善临床结局。
TLR9 agonists: double-edge sword for immune therapies
Toll-like receptor 9 (TLR9) senses unmethylated CpG dinucleotides, a hallmark of microbial DNA, that can be mimicked by synthetic oligonucleotides containing CpG motifs (CpG ODNs).
TLR9 stimulation by CpG DNA or CpG ODNs triggers intracellular signaling leading to the activation of macrophages, dendritic cells (DC) and B cells, and the production of cytokines, chemokines, and immunoglobulins. Subsequently, cytokines produced by DC, such as IL-12, induce the differentiation of naive T cells into T helper 1 (Th1) and cytotoxic T-cells (CTL).TLR9受体激动剂:免疫治疗的双刃剑
toll样受体9 (TLR9)感受未甲基化的CpG二核苷酸,这是微生物DNA的一个标志,可以被含有CpG基序的合成寡核苷酸(CpG ODNs)模仿。
CpG DNA或CpG ODNs刺激TLR9可触发细胞内信号,导致巨噬细胞、树突状细胞(DC)和B细胞的激活,并产生细胞因子、趋化因子和免疫球蛋白。随后,DC产生的细胞因子,如IL-12,诱导原始T细胞分化为T助手1 (Th1)和细胞毒性T细胞(CTL)。TLR9 and autoimmune diseases
Recent reports indicate that TLR9 may play a role in the pathogenesis of various autoimmune diseases, such as systemic lupus erythematosus (SLE). Under certain conditions,TLR9 is able to recognize self-DNA leading to the production of anti-DNA autoantibodies.
This discovery has prompted the development of specific inhibitors of TLR9. Paralleling the approach of stimulating TLR9 with CpG ODNs, it was found that suppressive ODNs exist that are able to inhibit TLR9 activation.The most potent inhibitory sequences contain TTAGGG multimers found in mammalian telomeres or a 5’ CCT, a C-free linker four to five bases long, and a GGG(G) tail. Some of these suppressive ODNs are able to inhibit an already ongoing immune response and therefore could be useful in the treatment of SLE [5].
This data illustrate the great potential of TLR9-based drugs for the treatment of infectious diseases, cancer and autoimmune diseases. However, as they can activate both the effector and suppressive arms of the immune system, more studies are needed to better understand the mechanisms involved allowing the development of safer and more effective TLR therapeutics.TLR9与自身免疫性疾病
最近的研究表明,TLR9可能在各种自身免疫性疾病的发病机制中发挥作用,如系统性红斑狼疮(SLE)。在一定条件下,TLR9能够识别自身dna,从而产生抗dna自身抗体。
这一发现促进了TLR9特异性抑制剂的开发。与CpG ODNs刺激TLR9的方法平行,研究发现存在能够抑制TLR9激活的抑制性ODNs。最有效的抑制序列包括哺乳动物端粒中发现的TTAGGG多聚体或5 ' CCT, 4到5个碱基长的不含c的连接子和GGG(G)尾巴。这些抑制性odn中的一些能够抑制已经进行的免疫反应,因此可能对SLE[5]的治疗有用。
这些数据说明了基于tlr9的药物在治疗传染病、癌症和自身免疫性疾病方面的巨大潜力。然而,由于它们可以同时激活免疫系统的效应臂和抑制臂,需要更多的研究来更好地了解涉及的机制,从而开发更安全、更有效的TLR疗法。https://www.invivogen.com/review-tlr9-agonists
Published 2009
Dendritic Cells as Danger-Recognizing Biosensors
Department of Bioscience and Biotechnology, College of Life Science, Sejong University, Seoul, 143-747, Korea
2.3.3. Autoimmune Diseases
Autoimmune diseases caused by autoreactive T cells or autoantibody-producing B cells can be initiated when self-antigens are presented by DCs. In addition, DCs in an autoimmune-prone condition can aggravate inflammatory immune responses by secreting IFNα/β and TNFα upon stimulation with necrotic cells caused by autoreactive T cells. TNFα plays a critical role in the pathogenesis of rheumatoid arthritis and psoriasis, and DCs are the primary source of TNFα [57]. Treatment of patients with TNFα-blocking therapeutic agents dramatically improved symptoms of arthritis and psoriasis. SLE is an autoimmune disease, characterized by the breakdown of immune tolerance by nuclear components and often gets worse by viral infection. Recently, it has been reported that pDCs are involved in the pathogenesis of SLE by secreting IFNα/β [58]. pDCs, the main producer of IFNα/β, induce activation of autoreactive T cells and the production of autoreactive antibodies by making immature DCs become mature DCs [59]. It is now believed that pDCs mediate IFNα/β production by signaling through TLR7 and TLR9, which recognize viral nucleic acid, or via DNA-binding proteins such as HMGB1 and RAGE [11,60].
2.3.4. Allergies
Although inhaled allergens are taken up by DCs and presented to T cells, tolerance is induced in healthy individuals. However, in allergic disease conditions, immune tolerance against inhaled allergens becomes broken, resulting in the presentation of allergens by DCs to T cells. Upon recognition of allergens, T cells differentiate toward Th2 cells, which give aid to B cells and produce allergen-specific immunoglobulin (Ig) E. Skin epithelial cells directly or indirectly influence DCs to induce allergic immune responses. Skin epithelial cells help DCs to sample allergens infiltrated into skin and also regulate cytokine production of DCs to help differentiation of naïve CD4+ T cells into Th2 cells. Thymic stromal lymphopoietin or granulocyte macrophage colony stimulating factor (GM-CSF), produced by epithelial cells, has been known to induce Th2 polarization [61,62]. In addition, it has been recently reported that inhaled allergens with enzymatic activity act on DCs to induce Th2 differentiation [62].
4. Conclusions
DCs play a critical role in determining the polarization of T cell immune responses, which have a direct impact on what types of antibody isotypes B cells produce. In other words, DCs can direct which adaptive immune responses are induced. Research on the functions of DCs should become a central focus of the immunology field because DCs are involved in various diseases, including infectious diseases, cancer, autoimmune diseases, and allergies.
Through recognition of appropriate danger signals, DCs help to protect the host from a variety of diseases and maintain a micro-environment for optimal immunity in healthy individuals. If DCs do not detect danger signals, the host immune system does not induce immune responses against pathogens and also may not induce immune tolerance upon induction of autoimmune responses. Considering the importance of DC functions in immunological regulation, DCs have been the focus for therapeutics for many diseases including cancers. Here, we have introduced the cytokine reporter mouse model as a tool for monitoring activation of DCs. To achieve better understanding of DC functions, it will be important to expand the studies of DC subsets in healthy and diseased states. To conclude, we anticipate a dramatic increase in the number of studies on DCs that will be based on populations that are isolated from mouse and human lymphoid organs or are cultured in vitro using DC growth factors.2.3.3。自身免疫性疾病
当DCs呈交自身抗原时,由自身反应性T细胞或产生自身抗体的B细胞引起的自身免疫性疾病就会发生。此外,在自身免疫倾向条件下,树突状细胞通过自身反应性T细胞引起的坏死细胞刺激分泌IFNα/β和TNFα,可加重炎症免疫反应。TNFα在类风湿关节炎和银屑病的发病机制中发挥重要作用,DCs是TNFα[57]的主要来源。TNFα阻断治疗药物显著改善关节炎和银屑病症状。SLE是一种自身免疫性疾病,其特征是通过核成分破坏免疫耐受,并经常因病毒感染而恶化。最近有报道称pDCs通过分泌IFNα/β[58]参与SLE的发病机制。pDCs是IFNα/β的主要产生者,通过使未成熟的树突状细胞变成成熟的树突状细胞[59],诱导自身反应性T细胞的活化和自身反应性抗体的产生。现在认为pDCs通过识别病毒核酸的TLR7和TLR9信号转导,或通过HMGB1和RAGE等dna结合蛋白介导IFNα/β的产生[11,60]。
2.3.4。过敏
虽然吸入的过敏原被树突状细胞吸收并呈递给T细胞,但在健康个体中可诱导耐受。然而,在过敏性疾病的条件下,免疫耐受吸入变应原被打破,导致DCs将变应原提交给T细胞。T细胞在识别过敏原后向Th2细胞分化,Th2细胞帮助B细胞产生过敏原特异性免疫球蛋白(Ig) E.皮肤上皮细胞直接或间接影响dc诱导过敏免疫应答。皮肤上皮细胞帮助树突状细胞对渗入皮肤的变应原进行取样,并调节树突状细胞的细胞因子生产,帮助原始CD4+ T细胞分化为Th2细胞。由上皮细胞产生的胸腺基质淋巴细胞生长期或粒细胞巨噬细胞集落刺激因子(GM-CSF)已被证实可诱导Th2极化[61,2]。此外,最近有报道称,吸入的具有酶活性的变应原作用于DCs,诱导Th2分化[62]。
4. 结论
DCs在决定T细胞免疫反应的极化过程中起着至关重要的作用,它直接影响B细胞产生何种类型的抗体同型。换句话说,树突状细胞可以指导哪些适应性免疫反应被诱导。由于DCs涉及多种疾病,包括传染病、癌症、自身免疫性疾病、过敏等,因此对DCs功能的研究应成为免疫学领域的重点。
通过识别适当的危险信号,DCs有助于保护宿主免受各种疾病的侵袭,并维持健康个体最佳免疫的微环境。如果DCs没有检测到危险信号,宿主免疫系统就不会诱导对病原体的免疫应答,也可能不会诱导自身免疫应答后的免疫耐受。由于DC功能在免疫调节中的重要性,DC已成为包括癌症在内的许多疾病治疗的重点。在这里,我们介绍了细胞因子报告小鼠模型作为监测DCs激活的工具。为了更好地理解直流电的功能,扩大健康和疾病状态下的直流电亚群的研究将是重要的。综上所述,我们预计DC研究的数量将显著增加,这些研究将基于从小鼠和人淋巴器官分离的群体,或使用DC生长因子体外培养的群体。http://www.mdpi.com/1424-8220/9/9/6730/htm
Cells, Mar 14, 2020
Glycogen Metabolism Supports Early Glycolytic Reprogramming and Activation in Dendritic Cells in Response to Both TLR and Syk-Dependent CLR Agonists
Dendritic cells (DCs) increase their metabolic dependence on glucose and glycolysis to support their maturation, activation-associated cytokine production, and T-cell stimulatory capacity. We have previously shown that this increase in glucose metabolism can be initiated by both Toll-like receptor (TLR) and C-type lectin receptor (CLR) agonists. In addition, we have shown that the TLR-dependent demand for glucose is partially satisfied by intracellular glycogen stores. However, the role of glycogen metabolism in supporting CLR-dependent DC glycolytic demand has not been formally demonstrated. In this work, we have shown that DCs activated with fungal-associated β-glucan ligands exhibit acute glycolysis induction that is dependent on glycogen metabolism. Furthermore, glycogen metabolism supports DC maturation, inflammatory cytokine production, and priming of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome in response to both TLR- and CLR-mediated activation. These data support a model in which different classes of innate immune receptors functionally converge in their requirement for glycogen-dependent glycolysis to metabolically support early DC activation. These studies provide new insight into how DC immune effector function is metabolically regulated in response to diverse inflammatory stimuli.糖原代谢支持早期糖酵解重编程和树突状细胞的活化,同时响应TLR和syk依赖性CLR激动剂
树突状细胞(dc)增加对葡萄糖和糖酵解的代谢依赖,以支持其成熟、激活相关细胞因子的产生和t细胞的刺激能力。我们之前已经证明,这种葡萄糖代谢的增加可以由toll样受体(TLR)和c型凝集素受体(CLR)激动剂启动。此外,我们发现依赖tlr的葡萄糖需求部分通过细胞内糖原存储得到满足。然而,糖原代谢在支持clr依赖性DC糖酵解需求中的作用尚未得到正式证明。在这项工作中,我们已经证明了被真菌相关β-葡聚糖配体激活的树突状细胞表现出依赖于糖原代谢的急性糖酵解诱导。此外,糖原代谢支持在TLR和clr介导的激活下,DC成熟,炎症细胞因子的产生,核苷结合域,富含亮氨酸的家族,pyrin区域包含-3 (NLRP3)炎性小体的启动。这些数据支持了一个模型,在这个模型中,不同种类的先天免疫受体在糖原依赖的糖酵解作用中功能趋同,以代谢方式支持早期DC激活。这些研究为DC免疫效应因子在不同炎症刺激下的代谢调节提供了新的见解。
Keywords: dendritic cells; glucose; glycogen; glycolysis; innate immunity; metabolism.Glycogen Metabolism Supports Early Glycolytic Reprogramming and Activation in Dendritic Cells in Response to Both TLR and Syk-Dependent CLR Agonists - PubMed
https://pubmed.ncbi.nlm.nih.gov/32183271/#:~:text=Dendritic%20cells%20%28DCs%29%20increase%20their%20metabolic%20dependence%20on,receptor%20%28TLR%29%20and%20C-type%20lectin%20receptor%20%28CLR%29%20agonists.
Metabolic Control of Dendritic Cell Functions: Digesting Information
Immunobiology Laboratory, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain
Metabolic Control of Dendritic Cell Development
Natural dendritic cells (DCs) present in steady state comprise type 1 conventional DCs (cDC1s), type 2 cDCs (cDC2s), double negative (CD8/CD103– CD11b–) DCs (DN-DCs), and plasmacytoid DCs (pDCs; Table 1). Natural DCs derive from myeloid progenitors in the bone marrow and require FMS-like tyrosine kinase 3 ligand (FLT3L) to differentiate via the common DC progenitor (CDP) and DC precursors (pre-DCs). In addition, other cells that are functionally similar to DCs, such as Langerhans cells (LCs), can derive from embryonic precursors. Moreover, during inflammatory settings, DCs can develop from blood monocytes (moDCs; Table 1).
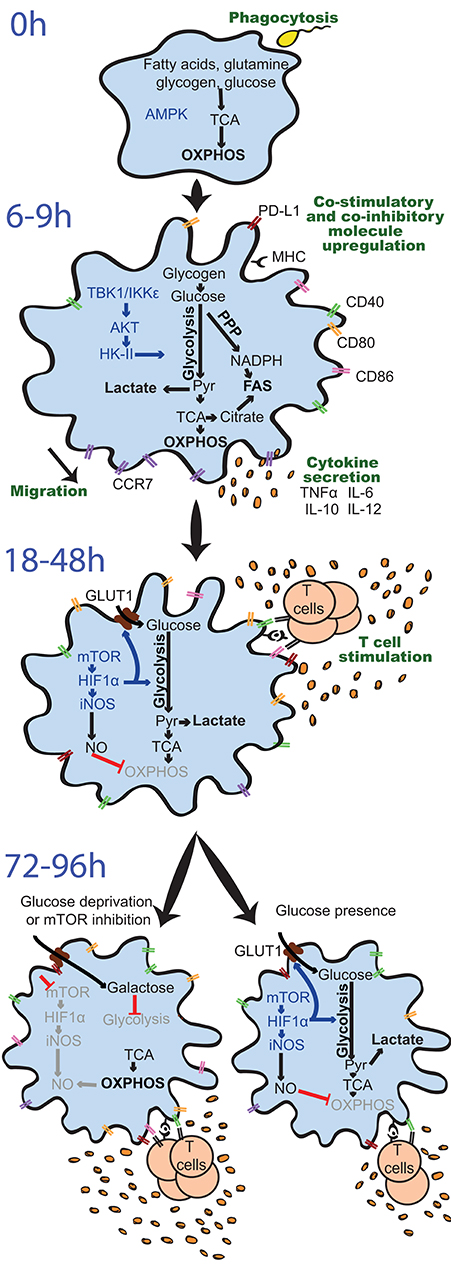
Figure 3. Differential regulation and effects of glycolysis induction in GM-DCs upon stimulation over time. Resting GM-DCs (top) display a basal metabolism with active AMPK (AMP-activated protein kinase) and fatty acids, glutamine, glycogen, and glucose being fully oxidized to generate energy by oxidative phosphorylation (OXPHOS). Upon early stimulation after 6–9 h, GM-DCs are activated and exhibit transiently enhanced OXPHOS/mitochondrial membrane potential and an increased glycolytic metabolism mainly using glucose from intracellular glycogen stores. The induction of glycolysis is predominantly driven by a TBK1-IKKε/AKT/HK-II axis and largely devoted to fatty acid synthesis (FAS). Moreover, enhanced early glycolytic activity of GM-DCs is vital for their migration and upregulation of co-stimulatory/inhibitory molecules as well as cytokines. At later time points about 18–48 h after robust stimulation, a mTOR/HIF1α/iNOS axis is activated in GM-DCs, leading to enforced glycolysis via upregulation of glucose importers such as GLUT1 and inhibition of OXPHOS via nitric oxide (NO). This fostered glycolytic activity appears crucial for the interaction of GM-DCs with T cells. Nevertheless, the sustained inhibition of OXPHOS by NO and reliance on glycolysis for energy generation can reduce the ability of GM-DCs to stimulate T cells in the long term. Glucose deprivation or mTOR inhibition can preserve metabolic flexibility and functional OXPHOS in GM-DCs, sustaining their activity at least during 72–96 h and extending their life span.
AKT, protein kinase B; CCR7, C-C chemokine receptor type 7; CD, cluster of differentiation; GLUT1, glucose transporter 1; GM-DC, GM-CSF, mouse GM-CSF-induced DCs; HIF1α, hypoxia-inducible factor 1-alpha; HK-II, hexokinase II; IKKε, IkB kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; MHC, major histocompatibility complex; mTOR, mammalian target of rapamycin; NADPH, nicotinamide adenine dinucleotide phosphate; PD-L1, programmed death-ligand 1, Pyr, pyruvate; PPP, pentose phosphate pathway; TBK1, TANK-binding kinase 1; TCA, Tricarboxcylic acid cycle; TNFα, tumor necrosis factor α.
Requirement of Glycolysis for Functions of Activated Dendritic Cells
Interrupting the glucose-to-pyruvate pathway significantly impairs DC maturation, upregulation of co-stimulatory molecules, cytokine secretion, and T cell stimulatory capacity in the long term (Figure 3). For example, pharmacological blockade of glycolysis using 2-deoxyglucose (2-DG), genetic deficiency of glycolytic enzymes such as α-enolase (ENO1), or overexpression of lactate dehydrogenase A (LDHA) or pyruvate dehydrogenase kinase 1 (PDK1) (Figure 2) prevents GM-DC maturation and immunogenicity upon stimulation with LPS or Chlamydia (13, 47, 49, 57) and can skew GM-DCs toward inducing Th17 and regulatory T cells (Treg) rather than Th1 and Th2 responses (49). In line, natural mouse cDC1s and cDC2s isolated from the spleen decrease expression of co-stimulatory molecules, IL-12 production, and activation of CD4+ and CD8+ T cells when activated by LPS in the presence of 2-DG (49). pRNA-stimulated human blood cDC2s require glycolytic activity for activation, evidenced by TNFα production, CD86, and programmed death ligand 1 (PD-L1) expression (53). Treatment of primary human pDCs with 2-DG upon influenza A virus stimulation also reduces co-stimulatory molecule and type I interferon (IFN-I) expression (52), while another study rather suggests induction of glutamine-fueled OXPHOS upon pRNA stimulation of human blood pDCs (53). However, the effects of inhibition of glycolysis by 2-DG in DCs have to be taken with caution, as 2-DG itself deregulates cytokine expression of human moDCs in vitro by activation of the endoplasmic reticulum (ER) stress response via the sensor inositol-requiring protein 1α (IRE1α) (50). In addition, 2-DG can impair the TCA cycle, OXPHOS, and ATP levels, as recently described in macrophages (58).
Other DC functions such as phagocytosis do not seem to be affected by inhibition of glycolysis during stimulation of human moDCs (50). However, reduced endocytic/phagocytic activity in aging mouse spleen cDC1s and DN-DCs [termed merocytic DCs (mcDCs)] and a resulting decline in antigen cross-presentation are linked to mitochondrial dysfunction with decreased basal OCR and ΔΨm as well as enhanced proton leakage and ROS. Importantly, inhibition of ATP synthase by oligomycin or the uncoupling agent carbonyl cyanide 4-(trifluromethoxy)phenyl-hydrazone (FCCP) corroborates the diminished phagocytosis of cDC1s and DN-DCs/mcDCs (22). Moreover, antigen uptake seems to decrease in GM-DCs in hypoxia, when glycolytic activity is increased by HIF1α stabilization, which is also observed in human moDCs after stimulation (47, 50).
In contrast, glucose and enhanced glycolytic activity are required for the ability of DCs to migrate (Figure 3). Independently of stimulation, glucose-deprived GM-DCs show reduced mobility, increased rounded morphology losing dendrites, and impaired oligomerization of CCR7, the chemokine receptor driving DC migration toward LNs. Subsequently, glucose limitation or 2-DG presence prevents migration of GM-DCs as well as splenic CD11c+ cDCs both in vitro and in vivo (49, 56). In line, HIF1α-deficient GM-DCs, which largely fail to induce glycolysis (see the section Sustained Glycolysis: The Role of HIF1α), display reduced CCR7 levels, and GM-DCs differentiated in hypoxic conditions exhibit elevated migratory potential in vitro and in vivo that is dependent on HIF1α (14).
Overall, early induction of glycolysis emerges as a general feature of immunogenic activation of most cultured DCs and primary DC subsets and appears necessary for several aspects of their maturation such as upregulation of co-stimulatory surface molecules and cytokine production, despite having no major effects on phagocytosis or antigen uptake. However, DC activation leads to cytoskeletal changes that support increased migratory capacity to migrate toward LNs and T cell zones, which is also affected by early induced glycolysis. Ultimately, in light of those findings, glycolytic increase in DCs upon stimulation is vital for adequate induction of adaptive T cell responses (59) and, hence, regulates immune homeostasis (Figure 3).
https://www.frontiersin.org/articles/10.3389/fimmu.2019.00775/full
Significance of DC-LAMP and DC-SIGN expression in psoriasis vulgaris lesions
In psoriasis vulgaris lesions, dendritic cells are massively recruited to the dermis, where these cells mature gradually and express a large amount of DC-LAMP and DC-SIGN, thereby activating T lymphocytes and participating in the process of immunological inflammation. The high expression levels of DC-LAMP and DC-SIGN are positively correlated.
Author: Ma Wei-yuan, Liu Wen-ting, Zhao Chen, Sun Qing
Cited by: 10
Publish Year: 2011
Significance of DC-LAMP and DC-SIGN expression in psoriasis vulgaris lesions - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0014480011000554#:~:text=In%20psoriasis%20vulgaris%20lesions%2C%20dendritic%20cells%20are%20massively,levels%20of%20DC-LAMP%20and%20DC-SIGN%20are%20positively%20correlated.
Front. Immunol., 19 June 2018
T Cell Hierarchy in the Pathogenesis of Psoriasis and Associated Cardiovascular Comorbidities
The key role of T cells in the pathogenesis of cutaneous psoriasis has been well described in the last decade and the knowledge of the relative role of the different subsets of T cells in psoriasis pathogenesis has considerably evolved. Now, it is clear that IL-17A-producing T cells, including Th17/Tc17, have a central role in the pathogenesis of cutaneous psoriasis and therapies blocking the IL-17A pathway show high clinical efficacy. By contrast, the contribution of IFNγ-producing T cells has progressively become less clear because of the lack of efficacy of anti-IFNγ antibodies in clinical studies. In parallel, the role of CD8+ T cells specific for self-antigens has been revived and increasing evidence now indicates that in psoriatic skin the majority CD8+ T cells are present in the form of epidermal tissue-resident memory T cells. In the last years it also emerged the possibility of a contribution of T cell recirculation in the pathogenesis of psoriasis and its systemic manifestations.前面。Immunol。2018年6月19日
T细胞等级在银屑病发病机制和相关心血管共病中的作用
T细胞在皮肤银屑病发病机制中的关键作用在过去十年中得到了很好的描述,并且对T细胞不同亚群在银屑病发病机制中的相对作用有了相当大的进展。现在已经明确IL-17A产生T细胞,包括Th17/Tc17,在皮肤银屑病的发病机制中起中心作用,阻断IL-17A通路的治疗显示出较高的临床疗效。相比之下,由于临床研究中缺乏抗IFNγ抗体的有效性,IFNγ产生T细胞的作用已逐渐变得不清楚。与此同时,针对自身抗原的CD8+ T细胞的作用也得到了恢复,越来越多的证据表明,在银屑病皮肤中,大多数CD8+ T细胞以表皮组织驻留记忆T细胞的形式存在。近年来,T细胞循环在银屑病发病机制及其全身表现中发挥作用的可能性也出现了。Frontiers | T Cell Hierarchy in the Pathogenesis of Psoriasis and Associated Cardiovascular Comorbidities | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2018.01390/full
Biomedicine & Pharmacotherapy
Volume 131, November 2020,
Luteolin attenuates imiquimod–induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response
School of Biomedical and Pharmaceutical Sciences, Guangdong University of Technology, 100 Waihuanxi Road, Guangzhou, 510006, PR China
b
Guangdong Provincial Key Laboratory of Emergency Test for Dangerous Chemicals, Guangdong Institute of Analysis, Guangzhou, 510070, PR China
Highlights
•
Luteolin ameliorated psoriasis-like skin lesions in an imiquimod-induced mouse model.
•
Luteolin suppressed the cutaneous macrophage infiltration and cytokine release in vivo.
•
Luteolin attenuated inflammatory response in a macrophage cell line.
Abstract
Psoriasis is considered as a common chronic immune-mediated skin disorder characterized by abnormal keratinocyte proliferation. Luteolin, an anti-inflammatory natural flavonoid with well-accepted inhibition effect against keratinocyte proliferation, was hypothesized to have a potential therapeutic effect for psoriasis. In this paper, we investigated the relieving effect of luteolin against imiquimod-induced psoriasis-like lesions on BALB/c mice and its possible anti-inflammatory mechanisms in lipopolysaccharide-stimulated macrophages (RAW264.7 cells). We found that luteolin ameliorated psoriasis-like skin lesions, suppressed the cutaneous infiltration of macrophages, T cells and neutrophils, and downregulated the expression of cytokines like IL-6, IL-1β, TNF-α, IL-17A and IL-23 in both skin lesions and eyeball blood of model mice. In vitro, we observed luteolin significantly suppressed the levels of psoriasis-related pro-inflammatory cytokines, such as IL-17A, IL-6, TNF-α and IL-23, and inflammatory mediators like nitric oxide NO, inducible NOS, COX-2 in RAW264.7 cells. The anti-inflammatory activity was accomplished by inhibiting NF-κB expression and activation. This study demonstrates luteolin is effective in alleviating psoriasis-like skin lesions and downregulating inflammatory response via NF-κB pathway, suggesting luteolin as a potential molecule for further therapeutic research of inflammation-related skin diseases like psoriasis.
木犀草素(luteolin)可通过抑制炎症反应减轻咪喹莫特诱导的BALB/c小鼠银屑病样皮损
突出了
• 木犀草素改善咪喹莫特诱导小鼠模型中银屑病样皮肤损伤。
• 木犀草素在体内抑制皮肤巨噬细胞浸润和细胞因子释放。
• 木犀草素可减弱巨噬细胞的炎症反应。
银屑病被认为是一种常见的慢性免疫介导的皮肤疾病,以异常的角质细胞增殖为特征。木犀草素是一种天然的抗炎类黄酮,对角质细胞增殖有良好的抑制作用,被认为对银屑病有潜在的治疗作用。本文研究木耳素对脂多糖刺激巨噬细胞(RAW264.7细胞)中吡喹莫特诱导的银屑病样皮损的缓解作用及其可能的抗炎机制。我们发现木犀草素可以改善银屑病样皮损,抑制巨噬细胞、T细胞和中性粒细胞的皮肤浸润,下调模型小鼠皮损和眼血中IL-6、IL-1β、TNF-α、IL-17A和IL-23等细胞因子的表达。在体外实验中,木犀草素显著抑制了RAW264.7细胞中与银屑病相关的促炎因子IL-17A、IL-6、TNF-α和IL-23的水平,以及炎症介质NO、诱导型NOS、COX-2的水平。其抗炎活性是通过抑制NF-κB的表达和活化来实现的。本研究表明木犀草素可通过NF-κB通路减轻银屑病样皮损,下调炎症反应,提示木犀草素有望成为银屑病等炎症相关皮肤病的进一步治疗研究的分子。
Luteolin attenuates imiquimod–induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0753332220308891#:~:text=Macrophages%20were%20found%20to%20accumulate%20in%20psoriatic%20skin,keratinocytes%20and%20the%20skin%20inflammation%20observed%20in%20psoriasis.
Cytokine Pathways in Psoriasis and Psoriatic Arthritis
Psoriatic disease is a systemic autoimmune disease mostly associated with skin and joint involvement (psoriatic arthritis). Strong evidences from clinical studies and experimental models suggest that both innate and adaptive immune responses are involved in its pathogenesis. Psoriatic disease used to be regarded as a Th1-driven disease, now there are substantial evidences to suggest regulatory role of Th17 cells as well in the pathogenesis of psoriasis and psoriatic arthritis. Cytokines play a critical role; besides IFN-γ and TNFα, IL-23/Th17 pathway plays a dominant role in the inflammatory and proliferative cascades of both the skin and joint tissues. Recently in a series of elegant experiments using mouse models and human tissues it has been demonstrated that IL-23 induced Th17 cytokines (IL-17 and IL-22) can contribute to all four pathologic events in a psoriatic disease: development of psoriatic plaque, pannus formation in the joint, joint erosion and new bone formation.
银屑病和银屑病关节炎中的细胞因子通路
银屑病是一种全身自身免疫性疾病,多与皮肤和关节受累相关(银屑病关节炎)。临床研究和实验模型的有力证据表明,先天性和适应性免疫反应均参与其发病机制。银屑病曾被认为是th1驱动的疾病,现在有大量证据表明Th17细胞在银屑病和银屑病关节炎的发病机制中也发挥调节作用。细胞因子起关键作用;除了IFN-γ和TNFα外,IL-23/Th17通路在皮肤和关节组织的炎症和增殖级联中起主导作用。最近的一系列优雅实验用小鼠模型和人体组织已经证明IL-23感应Th17细胞因子(IL-17和il - 22生成)可以为所有四个病理事件牛皮癣疾病:银屑病斑块的发展,血管翳形成联合,共同侵蚀和新骨形成。
Cytokine Pathways in Psoriasis and Psoriatic Arthritis | SpringerLink
https://link.springer.com/chapter/10.1007/978-3-319-19530-8_9
Frontier in Immunology Oct 6, 2020
Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-κB Activation via GPR81-Mediated Signaling
Kun Yang1,2†, Jingjing Xu1†‡, Min Fan1,2, Fei Tu1,2, Xiaohui Wang1, Tuanzhu Ha1,2, David L. Williams1,2 and Chuanfu Li1,2*
1Department of Surgery, Quillen College of Medicine, East Tennessee State University, Johnson City, TN, United States
2Center of Excellence for Inflammation, Infectious Disease and Immunity, Quillen College of Medicine, East Tennessee State University, Johnson City, TN, United StatesRecent evidence from cancer research indicates that lactate exerts a suppressive effect on innate immune responses in cancer. This study investigated the mechanisms by which lactate suppresses macrophage pro-inflammatory responses. Macrophages [Raw 264.7 and bone marrow derived macrophages (BMDMs)] were treated with LPS in the presence or absence of lactate. Pro-inflammatory cytokines, NF-κB and YAP activation and nuclear translocation were examined. Our results show that lactate significantly attenuates LPS stimulated macrophage TNF-α and IL-6 production. Lactate also suppresses LPS stimulated macrophage NF-κB and YAP activation and nuclear translocation in macrophages. Interestingly, YAP activation and nuclear translocation are required for LPS stimulated macrophage NF-κB activation and TNFα production. Importantly, lactate suppressed YAP activation and nuclear translocation is mediated by GPR81 dependent AMKP and LATS activation which phosphorylates YAP, resulting in YAP inactivation. Finally, we demonstrated that LPS stimulation induces an interaction between YAP and NF-κB subunit p65, while lactate decreases the interaction of YAP and NF-κB, thus suppressing LPS induced pro-inflammatory cytokine production. Our study demonstrates that lactate exerts a previously unknown role in the suppression of macrophage pro-inflammatory cytokine production via GPR81 mediated YAP inactivation, resulting in disruption of YAP and NF-κB interaction and nuclear translocation in macrophages.
乳酸通过gpr81介导的信号通路抑制YAP和NF-κB活化,从而抑制巨噬细胞对LPS刺激的促炎症反应
最近癌症研究的证据表明,乳酸对癌症的先天免疫反应有抑制作用。本研究探讨了乳酸盐抑制巨噬细胞促炎反应的机制。巨噬细胞[Raw 264.7和骨髓来源巨噬细胞(bone marrow derived Macrophages, BMDMs)]在乳酸存在或不存在的情况下被LPS处理。检测促炎细胞因子、NF-κB、YAP活化及核转位。我们的研究结果表明,乳酸可以显著降低LPS刺激的巨噬细胞TNF-α和IL-6的产生。乳酸还可以抑制LPS刺激的巨噬细胞NF-κB和YAP的活化以及巨噬细胞的核转位。有趣的是,LPS刺激的巨噬细胞NF-κB激活和TNF -α产生需要YAP激活和核转位。重要的是,乳酸抑制YAP激活和核转位是由GPR81依赖的AMKP和LATS激活介导的,这些激活使YAP磷酸化,导致YAP失活。最后,我们发现LPS刺激诱导YAP和NF-κB亚基p65相互作用,而乳酸降低YAP和NF-κB相互作用,从而抑制LPS诱导的促炎症细胞因子的产生。我们的研究表明,乳酸通过GPR81介导的YAP失活在抑制巨噬细胞促炎性细胞因子产生中发挥着以前未知的作用,导致巨噬细胞中YAP和NF-κB相互作用的中断以及核转位。
Frontiers | Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-κB Activation via GPR81-Mediated Signaling | Immunology
https://www.frontiersin.org/articles/10.3389/fimmu.2020.587913/full
Lactic acid delays the inflammatory response of human monocytes
a Department of Internal Medicine III, University Hospital Regensburg, Franz-Josef-Strauß-Allee 11, 93053 Regensburg, Germany
b RCI Regensburg Center for Interventional Immunology, University Hospital Regensburg, Franz-Josef-Strauß-Allee 11, 93053 Regensburg, Germany
Received 20 December 2014, Available online 9 January 2015.
Highlights
• Lactic acid broadly delays LPS-induced gene expression in human monocytes.
• Expression of important monocyte effector molecules is affected by lactic acid.
• Interference of lactic acid with TLR signaling causes the delayed gene expression.
•The profound effect of lactic acid might contribute to immune suppression in tumors.
Abstract
Lactic acid (LA) accumulates under inflammatory conditions, e.g. in wounds or tumors, and influences local immune cell functions. We previously noted inhibitory effects of LA on glycolysis and TNF secretion of human LPS-stimulated monocytes. Here, we globally analyze the influence of LA on gene expression during monocyte activation. To separate LA-specific from lactate- or pH-effects, monocytes were treated for one or four hours with LPS in the presence of physiological concentrations of LA, sodium lactate (NaL) or acidic pH. Analyses of global gene expression profiles revealed striking effects of LA during the early stimulation phase. Up-regulation of most LPS-induced genes was significantly delayed in the presence of LA, while this inhibitory effect was attenuated in acidified samples and not detected after incubation with NaL. LA targets included genes encoding for important monocyte effector proteins like cytokines (e.g. TNF and IL-23) or chemokines (e.g. CCL2 and CCL7). LA effects were validated for several targets by quantitative RT-PCR and/or ELISA. Further analysis of LPS-signaling pathways revealed that LA delayed the phosphorylation of protein kinase B (AKT) as well as the degradation of IκBα. Consistently, the LPS-induced nuclear accumulation of NFκB was also diminished in response to LA. These results indicate that the broad effect of LA on gene expression and function of human monocytes is at least partially caused by its interference with immediate signal transduction events after activation. This mechanism might contribute to monocyte suppression in the tumor environment.乳酸延缓人类单核细胞的炎症反应
突出了
•乳酸广泛延迟lps诱导的人类单核细胞基因表达。
•重要单核细胞效应分子的表达受到乳酸的影响。
•乳酸对TLR信号的干扰导致基因表达延迟。
•乳酸的深远影响可能有助于肿瘤的免疫抑制。
摘要
乳酸(LA)在炎症条件下积累,如在伤口或肿瘤中,并影响局部免疫细胞功能。我们先前注意到LA对lps刺激的人单核细胞的糖酵解和TNF分泌的抑制作用。在这里,我们全面分析LA在单核细胞激活过程中对基因表达的影响。为了将LA特异性与乳酸或ph效应区分开来,在生理浓度为LA、乳酸钠(NaL)或酸性ph的情况下,单核细胞用LPS处理1或4小时。对整体基因表达谱的分析显示,在早期刺激阶段,LA具有显著的作用。在LA存在的情况下,大多数lps诱导基因的上调被显著延迟,而这种抑制作用在酸化样品中减弱,在与NaL孵育后没有检测到。LA的靶标包括编码重要单核细胞效应蛋白的基因,如细胞因子(如TNF和IL-23)或趋化因子(如CCL2和CCL7)。通过RT-PCR和/或ELISA方法验证LA对多个靶点的作用。进一步分析lps信号通路发现LA延缓了蛋白激酶B (AKT)的磷酸化以及IκBα的降解。同样,lps诱导的NF -κB的核积累也在LA的作用下减少。这些结果表明,LA对人单核细胞基因表达和功能的广泛影响至少部分是由于其在激活后直接干扰信号转导事件所致。这一机制可能有助于单核细胞在肿瘤环境中的抑制。
Lactic acid delays the inflammatory response of human monocytes - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0006291X1500011X
IMMUNOBIOLOGY| MARCH 1, 2006
Tumor-derived lactic acid modulates dendritic cell activation and antigen expressionThe Department of Hematology and Oncology, Institute of Pathology, University of Regensburg; and the Institute of Physiology and Pathophysiology, University of Mainz, Germany.
The tumor milieu can influence dendritic cell (DC) differentiation. We analyzed DC differentiation in a 3-dimensional tumor model and propose a new mechanism of DC modulation by the tumor environment. Monocytes were cultured in the presence of IL-4 and GM-CSF within multicellular tumor spheroids (MCTSs) generated from different tumor cell lines. Monocytes invaded the MCTSs and differentiated into tumor-associated dendritic cells (TADCs). The antigen expression was altered on TADCs independent of the culture conditions (immature/mature DCs, Langerhans cells) and IL-12 secretion was reduced. Supernatants of MCTSs could partially transfer the suppressive effect. Conditioned media from urothelial carcinoma cell lines contained high levels of M-CSF and IL-6, both cytokines known to modulate DC differentiation. In contrast, melanoma and prostate carcinoma MCTS cocultures produced little M-CSF and IL-6, but high levels of lactic acid. Indeed, addition of lactic acid during DC differentiation in vitro induced a phenotype comparable with TADCs generated within melanoma and prostate carcinoma MCTSs. Blocking of lactic acid production in melanoma MCTS cocultures reverted the TADC phenotype to normal. We therefore conclude that tumor-derived lactic acid is an important factor modulating the DC phenotype in the tumor environment, which may critically contribute to tumor escape mechanisms.Tumor-derived lactic acid modulates dendritic cell activation and antigen expression | Blood | American Society of Hematology
https://ashpublications.org/blood/article/107/5/2013/133421/Tumor-derived-lactic-acid-modulates-dendritic-cell
Vitamin C as therapy for Psoriasis?? - Vitamin C Forum
vitamincfoundation.com/forum/viewtopic.php?t=3174
Dec 24, 2019 · So iI Applied it topically and it cleared it up. Then I realized psoriasis is a fungal infection!!! There's no way I have an autoimmune disease without an explanation. So I mix my vitamin c concoction and day 2 now there is no itching and the patches are shrinking and no longer red. I'm amazed that vitamin c has come through for me again!!!I have been battling psoriasis for 6 months. I've tried all sorts of things including bowel tolerance doses of vitamin c for half a yr to no avail. I tried omega 3 two weeks ago and it improved for a day and got worse again. Then I started thinking the only thing I have not been able to cure with bowel tolerance doses of vitamin c is ringworm. So iI Applied it topically and it cleared it up. Then I realized psoriasis is a fungal infection!!! There's no way I have an autoimmune disease without an explanation. So I mix my vitamin c concoction and day 2 now there is no itching and the patches are shrinking and no longer red. I'm amazed that vitamin c has come through for me again!!! So topically apply it to your psoriasis!
J Immunol, 2009 May 1
Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis
Department of Dermatology, Erasmus Medical Center, Rotterdam, The Netherlands.
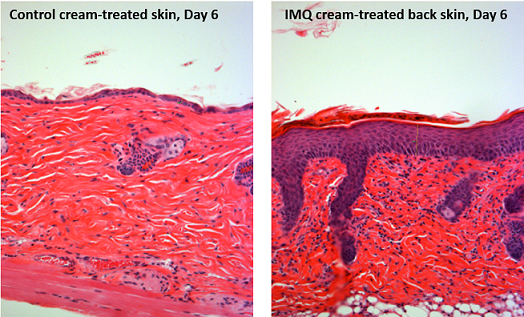
Topical application of imiquimod (IMQ), a TLR7/8 ligand and potent immune activator, can induce and exacerbate psoriasis, a chronic inflammatory skin disorder. Recently, a crucial role was proposed for the IL-23/IL-17 axis in psoriasis. We hypothesized that IMQ-induced dermatitis in mice can serve as a model for the analysis of pathogenic mechanisms in psoriasis-like dermatitis and assessed its IL-23/IL-17 axis dependency. Daily application of IMQ on mouse back skin induced inflamed scaly skin lesions resembling plaque type psoriasis. These lesions showed increased epidermal proliferation, abnormal differentiation, epidermal accumulation of neutrophils in microabcesses, neoangiogenesis, and infiltrates consisting of CD4(+) T cells, CD11c(+) dendritic cells, and plasmacytoid dendritic cells. IMQ induced epidermal expression of IL-23, IL-17A, and IL-17F, as well as an increase in splenic Th17 cells. IMQ-induced dermatitis was partially dependent on the presence of T cells, whereas disease development was almost completely blocked in mice deficient for IL-23 or the IL-17 receptor, demonstrating a pivotal role of the IL-23/IL-17 axis. In conclusion, the sole application of the innate TLR7/8 ligand IMQ rapidly induces a dermatitis closely resembling human psoriasis, critically dependent on the IL-23/IL-17 axis. This rapid and convenient model allows further elucidation of pathogenic mechanisms and evaluation of new therapies in psoriasis.
免疫杂志,2009年5月1日
小鼠银屑病样皮肤炎症是通过IL-23/IL-17轴介导的
荷兰鹿特丹伊拉斯谟医学中心皮肤科。
局部应用亚咪喹莫特(IMQ),一种TLR7/8配体和强免疫激活剂,可诱发和加重银屑病(一种慢性炎症性皮肤病)。最近,IL-23/IL-17轴在银屑病中的重要作用被提出。我们假设imq诱导的小鼠皮炎可作为分析银屑病样皮炎致病机制的模型,并评估其IL-23/IL-17轴依赖性。每天应用IMQ在小鼠背部皮肤诱导的类似斑块型银屑病的炎症鳞状皮损上。这些病变表现为表皮细胞增殖增加,分化异常,微上皮中中性粒细胞表皮聚集,新生血管生成,CD4(+) T细胞、CD11c(+)树突状细胞和浆细胞样树突状细胞浸润。IMQ诱导IL-23、IL-17A和IL-17F的表皮表达,并增加脾Th17细胞。imq诱导的皮炎部分依赖于T细胞的存在,而在IL-23或IL-17受体缺失的小鼠中,疾病的发展几乎完全被阻断,这表明IL-23/IL-17轴的关键作用。总之,仅使用天然TLR7/8配体IMQ,就会迅速诱发与人类银屑病非常相似的皮炎,严重依赖于IL-23/IL-17轴。这种快速、方便的模型允许进一步阐明银屑病的致病机制和评价新的治疗方法。
https://pubmed.ncbi.nlm.nih.gov/19380832/IL-23 from Langerhans cells is required for the development of imiquimod-induced psoriasis-like dermatitis by induction of IL-17A-producing γδ T cells.
Yoshiki R, Kabashima K, Honda T, Nakamizo S, Sawada Y, Sugita K, Yoshioka H, Ohmori S, Malissen B, Tokura Y, Nakamura M.
J Invest Dermatol. 2014 Jul;134(7):1912-1921. doi: 10.1038/jid.2014.98. Epub 2014 Feb 25.
PMID: 24569709
Azithromycin impairs TLR7 signaling in dendritic cells and improves the severity of imiquimod-induced psoriasis-like skin inflammation in mice.
Huang SW, Chen YJ, Wang ST, Ho LW, Kao JK, Narita M, Takahashi M, Wu CY, Cheng HY, Shieh JJ.
J Dermatol Sci. 2016 Oct;84(1):59-70. doi: 10.1016/j.jdermsci.2016.07.007. Epub 2016 Jul 12.
PMID: 27449383
Mechanism of pathogenesis of imiquimod-induced skin inflammation in the mouse: a role for interferon-alpha in dendritic cell activation by imiquimod.
Ueyama A, Yamamoto M, Tsujii K, Furue Y, Imura C, Shichijo M, Yasui K.
J Dermatol. 2014 Feb;41(2):135-43. doi: 10.1111/1346-8138.12367. Epub 2014 Jan 3.
PMID: 24387343
Development of psoriasis by continuous neutrophil infiltration into the epidermis.
Katayama H.
Exp Dermatol. 2018 Oct;27(10):1084-1091. doi: 10.1111/exd.13746. Epub 2018 Aug 14.
PMID: 30019426 Review.
IL-17 and IL-17R: an auspicious therapeutic target for psoriatic disease.
Mitra A, Raychaudhuri SK, Raychaudhuri SP.
Actas Dermosifiliogr. 2014 Oct;105 Suppl 1:21-33. doi: 10.1016/S0001-7310(14)70015-8.
PMID: 25398489 Review.
Imiquimod-Induced Psoriasis Model
Psoriasis is a chronic skin condition associated with multiple contributing factors including autoimmune disease. The imiquimod-induced Psoriasis model is particularly translational into the clinic as it has many of the significant markers of human disease, including histopathology of lesions and strong activation of the immune system. Charles River’s extensive immunology expertise allows our scientists to implement these translational models to mimic human disease, and our experienced pathologists can analyze the pathology associated with the Psoriasis disease model.
Psoriasis Mouse Model
Psoriasis is an immune-mediated inflammatory skin disease characterized by skin thickening, red plaques and dry scales. Psoriasis can be triggered by many factors, including injury, trauma, infection and medications. The disease is assessed clinically using the Psoriasis Activity and Severity Index (PASI) scale which ranks severity of erythema (redness), induration (thickness) and desquamation (scale).
Histologically, the disease is characterized by epidermal thickening due to hyperkeratosis, infiltration of immune cells in dermis and epidermis, parakeratosis and neovascularization. A hallmark of the disease in humans is the involvement of IL-23/IL17 cytokine axis.
Charles River has characterized a clinically relevant psoriasis model in mice through the topical application of 5% imiquimod (IMQ) cream. This imiquimod-induced psoriasis models human plaque-type psoriasis in which the IL-23/IL-17 cytokine axis plays a pivotal role.
Study parameters include in-life clinical evaluation of skin, histopathological evaluation of skin sections and optional cytokine analysis in skin and/or internal immune organs.
H&E stained sections of back skin of mice treated with vehicle Vaseline and 5% imiquimod cream
Figure 1: H&E stained sections of back skin of mice treated with vehicle Vaseline and 5% imiquimod cream.Imiquimod-Induced银屑病模型
银屑病是一种与包括自身免疫性疾病在内的多种因素相关的慢性皮肤疾病。吡喹莫特诱导的银屑病模型特别适合临床应用,因为它具有许多人类疾病的重要标志物,包括病变的组织病理学和免疫系统的强烈激活。Charles River丰富的免疫学专业知识使我们的科学家能够实现这些转化模型来模拟人类疾病,我们经验丰富的病理学家可以分析与牛皮癣疾病模型相关的病理。牛皮癣小鼠模型
牛皮癣是一种免疫介导的炎症性皮肤病,其特征是皮肤增厚、红斑和干燥鳞屑。银屑病可由多种因素引发,包括损伤、创伤、感染和药物。临床上使用银屑病活动性和严重程度指数(PASI)量表来评估银屑病,该量表将红斑(发红)、硬结(厚度)和脱屑(鳞片)的严重程度进行分级。
在组织学上,本病的特征是表皮因角化过度而增厚,真皮和表皮内免疫细胞浸润,角化不全和新生血管形成。IL-23/IL17细胞因子轴的参与是该疾病在人类中的一个标志。
Charles River通过外用5%咪喹莫特(IMQ)乳膏,建立了一种与临床相关的小鼠银屑病模型。吡喹莫特诱导的银屑病模型是IL-23/IL-17细胞因子轴起关键作用的人斑型银屑病模型。
研究参数包括皮肤的活体临床评估、皮肤切片的组织病理学评估和皮肤和/或内部免疫器官的细胞因子分析。
用载体凡士林和5%咪喹莫特乳膏处理的小鼠背部皮肤切片的H&E染色
图1:使用vehicle凡士林和5%咪喹莫特乳膏处理的小鼠背部皮肤的H&E染色切片。
Imiquimod-induced psoriasis-like skin inflammation in mice ...
https://pubmed.ncbi.nlm.nih.gov/19380832
Topical application of imiquimod (IMQ), a TLR7/8 ligand and potent immune activator, can induce and exacerbate psoriasis, a chronic inflammatory skin disorder. Recently, a crucial role was proposed for the IL-23/IL-17 axis in psoriasis. We hypothesized that IMQ-induced dermatitis in mice can serve as a model for the analysis of pathogenic mechanisms in psoriasis-like dermatitis and assessed its IL-23/IL-17 …
Cited by: 1571
Publish Year: 2009
Author: Leslie van der Fits, Sabine Mourits, Jane S. A. Voerman, Marius Kant, Louis Boon, Jon D. Laman, Ferr...
Imiquimod-Induced Psoriasis Model - Charles River
https://www.criver.com/.../imiquimod-induced-psoriasis-model
The Imiquimod (IMQ)-induced Psoriasis model is translational into the clinic as it exhibits markers of human disease including histopathology and activation of the immune system, with a strong T-cell element. IMQ is a ligand for TLRs (Toll-like receptors) of immune cells (including macrophages, monocytes and plasmacytoid dendritic cells), and therefore contributes to strong activation of the immune …
Published Jan 11, 2019.
The Imiquimod Induced Psoriatic Animal “Model: Scientific Implications
1 Korea Institute of Science & Technology, Gangneung, Republic of Korea
2 KIST School, Korea University of Science and Technology (UST), Daejeon, Republic of Korea咪喹莫特诱发银屑病动物模型:科学意义
银屑病是一种慢性炎症性自身免疫性疾病,可导致严重的皮肤病变、棘皮化和角化不全,对我们的外观和生活质量造成长期的不良影响。尽管在过去的几十年里,在许多专家的宝贵实验和努力下,积累了大量关于银屑病相关机制和病理分析的科学信息。然而,缺乏可靠的小鼠模型一直是银屑病研究进展的主要障碍。近年来,在银屑病动物模型上局部应用经典toll样受体(TLR)-7激动剂咪喹莫特(IMQ),为银屑病的研究提供了坚实的基础,因为它能刺激小鼠皮肤表型,类似于人银屑病。IMQ介导的皮肤炎症在人银屑病上常常表现出轻微的差异,为了获得更适合的银屑病模型,正在进行广泛的研究。本文综述了近年来在改进现有IMQ模型和发现银屑病新模型方面取得的新突破,并讨论了其在银屑病研究中的重要意义。
关键词:牛皮癣;咪喹莫特;动物模型;皮肤炎症;TLR
缩写:IMQ:咪喹莫特;TLR: toll样受体;柯:mice-Knockout老鼠;DC:树突细胞
IMQ诱导小鼠模型的表型
咪喹莫特(IMQ)是小鼠toll样受体(TLR)-7和人TLR7/8的标志性配体,已被证实对人乳头瘤病毒引起的疣、光化性角化病和基底细胞癌具有保护作用。由于其化学结构小,亲脂性好,非常适合外用,成功应用于上述疾病的软膏[1,2]。IMQ治疗很快成为银屑病易感人群患银屑病的一个原因。自2009年imq诱导小鼠炎症模型发表以来,该模型成为银屑病研究中最引人注目的动物模型之一,科学家将该模型用于研究实践和实验。IMQ长期局部应用于皮肤耳部或剃后皮肤导致银屑病样皮肤炎症,表现为皮肤红斑和脱屑增多,表皮增厚,角质细胞分化改变,免疫细胞向皮肤募集,与人类银屑病发病机制相似[3,4]。
IMQ诱导小鼠模型的病理机制
IMQ一般通过激活巨噬细胞和树突状细胞(DC)[5]中的细胞内受体TLR7和TLR8发挥抗病毒和抗肿瘤活性。巨噬细胞和树突状细胞中与TLR7信号结合的IMQ刺激这些细胞浸润到感染/炎症部位,表达促炎细胞因子和趋化因子,如IL-1α、IL-6、IL-23和IFN-α,导致DCs成熟和Th1/Th17应答[6]。此外,IMQ治疗增强了中性粒细胞浸润、角化细胞增殖和肥大细胞活动的改变。炎症第一阶段,TLR7/8下游靶点MyD88的激活主要导致CD11c+髓系DCs产生IL-23和浆细胞样DCs产生INF-α,通过上调NF-κB和MAPK信号通路放大炎症反应,并开始分化初发T细胞[7,8]。在IL-23的分泌下,γδT细胞主要释放IL-17和IL-22,激活角质形成细胞增殖,增加趋化因子和抗菌蛋白的产生,并增加中性粒细胞的招募,从而吸引其他免疫细胞维持局部炎症。
另一方面,IMQ也通过MyD88独立触发角化细胞增殖和细胞凋亡通路,可能腺苷受体介导营地通路,因为角质细胞缺乏细胞内TLR7/8受体在初始阶段的炎症和之后,角化细胞活动受IL-17和il - 22生成表达式通过打开inflammasome复杂加强S100蛋白水平和LL-37(9、10)。反复给药IMQ诱导的多个细胞和分子的炎症作用均在7天后达到高峰,并可能持续到治疗结束,与人类银屑病具有显著的相似性,是最可靠的模型。Psoriasis is a chronic inflammatory auto-immune disease, which causes serious skin lesions, acanthosis and parakeratosis, leaving a long-lasting detrimental influence on our appearances and life quality. Though a huge scientific information regarding psoriasis-related mechanism and pathological analysis is getting accumulated over the last decades in the price of valuable experiments and efforts of many experts. However, an absence of reliable mouse model has been a major obstacle to further advances in psoriasis research. Recently, a topical application of imiquimod (IMQ), classical Toll-like Receptor (TLR)-7 agonist, has provided a strong foundation of psoriasis study on the animal model as it stimulates cutaneous phenotype similar to human psoriasis in the mouse. IMQ-mediated skin inflammation often exhibits slight divergences on human psoriasis and extensive investigations to yield more suitable psoriatic model are taking place. In this review, we summarized the recent novel breakthroughs on improving existing IMQ model and discovering a new model of psoriasis and discussed their importance on psoriasis research.
Keywords: Psoriasis; Imiquimod; Animal model; Skin inflammation; TLR
Abbreviations: IMQ: Imiquimod; TLR: Toll-like Receptor; KO: mice-Knockout mice; DC: Dendritic cell
The phenotypes of IMQ-Induced Mouse Model
Imiquimod (IMQ), hallmark ligand of Toll-like Receptor (TLR)-7 in mouse and TLR7/8 in human has been known to be protective against human papilloma virus-caused warts, actinic keratosis and basal cell carcinomas. Due to its small chemical structure and lipophilicity, it is really suitable for topical administration, thus successfully used as an ointment for the aforementioned diseases [1,2]. Sooner IMQ treatment appeared to be a reason for psoriasis for people who are prone to psoriasis. Since the first article of IMQ-induced mouse inflammatory model was published in 2009, it emerged as one of the most prominent animal models in psoriasis study as scientist have adopted this model to their research practice and experiments. The long-term topical administration of IMQ on skin ear or shaved back skin results psoriasis-like skin inflammation, characterized by increased skin erythema and scaling, thickened epidermis, alteration of keratinocytes differentiation and recruitment of immune cells to skin, mimicking psoriatic pathogenesis in human [3,4].
Pathomechanism of IMQ Induced Mouse Model
Generally, IMQ exerts its antiviral and antitumor activity through activation of TLR7 and TLR8, intracellular receptors in macrophages and dendritic cells (DC) [5]. IMQ binding to TLR7 signals in macrophage and dendritic cells thrusts those cells to infiltrate to infection/inflammatory sites and to express pro-inflammatory cytokines and chemokines such as IL-1α, IL-6, IL-23, and IFN-α, resulting in maturation of DCs and initiation of Th1/Th17 responses [6]. Moreover, IMQ treatment enhance infiltration of neutrophil, the proliferation of keratinocytes and changes in mast cells action. During the first phase of inflammation, the activation of MyD88, downstream target of TLR7/8 primarily causes the production of IL-23 from CD11c+ myeloid DCs and INF-α from plasmacytoid DCs to amplify inflammatory reaction and start differentiation of naive T cells via upregulation of NF-κB and MAPK signaling [7,8]. In response to IL-23 secretion, γδ T cells predominantly release IL-17 and IL-22, which activate keratinocytes to proliferate and increase a production of chemokines and antimicrobial proteins as well as recruitment of neutrophils, thereby attracting other immune cells to maintain local inflammation.
On the other hand, IMQ also triggers keratinocyte proliferation and apoptosis through MyD88 independent pathway, possibly adenosine receptor-mediated cAMP pathway, since keratinocytes lack intracellular TLR7/8 receptor during the initial phase of inflammation and later, keratinocyte activity is regulated by IL-17 and IL-22 expression via switching on inflammasome complex to enhance level of S100 proteins and LL-37 [9,10]. All sequential and balanced inflammatory action of multiple cells and molecules, which is inducible by repeated administration of IMQ reach its peak after 7 days and likely to be maintained as long as if treatment continues and bear remarkable resemblance with human psoriasis, thereby making it most reliable model.https://biomedres.us/fulltexts/BJSTR.MS.ID.002347.php
5. Lactate transporters in immunometabolism
Although lactate was previously believed to be only a dead-end waste product of glycolysis, substantial evidence highlighted its critical role not only in regulating tumor immune surveillance but also in the regulation of immune function138, 139, 140.
Lactic acid is transported across biological membranes through four reversible monocarboxylate transporters (MCT) (lactate–H+ symporter) that belong to the SLC16 family of solute carriers comprising a total 14 members that share highly conserved characteristic motifs141. The transport direction of MCTs is determined by the concentration gradients of both monocarboxylate ions and protons. There is evidence that different MCTs facilitate lactic acid transport between cells. The most ubiquitously expressed family member, MCT1, facilitates lactate and pyruvate exchange and is induced by c-MYC142. Very similar to MCT1 but less expressed in human tissues, MCT2 displays a higher affinity for l-lactic acid and pyruvate. By contrast, MCT3 and MCT4 share similar functions and are efficient lactate exporters, fulfilling a key function in glycolytic cells and the retinal pigment epithelium141. Similarly, sodium-coupled lactate transport is also performed by the widely expressed high-affinity transporter SLC5A8 or the low-affinity transporter SLC5A12143.
The physiological concentration of lactate in normal tissues is about 1.5–3 mmol/L143, but can increase to 10–12 mmol/L at sites of inflammation such as atherosclerotic plaques or rheumatic synovial fluid, and even rise up to 20–30 mmol/L in tumors139,144,145.
Under high extracellular lactate concentrations, human cytotoxic T lymphocytes (CTL) were demonstrated to internalize lactate through MCT1146,147. It was later reported that CD4+ and CD8+ T cell subsets sense high lactate concentrations upon entering inflammatory sites via SLC5A12 and MCT1, respectively, and detrimentally undergo T cell motility inhibition, produce higher amounts of IL-17 and lose cytolytic activity due to interference with glycolysis via inhibition of phosphofructokinase (PFK) or downregulation of hexokinase-1 (HK1)144,148 (Fig. 1). Notably, these effects on T cell migration were not only observed in in vitro experiments, but also in a peritonitis model144. Although lactate can generally inhibit the activity of effector T cells, it has limited effects on the function of Tregs149.
Crucially, Zhang et al.150 found that downregulation of anaerobic glycolysis is required for the promotion of RIG-I-like receptor (RLR)-induced production of type I IFN. However, without sufficient surface expression of monocarboxylate transporter 1 (MCT1), cells stimulated upon poly (I:C) transfection were unable to properly upregulate IFN-β expression induced by lactate dehydrogenase A (LDHA) inhibitors, indicating that lactate uptake is essential for the inhibitory effect of glycolysis on RLR signaling.
MCT1 is only minimally expressed in the cell membrane of macrophages151,152 while MCT4 is required for macrophage activation by TLR2 and TLR4 agonists, where it helps sustain high glycolysis and expression of proinflammatory mediators153.
In the high-lactate tumor microenvironment, the cellular uptake of lactate produced by tumor cells is mediated by MCTs in tumor-associated macrophages (TAMs), where it promotes polarization towards the M2-like phenotype and the resulting increased secretion of vascular endothelial growth factor (VEGF) through hypoxia-inducible factor 1-alpha (HIF-1α)139. The information reviewed above lactate transporters involved in immune cells are summarized in Table 1.
6.1. Zinc transporters-mediated zinc homeostasis
As an indispensable component of over 300 different enzymes, zinc (Zn2+) participates in scores of essential biochemical processes in addition to its structural roles175,176. Zinc released from internal stores may act as a second massager to promote cellular mobility177. Many zinc-related enzymes and metalloproteins are present in immune cells. Zinc deficiency leads to impaired immune responses both in innate immunity and antibody-mediated adaptive immunity. In zinc-deficient pro-myeloid cells, interleukin (IL)-1β and tumor necrosis factor alpha (TNFα) are upregulated, due to improved posttranscriptional processing via nicotinamide adenine dinucleotide phosphate (NADPH), ROS-mediated redox signaling, and the activation of the p38 mitogen-activated protein kinase (MAPK) phosphorylation mechanism178. Consistent with these observations, zinc deficiency increases the phagocytosis and oxidative burst in human peripheral blood mononuclear cells, whereas the production of TNF-α and IL-6 is reduced after zinc deprivation for three days178. In zinc-deficient mice, T cells show more apoptosis and impaired thymulin signaling, which is rescued by zinc supplementation. High levels of zinc supplementation, about four times the physiological concentration, suppress the alloreactivity of mixed lymphocytes179. As indicated, zinc performs its function in a concentration-dependent manner, which reflects its important roles in a complex network of cellular activities.
The SLC39A (zrt/irt-like proteins; ZIP) family and SLC30A (cation diffusion; ZnT) Zn2+ family are two major Zn2+ transporters families. The 14 SLC39A family members mediate the influx of zinc from the extracellular or luminal side into the cytoplasm and the 10 identified SLC30A family members mediate the efflux of zinc154. Their activity is tightly correlated with the immune system because of the essential status of zinc. SLC39A7 encodes the ZIP7 protein, which is expressed ubiquitously regulating the influx of zinc from the Golgi or endoplasmic reticulum (ER) into the cytosol180,181 (Fig. 2156,182, 183, 184, 185). Multiple loss of function alleles of SLC39A7 result in a human immunodeficiency syndrome featured by reduced B cell signaling at the positive selection checkpoints156. Developing B cells are prone to be affected by partial SLC39A7 deficiency, which is reflected in impeded development beyond the pre-B cell stage156. Since the B cell receptor (BCR)-initiated pathways are mediated by various kinases and phosphatases186, the inhibitory effect of zinc on phosphatases is likely the proximal cause of impaired BCR signaling (Fig. 2). Recently, a compound has been identified in a drug screen that is able to rescue the impairment of cell proliferation and endoplasmic reticulum stress caused by ZIP7 ablation in human osteosarcoma cell line MG-63 in a zinc-independent manner187. This makes ZIP7 a potential therapeutic target in diseases related to zinc dysregulation. Similar to SLC39A7, ZIP10, encoded by SLC39A10, is another zinc transporter that regulates BCR transduction. When stimulated with IL-7, the signal transducer and activator of transcription (STAT), like STAT5, are activated in the early B cells. Then the signal upregulating of ZIP10 promotes early B-cell survival by inhibiting caspase activation (Fig. 2). ZIP10 also functions as a positive regulator of CD45R that alters the signal strength of the BCR188 (Fig. 2). In innate immunity, Slc39a10-deficency leads to reduced numbers of macrophages and monocytes in the LPS-induced inflammatory response, which is caused by increased mortality in a P53-dependent manner189. However, how reduced cytoplasmic zinc levels lead to the accumulation of P53 and apoptosis-inducing factor (AIF), and whether there is a complementary function of other zinc transporters, which may explain the considerable number of normal infiltrating macrophages in inflammatory responses, is still not fully clarified. Although the relationship between Zn2+ deficiency and cell death has been studied in detail, these findings highlight the critical roles of zinc transporters in the immune response as well as the potential drug targets in some human diseases, like thymic atrophy and lymphopenia, which are tightly related to zinc deficiency190,191.
Figure 2
Download : Download high-res image (390KB)Download : Download full-size image
Figure 2. The roles of zinc transporters in B cells of different stages. In the developmental stages of early B cells, STAT5 activation in the BCR signaling pathway triggered by cytokines like IL-7 upregulates SLC39A10, which subsequently increases the cytoplasmic zinc levels. As an inhibitor of effort caspases such as caspase 3 and caspase 9182,183, zinc may regulate the early B cell survival, which is consistent with the phenomenon that SLC39A10 deficiency leads to a reduced population of both pro- and pre-B-cell, accompanied with reduced intracellular zinc concentrations184. In mature B cells, SLC39A10 acts as a positive regulator of CD45R, which has inhibitory effect on the Lyn by dephosphorylating regulatory tyrosine residues of the Lyn in a dynamic behavior185. In the pre- and immature-B cells, SLC39A7 deficiency is associated with reduced cytoplasm zinc level. Since zinc is a negative regulator of phosphatases, their activity is elevated in SLC39A7-deficient B cells, which inhibits kinase activity and consequently leads to impaired BCR-dependent signaling. This impairment finally leads to reduced numbers of pre-, immature, and mature B cells, with a developmental block in pre-B stage156. STAT5, signal transducer and activator of transcription 5; BCR, B cell receptor; IL-7, interleukin 7; CD45R, a receptor-type protein tyrosine phosphatase; Lyn, tyrosine-protein kinase.
Local zinc accumulation mediated by SLC39A6 (ZIP6) in dendritic cells (DC) and T cells can indirectly activate the TCR-activation pathway, followed by cell proliferation and cytokine production192. The zinc transporters ZIP6 and ZIP10 are downregulated, while the zinc the exporters ZnT1 and ZnT6 are upregulated in the DCs when treated with LPS, thereby promoting their maturation193. SLC39A8 (ZIP8) is highly induced in response to LPS and TNFα, leading to a rapid increase of intracellular zinc levels194. The upregulation of ZIP8 is directly induced by transcription factor NF-κB that is activated through phosphorylation when the IκB kinase (IKK) complex is activated in the toll-like receptor (TLR) signaling pathway. Conversely, the increased zinc concentration negatively regulates NF-κB by inhibiting the IκB kinase beta subunit (IKKβ) in the kinase domain, thus suppressing inflammation and improving survival of the immune cells194. This feedback effect of the zinc transporter in innate immunity shows how the action of zinc coordinates with the immune responses to protect the host cells. However, before a specialized role of ZIP8 can be postulated, more evidence is needed to determine its correlation with other zinc transporters in certain immune responses and the regulatory relationship with other inhibitors of the NF-κB pathway. The roles of SLC30A (ZnT) family members in immunity are less studied, but some of them are nevertheless known to be involved in the maintenance of metabolic homeostasis. Zinc transporter protein member 8 (ZnT8; SLC30A8), an islet-specific cell-membrane zinc transporter involved in the assembly of insulin hexamers195, is reported as a major autoantigen and a potential diagnostic marker for type 1 diabetes, that is detected in different populations at varying levels157,196; while ZnT5, which is responsible for NF-κB-dependent cytokine production in mast cells, has been associated with the allergic response197. Since the mRNA level of ZnT1, ZnT4, ZnT6, and ZnT7 are strongly reduced in T cells following stimulation by phytohemagglutinin, it stands to reason that the downregulation of the ZnTs can be used as an intervention to maintain the intracellular zinc concentration during T cell activation198. However, the regulatory mechanisms underpinning zinc homeostasis still remain to be clearly elucidated.
https://www.sciencedirect.com/science/article/pii/S2211383519309360
J Allergy Clin Immunol. 2017 Sep
Psoriasis pathogenesis and the development of novel targeted immune therapies
Jason E Hawkes 1, Tom C Chan 1, James G Krueger 2
Laboratory for Investigative Dermatology, Rockefeller University, New York, NY.
Psoriasis is caused by a complex interplay between the immune system, psoriasis-associated susceptibility loci, autoantigens, and multiple environmental factors. Over the last 2 decades, research has unequivocally shown that psoriasis represents a bona fide T cell-mediated disease primarily driven by pathogenic T cells that produce high levels of IL-17 in response to IL-23. The discovery of the central role for the IL-23/type 17 T-cell axis in the development of psoriasis has led to a major paradigm shift in the pathogenic model for this condition. The activation and upregulation of IL-17 in prepsoriatic skin produces a "feed forward" inflammatory response in keratinocytes that is self-amplifying and drives the development of mature psoriatic plaques by inducing epidermal hyperplasia, epidermal cell proliferation, and recruitment of leukocyte subsets into the skin. Clinical trial data for mAbs against IL-17 signaling (secukinumab, ixekizumab, and brodalumab) and newer IL-23p19 antagonists (tildrakizumab, guselkumab, and risankizumab) underscore the central role of these cytokines as predominant drivers of psoriatic disease. Currently, we are witnessing a translational revolution in the treatment and management of psoriasis. Emerging bispecific antibodies offer the potential for even better disease control, whereas small-molecule drugs offer future alternatives to the use of biologics and less costly long-term disease management.银屑病发病机制与新型靶向免疫治疗的研究进展
银屑病是由免疫系统、银屑病相关易感性位点、自身抗原和多种环境因素之间复杂的相互作用引起的。在过去的20年里,研究明确表明银屑病是一种真正的T细胞介导疾病,主要由致病性T细胞产生高水平的IL-17来响应IL-23。IL-23/type 17 t细胞轴在银屑病发展中的核心作用的发现,导致银屑病致病模型的主要范式转变。上皮前皮肤中IL-17的激活和上调在角质形成细胞中产生一种“前馈”炎症反应,通过诱导表皮增生、表皮细胞增殖和皮肤白细胞亚群募集,自我扩增并驱动成熟的银屑病斑块的发展。针对IL-17信号(secukinumab、ixekizumab和brodalumab)单克隆抗体和新的IL-23p19拮抗剂(tildrakizumab、guselkumab和risankizumab)的临床试验数据强调了这些细胞因子作为主要的银屑病驱动因子的中心作用。目前,我们正在见证牛皮癣治疗和管理的转化革命。双特异性抗体的出现为更好地控制疾病提供了可能,而小分子药物则为生物制剂和成本更低的长期疾病管理提供了未来的替代品。
Keywords: IL-17; IL-23; Psoriasis; autoantigens; brodalumab; ixekizumab; psoriatic arthritis; secukinumab; tildrakizumab, guselkumab, and risankizumab.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Psoriasis pathogenesis and the development of novel targeted immune therapies - PubMed
https://pubmed.ncbi.nlm.nih.gov/28887948/
J Immunol. 2018 Sep 15
Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis
Jason E Hawkes 1, Bernice Y Yan 1, Tom C Chan 1 2, James G Krueger 31Laboratory for Investigative Dermatology, The Rockefeller University, New York, NY 10065; and.
2Department of Dermatology, National Taiwan University Hospital and College of Medicine, Taipei 10002, Taiwan.
3Laboratory for Investigative Dermatology, The Rockefeller University, New York, NY 10065; and kruegej@rockefeller.edu.
Psoriasis vulgaris is a common, heterogeneous, chronic inflammatory skin disease characterized by thickened, red, scaly plaques and systemic inflammation. Psoriasis is also associated with multiple comorbid conditions, such as joint destruction, cardiovascular disease, stroke, hypertension, metabolic syndrome, and chronic kidney disease. The discovery of IL-17-producing T cells in a mouse model of autoimmunity transformed our understanding of inflammation driven by T lymphocytes and associations with human inflammatory diseases, such as psoriasis. Under the regulation of IL-23, T cells that produce high levels of IL-17 create a self-amplifying, feed-forward inflammatory response in keratinocytes that drives the development of thickened skin lesions infiltrated with a mixture of inflammatory cell populations. Recently, the Food and Drug Administration approved multiple highly effective psoriasis therapies that disrupt IL-17 (secukinumab, ixekizumab, and brodalumab) and IL-23 (guselkumab and tildrakizumab) signaling in the skin, thus leading to a major paradigm shift in the way that psoriatic disease is managed.
Copyright © 2018 by The American Association of Immunologists, Inc.Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis - PubMed
https://pubmed.ncbi.nlm.nih.gov/30181299/
Regulation of Dendritic Cell Immune Function and Metabolism by Cellular Nutrient Sensor Mammalian Target of Rapamycin (mTOR)
Julia P. Snyder1 and Eyal Amiel2* 1Predoctoral student of the Cellular, Molecular, and Biomedical (CMB) Sciences Graduate Program at the University of Vermont, Burlington, VT, United States
2Department of Biomedical and Health Sciences, College of Nursing and Health Sciences, University of Vermont, Burlington, VT, United States
Dendritic cell (DC) activation is characterized by an acute increase in glucose metabolic flux that is required to fuel the high anabolic rates associated with DC activation. Inhibition of glycolysis significantly attenuates most aspects of DC immune effector function including antigen presentation, inflammatory cytokine production, and T cell stimulatory capacity. The cellular nutrient sensor mammalian/mechanistic Target of Rapamycin (mTOR) is an important upstream regulator of glycolytic metabolism and plays a central role in coordinating DC metabolic changes and immune responses. Because mTOR signaling can be activated by a variety of immunological stimuli, including signaling through the Toll-like Receptor (TLR) family of receptors, mTOR is involved in orchestrating many aspects of the DC metabolic response to microbial stimuli. It has become increasingly clear that mTOR's role in promoting or attenuating inflammatory processes in DCs is highly context-dependent and varies according to specific cellular subsets and the immunological conditions being studied. This review will address key aspects of the complex role of mTOR in regulating DC metabolism and effector function.
The Role of mTOR in Cellular Metabolism
The hierarchical regulation of cellular metabolism and energy homeostasis can be functionally partitioned into two opposing “programs,” anabolism and catabolism, each governed by a distinct central upstream regulator. Energetic anabolism, generically characterized by reduced metabolic activity coupled to energy conservation and production, is controlled by AMP-activated protein kinase (AMPK) in response to low cellular ATP levels or nutrient starvation [reviewed in (25)]. Catabolism, contrastingly comprised by high rates of energy expenditure for nutrient breakdown and molecular biosynthesis, is controlled by mTOR complex activity [reviewed in (26)]. Not surprisingly, these two processes cross-regulate each other, most notably by AMPK inhibition of mTOR activation. While AMPK has been implicated in important aspects of DC biology (10, 27, 28), it is a notably understudied aspect of DC metabolic biology and this review will focus primarily on mTOR-mediated metabolic regulation of DCs.
The mTOR protein itself functions as a required component of two major signaling complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). While mTORC1 is primarily responsible for cellular energy expenditure and protein translation, mTORC2 serves an important role as a positive regulator of mTORC1. For the purposes of this review, “mTOR activity” will refer to mTORC1 functions unless otherwise noted. It is notable that while mTOR promotes sustained catabolism of carbohydrates, it concurrently supports the de novo synthesis of lipids, proteins, and amino acids, serving as an important checkpoint in converting increased cellular fuel consumption into processes such as cell division and protein production that have obvious implications for broad physiological responses, including those carried out by immune cells (29). As a downstream target of the PI3K/Akt signaling axis, mTOR activation in DCs can be initiated by a number of immunologically relevant factors, including cytokine signaling, growth factor signaling, and PRR signaling. In light of this, mTOR is positioned as a critical molecule integrating immunological stimuli into changes in cellular metabolism that regulate protein translation events required for the immunological function of these cells. The role of mTOR in governing immune cell homeostasis and the use of mTOR inhibitors as viable immunoregulatory strategies continue to be of intense interest to the field (23).DC Commitment to Aerobic Glycolysis
Activation of DCs via TLRs promotes significant upregulation of aerobic glycolysis, which regulates the immune function of both human and mouse DCs (10–12, 18, 20, 21, 30, 31). To date, ligands for both MyD88 -dependent and -independent TLR members have been shown to result in an acute upregulation of glycolysis (20), as well as ligands for the C-type Lectin Receptors Dectin-1/2 (21), suggesting that this metabolic reprogramming may be a broadly conserved feature of PRR signaling. A wide variety of approaches, including inhibition of glycolysis through culture with 2-deoxy-glucose (2DG), pharmacological inhibition of glycolysis-regulating signaling pathways, and genetic silencing of rate-limiting glycolysis enzymes, have demonstrated that loss of glycolytic capability significantly impairs DC effector functions, including antigen presentation, co-stimulatory molecule expression, chemotaxis, cytokine secretion, and T lymphocyte stimulatory capacity (10–12, 18, 20, 21, 30, 31). The prevailing consensus has emerged that acute, and in some cases sustained, metabolic commitment to elevated rates of glucose catabolism are an essential metabolic requirement for proper DC activation. We have previously argued that DC metabolic reprogramming can be functionally partitioned into two temporal phases governed by distinct signaling events (32): (1) an acute induction of glycolysis occurring within minutes of TLR activation that supports the high biosynthetic demand associated with early DC maturation for several hours (20); (2) a long-term commitment to glycolysis in subsets of nitric oxide (NO) -producing DCs that is required for their metabolic adaptation to NO-mediated mitochondrial toxicity (12, 15).DCs中的急性糖酵解重编程与mtor无关
TLR刺激几分钟后,DCs中糖酵解的快速诱导受到PI3K/TBK1/IKKε/Akt信号轴的控制,PI3K/TBK1/IKKε/Akt信号轴可促进己糖激酶2 (HK2)快速转位到线粒体,从而支持与DC成熟相关的葡萄糖分解代谢的快速通量(20)。葡萄糖正迅速被激活DCs和单糖进行碳主要是投资于磷酸戊糖途径(PPP)新陈代谢,生产乳酸,柠檬酸和柠檬酸合成通过线粒体航天飞机,后者可能支持脂肪酸合成与内质网,高尔基体端依赖翻译和控制炎症细胞因子分泌途径生产(16日20)。葡萄糖的来源既来自细胞外葡萄糖的输入,也来自这些细胞处于静止状态时细胞内糖原库的分解代谢(18)。PI3K/TBK1/IKKε/Akt信号轴介导的糖酵解的急性诱导,在小鼠和人类系统的多个DC亚群中都是保守的(20,21,31),这是短暂的(持续约6-8小时),之后糖酵解水平逐渐下降,接近其前激活水平(20)。mTOR抑制剂不能减弱这种早期糖酵解的波,这表明mTOR激活位于DCs早期糖酵解作用的下游(12,15,20)。
DCs中持续的糖酵解重编程依赖于mtor
而mTOR的研究已经得出结论,数量,和它的一个下游转录因子HIF1α,所需直流糖酵解重组(9三十到三十五),我们和其他人的情况表明,这主要是长期致力于糖酵解中观察到NO-producing DCs表达诱导一氧化氮合酶(间接宾语)和独立于上述急性糖酵解重组事件(12、15)。在表达iNOS的DCs中,主要局限于小鼠中炎症单核细胞来源的DCs和人类DCs的次要亚群[先前在(32)中综述过],mtor依赖的HIF1α活性促进tlr激活的DCs中iNOS的表达(30,36)。当糖酵解的急性诱导开始减弱时,iNOS蛋白表达开始可检测到(12,15),然后通过可逆抑制线粒体细胞色素c氧化酶功能,no介导的DC线粒体活性抑制(12,15)(37,38)。mTOR通过调节iNOS的表达,以no依赖的方式调节这些细胞对糖酵解代谢的长期承诺(12,15)。值得注意的是,mTOR抑制降低了表达inos的DCs中NO的生成并恢复了线粒体功能,从而增加了这些细胞的代谢灵活性并增强了炎症活性(11)。下文将更详细地讨论mTOR对DC功能的多方面、高度复杂的非独立影响,但不能忽视mTOR在调节DC iNOS表达方面的重要作用及其对DC代谢的影响。https://www.frontiersin.org/articles/10.3389/fimmu.2018.03145/full
PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis
Functional Genomics Unit, CSIR-Institute of Genomics and Integrative Biology, Mall Road, Delhi 110007, India
Academy of Scientific & Innovative Research, CSIR-Institute of Genomics and Integrative Biology, Delhi 110007, India
在IL-17A介导的银屑病炎症反应中,PI3K/AKT/mTOR激活和自噬抑制在胆固醇升高中起关键作用
银屑病是一种由免疫介导的皮肤炎症性疾病。包括我们在内的前期研究均表明IL-17A在其发病机制中起重要作用;然而,其确切的分子作用机制尚不清楚。TNFα和IL-23等细胞因子也在介导疾病中发挥重要作用,一些研究也报道了自噬作为细胞因子控制免疫反应的一种新机制。在此,我们研究了IL-17A对角质形成细胞自噬的影响,并揭示了自噬和角质形成细胞中胆固醇信号之间的交互作用。我们的结果表明,IL-17A刺激角质形成细胞激活PI3K/AKT/mTOR信号,并通过同时抑制自噬体形成和增强自噬通量来抑制自噬。Western blotting检测自噬标志物(LC3和p62)、PI3K、mTOR和AKT的表达。mTOR抑制剂雷帕霉素诱导自噬和/或饥饿也抑制角质形成细胞分泌IL-17A、IL-8、CCL20和S100A7的水平。在此,我们还观察到IL-17A抑制自噬伴随着细胞胆固醇水平的升高,而细胞胆固醇水平反过来调节自噬通量。为了研究细胞自噬和细胞胆固醇之间的相互干扰,我们使用甲基β-环糊精(MβCD),它通过消耗细胞胆固醇来破坏清净不溶性微区(DIMs),并检查自噬。与非损伤性皮肤相比,银屑病皮损性皮肤LC3-II表达降低,抗银屑病药物甲氨蝶呤诱导角质细胞自噬进一步证实了自噬在银屑病中的作用。我们的研究结果表明,自噬和/或胆固醇水平的调节剂可能被开发出来,也可能导致新的治疗银屑病的药物。
在IL-17A介导的银屑病炎症反应中,PI3K/AKT/mTOR激活和自噬抑制在胆固醇升高中起关键作用
https://www.sciencedirect.com/science/article/pii/S0925443918300462
Pathogenesis of psoriasis and development of treatment
The pathogenesis of psoriasis can be explained by dysregulation of immunological cell function as well as keratinocyte proliferation/differentiation. Recently, the immunological pathomechanism has been clarified substantially. Whereas T‐helper (Th)1 overactivation was thought to induce occurrence of psoriasis, it has been demonstrated that Th17 cells play a key role. Th17 development is maintained by interleukin (IL)‐23 mainly produced by dendritic cells. Th17 cells produce various cytokines, including IL‐17A, IL‐17F and IL‐22. IL‐17A and IL‐22 induce not only keratinocyte proliferation, but also tumor necrosis factor (TNF)‐α, chemokine (C‐X‐C motif) ligand (CXCL)1 and CXCL8 production. TNF‐α accelerates the infiltration of inflammatory cells, including lymphocytes, monocytes and neutrophils, from the peripheral blood into skin with dendritic cell activation. In addition, antimicrobial peptides are overexpressed in psoriatic skin lesions, and the antimicrobial peptide, LL‐37, activates dendritic cells, which leads to the development of inflammation. Furthermore, activation of nuclear factor‐κB signal induces the expression of keratins 6 and 16 in keratinocytes, which are associated with acanthosis and reduced turnover time in the epidermis. The progression of the pathomechanism contributes to the development of new therapies for psoriasis.银屑病的发病机制及治疗进展
银屑病的发病机制可能是免疫细胞功能失调和角质细胞增殖/分化失调。近年来,免疫病理机制已基本阐明。尽管T‐helper (Th)1过度激活被认为是导致银屑病发生的原因,但Th17细胞在其中发挥了关键作用。Th17的发育由主要由树突状细胞产生的白细胞介素(IL)‐23维持。Th17细胞产生各种细胞因子,包括IL - 17A、IL - 17F和IL - 22。IL - 17A和IL - 22不仅能诱导角质细胞增殖,还能诱导肿瘤坏死因子(TNF)α、趋化因子(C - X - C基序)配体(CXCL)1和CXCL8的产生。TNF‐α激活树突状细胞,加速炎症细胞(包括淋巴细胞、单核细胞和中性粒细胞)从外周血向皮肤的浸润。此外,抗菌肽在银屑病皮损中过度表达,抗菌肽LL - 37激活树突状细胞,从而导致炎症的发展。此外,核因子κB信号的激活诱导角质形成细胞角蛋白6和16的表达,这与表皮的棘皮化(acanthosis)和减少周转时间有关。银屑病病理机制的发展有助于银屑病新疗法的开发。The close relation between IL‐17 and psoriasis was reported. High levels of IL‐17 mRNA were detected in psoriatic lesional skin, but not in non‐lesional skin.25 In keratinocytes, IL‐17 enhanced the expression of IL‐6 and IL‐8, which are known as pro‐inflammatory cytokines and exacerbate psoriasis.26 In addition, topical application of imiquimod, a Toll‐like receptor (TLR)7/8 ligand and potent immune activator, induced psoriasis‐like dermatitis in mice together with the expression of IL‐17A and IL‐17F.27 Furthermore, both CsA and anti‐TNF‐α agents decreased the levels of IL‐17A, IFN‐γ, IL‐23p19 and chemokine (C‐C motif) ligand 20 in psoriatic lesions in conjunction with improvement of psoriatic eruptions, indicating that pro‐inflammatory cytokines, including IL‐17A, are involved in the development of psoriasis.26, 28-30 These findings suggest that the IL‐17 family plays an important role in psoriasis.
IL - 17与银屑病密切相关。IL - 17 mRNA在银屑病皮损性皮肤中检测到高水平,而在非皮损性皮肤中检测不到。在角质形成细胞中,IL - 17增强了IL - 6和IL - 8的表达,这两种细胞因子被称为促炎性细胞因子,并加剧了银屑病。26此外,局部应用Toll样受体(TLR)7/8配体和强免疫激活剂咪喹莫特可诱导小鼠银屑病样皮炎并表达IL - 17A和IL - 17F。27此外,CsA和anti - TNF -α均能降低银屑病皮损中IL - 17A、IFN -γ、IL - 23p19和趋化因子(C - C基序)配体20的水平,同时改善银屑病出血量,这表明包括IL - 17A在内的促炎细胞因子参与了银屑病的发生发展。26,28 -30这些结果表明IL - 17家族在银屑病发病中起重要作用。
Pathogenesis of psoriasis and development of treatment - Ogawa - 2018 - The Journal of Dermatology - Wiley Online Library
https://onlinelibrary.wiley.com/doi/full/10.1111/1346-8138.14139
膳食抗氧化剂Delphinidin双重抑制PI3K/Akt和mTOR可改善体外实验和小鼠亚咪喹莫特诱导的银屑病样疾病的特征
目的:银屑病的治疗仍然是未知的,强调需要确定新的疾病靶点和基于机制的治疗方法。我们最近报道,PI3K/Akt/mTOR信号通路在许多恶性肿瘤中经常失调,这也与银屑病的临床相关。我们还为开发delphinidin (Del)提供了理论依据,Del是一种用于治疗牛皮癣的膳食抗氧化剂。本研究利用高通量生物物理和生物化学方法以及体内外模型,识别Del调控的银屑病分子靶点。
结果:对102个人类激酶靶点进行的激酶水平筛选和Kds分析显示,Del与三种脂质(PIK3CG、PIK3C2B和PIK3CA)和六种丝氨酸/苏氨酸(PIM1、PIM3、mTOR、S6K1、PLK2和AURKB)激酶结合,其中五种属于PI3K/Akt/mTOR通路。表面等离子体共振和硅分子模拟证实了Del与三个PI3Ks(α/c2β/γ),mTOR和p70S6K的直接相互作用。Del治疗白细胞介素-22或tpa刺激的正常人表皮角质形成细胞(NHEKs)显著抑制增殖,活化PI3K/Akt/mTOR成分,并分泌促炎细胞因子和趋化因子。为了建立这些发现的体内相关性,我们采用了IMQ诱导的Balb/c小鼠银屑病样皮肤模型。与对照组相比,Del的局部治疗显著降低了(i)增殖和表皮厚度,(ii)免疫细胞对皮肤的浸润,(iii)银屑病相关细胞因子/趋化因子,(iv) PI3K/Akt/mTOR通路激活,以及(v)分化程度增加。
创新与结论:我们观察到,Del抑制银屑病发病机制中的关键激酶,减轻imq诱导的小鼠银屑病样疾病,提示一种新的PI3K/AKT/mTOR通路调节剂可能被开发用于治疗银屑病。Antioxid。氧化还原信号26 49-69。Dual Inhibition of PI3K/Akt and mTOR by the Dietary Antioxidant, Delphinidin, Ameliorates Psoriatic Features In Vitro and in an Imiquimod-Induced Psoriasis-Like Disease in Mice | Antioxidants & Redox Signaling
https://www.liebertpub.com/doi/10.1089/ars.2016.6769
Compounds from Natural Sources as Protein Kinase Inhibitors
https://www.mdpi.com/2218-273X/10/11/1546/htm
Ellagic acid is a dimer of gallic acid and can be found in several fruits and vegetables. ... It displays effects by the inhibition of the PI3K/Akt/mTOR pathway and regulation of the EGFR signaling pathway through suppression of EGFR expression and phosphorylation.槲皮素QUERCETIN自然存在于不同的水果和蔬菜中,比如苹果、葡萄、浆果和洋葱。此外,黄酮类化合物是最早被描述的对蛋白激酶具有抑制活性的化合物之一[141]。研究表明槲皮素作用于多种激酶靶点,这些靶点主要参与癌细胞的细胞增殖。PI3K-Akt / PKB通路通过绑定的槲皮素抑制PI3Kγ(IC50 = 3.8µM)没有针对一种蛋白激酶/ PKB[142143]。另一个参与相同途径的激酶是CK2。槲皮素是一种著名的atp竞争CK2抑制剂,常被用作动力学分析的标准。CK2是抑制IC50值介于0.2和1.8µM,根据检查同种型和磷酸化蛋白质底物[144145146147]。其他激酶,包括MEK-1, GSK-3β,Hck和IKKα/β,也被发现在低微摩尔水平被抑制。对于Hck和GSK-3β,槲皮素结合的腺苷结合口袋的IC50值2µM[148149]。另一方面,MEK-1通过另一种机制被抑制,即槲皮素与atp结合口袋附近的活化环结合[142,150]。在这些激酶,槲皮素展品最弱的效应对IKK IKK和αβ的IC50值11和4µM[151]。动力学分析表明,这种抑制方式可能是通过与atp结合袋和蛋白质底物结合位点的结合实现的。在其他的研究中,槲皮素被证实对PKC抑制潜在的减少和MAPK抑制9至24%的抑制剂浓度50µM[152]。该研究还描述槲皮素是酪氨酸激酶的一种重要抑制剂,如Syk、Src、Fyn和Lyn[152]。在本研究中,假单胞菌产生的异黄酮染料木素与一组代表酪氨酸和丝氨酸/苏氨酸的蛋白激酶进行了测试
https://www.mdpi.com/2218-273X/10/11/1546/htm
Exp Mol Pathol, 2003 Dec;
Dendritic cell differentiation and proliferation: enhancement by tumor necrosis factor-alpha
Robert S Morrison 3rd 1, Julius M Cruse, Huan Wang, Robert E LewisDepartment of Pathology, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MI 39216, USA.
Dendritic cells (DCs) are a diverse group of hematopoietic-derived cells that play a prominent role in initiating the body's immune response. Tumor necrosis factor-alpha (TNFalpha) aids CD34+ hematopoietic stem cells in the development of DCs. In this study, we aimed to further define the relationship between TNFalpha and DC maturation. CD34+ stem cells were isolated from umbilical cord blood and cultured using granulocyte-macrophage colony stimulating factor, stem cell factor, and varying concentrations of TNFalpha. An anti-TNF receptor 1 (anti-TNFR1) antibody was used to show the specificity of TNFalpha. Flow cytometry and light microscopy analyses were performed at days 0, 7, and 14 of culture, revealing mature DCs at all concentrations of TNFalpha by day 14, excluding those with anti-TNFR1 bound to the cell's TNF receptor 1. DCs possessed a characteristic veiled appearance and were consistent with a DC panel of surface markers. TNFalpha was essential to the development of DCs, as those with bound anti-TNFR1 were virtually unable to develop into DCs. Increasing TNFalpha enhanced the survival of culturing stem cells and resulted in a parallel increase in day 14 DCs. Although increases in TNFalpha produced more DCs, these cells were not as phenotypically mature, expressing less CD80 than those receiving only a single initial dosage of TNFalpha. These studies support the prevalence of large numbers of DCs under inflammatory conditions, such as the rheumatoid joint, where local concentrations of TNFalpha are high.树突状细胞分化和增殖:肿瘤坏死因子增强
树突状细胞(DCs)是一组多样的造血细胞,在机体免疫反应的启动中发挥着重要作用。肿瘤坏死因子-α(TNFalpha)辅助CD34+造血干细胞在树突状细胞发育中的作用。在本研究中,我们旨在进一步明确falpha与DC成熟之间的关系。从脐带血分离CD34+干细胞,使用粒细胞-巨噬细胞集落刺激因子、干细胞因子和不同浓度的falpha进行培养。tnf受体1 (anti-TNFR1)抗体用于显示tnpha的特异性。在培养的第0天、第7天和第14天进行流式细胞术和光学显微镜分析,在第14天显示所有浓度的三叉树的树突状细胞成熟,不包括那些与细胞TNF受体1结合的抗tnfr1的树突状细胞。DCs具有特征性的隐蔽性外观,与表面标记的DC面板一致。TNFalpha对dc的发展至关重要,因为那些绑定了anti-TNFR1的细胞实际上无法发展成dc。增加TNFalpha提高了培养干细胞的存活率,并导致14天树突状细胞的平行增加。尽管falpha的增加产生了更多的dc,但这些细胞的表型并不成熟,表达的CD80少于那些只接受单一初始剂量falpha的细胞。这些研究支持在炎症条件下大量树突状细胞的流行,如类风湿关节,局部集中度高的falpha。
Dendritic cell differentiation and proliferation: enhancement by tumor necrosis factor-alpha - PubMed
https://pubmed.ncbi.nlm.nih.gov/14611814/
Arch Pharm Res, 2004 Oct;
Vitamin C blocks TNF-alpha-induced NF-kappaB activation and ICAM-1 expression in human neuroblastoma cellsDept. of Pharmacognosy Material Development, Samcheok National University, Samcheok, Kangwon-do 245-711, Korea.
Interactions of the cell adhesion molecules are known to play important roles in mediating inflammation. The proinflammatory cytokine, tumor necrosis factor-alpha (TNF-alpha), activates the NF-kappaB signaling pathway, which induces the expression of various genes, such as intercellular adhesion molecule-1 (ICAM-1). In this study, the effect of vitamin C on the ICAM-1 expression induced by TNF-alpha in a human neuroblastoma cell line, SK-N-SH was investigated. Treatment with vitamin C resulted in the downregulation of the TNF-alpha-induced surface expression and ICAM-1 mRNA levels in a concentration-dependent manner. Moreover, a gel shift analysis indicated that vitamin C dose-dependently inhibited the NF-kappaB activation and IkappaBalpha degradation induced by TNF-alpha. Taken together, these results suggest that vitamin C downregulates TNF-alpha-induced ICAM-1 expression via the inhibition of NF-kappaB activation.Vitamin C blocks TNF-alpha-induced NF-kappaB activation and ICAM-1 expression in human neuroblastoma cells - PubMed
https://pubmed.ncbi.nlm.nih.gov/15554267/
Role of TNFα in pulmonary pathophysiology | Respiratory ...
https://respiratory-research.biomedcentral.com/...
Oct 11, 2006 · TNFα is produced by many different cell types. The main sources in vivo are stimulated monocytes, fibroblasts, and endothelial cells. Macrophages, T-cells, B-lymphocytes, granulocytes, smooth muscle cells, eosinophils, chondrocytes, osteoblasts, mast cells, glial cells, and keratinocytes also produce TNFα after stimulation.
Endocrine. 2008 Jun;
Berberine inhibits the expression of TNFalpha, MCP-1, and IL-6 in AcLDL-stimulated macrophages through PPARgamma pathway
Department of Endocrinology, Huashan Hospital, Fudan University, 12# Middle Urumqi Road, Shanghai 200040, China.
Macrophages are the main source of cytokines in atherosclerotic plaques. Modified low-density lipoproteins may stimulate macrophages to produce large quantities of proinflammatory cytokines that promote atherosclerosis. Berberine is the main component of the traditional Chinese medicine umbellatine, which has a widespread effect and was used to treat many diseases clinically. Our previous study found that berberine could increase adipophilin expression in macrophages, which is a target gene of PPARgamma. PPARgamma agonist could decrease proinflammatory cytokines in macrophage. In this study, we investigated the effects and the mechanism of action of berberine on the expression and secretion of TNFalpha, MCP-1, and IL-6 in vitro to identify new pharmacological actions of berberine. The results of RT-PCR and ELISA shows that berberine may inhibit the expression and secretion of the tumor necrosis factor alpha (TNFalpha), monocyte chemoattractant protein 1 (MCP-1), and interleukin-6 (IL-6) in macrophages stimulated by acetylated low-density lipoprotein (AcLDL), whereas the peroxisome proliferator-activated receptor gamma (PPARgamma) inhibitor GW9662 could attenuate this effect of berberine. This study demonstrates that berberine may inhibit the expression and production of TNF-alpha, MCP-1, and IL-6 in AcLDL-stimulated macrophages. This effect might be partially mediated through PPARgamma activity.内分泌。2008年6月;
黄连素通过PPARgamma通路抑制acldl刺激巨噬细胞中TNFalpha、MCP-1、IL-6的表达
巨噬细胞是动脉粥样硬化斑块细胞因子的主要来源。修饰的低密度脂蛋白可能刺激巨噬细胞产生大量促炎细胞因子,促进动脉粥样硬化。小檗碱是中药umbellatine的主要成分,其作用广泛,临床上用于治疗多种疾病。我们前期研究发现黄连素可以增加巨噬细胞中脂质素的表达,这是PPARgamma的靶基因。PPARgamma激动剂能降低巨噬细胞的促炎细胞因子。本研究通过体外研究小檗碱对TNFalpha、MCP-1、IL-6表达和分泌的影响及其作用机制,以确定小檗碱新的药理作用。rt - pcr和ELISA结果表明,小檗碱可能抑制肿瘤坏死因子-α的表达和分泌(TNFalpha)、单核细胞化学引诱物蛋白1 (MCP-1),刺激巨噬细胞和白细胞介素- 6 (il - 6)乙酰化低密度脂蛋白(AcLDL),而过氧物酶体proliferator-activated受体γ(PPARgamma)抑制剂GW9662可以减弱这种影响的小檗碱。本研究表明黄连素可以抑制acldl刺激的巨噬细胞中tnf -α、MCP-1和IL-6的表达和产生。这种效应可能部分通过PPARgamma活性介导。Berberine inhibits the expression of TNFalpha, MCP-1, and IL-6 in AcLDL-stimulated macrophages through PPARgamma pathway - PubMed
https://pubmed.ncbi.nlm.nih.gov/19034703/
Fatty acid-binding protein regulates LPS-induced TNF-α ...
https://www.sciencedirect.com/science/article/pii/S0952327808000902
Jul 01, 2008 · 1. Introduction. Mast cells (MCs) induce profound allergic responses through release of a variety of inflammatory mediators, including histamine and a number of immuno-regulatory cytokines, by cross-linking high-affinity receptors for IgE (FcεRI) on their plasma membrane and appropriate antigens , .In contrast to the harmful role of MCs, recent studies have revealed that MCs that reside in ...
TLR signaling in mast cells: common and unique features
https://pubmed.ncbi.nlm.nih.gov/22783258
Mast cells respond to TLR ligands by secreting cytokines, chemokines, and lipid mediators, and some studies have found that TLR ligands can also cause degranulation, although this finding is contentious.
Cited by: 220
Publish Year: 2012
Author: Hilary Sandig, Silvia Bulfone-Paus
Cell Res. 2019 May;
LKB1 expressed in dendritic cells governs the development and expansion of thymus-derived regulatory T cells
Liver Kinase B1 (LKB1) plays a key role in cellular metabolism by controlling AMPK activation. However, its function in dendritic cell (DC) biology has not been addressed. Here, we find that LKB1 functions as a critical brake on DC immunogenicity, and when lost, leads to reduced mitochondrial fitness and increased maturation, migration, and T cell priming of peripheral DCs. Concurrently, loss of LKB1 in DCs enhances their capacity to promote output of regulatory T cells (Tregs) from the thymus, which dominates the outcome of peripheral immune responses, as suggested by increased resistance to asthma and higher susceptibility to cancer in CD11cΔLKB1 mice. Mechanistically, we find that loss of LKB1 specifically primes thymic CD11b+ DCs to facilitate thymic Treg development and expansion, which is independent from AMPK signalling, but dependent on mTOR and enhanced phospholipase C β1-driven CD86 expression. Together, our results identify LKB1 as a critical regulator of DC-driven effector T cell and Treg responses both in the periphery and the thymus.
LKB1 expressed in dendritic cells governs the development and expansion of thymus-derived regulatory T cells - PubMed
https://pubmed.ncbi.nlm.nih.gov/30940876/
Cell Res. 2019 May;
LKB1 orchestrates dendritic cell metabolic quiescence and anti-tumor immunity
Dendritic cells (DCs) play a pivotal role in priming adaptive immunity. However, the involvement of DCs in controlling excessive and deleterious T cell responses remains poorly defined. Moreover, the metabolic dependence and regulation of DC function are unclear. Here we show that LKB1 signaling in DCs functions as a brake to restrain excessive tumor-promoting regulatory T cell (Treg) and Th17 cell responses, thereby promoting protective anti-tumor immunity and maintaining proper immune homeostasis. LKB1 deficiency results in dysregulated metabolism and mTOR activation of DCs. Loss of LKB1 also leads to aberrant DC maturation and production of cytokines and immunoregulatory molecules. Blocking mTOR signaling in LKB1-deficient DCs partially rectifies the abnormal phenotypes of DC activation and Treg expansion, whereas uncontrolled Th17 responses depend upon IL-6-STAT3 signaling. By coordinating metabolic and immune quiescence of DCs, LKB1 acts as a crucial signaling hub in DCs to enforce protective anti-tumor immunity and normal immune homeostasis.
LKB1 orchestrates dendritic cell metabolic quiescence and anti-tumor immunity - PubMed
https://pubmed.ncbi.nlm.nih.gov/30911060/
Cell Res. 2019 Jun; 29
LKB1 restrains dendritic cell function
Three independent recent studies support the notion that liver kinase B1 (LKB1), a key nutrient sensor, controls dendritic cell (DC) function. Selective loss of LKB1 in DCs leads to their increased ability to prime effector T cell responses, but the prevailing effect is the expansion of thymus-derived natural regulatory T cells, creating a dominant immunosuppressive environment.
Tight regulation of T cell priming by dendritic cells (DCs) is key to maintain tissue homeostasis and orchestrate immunity. As immune sentinels, DCs control the activation of different flavors of immunity including effector CD8+ T cells and CD4+ helper T (Th) cells, the latter comprising IFNγ-producing Th1, IL-4-producing Th2, IL-17-producing Th17 cells and follicular helper T cells (Tfh) that promote B cell differentiation in germinal centers.1,2 However, DCs also contribute to homeostasis and self-tolerance through the induction of regulatory T cells (Tregs), which can be Helios+ thymus-derived/natural (tTregs) or generated upon antigen exposure in the periphery (Helios− pTregs).1,3
DCs integrate environmental cues such as pathogen- or danger-associated molecular patterns and cytokines to activate different intracellular signaling pathways. Those adaptions lead to adjustment of antigen uptake, processing and presentation on MHC molecules (signal 1), expression of co-stimulatory molecules (signal 2) and production of specific cytokines (signal 3) by DCs to modulate induction of effector and regulatory T cell responses. Different DC subsets include plasmacytoid (pDCs), conventional type 1 (cDC1s) and 2 (cDC2s) DCs, and express a varying repertoire of pattern recognition receptors sensing those extracellular cues culminating in diverse functional features.1
Metabolic alterations in DCs upon sensing the environment have emerged as an essential mechanism for control of DC function in the regulation of adaptive immune responses.2,4 Resting or tolerogenic DCs preferentially display an active catabolic energy metabolism via the Krebs cycle and oxidative phosphorylation (OXPHOS) that can be fueled by fatty acid oxidation. Immunogenic activation of DCs generally fosters an anabolic metabolism characterized by enhanced glycolysis, and fatty acid synthesis to drive the extension of the Golgi apparatus and endoplasmic reticulum for cytokine production.4 The early induction of glycolysis regulates many aspects of immunogenic DC activation, including migration, upregulation of MHC and co-stimulatory molecules, and T cell stimulation.2 The metabolic adaption of DCs upon stimulation is, at least partially, regulated by a balance of AKT/mTOR/HIF and AMPK signaling pathways.2,4 Activation of mTOR can sustain immunogenic DC activation, while active AMPK is associated with resting and tolerized DCs. However, the precise pathways regulating the metabolic state that drive tolerogenic DC function are poorly understood.2,4
LKB1 is a serine/threonine kinase that can activate AMPK upon low intracellular ATP to induce catabolic oxidative metabolism. As such, LKB1 is implicated in modulating metabolism, survival, differentiation and functional features of hematopoietic stem cells, effector CD4+ and CD8+ T cells as well as Tregs in AMPK-dependent and -independent fashions.5–7 Also, LKB1 is phosphorylated on Ser428 in lipopolysaccharide (LPS)-stimulated macrophages and restricts their pro-inflammatory functions by inhibition of NF-κB signaling, likely via binding to IκB kinase (IKK) β.8
To dissect the contribution of LKB1 to DC function, three independent studies have analyzed CD11c-Cre LKB1f/f (CD11cΔLKB1) mice (Fig. 1).9–11 They find an enlarged tTreg pool throughout the body of CD11cΔLKB1 mice, including the thymus, which protects them from experimental allergy or autoimmunity but makes them more susceptible to grafted tumors. LKB1 is phosphorylated in intratumoral DCs,11 while Escherichia coli or LPS stimulation downregulates LKB1 expression in DCs, which associates with expansion of tTregs.9 Two studies show that Tregs of CD11cΔLKB1 mice express higher levels of immune-suppressive molecules and display an enhanced suppressive activity limiting T cell proliferation.10,11 Further, Wang et al.11 demonstrate that LKB1-deficient DCs express higher levels of Treg-inducing IL-2, indoleamine 2, 3-dioxygenase (IDO) 1, arginase (Arg) 2 and integrin beta (Itgb) 8 than wild-type DCs. These factors contribute to induction of mTOR signaling in tTregs and enhance tTreg proliferation....
LKB1 restrains dendritic cell function
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6796839/
T helper type 17 in psoriasis: From basic immunology to clinical practice
Psoriasis is a chronic inflammatory disease mediated by a complex interplay between immune system and keratinocytes. Initially considered as a keratinocyte proliferation/differentiation disorder, an immune dysregulation was confirmed after the successful treatment of psoriasis with cyclosporine. The ying–yang theory, or T helper type 1 (Th1)/Th2 concept, was then introduced to explain the rarity of atopic dermatitis in patients with psoriasis and the aggravation of psoriasis after interferon-γ treatment. However, recent advances have revised the Th1/Th2 paradigm after the discovery of a novel subset of T cells, called Th17 cells. Th17 cells produce interleukin (IL)-17 and IL-22, and have other important downstream proinflammatory effects on skin, leading to clinical and pathological features typical of psoriasis. Nowadays, emerging evidence suggests integrative and complicated inflammatory circuits among Th1 and Th17 cells and keratinocytes in the pathogenesis of psoriasis, with Th17 cells playing a central role. Herein, we review the biology of Th17 cells as well as the reciprocal interplay between Th17 and regulatory T cells in psoriasis. Integration of the IL-23/Th17 axis into a revised concept of psoriasis has already been translated into novel therapeutic strategies. Studies investigating the effect and molecular mechanism of conventional and biological therapy for psoriasis on the IL-23/Th17 pathway were also discussed.https://www.sciencedirect.com/science/article/pii/S102781171200081X
J Immunology. 2015 Jun 1
High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells
1Institute of Molecular and Cell Biology, Agency for Science, Technology and Research, Singapore 138673; Singapore Immunology Network, Singapore 138648; and.
2Singapore Immunology Network, Singapore 138648; and.
3Institute of Molecular and Cell Biology, Agency for Science, Technology and Research, Singapore 138673; Singapore Immunology Network, Singapore 138648; and Institute of Biomedical Studies, Baylor University,
Human dendritic cells (DCs) regulate the balance between immunity and tolerance through selective activation by environmental and pathogen-derived triggers. To characterize the rapid changes that occur during this process, we analyzed the underlying metabolic activity across a spectrum of functional DC activation states, from immunogenic to tolerogenic. We found that in contrast to the pronounced proinflammatory program of mature DCs, tolerogenic DCs displayed a markedly augmented catabolic pathway, related to oxidative phosphorylation, fatty acid metabolism, and glycolysis. Functionally, tolerogenic DCs demonstrated the highest mitochondrial oxidative activity, production of reactive oxygen species, superoxide, and increased spare respiratory capacity. Furthermore, assembled, electron transport chain complexes were significantly more abundant in tolerogenic DCs. At the level of glycolysis, tolerogenic and mature DCs showed similar glycolytic rates, but glycolytic capacity and reserve were more pronounced in tolerogenic DCs. The enhanced glycolytic reserve and respiratory capacity observed in these DCs were reflected in a higher metabolic plasticity to maintain intracellular ATP content. Interestingly, tolerogenic and mature DCs manifested substantially different expression of proteins involved in the fatty acid oxidation (FAO) pathway, and FAO activity was significantly higher in tolerogenic DCs. Inhibition of FAO prevented the function of tolerogenic DCs and partially restored T cell stimulatory capacity, demonstrating their dependence on this pathway. Overall, tolerogenic DCs show metabolic signatures of increased oxidative phosphorylation programing, a shift in redox state, and high plasticity for metabolic adaptation. These observations point to a mechanism for rapid genome-wide reprograming by modulation of underlying cellular metabolism during DC differentiation.High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells - PubMed
https://pubmed.ncbi.nlm.nih.gov/25917094/
Current Opinion in Immunology
Volume 58, June 2019
Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization
Highlights
• DCs with different Th cell-polarizing properties have distinct metabolic profiles.
• Metabolic regulators mTOR, AMPK, and PPAR-γ play key roles in Th cell polarization by DCs.
• Targeting specific metabolic pathways in DCs alters their Th cell-polarizing properties.
Dendritic cells (DCs) are professional antigen-presenting cells that play a crucial role in the priming and differentiation of CD4+ T cells into several distinct subsets including effector T helper (Th) 1, Th17 and Th2 cells, as well as regulatory T cells (Tregs). It is becoming increasingly clear that cellular metabolism shapes the functional properties of DCs. Specifically, the ability of DCs to drive polarization of different Th cell subsets may be orchestrated by the engagement of distinct metabolic pathways. In this review, we will discuss the recent advances in the DC metabolism field, by focusing on how cellular metabolism of DCs shapes their priming and polarization of distinct Th cell responses.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that play a crucial role in the development of adaptive immune responses by governing the priming and maintenance of CD4+ and CD8+ T cell responses. Classically, DCs reside in a quiescent state in peripheral tissues acting as sentinels of the immune system. Upon capturing pathogen-derived antigens or detecting tissue-derived danger signals, DCs become activated and migrate to draining lymph nodes (LNs). Herein, processed antigens are presented to T cells to initiate an adaptive immune response. Depending on the DC subset involved and the nature of the activation signal received, DCs control the priming and differentiation of CD4+ T cells into several distinct subsets including effector T helper (Th) 1, Th17, and Th2 cells, as well as regulatory T cells (Tregs) [1].
It is becoming increasingly clear that immune cell activation and function, including that of DCs, are coupled to, and underpinned by, profound changes in cellular metabolism [2,3]. There is a growing body of literature showing that acquisition of an immunogenic phenotype by DCs, characterized by enhanced migratory-capacity and overall T cell priming-capacity, is accompanied by, and dependent on a switch from oxidative phosphorylation (OXPHOS) to glycolysis [4•,5,6••,7, 8, 9, 10, 11, 12, 13]. In addition, more recent studies show that the ability of DCs to drive polarization of different Th cell subsets may be underpinned by engagement of distinct metabolic pathways. In this review, we will discuss these recent advances in the DCs metabolism field, by specifically focusing on how DC metabolism shapes the priming and polarization of distinct CD4+ T cell responses. For a discussion of the metabolic requirements of DCs to shape CD8+ T cell responses please refer to the following recent studies/reviews [14•,15••,16, 17, 18].
Metabolic regulation of MHC II-mediated antigen presentation and costimulation
A prerequisite for priming of CD4+ T cell responses is antigen presentation in the context of MHC II. TLR-driven upregulation of surface expression of MHC II by murine DCs has been shown to depend on glycolysis [4•,6••,19]. DC activation involves acidification of the lysosomal compartment, which is required for efficient generation of peptides for loading into MHC II and that is dependent on activity of ATP-driven proton pumps [20]. In addition, TLR-driven surface expression of MHC II by DCs is primarily thought to arise from redistribution of molecules from endocytic compartments through an energy-dependent process called lysosome tubulation [21,22]. Therefore, it is conceivable that glycolysis is required for efficient upregulation of surface expression of MHC II because it serves as a key source of ATP to support these two steps in antigen presentation (Figure 1). Mammalian target of rapamycin complex 1 (mTORC1) is an important nutrient sensor that promotes glycolysis, anabolic metabolism and translation [23]. Consistent with a role for glycolysis in regulation of MHC II expression, mTORC1 is activated in DCs upon TLR stimulation [14•] and is implicated in TLR-induced MHC II surface expression by DCs [19,24], through its ability to promote lysosome acidification and tubulation [24,25]. However, constitutive activation of mTORC1 in murine DCs has been shown to result in impaired MHC II expression, via mTORC1-driven suppression expression of complex transactivator (CIITA), a protein that directly drives the expression of MHC II [26]. In this scenario, as a consequence of constitutive mTORC1 activation, low CIITA expression already in immature DCs leads to reduced synthesis of MHC II molecules and thereby lower levels of the molecules on the surface on DCs following TLR stimulation, despite the promotion of lysosome acidification and tubulation [25]. Finally, mTORC1 may be involved in shaping the antigen repertoire loaded into MHC II. In immature DCs, autophagy – a process classically activated under bioenergetic stress that degrades cellular components to restore cellular energy levels – is thought to allow for cytosolic proteins to enter endosomes and thereby the MHC II-restricted presentation pathway [27]. Upon TLR stimulation, mTORC1 activity is increased and is likely to reduce basal levels of autophagy in activated DCs to limit endogenous but favor exogenous antigen presentation [28]. Together, this implicates glycolysis and mTOR signaling in the regulation of peptide-loading into, and surface expression of MHC II (Figure 1), although to what extent mTOR regulates these processes through control of glycolysis or other metabolic pathways is currently unclear.
Figure 1
Download : Download high-res image (645KB)Download : Download full-size image
Figure 1. Role of mTOR and cellular metabolism in the regulation of MHC II-dependent antigen presentation by DCs.
Metabolic pathways and upstream signaling pathways regulating these are indicated in red and black, respectively. The left and the right sides of the figure depict how MHC II-dependent antigen presentation is metabolically regulated in unactivated (immature) and TLR-stimulated (mature) DCs, respectively.
In addition to antigen presentation in the context of MHC II, the upregulation of co-stimulatory molecules is also essential for efficient priming of Th cell responses by DCs. TLR-induced CD40 and CD86 expression has been shown to critically depend on glycolysis [4•,6••]. In line with this observation, blocking glycolysis in TLR-activated DCs reduced their overall CD4+ T cell-priming capacity [6••]. Mechanistically, it was found that increased glycolysis, by fueling the TCA cycle, supports de novo synthesis of fatty acids for the expansion of the endoplasmic reticulum (ER) and Golgi allowing for effective translation of those molecules [6••]. This highlights the importance of anabolic metabolism fueled by glycolysis to support expression of costimulatory molecules by DCs that is required for effective priming of effector CD4+ T cell responses. Yet to what extent Th polarization by DCs is controlled by metabolism through regulation of expression of MHC II and costimulatory molecules is unclear at this point.
Metabolic regulation of DC-driven Th1 polarization
A key Th1-polarizing cytokine produced by DCs is IL-12 [29]. IL-12 expression by DCs has been shown to critically depend on glycolysis by supporting the de novo synthesis of fatty acids for the expansion of the endoplasmic reticulum (ER) and Golgi required for effective translation [6••]. Concordantly, inhibition of glycolysis in murine LPS-activated DCs impaired their ability to promote IFN-γ secretion by CD4+ T cells. Moreover, type 1 conventional DCs (cDC1s), the primary DC subset that produces IL-12 and drives Th1 responses in vivo [30,31], are more glycolytic than cDC2s [15••]. Despite the importance of mTORC1 in driving glycolytic metabolism and translation, interfering with mTORC1 signaling has been shown to augment IL-12 secretion [32, 33, 34]. In line with this, hyperactivation of mTORC1 in CD11c+ cells, by ablation of its negative regulator tuberous sclerosis 1 (TSC1), resulted in downregulation of il12a and consequently in impaired Th1 priming by BMDCs generated with FLT3L [35]. Presumably this suppressive effect of mTORC1 signaling on IL-12 production is mediated by a an mTORC1-driven negative feedback loop involving IL-10 [34,36]. Nonetheless, favoring anabolic metabolism, by inactivation of AMP-activated kinase (AMPK), which normally promotes OXPHOS and catabolic metabolism in part by suppressing mTOR activity, increases IL-12 production by DCs [4•,37]. This points toward an important role for anabolic metabolism supported by glycolysis in supporting the ability of DCs to promote Th1 differentiation (Figure 2a).
Figure 2
Download : Download high-res image (917KB)Download : Download full-size image
Figure 2. Metabolic characteristics of DCs that prime different Th cell responses.
Metabolic pathways and upstream signaling pathways regulating these in DCs that prime Th1 (a), Th2 (b), Th17 (c) and Treg (d) responses are indicated in red and black, respectively.
Metabolic regulation of DC-driven Th2 polarization
Despite the evidence that DCs are crucially important for induction of Th2 responses, the nature of the polarizing signals derived from DCs to drive these responses are not fully understood, although reduced TCR signal strength, absence of IL-12 and secretion of type 2 cytokines by other innate type 2 immune cells have been suggested [38,39]. Likewise, the metabolic properties of Th2-priming DCs are not well defined.
In contrast to activation with LPS, stimulation of DCs with house dust mite extract (HDM), an antigen mixture known to promote DC-dependent Th2 responses in the lung resulting in allergic asthma, was recently found to induce only a mild increase in glycolysis with no loss of OXPHOS over time [19]. This suggests a more catabolic metabolic state of Th2-priming DCs, similar to what has been implied for other cells involved in type 2 immune responses [40]. Consistent with this, increased phosphorylation of AMPK was found in CD11b+ lung cDC2s of mice infected with hookworm that promotes strong Type 2 immune responses in the lung [37]. Moreover, mice with a specific deletion of AMPKα1 in CD11c-expressing cells fail to mount protective Th2 responses against this parasitic worm infection, suggesting AMPK-driven catabolic metabolism in DCs may be functionally relevant for Th2 priming. This would be supported by the observations that acute mTOR inhibition potentiates Th2 priming by human DCs in vitro [41] and that mice with a DC-specific deletion of mTOR have higher circulating titers of type 2 cytokine-dependent antibodies IgE and antigen-specific IgG1, in a model of allergic asthma, although this is not mirrored by an enhanced Th2 response in the lungs [42•].
Lipid metabolism, regulated by PPAR-γ, may also play an important role in Th2 priming by DCs. Mice with a deletion of PPAR-γ in CD11c-expressing cells are resistant to induction of allergic asthma [43]. In contrast, stimulation of DCs with PPAR-γ activator rosiglitazone, ameliorates airway inflammation in a mouse model of asthma [44]. In agreement with this, deletion of Sirtuin-1 (SIRT1) in DCs restricts an allergen-induced Th2 response in the lung by activating PPAR-γ [45]. This may indicate that a certain level of PPAR-γ activity is required for Th2 priming by DCs, but strong activation may interfere with this, possibly by rendering DCs tolerogenic [46,47]. Taken together, these studies provide a first indication that Th2-priming DCs, have a more oxidative metabolic profile dependent on AMPK and low PPAR-γ signaling (Figure 2b). One could hypothesize that this oxidative/catabolic metabolic profile limits high expression of IL-12 and MHC II, thereby favoring Th2 polarization by DCs. However, this link remains to be experimentally addressed.
Metabolic regulation of DC-driven Th17 polarization
The polarization of naïve CD4+ T cells into Th17 cells is driven by TGF-β and IL-6, while IL-23 and IL-1β play a prominent role in the expansion and survival of Th17 cells [48,49]. Little is known about the metabolic regulation of TGF-β production by DCs. However, both the secretion of IL-6 by murine DCs in response to TLR stimulation [6••,50] and the secretion of IL-23 and IL-1β by human DCs following the combined engagement of TLRs and FcαR [51,52] seem to be dependent on anabolic metabolism characterized by de novo fatty acid synthesis fueled by glycolysis. However, another study found that blocking glycolysis during TLR stimulation increased IL-23 expression by human DCs due to enhanced expression of the ER stress sensor IRE1α [53]. Consistent with this latter observation, treatment of murine DCs with glycolysis inhibitor 2-deoxyglucose (2-DG) can enhance their Th17-priming potential [6••] (Figure 2c). These opposing may suggest that the metabolic requirements for cytokine production can be context specific and depend on the DC subset and type of stimulus involved, as has been demonstrated for different TLR ligands in monocytes [54].
The metabolic pathways involved in Th17 differentiation by DCs in vivo are not well defined. Mice with a defect in mTORC1 in CD11c-expressing cells, show a switch toward Th17 instead of Th2 polarization in response to HDM-induced asthma, that is accompanied by changes in the frequency and metabolic profile of lung DCs [42•]. This was found to be mediated by inflammatory DCs, that expressed higher levels of IL-23, IL-6, and IL-1β, which the authors postulated to be due to enhanced fatty acid oxidation in these cells. However, it is conceivable that loss of mTORC1-driven IL-10 secretion, that could otherwise suppress expression of these pro-inflammatory cytokines, also contributes to this phenotype [34,36]. Of note, susceptibility to develop Th17-driven experimental autoimmune encephalomyelitis (EAE), a murine model for multiple sclerosis, was not increased, implying that mTOR in DCs restricts Th17 responses in a tissue-specific manner [42•]. Furthermore, DCs in which cholesterol accumulated as a consequence of deletion of ATP binding cassette transporters A1 and G1 (ABCA1 and ABCG1), two transporters responsible for cholesterol efflux, displayed increased secretion of IL-23, IL-6, and IL-1β and a potentiated ability to drive Th17 polarization [55]. The known stimulating effect of cholesterol on pro-inflammatory TLR signaling and inflammasome activation is likely to explain these effects [56]. However, whether cholesterol synthesis and/or regulation of its efflux is a metabolic process general employed by DCs to prime Th17 responses such as following exposure to Th17-priming pathogens is unknown and warrants further investigation.
Metabolic regulation of DC-driven Treg polarization
DCs are also key regulators of maintenance of immune tolerance by governing the development of regulatory T cells (Tregs). Tregs can be induced in the thymus, referred to as thymic-derived Tregs (tTregs), as well as in the periphery (pTregs) or in vitro (iTregs) from naïve T cells [57]. A diverse set of mechanisms has been identified through which pTreg and iTreg differentiation from naïve T cells can be induced by DCs, including low costimulatory signal strength, increased expression of IL-10 and TGF-β and enhanced activity of retinaldehyde dehydrogenase (RALDH) and indoleamine 2,3-dioxygenase (IDO) [58,59]. Now, the metabolic characteristics of tolerogenic DCs and requirements for their tolerogenic phenotype leading to pTreg and iTreg differentiation are also starting to be elucidated.
It has long been appreciated that differentiation of human monocytes toward DCs in the presence of mTORC1 inhibitor rapamycin, generates tolerogenic DCs [60], providing a first indication that inhibition of anabolic metabolism could favor acquisition of a tolerogenic DC phenotype. Consistent with this notion, two recent studies characterizing the metabolic properties of human moDCs rendered tolerogenic with 1,25(OH)2D3 (VitD3) alone [61•] or together with dexamethasone [62], reported increased mitochondrial activity evidenced by heightened OXPHOS. In the latter study, acquisition of tolerogenic phenotype by the DCs was partly dependent on increased FAO [62]. Interestingly, VitD3–DCs additionally displayed increased mTOR/hypoxia induced factor (HIF)-1α-dependent glycolytic rates [61•]. This increased glycolysis was functionally relevant as several markers of a tolerogenic phenotype (i.e. reduced expression of costimulatory molecules CD86 and CD80 and increased production of IL-10) was lost by VitD3–DCs in which glycolysis was inhibited [61•]. Concordantly, glycolysis inhibition limited their ability to suppress CD4+ T cell proliferation. Interestingly, the observations that these cells show an increased AMPK activation [61•] and elevated glucose carbon tracing into the TCA cycle [63], suggest that this increased glycolytic flux, in contrast to immunogenic DCs, may primarily serve a catabolic role by fueling mitochondrial OXPHOS.
Largely consistent with these human DC data, a recent in vivo study focusing on the metabolic properties of DCs in tumors [64••], a microenvironment that is a well-known to render DCs tolerogenic [65], revealed that these cells displayed increased FAO-dependent OXPHOS. This metabolic shift as well as IDO activity was driven by tumor cell-derived Wnt5a and dependent on β-catenin and PPAR-γ signaling. Importantly, when FAO was blocked in DCs, Wnt5a failed to enhance IDO activity in these cells as well as to instruct them to promote FoxP3+ pTreg differentiation and as a consequence enhanced anti-tumor immunity in vivo. Interestingly, a similar β-catenin-PPAR-γ-dependent pathway is required for the maintenance tolerogenic DCs in visceral adipose tissue [66]. This, together with the findings that tolerogenic DCs in mucosal tissues depend on PPAR-γ signaling for RALDH expression and activity [46,47], points toward a crucial role for PPAR-γ in supporting tolerogenic properties of DCs in various settings (Figure 2d). However, to what extent these PPAR-γ-driven effects are mediated by controlling lipid metabolism remains to be determined.
Conversely, a switch from OXPHOS toward aerobic glycolysis by stabilization of hypoxia induced factor (HIF)-1α, as seen in SIRT1-deficient DCs, redirects pTreg toward Th1 priming by enhancing IL-12 production and reducing TGF-beta expression [67], providing further support for a key role in catabolic/oxidative metabolism in pTreg induction by DCs. Given the key role for AMPK signaling in promoting this type of metabolism it is tempting to speculate that this kinase is important in DC-driven iTreg and pTreg polarization. The fact that SIRT1 can promote the activation of AMPK, through deacetylation of LKB1 [68], and that VitD3-treated human DCs showed increased AMPK activation [61•], would be consistent with this idea and warrants a more direct assessment of the role of AMPK signaling in regulating the tolerogenic properties in DCs. The aforementioned studies have focused on the metabolic requirements for DCs to promote iTreg or pTreg differentiation. To what extent these observations, can be extrapolated to DC-driven tTreg differentiation in the thymus remains an open question.It is becoming increasingly clear that immune cell activation and function, including that of DCs, are coupled to, and underpinned by, profound changes in cellular metabolism [2,3]. There is a growing body of literature showing that acquisition of an immunogenic phenotype by DCs, characterized by enhanced migratory-capacity and overall T cell priming-capacity, is accompanied by, and dependent on a switch from oxidative phosphorylation (OXPHOS) to glycolysis [4•,5,6••,7, 8, 9, 10, 11, 12, 13]. In addition, more recent studies show that the ability of DCs to drive polarization of different Th cell subsets may be underpinned by engagement of distinct metabolic pathways.
Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0952791518301080
Nihon Yakurigaku Zasshi,
1987 Mar;
[Effects of magnesium deficiency on dermal mast cells in rats]
The effects of dietary magnesium (Mg) deficiency on dermal mast cells were studied in young Wistar rats weighing about 50 g. The rats fed with a Mg-deficient diet (0.001% Mg) showed hyperemia on the 3rd or the 4th day after they were fed the diet. The dermal mast cells of the control rats were filled with granules, while the cells of rats fed the Mg-deficient diet for 4 days contained less granules than the controls, but contained extensively dilated rough surfaced endoplasmic reticulum, well-developed Golgi complexes, many mitochondria and ribosomes. These data suggest that hypomagnesemia could induce a release of histamine from dermal mast cells. So, the effect of a low Mg medium on the release of histamine was studied using peritoneal mast cells in vitro. A low Mg medium (0.2 mM Mg) induced much more histamine release than control medium (1 mM Mg) from the peritoneal mast cells obtained from both control and Mg-deficient rats fed with the Mg-deficient diet for 2 days. The peritoneal mast cells obtained on the 8th day of Mg-deficiency released much more histamine than controls in 1 mM Mg medium. These results suggest that hyperemia observed in Mg-deficient rats depends partly on histamine released from dermal mast cells.
[Effects of magnesium deficiency on dermal mast cells in rats] - PubMed
https://pubmed.ncbi.nlm.nih.gov/2438197/
Inflammatory response following acute magnesium deficiency in the rat
The importance of inflammatory processes in the pathology of Mg deficiency has been recently reconsidered but the sequence of events leading to the inflammatory response remains unclear. Thus, the purpose of the present study was to characterize more precisely the acute phase response following Mg deficiency in the rat. Weaning male Wistar rats were pair-fed either a Mg-deficient or a control diet for either 4 or 8 days. The characteristic allergy-like crisis of Mg-deficient rats was accompanied by a blood leukocyte response and changes in leukocytes subpopulations. A significant increase in interleukin-6 (IL-6) plasma level was observed in Mg-deficient rats compared to rats fed a control diet. The inflammatory process was accompanied by an increase in plasma levels of acute phase proteins. The concentrations of α2-macroglobulin and α1-acid glycoprotein in the plasma of Mg-deficient rats were higher than in control rats. This was accompanied in the liver by an increase in the level of mRNA coding for these proteins. Moreover, Mg-deficient rats showed a significant increase in plasma fibrinogen and a significant decrease in albumin concentrations. Macrophages found in greater number in the peritoneal cavity of Mg-deficient rats were activated endogenously and appeared to be primed for superoxide production following phorbol myristate acetate stimulation. A high plasma level of IL-6 could be detected as early as day 4 for the Mg-deficient diet. Substance P does not appear to be the initiator of inflammation since IL-6 increase was observed without plasma elevation of this neuropeptide. The fact that the inflammatory response was an early consequence of Mg deficiency suggests that reduced extracellular Mg might be responsible for the activated state of immune cells.
Inflammatory response following acute magnesium deficiency in the rat - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0925443900000181
cell reports, 2020
Extracellular Acidosis and mTOR Inhibition Drive the Differentiation of Human Monocyte-Derived Dendritic Cells
• Low pH promotes monocyte differentiation into dendritic cells (mo-DCs)
• Low pH inhibits mTORC1 activity
• mTORC1 inhibition promotes mo-DC differentiation at neutral pH
• mTORC1 inhibition turns GM-CSF into a strong inducer of mo-DC differentiation
Summary
During inflammation, recruited monocytes can differentiate either into macrophages or dendritic cells (DCs); however, little is known about the environmental factors that determine this cell fate decision. Low extracellular pH is a hallmark of a variety of inflammatory processes and solid tumors. Here, we report that low pH dramatically promotes the differentiation of monocytes into DCs (monocyte-derived DCs [mo-DCs]). This process is associated with a reduction in glucose consumption and lactate production, the upregulation of mitochondrial respiratory chain genes, and the inhibition of mTORC1 activity. Interestingly, we also find that both serum starvation and pharmacological inhibition of mTORC1 markedly promote the differentiation of mo-DCs. Our study contributes to better understanding the mechanisms that govern the differentiation of monocytes into DCs and reveals the role of both extracellular pH and mTORC1 as master regulators of monocyte cell fate.Low pH Induces a Fall in Intracellular pH and a Starvation-like Response
Previous studies in cell lines have shown that acidosis inhibits glycolysis (Corbet et al., 2016, Lamonte et al., 2013). We found that low pH as well as amiloride suppressed both glucose consumption and lactate production in monocytes cultured with M-CSF, IL-4, and TNF-α (Figure 2C). We hypothesized that inhibition of glycolysis might explain, at least partially, the ability of low pH to promote mo-DC differentiation. However, results in Figure 2D showed that two glycolytic inhibitors, 2-deoxy-D-glucose (2DG) and dichloroacetate (DCA), did not promote DC differentiation. In fact, 2DG partially prevented mo-DC differentiation, without affecting cell viability (data not shown).
Studies performed in cell lines have shown that the starvation response induced by low pH involves not only the inhibition of glycolysis but also the inhibition of the cellular nutrient sensor mTORC1 (Balgi et al., 2011, Walton et al., 2018). As expected, we found that the mammalian target of rapamycin (mTOR) inhibitor temsirolimus markedly inhibited both glucose consumption and lactate production (Figure 2E). Interestingly, low pH and amiloride strongly inhibited mTORC1 activity (Figures 2F and 2G), raising the possibility that inhibition of mTORC1 might contribute to the ability of low pH to promote mo-DC differentiation.DISCUSSION
A large body of evidence from studies performed in cell lines show that acidic pH profoundly reprograms cellular metabolism from aerobic glycolysis toward fatty acid oxidation in the mitochondria (Corbet et al., 2016, Khacho et al., 2014, Lamonte et al., 2013). In line with these observations, we found not onlythe course of DC differentiation but also that monocytes cultured at low pH upregulated genes of the mitochondrial respiratory chain. Also consistent with observations made in cell lines (Balgi et al., 2011, Corbet et al., 2016, Lamonte et al., 2013, Walton et al., 2018), we found that low pH markedly inhibited the activity of mTORC1, a cellular nutrient sensor that plays an essential role in cell growth and survival (Kim and Guan, 2019, Saxton and Sabatini, 2017). Interestingly, the NHE inhibitor amiloride reproduced all the effects, suggesting that a drop in cytoplasmic pH might explain, at least partially, the ability of extracellular pH to promote mo-DC differentiation.
Extracellular Acidosis and mTOR Inhibition Drive the Differentiation of Human Monocyte-Derived Dendritic Cells: Cell Reports
https://www.cell.com/cell-reports/fulltext/S2211-1247(20)30562-3
IMMUNOBIOLOGY. 2012
Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells
TLR agonists initiate a rapid activation program in dendritic cells (DCs) that requires support from metabolic and bioenergetic resources. We found previously that TLR signaling promotes aerobic glycolysis and a decline in oxidative phosphorylation (OXHPOS) and that glucose restriction prevents activation and leads to premature cell death. However, it remained unclear why the decrease in OXPHOS occurs under these circumstances. Using real-time metabolic flux analysis, in the present study, we show that mitochondrial activity is lost progressively after activation by TLR agonists in inflammatory blood monocyte–derived DCs that express inducible NO synthase. We found that this is because of inhibition of OXPHOS by NO and that the switch to glycolysis is a survival response that serves to maintain ATP levels when OXPHOS is inhibited. Our data identify NO as a profound metabolic regulator in inflammatory monocyte–derived DCs.the increase in glycolytic rate in DCs was found to be dependent on the PI3K/Akt pathway,4 which is one of the most commonly mutated signaling pathways in tumors.8 We reasoned that glycolysis could serve essentially the same purpose in active DCs as it is thought to do in tumors.4 This view was supported by the fact that glucose restriction inhibits severely the activation and life span of DCs exposed to TLR agonists.4 However, unlike in most cancers, which continue to consume oxygen at rates comparable to normal tissues despite increased glycolytic rates,9 activated DCs use significantly less oxygen than do resting DCs.4
To address these issues, we have in the present study, undertaken a detailed analysis of mitochondrial function in DCs after TLR stimulation. Using extracellular flux analysis to measure changes in oxygen consumption in real time, we found that 6 hours after stimulation, mitochondrial oxygen consumption was progressively lost due to the initiation of NO production. By 24 hours after exposure to TLR agonists, mitochondria in NO-producing DCs exhibit no evidence of oxidative phosphorylation (OXPHOS) or of an active tricarboxylic acid (TCA) cycle. We show that the switch to sustained glycolytic metabolism by activated DCs in vitro and monocyte-derived inflammatory DCs (moDCs) in vivo is a response to the inhibition of respiration by NO and is essential for DC function and survival because it provides essential ATP in the absence of a functioning respiratory chain.
Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells | Blood | American Society of Hematology
https://ashpublications.org/blood/article/120/7/1422/30765/Commitment-to-glycolysis-sustains-survival-of-NO
Science. 2018 Apr 27;
Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity
Johns Hopkins University School of Medicine
Activated immune cells undergo a metabolic switch to aerobic glycolysis akin to the Warburg effect, thereby presenting a potential therapeutic target in autoimmune disease. Dimethyl fumarate (DMF), a derivative of the Krebs cycle intermediate fumarate, is an immunomodulatory drug used to treat multiple sclerosis and psoriasis. Although its therapeutic mechanism remains uncertain, DMF covalently modifies cysteine residues in a process termed succination. We found that DMF succinates and inactivates the catalytic cysteine of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in mice and humans, both in vitro and in vivo. It thereby down-regulates aerobic glycolysis in activated myeloid and lymphoid cells, which mediates its anti-inflammatory effects. Our results provide mechanistic insight into immune modulation by DMF and represent a proof of concept that aerobic glycolysis is a therapeutic target in autoimmunity.富马酸二甲酯靶向GAPDH和有氧糖酵解以调节免疫力
约翰霍普金斯大学医学院
活化的免疫细胞经历类似于Warburg效应的代谢转换为有氧糖酵解,从而在自身免疫性疾病中提供了潜在的治疗靶点。富马酸二甲酯(DMF)是Krebs循环富马酸酯的衍生物,是一种免疫调节药物,用于治疗多发性硬化症和牛皮癣。尽管其治疗机制仍不确定,但DMF在称为琥珀酸的过程中共价修饰半胱氨酸残基。我们发现,DMF可在小鼠和人类体内和体外使琥珀酸琥珀酸并灭活糖酵解酶甘油醛3-磷酸脱氢酶(GAPDH)的催化半胱氨酸。因此,它在激活的髓样和淋巴样细胞中下调有氧糖酵解,介导其抗炎作用。我们的结果提供了对DMF免疫调节机制的深入了解,并代表了有氧糖酵解是自身免疫性治疗靶点的概念证明。Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity - PubMed
https://pubmed.ncbi.nlm.nih.gov/29599194/#:~:text=Activated%20immune%20cells%20undergo%20a%20metabolic%20switch%20to,drug%20used%20to%20treat%20multiple%20sclerosis%20and%20psoriasis.
Finally, dimethyl fumarate, a derivative of the Krebs cycle intermediate fumarate that inactivates glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) and therefore inhibits both branches of glycolysis, altered the differentiation and function of Th1 and Th17 cells, attenuating disease in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis 46, stressing again the potentials of glucose metabolic inhibitors to target pathogenic autoreactive T cells.
Immune cell metabolism in autoimmunity - Teng - 2019 - Clinical & Experimental Immunology - Wiley Online Library
https://onlinelibrary.wiley.com/doi/full/10.1111/cei.13277
Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany.
Disrupting metabolism to treat autoimmunity - PubMed
https://pubmed.ncbi.nlm.nih.gov/29700251/
Journal of Allergy and Clinical Immunology
January 2008
Epigallocatechin gallate affects human dendritic cell differentiation and maturation
Department of Surgical Oncology, Faculty of Medicine, University of Tokyo, Tokyo, Japan
Department of Transfusion Medicine, Faculty of Medicine, University of Tokyo, Tokyo, Japan
Background
Epigallocatechin gallate (EGCG), a component of green tea catechin with the strongest biological activity, has been focused in recent years because of its anti-inflammatory and immunomodulatory activities. Dendritic cells (DCs) are professional antigen-presenting cells, capable of priming naive T cells, and play the key roles in the activation of T-cell–mediated immune responses.
Objective
We aimed to investigate the effect of EGCG on human monocyte-derived DCs (MODCs) and, consequently, on the T-cell–mediated immune response.
Methods
The induction of apoptosis, and the detailed phenotypic and functional changes of MODCs, generated by culture of peripheral blood monocytes in the presence of GM-CSF and IL-4, induced by EGCG was investigated and compared with the effects of dexamethasone.
Results
Epigallocatechin gallate induced apoptosis and affected the phenotype of the developing DCs. The expressions of CD83, CD80, CD11c, and MHC class II, which are molecules essential for antigen presentation by DCs, were downregulated by EGCG. EGCG also suppressed the endocytotic ability of immature DCs, whereas dexamethasone-treated DCs had higher endocytotic ability than control DCs. Most importantly, mature DCs treated with EGCG inhibited stimulatory activity toward allogeneic T cells while secreting high amounts of IL-10.
Conclusion
Epigallocatechin gallate induces immunosuppressive alterations on human MODCs, both by induction of apoptosis and suppression of cell surface molecules and antigen presentation.Epigallocatechin gallate affects human dendritic cell differentiation and maturation - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0091674907015928
BMC Complementary Medicine and Therapies
Published: 31 August 2016
Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice
Background
Psoriasis is a chronic inflammatory immune disease with undefined pathogenesis. It is associated with T cells, and the IL-23/IL17 axis is believed to be crucial in the pathogenesis. The present treatments have side effects that influence the compliance of patients. Tea polyphenol is extracted from tea polyphenols, and its main active ingredient is Epigallocatechin-3-gallate (EGCG), which has been shown to have antioxidant, anti-tumor, and anti-ultraviolet radiation effects. Here, we aim to report that EGCG can inhibit imiquimod (IMQ)-induced psoriasis-like inflammation.
Methods
We used BALB/c mice, which were topically treated with IMQ for 6 consecutive days, as a psoriasis mouse model. Topical application of EGCG and treatment with EGCG were conducted in the experiments. Then observed the effects of the two methods on psoriasis-like mice dermatitis. Statistics are presented as the means ± standard error of mean (SEM) and compared using unpaired two-tailed Student’s t tests or one-way ANOVA.
Results
Topical application of EGCG alleviated psoriasiform dermatitis, improved the skin pathological structure by reduce the expression of epidermal PCNA, promoted the expression of caspase-14. Treatment with EGCG attenuated skin inflammation, accompanied by reduced infiltrations of T cells; reduced percentages of CD11c+ DC in the composition of immunocytes of spleens; reduced levels of interleukin (IL)-17A, IL-17F, IL-22, IL-23 and malondialdehyde (MDA) in plasma; increased percentages of CD4+ T cells in the composition of immunocytes of spleens; and increased bioactivities of superoxide dismutase (SOD) and catalase (CAT) in plasma.
Conclusions
All the results demonstrated that EGCG had anti-inflammatory, immune regulatory and antioxidant effects. It is a promising intervention in psoriasis in the future.Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice | BMC Complementary Medicine and Therapies | Full Text
https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-016-1325-4
Experimental biology symposia, 2016
Green tea EGCG, T-cell function, and T-cell-mediated autoimmune encephalomyelitis
Dayong Wu
Correspondence to Dr Dayong Wu, Nutritional Immunology Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, 711 Washington Street, Boston, MA 02111, USA; dayong.wu@tufts.edu
Abstract
Autoimmune diseases are common, disabling immune disorders affecting millions of people. Recent studies indicate that dysregulated balance of different CD4+ T-cell subpopulations plays a key role in immune pathogenesis of several major autoimmune diseases. Green tea and its active ingredient, epigallocatechin-3-gallate (EGCG), have been shown to modulate immune cell functions and improve some autoimmune diseases in animal models. In a series of studies we determined EGCG's effect on T-cell functions and its application in autoimmune diseases. We first observed that EGCG inhibited CD4+ T-cell expansion induced by polyclonal (mitogens or anti-CD3/CD28) or antigen-specific stimulation. We then showed that EGCG suppressed expansion and cell cycle progression of naïve CD4+ T by modulating cell cycle-related proteins. EGCG also inhibited naive CD4+ T-cell differentiation into Th1 and Th17 effector subsets by impacting their respective signaling transducers and transcription factors. These results suggest that EGCG may improve T-cell-mediated autoimmune diseases. Using the experimental autoimmune encephalomyelitis (EAE) mice, an animal model for human multiple sclerosis, we found that dietary supplementation with EGCG attenuated the disease's symptoms and pathology. These EGCG-induced changes are associated with findings in the immune and inflammation profiles in lymphoid tissues and the central nervous system: a reduction in proliferation of autoreactive T cells, production of proinflammatory cytokines, and Th1 and Th17 subpopulations, and an increase in regulatory T-cell populations. These results suggest that green tea or its active components may have a preventive and therapeutic potential in dealing with T-cell-mediated autoimmune diseases. However, the translational value of these findings needs to be validated in future human studies.Green tea EGCG, T-cell function, and T-cell-mediated autoimmune encephalomyelitis | Journal of Investigative Medicine
https://jim.bmj.com/content/64/8/1213
Int Immunopharmacology. 2017 Aug;
Anti-inflammatory effect of epigallocatechin gallate (EGCG) in a mouse model of ovalbumin-induced allergic rhinitis
Currently, a variety of studies have demonstrated that green tea has anti-allergic properties, and the major polyphenolic compound, epigallocatechin gallate (EGCG), plays a significant role. Some research indicates that EGCG reduces the production and expression of allergy-related substances. Therefore, EGCG has a potential effect of reducing allergic rhinitis (AR). In this study, the effect of EGCG on allergic rhinitis in an ovalbumin (OVA)-induced mouse model was investigated. After administration of EGCG, the number of sneezes and the occurrence of nasal rubbing were significantly decreased, the concentrations of immunoglobulin E (IgE) and histamine were suppressed in AR mouse serum, the levels of interleukin (IL)-1β, IL-4, and IL-6 were reduced in AR mice nasal lavage fluid (NLF), and the nasal mucosa mRNA and protein expression of cyclooxygenase 2 (COX-2), IL-1β, IL-4, and IL-6 were inhibited. The data indicate that EGCG has a beneficial effect of reducing allergic rhinitis.
Anti-inflammatory effect of epigallocatechin gallate in a mouse model of ovalbumin-induced allergic rhinitis - PubMed
https://pubmed.ncbi.nlm.nih.gov/28558302/Another Green Tea Miracle: How Japanese Green Tea Can Cure Allergies | Yoga Digest
https://yogadigest.com/another-green-tea-miracle-japanese-green-tea-can-cure-allergies/
Int Immunopharmacology. 2019 May
Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis
Allergic rhinitis (AR) is an allergic nasal disease characterized by nasal obstruction, rhinorrhea, sneezing, and itching. Type 1 helper T cells (Th1)/type 2 helper T cells (Th2) imbalance has been identified as an important immunological mechanism of AR. In addition, up-regulation of type 17 helper T cells (Th17) also increase the risk of developing AR. Gallic acid (3, 4, 5-trihydroxybenzoic acid, GA), a polyphenol natural product, is obtained from various herbs, red wine, and green tea. It is known to have diverse biological effects such as anti-oxidation, anti-inflammation, anti-microbial and anti-cancer. In the present study, the effect of GA on airway inflammation and expression of Th1, Th2 and Th17 cytokines in an ovalbumin (OVA)-induced AR mouse model were investigated. GA alleviated the nasal allergic symptoms, reduced the thickness of nasal mucosa, attenuated goblet cell hyperplasia and eosinophil cell infiltration in the nasal mucosa, decreased the levels of interleukin (IL)-4, IL-5, IL-13 and IL-17 in nasal lavage fluid (NALF), and diminished the levels of OVA-specific IgE, OVA-specific IgG1 and OVA-specific IgG2a in serum. However, GA increased the expression of interferon-gamma and IL-12 in NALF. Taken together, it suggests that GA may be used as a therapeutic agent for AR.
Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis - PubMed
https://pubmed.ncbi.nlm.nih.gov/30884431/
Frontiers |
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses
Induction of Interleukin-10 Producing ...
https://www.frontiersin.org/articles/10.3389/fimmu.2018.00455
Mechanistically, IL-10 inhibits the function of APCs, including macrophages and DCs, by downregulating their maturation status and reducing the associated production of pro-inflammatory cytokines (such as IL-1β, IL-6, or TNF-α), while increasing the expression of inhibitory genes (23, 36).
Cited by: 70
Publish Year: 2018
Author: Stefan Schülke
Jounard of Allergy Clinical Immunology. 2008 Jan;
Epigallocatechin gallate affects human dendritic cell differentiation and maturation
Department of Surgical Oncology, Faculty of Medicine, University of Tokyo, Tokyo, Japan.
Abstract
Background: Epigallocatechin gallate (EGCG), a component of green tea catechin with the strongest biological activity, has been focused in recent years because of its anti-inflammatory and immunomodulatory activities. Dendritic cells (DCs) are professional antigen-presenting cells, capable of priming naive T cells, and play the key roles in the activation of T-cell-mediated immune responses.
Objective: We aimed to investigate the effect of EGCG on human monocyte-derived DCs (MODCs) and, consequently, on the T-cell-mediated immune response.
Methods: The induction of apoptosis, and the detailed phenotypic and functional changes of MODCs, generated by culture of peripheral blood monocytes in the presence of GM-CSF and IL-4, induced by EGCG was investigated and compared with the effects of dexamethasone.
Results: Epigallocatechin gallate induced apoptosis and affected the phenotype of the developing DCs. The expressions of CD83, CD80, CD11c, and MHC class II, which are molecules essential for antigen presentation by DCs, were downregulated by EGCG. EGCG also suppressed the endocytotic ability of immature DCs, whereas dexamethasone-treated DCs had higher endocytotic ability than control DCs. Most importantly, mature DCs treated with EGCG inhibited stimulatory activity toward allogeneic T cells while secreting high amounts of IL-10.
Conclusion: Epigallocatechin gallate induces immunosuppressive alterations on human MODCs, both by induction of apoptosis and suppression of cell surface molecules and antigen presentation.
Epigallocatechin gallate affects human dendritic cell differentiation and maturation - PubMed
https://pubmed.ncbi.nlm.nih.gov/17935769/
Molecular Aspects Medicine. 2012 Feb;
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases
1Nutritional Immunology Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA 02111, United States.
One of the proposed health benefits of consuming green tea is its protective effect on autoimmune diseases. Research on the immunopathogenesis of autoimmune diseases has made significant progression in the past few years and several key concepts have been revised.T cells, particularly CD4(+) T helper (Th) cells, play a key role in mediating many aspects of autoimmune diseases. Upon antigenic stimulation, naïve CD4(+) T cells proliferate and differentiate into different effector subsets. Th1 and Th17 cells are the pro-inflammatory subsets of Th cells responsible for inducing autoimmunity whereas regulatory T cells (Treg) have an antagonistic effect. Green tea and its active ingredient, epigallocatechin-3-gallate (EGCG), have been shown to improve symptoms and reduce the pathology in some animal models of autoimmune diseases.
Whether or not EGCG's effect is mediated through its impact on Th17 and Treg development has not been studied. We conducted a series of studies to investigate EGCG's effect on CD4(+) T cell proliferation and differentiation as well as its impact on the development of autoimmune disease. We first observed that EGCG inhibited CD4(+) T cell expansion in response to either polyclonal or antigen specific stimulation. We then determined how EGCG affects naïve CD4(+) T cell differentiation and found that it impeded Th1 and Th17 differentiation and prevented IL-6-induced inhibition on Treg development. We further demonstrated that EGCG inhibited Th1 and Th17 differentiation by downregulating their corresponding transcription factors (STAT1 and T-bet for Th1, and STAT3 and RORγt for Th17). These effects provide further explanation for previous findings that administration of EGCG by gavage to experimental autoimmune encephalomyelitis (EAE) mice, an animal model for human multiple sclerosis (MS), reduced the clinical symptoms, brain pathology, and proliferation and TNF-α production of encephalitogenic T cells. Upon further investigating the working mechanisms for EGCG's protective effect in the EAE model, we showed that dietary EGCG dose-dependently attenuated the disease's severity. This protective effect of EGCG is associated with the suppressed proliferation of autoreactive T cells, reduced production of pro-inflammatory cytokines, decreased Th1 and Th17, and increased Treg populations in lymphoid tissues and central nervous system. EGCG-induced shifts in CD4(+) T cell subsets in EAE mice are accompanied by the corresponding changes in their regulator molecules. Recent studies have also highlighted the critical role of Th17/Treg balance in the pathogenesis of rheumatoid arthritis (RA). EGCG has been shown to be anti-inflammatory and protective in several studies using animal models of inflammatory arthritis, but research, at the best, only to start looking into the mechanisms with a focus on T cells. Overall, future research should fully incorporate the current progress in autoimmunity into the study design to expand the power of evaluating EGCG's efficacy in treating autoimmune diseases. Data from human studies are essentially absent and thus are urgently needed.
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases - PubMed
https://pubmed.ncbi.nlm.nih.gov/22020144/
Jounard of Allergy Clinical Immunology. 2005 Jan;
Epigallocatechin gallate induces apoptosis of monocytes
1Department of Surgical Oncology, Faculty of Medicine, University of Tokyo, Japan.
Background: Monocytes are the main effector cells of the immune system, and the regulation of their survival and apoptosis is essential for monocyte-involved immune responses. Green tea polyphenol catechin has been reported to have antiallergic and anti-inflammatory activities, but its effect on monocytes has not yet been explored.
Objective: To elucidate the mechanisms of the anti-inflammatory effect of catechin, we studied the effect of catechin, especially epigallocatechin gallate (EGCG), on the apoptosis of monocytes.
Methods: Isolated peripheral blood monocytes were incubated without or with catechin, and apoptosis was evaluated by annexin V and propidium iodide double-staining or terminal deoxynucleotidyl assay. The activation of caspases 3, 8, and 9 was also evaluated by flow cytometry. The influence of GM-CSF or LPS, the known monocyte survival factors, on the EGCG-induced apoptosis of monocytes was investigated.
Results: Among the 4 catechin derivatives tested, EGCG and epicatechin gallate induced apoptosis of monocytes. Caspases 3, 8, and 9, which play a central role in the apoptotic cascade, were dose-dependently activated by EGCG treatment. The EGCG-induced apoptosis of monocytes was not affected by GM-CSF or LPS.
Conclusion: Catechin, especially EGCG, by promoting monocytic apoptosis, may be a new promising anti-inflammatory agent, and should be tested in clinical trials.Epigallocatechin gallate induces apoptosis of monocytes - PubMed
https://pubmed.ncbi.nlm.nih.gov/15637567/
Biochem Soc Transi2004 Jun;
The role of oxidants and vitamin C on neutrophil apoptosis and clearance
We have investigated the role of neutrophil oxidants in the surface changes that result in recognition and uptake of neutrophils by macrophages. We have shown that H2O2 produced by stimulated neutrophils is essential for the surface expression of phosphatidylserine. This does not occur in neutrophils from mice with chronic granulomatous disease and may explain the formation of granuloma in this condition. We have also investigated the role of intracellular vitamin C on neutrophil apoptosis. Cells from vitamin C-deficient mice were found to be less likely to undergo both spontaneous and oxidant-induced apoptosis, with eventual necrosis being the most probable outcome.The role of oxidants and vitamin C on neutrophil apoptosis and clearance - PubMed
https://pubmed.ncbi.nlm.nih.gov/15157171/
J Leukocytes. Biology. 2007 May;
Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha
1Free Radical Research Group, Pathology Department, Christchurch School of Medicine and Health Sciences, P.O. Box 4345, Christchurch, New Zealand.
Some cells, including neutrophils, accumulate high intracellular ascorbate concentrations, which suggests that they have an important function in these cells. In this study we have used L-gulono-gamma-lactone oxidase (Gulo)-/- mice, which are unable to synthesize ascorbate, to generate ascorbate-deficient neutrophils and have used these to investigate the effect of ascorbate on neutrophil function. Peritoneal neutrophils from ascorbate-deficient animals had normal morphology and respiratory burst activity but failed to undergo spontaneous apoptosis, determined by morphology and the surface expression of phosphatidylserine. Initially, there was increased cell survival, but death eventually occurred by necrosis within 48 h. Neutrophils persisted in thioglycollate-induced inflammation in Gulo-/- mice with the later appearance of necrotic cells, suggesting that apoptosis was also affected in vivo. Also, ascorbate-deficient neutrophils were not recognized by macrophages in an in vitro assay for phagocytosis, providing further evidence for defective apoptosis and clearance. Neutrophils from Gulo-/- mice had elevated levels of hypoxia-inducible factor (HIF)-1alpha, a transcription factor regulated by Fe2+-dependent hydroxylases which require ascorbate for optimal activity. HIF-1alpha has been shown previously to inhibit neutrophil apoptosis under hypoxic conditions. Our results suggest that in ascorbate deficiency, up-regulation of HIF-1alpha blocks neutrophil apoptosis under normoxic conditions and that this represents a novel and important function for vitamin C in inflammatory cells.Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha - PubMed
https://pubmed.ncbi.nlm.nih.gov/17264304/
Immunological Research. 2017 Jun;
Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells
Glycyrrhizin, a component of Chinese medicine licorice root, has the ability to inhibit the functions of high-mobility group box 1 (HMGB1). While glycyrrhizin is known to have anti-inflammatory activities, the underlying mechanisms by which glycyrrhizin inhibits inflammation during the development of trinitrobenzenesulfonic acid (TNBS)-induced experimental colitis are not well understood. This study systemically examined the regulatory effects of glycyrrhizin on inflammatory response in TNBS-induced murine colitis and explored the potential mechanisms involved in this process. We reported that glycyrrhizin treatment ameliorated colitis and decreased the production of inflammatory mediators HMGB1, IFN-γ, IL-6, TNF-α, and IL-17. In addition, glycyrrhizin regulated responses of dendritic cells (DCs) and macrophages during the development of colitis. Furthermore, administration of glycyrrhizin suppressed the proliferation of Th17 cells in colitis. Moreover, the ability of DCs and macrophages to induce the differentiation of Th17 cells was enhanced in presence of HMGB1, which was inhibited by glycyrrhizin. These results demonstrated that glycyrrhizin alleviated colitis by inhibiting the promotive effect of HMGB1 on DC/macrophage-mediated Th17 proliferation. In conclusion, HMGB1 plays an important role in the development of colitis. As an inhibitor of HMGB1, glycyrrhizin might be a novel therapy for colitis.
Keywords: Antigen-presenting cells; Colitis; Glycyrrhizin; HMGB1; Th17.Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells - PubMed
https://pubmed.ncbi.nlm.nih.gov/28108937/
J Leukocyte Biology. 2007 Jan;
High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin
1Laboratory of Molecular Immunoregulation, Center for Cancer Research, and Basic Research Program, Rm. 31-19/Bldg. 560, 1050 Boyles Street, Frederick, MD 21702, USA.
High mobility group box-1 (HMGB1) protein is a nonhistone, DNA-binding protein that plays a critical role in regulating gene transcription. Recently, HMGB1 has also been shown to act as a late mediator of endotoxic shock and to exert a variety of proinflammatory, extracellular activities. Here, we report that HMGB1 simultaneously acts as a chemoattractant and activator of dendritic cells (DCs). HMGB1 induced the migration of monocyte-derived, immature DCs (Mo-iDCs) but not mature DCs. The chemotactic effect of HMGB1 on iDCs was pertussis toxin-inhibitable and also inhibited by antibody against the receptor of advanced glycation end products (RAGE), suggesting that HMGB1 chemoattraction of iDCs is mediated by RAGE in a Gi protein-dependent manner. In addition, HMGB1 treatment of Mo-iDCs up-regulated DC surface markers (CD80, CD83, CD86, and HLA-A,B,C), enhanced DC production of cytokines (IL-6, CXCL8, IL-12p70, and TNF-alpha), switched DC chemokine responsiveness from CCL5-sensitive to CCL21-sensitive, and acquired the capacity to stimulate allogeneic T cell proliferation. Based on its dual DC-attracting and -activating activities as well as its reported capacity to promote an antigen-specific immune response, we consider HMGB1 to have the properties of an immune alarmin.
High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin - PubMed
https://pubmed.ncbi.nlm.nih.gov/16966386/
Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products.
Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P.
J Immunol. 2005 Jun 15;174(12):7506-15. doi: 10.4049/jimmunol.174.12.7506.
PMID: 15944249
甘草甜素对树突状细胞成熟和T细胞刺激活性的影响
The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells
Glycyrrhizin (GL), a main component of the plant Glycyrrhiza glabra has shown various immunomodulatory activities that can interfere with immune responses by targeting the dendritic cells (DCs). In this study, the effects of GL on the maturation and function of mouse splenic DCs was investigated. The results of flow cytometry analysis showed that GL was able to up-regulate the expression of CD40, CD86 and MHC-ІІ maturation markers on DCs. This component increased the production of IL-12 by these cells. The capacity of treated DCs to stimulate allogenic T cells and secretion of cytokines was examined in mixed lymphocyte reaction. DCs treated with GL enhanced proliferation of allogenic T cells along with the production of IFN-γ and IL-10 cytokines and reduced IL-4 production. These data indicated that GL has the capacity to up-regulate allostimulatory activity of professional antigen presenting DCs and conduct immune responses toward a T helper 1 response.
Highlights
► Glycyrrhizin (GL) up-regulates the expression of maturation markers on DCs. ► Treatment of DCs with GL enhances allogenic T cells proliferation. ► Treatment of DCs with GL increases IFN-γ and IL-10 and reduces IL-4 production. ► GL conducts immune responses toward a T helper 1 response.
甘草甜菜碱(GL)是植物甘草的主要成分,它具有多种免疫调节活性,可通过靶向树突状细胞(DC)来干扰免疫反应。在这项研究中,研究了GL对小鼠脾脏DC的成熟和功能的影响。流式细胞仪分析结果表明,GL能够上调DCs上CD40,CD86和MHC-β成熟标记的表达。该组分增加了这些细胞的IL-12产生。在混合淋巴细胞反应中检查了处理过的DC刺激同种异体T细胞和细胞因子分泌的能力。用GL处理的DC增强了同种T细胞的增殖以及IFN-γ和IL-10细胞因子的产生,并降低了IL-4的产生。这些数据表明,GL具有上调专业抗原呈递DC的同素刺激活性并向T辅助1应答进行免疫应答的能力。
The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0008874912002079
Mol Med Rep. 2019 Nov;
Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress
The present study aimed to investigate the anti‑ferroptosis effects of the HMGB1 inhibitor glycyrrhizin (GLY). The present study used a cell and animal model of acute liver failure (ALF), induced using tumor necrosis factor‑α, lipopolysaccharide and D‑galactosamine, to investigate the effects of GLY. The expression of glutathione peroxidase 4 (GPX4) and high mobility group protein B1 (HMGB1), heme oxygenase‑1 (HO‑1) and nuclear factor erythroid 2‑related factor 2 (Nrf2) were detected were detected by western blotting in L02 hepatocytes and mouse liver. The expression of GPX4 and HMGB1 in L02 hepatocytes and mouse liver was detected by immunofluorescence. The pathological changes to liver tissues were determined by hematoxylin and eosin staining. The levels of lactate dehydrogenase (LDH), Fe2+, reactive oxygen species (ROS) and glutathione (GSH) were tested using kits. Compared with the normal group, the degree of liver damage and liver function in the model animal group was severe. The protein levels of HMGB1 in L02 cells and liver tissues were significantly increased. The expression of NRF2, HO‑1 and GPX4 was significantly decreased. The levels of LDH, Fe2+, malondialdehyde (MDA) and ROS were increased, whereas the level of GSH was decreased. Treatment with GLY reduced the degree of liver damage, the expression of HMGB1 was decreased, and the levels of Nrf2, HO‑1 and GPX4 were increased. The levels of LDH, Fe2+, MDA, ROS were decreased, while the level of GSH was increased by GLY treatment. The results of the present study indicated that HMGB1 is involved in the process of ferroptosis. The HMGB1 inhibitor GLY significantly reduced the degree of ferroptosis during ALF by inhibiting oxidative stress.
Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress - PubMed
https://pubmed.ncbi.nlm.nih.gov/31545489/
Journal of Gastroenterology ,Published: October 2003
Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis
Glycyrrhizin (GL), an aqueous extract of licorice root, is known to have various immune-modulating and biological response-modifier activities. GL is used in patients with hepatitis to reduce the activity of liver inflammation; however, the mechanism underlying the anti-inflammatory activity of GL is poorly understood. As antigen-presenting dendritic cells (DC) in the tissue play a major role in the regulation of the inflammatory mucosal milieu during tissue inflammation, we studied whether the function of liver DC was altered by GL therapy in a murine model of concanavalin-A (Con A)-induced hepatitis.
Methods
Liver DC were propagated from control mice or mice with Con-A-induced hepatitis, and the effect of GL on liver DC was evaluated in vivo and in vitro.
Results
The levels of interleukin (IL)-10 produced by liver DC were significantly lower in mice with Con-A-induced hepatitis compared with control mice. However, treatment with GL caused increased production of IL-10 in mice with Con A-induced hepatitis. The increased production of IL-10 by mice with Con A-induced hepatitis was also confirmed in vitro by culturing liver DC with GL.
Conclusions
This study indicates that increased production of IL-10 by liver DC due to GL administration may be involved in downregulation of the levels of liver inflammation in mice with Con A-induced hepatitis.Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis | SpringerLink
https://link.springer.com/article/10.1007/s00535-003-1179-7
Effect of Glycyrrhizin on Pseudomonal Skin Infections in Human-Mouse Chimeras
In our previous studies, peripheral blood lineage−CD34+CD31+ cells (CD31+ IMC) appearing in severely burned patients have been characterized as inhibitor cells for the production of β-defensins (HBDs) by human epidermal keratinocytes (NHEK). In this study, the effect of glycyrrhizin on pseudomonal skin infections was studied in a chimera model of thermal injury. Two different chimera models were utilized. Patient chimeras were created in murine antimicrobial peptide-depleted NOD-SCID IL-2rγnull mice that were grafted with unburned skin tissues of severely burned patients and inoculated with the same patient peripheral blood CD31+ IMC. Patient chimera substitutes were created in the same mice that were grafted with NHEK and inoculated with experimentally induced CD31+ IMC. In the results, both groups of chimeras treated with glycyrrhizin resisted a 20 LD50 dose of P. aeruginosa skin infection, while all chimeras in both groups treated with saline died within 3 days of the infection. Human antimicrobial peptides were detected from the grafted site tissues of both groups of chimeras treated with glycyrrhizin, while the peptides were not detected in the same area tissues of controls. HBD-1 was produced by keratinocytes in transwell-cultures performed with CD31+ IMC and glycyrrhizin. Also, inhibitors (IL-10 and CCL2) of HBD-1 production by keratinocytes were not detected in cultures of patient CD31+ IMC treated with glycyrrhizin. These results indicate that sepsis stemming from pseudomonal grafted site infections in a chimera model of burn injury is controllable by glycyrrhizin. Impaired antimicrobial peptide production at the infection site of severely burned patients may be restored after treatment with glycyrrhizin.甘草甜素对人鼠嵌合体假性皮肤感染的影响
在我们以前的研究中,严重烧伤患者中出现的外周血谱系CD34 + CD31 +细胞(CD31 + IMC)被表征为人类表皮角质形成细胞(NHEK)产生β-防御素(HBD)的抑制剂细胞。在这项研究中,在热损伤的嵌合模型中研究了甘草甜素对假性皮肤感染的影响。利用了两种不同的嵌合体模型。在鼠类抗微生物肽缺乏的NOD-SCIDIL-2rγnull小鼠中创建患者嵌合体,将其移植至严重烧伤患者的未烧伤皮肤组织中,并接种相同的患者外周血CD31 + IMC。在与NHEK移植并用实验诱导的CD31 + IMC接种的同一只小鼠中创建患者嵌合体替代物。结果,两组用甘草甜素处理的嵌合体均抵抗20 LD50剂量的铜绿假单胞菌皮肤感染,而两组用盐水处理的所有嵌合体均在感染后3天内死亡。从用甘草甜素处理的两组嵌合体的移植部位组织中检测到人抗菌肽,而在对照的相同区域组织中未检测到肽。 HBD-1由角质形成细胞在CD31 + IMC和甘草甜素进行的跨孔培养中产生。同样,在用甘草甜素治疗的患者CD31 + IMC的培养物中未检测到角质形成细胞产生HBD-1的抑制剂(IL-10和CCL2)。这些结果表明,在烧伤损伤的嵌合体模型中,源自假单核移植位点感染的败血症可由甘草甜素控制。重度烧伤患者感染部位的抗菌肽产生受损,可通过甘草甜素治疗后恢复。Effect of Glycyrrhizin on Pseudomonal Skin Infections in Human-Mouse Chimeras
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0083747
Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats
Background
High mobility group box-1 (HMGB1), a proinflammatory cytokine, plays a pivotal role in tissue remodeling and angiogenesis, both of which are crucial for the pathogenesis of pulmonary arterial hypertension. In this study, we explored the relationship between HMGB1 and pulmonary hypertension and whether glycyrrhizin, an inhibitor of HMGB1, attenuates disease progression in an animal model of pulmonary hypertension induced by monocrotaline sodium (MCT).
Methods
After inducing pulmonary hypertension through a single subcutaneous injection of MCT (60 mg/kg) to Sprague–Dawley rats, we administered daily intraperitoneal injections of either glycyrrhizin (GLY, 50 mg/kg), an inhibitor of HMGB1, or saline (control) for either 4 or 6 weeks.
Results
Expression levels of HMGB1 in serum increased from the second week after MCT injection and remained elevated throughout the experiment periods. Lung tissue levels of HMGB1 assessed by immunohistochemical staining at 4 weeks after MCT injection also increased. Chronic inhibition of HMGB1 by GLY treatment reduced the MCT-induced increase in right ventricular (RV) systolic pressure, RV hypertrophy (ratio of RV to [left ventricle + septum]), and pulmonary inflammation. MCT-induced muscularization of the pulmonary artery was also attenuated in the GLY-treated group. As assessed 6 weeks after MCT injection, the GLY-treated group exhibited increased survival (90% [18 of 20]) when compared with the control group (60% [12 of 20]; p =0.0027).
Conclusions
Glycyrrhizin, an inhibitor of HMGB1, attenuates pulmonary hypertension progression and pulmonary vascular remodeling in the MCT-induced pulmonary hypertension rat model. Further studies are needed to confirm the potential of HMGB1 as a novel therapeutic target for pulmonary hypertension.Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats | Respiratory Research | Full Text
https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-014-0148-4
Effect of Glycyrrhizin on Pseudomonal Skin Infections in Human-Mouse Chimeras
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0083747
Metabolic Control of Th17 Cell Generation and CNS Inflammation
Multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system (CNS), results from uncontrolled auto reactive T cells that infiltrate the CNS and attack the myelin sheath. Th17 cells play a prominent role in the pathogenesis of MS and experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. Extensive studies have focused on understanding the roles of cytokine signaling and transcriptional network in the differentiation of Th17 cells and their pathogenicity in CNS inflammation. Aside from these events, activated T cells dynamically reprogram their metabolic pathways to fulfill the bioenergic and biosynthetic requirements for proper T cell functions. Emerging evidence indicates that modulation of these metabolic pathways impinges upon the differentiation of Th17 cells and the pathogenesis of EAE. Thus, a better understanding of the functions and mechanisms of T cell metabolism in Th17 cell biology may provide new avenues for therapeutic targeting of MS. In this review, we discuss the recent advances in our understanding of T cell metabolic pathways involved in Th17 cell differentiation and CNS inflammation.Metabolic Control of Th17 Cell Generation and CNS Inflammation
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4334382/
Biomedical Research (2016) Volume 27, Issue 3
Expression and correlation of HIF-1α, MIF, COX-2 and VEGF in psoriasis lesions.
Psoriasis is a common chronic, inflammatory and proliferative skin disease that is widely associated with multiple factors under a polygenic background. The expression of hypoxia-inducible factor-1alpha (HIF-1alpha), macrophage migration inhibitory factor (MIF), cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) is associated with psoriasis; however, there have been no systematic studies analyzing the mRNA and protein levels in psoriasis vulgaris tissues. The aim of the present study was to investigate the mRNA and protein levels of these genes in psoriasis vulgaris tissues and assess their correlation. Tissue samples from 45 cases of psoriasis vulgaris lesions and 45 cases of normal skin were collected. The study used semi-quantitative polymerase chain reaction and western blot analysis to detect the mRNA and protein levels in the psoriatic lesions and normal skin tissue. The mRNA and protein levels of HIF-1alpha, MIF, COX-2 and VEGF were significantly higher in psoriasis vulgaris tissues compared with those in normal skin tissue, all P-values were <0.05. Additionally, the mRNA and protein levels in the psoriatic lesions were positively correlated with each other. In conclusion, these genes may have important roles in the development of psoriasis.Expression and correlation of HIF-1α, MIF, COX-2 and VEGF in psoriasis lesions.
https://www.alliedacademies.org/articles/expression-and-correlation-of-hif1alpha-mif-cox2-and-vegf-in-psoriasis-lesions.html
Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor
a Department of Cancer Research, GlaxoSmithKline, Collegeville, PA 19426, USA
b Discovery Biology, BioDuro, No. 29 Life Science Park Road, Changping, Beijing, ChinaThe PI3K signaling pathway is activated in a broad spectrum of human cancers, either directly by genetic mutation or indirectly via activation of receptor tyrosine kinases or inactivation of the PTEN tumor suppressor. The key nodes of this pathway have emerged as important therapeutic targets for the treatment of cancer. In this study, we show that (−)-epigallocatechin-3-gallate (EGCG), a major component of green tea, is an ATP-competitive inhibitor of both phosphoinositide-3-kinase (PI3K) and mammalian target of rapamycin (mTOR) with Ki values of 380 and 320 nM respectively. The potency of EGCG against PI3K and mTOR is within physiologically relevant concentrations. In addition, EGCG inhibits cell proliferation and AKT phosphorylation at Ser473 in MDA-MB-231 and A549 cells. Molecular docking studies show that EGCG binds well to the PI3K kinase domain active site, agreeing with the finding that EGCG competes for ATP binding. Our results suggest another important molecular mechanism for the anticancer activities of EGCG.
Research highlights
► Epigallocatechin-3-gallate (EGCG) is an ATP-competitive inhibitor of PI3K and mTOR with Ki values around 300 nM.
► EGCG inhibits cell proliferation and AKT phosphorylation at Ser473 in MDA-MB-231and A549 cells.
► Molecular docking studies show that EGCG binds well to the PI3K kinase domain active site.
► These results suggest another important molecular mechanism for the anticancer activities of EGCG.
Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0006291X11001938
Front Bioscience. 2008 Jan 1
EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer
1Department of Biochemistry, University of Texas Health Science Center at Tyler, Tyler, Texas 75703, USA.
We have shown that epigallocatechin-3-gallate (EGCG), a polyphenolic compound from green tea, inhibits growth and induces apoptosis in human pancreatic cancer cells. However, the preclinical potential of EGCG in a suitable mouse model has not been examined. In this study, we examined the molecular mechanisms by which EGCG inhibited growth, invasion, metastasis and angiogenesis of human pancreatic cancer cells in a xenograft model system. EGCG inhibited viability, capillary tube formation and migration of HUVEC, and these effects were further enhanced in the presence of an ERK inhibitor. In vivo, AsPC-1 xenografted tumors treated with EGCG showed significant reduction in volume, proliferation (Ki-67 and PCNA staining), angiogenesis (vWF, VEGF and CD31) and metastasis (MMP-2, MMP-7, MMP-9 and MMP-12) and induction in apoptosis (TUNEL), caspase-3 activity and growth arrest (p21/WAF1). EGCG also inhibited circulating endothelial growth factor receptor 2 (VEGF-R2) positive endothelial cells derived from xenografted mice. Tumor samples from EGCG treated mice showed significantly reduced ERK activity, and enhanced p38 and JNK activities. Overall, our data suggest that EGCG inhibits pancreatic cancer growth, invasion, metastasis and angiogenesis, and thus could be used for the management of pancreatic cancer prevention and treatment.EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer - PubMed
https://pubmed.ncbi.nlm.nih.gov/17981559/#:~:text=Tumor%20samples%20from%20EGCG%20treated%20mice%20showed%20significantly,the%20management%20of%20pancreatic%20cancer%20prevention%20and%20treatment.
Eur Rev Med Pharmacology Science. 2019 Jan.
Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state
1Department of Digestive Disease, People's Hospital of Rizhao, Affiliated Clinical Hospital of Jining Medical Univerity, Jining Medical University, Rizhao, Shandong, China.
Objective: To investigate the effects of epigallocatechin-3-gallate (EGCG) on proliferation and apoptosis of human gastric cancer SGC7901 cells under a hypoxic state.
Materials and methods: Human gastric cancer SGC7901 cells were sub-cultured, and the cobalt chloride (CoCl2) hypoxia model was established. The blank control group (normoxia group), hypoxia control group (hypoxia group) and hypoxia + different concentrations of EGCG subgroups (20, 40, 60, 80, 100 μg/mL EGCG) were set up. Cell viability was detected via methyl thiazolyl tetrazolium (MTT) assay, apoptosis was detected via flow cytometry, and expressions of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) were detected via reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting.
Results: Relatively low concentrations of EGCG (20-80 μg/mL) presented no significant inhibiting effect on SGC7901 cell growth within a short time (24 h) (p>0.05). The increasing concentration of EGCG inhibited cell proliferation under a hypoxia state (p<0.05). EGCG induced apoptosis in a dose-dependent manner under hypoxia (p<0.05). EGCG could significantly impede expressions of HIF-1α and VEGF proteins (p<0.05), and down-regulate the level of VEGF mRNA (p<0.05), but it showed no significant effect on the HIF-1α mRNA expression (p>0.05).
Conclusions: EGCG inhibited cell proliferation under hypoxia via the downregulation of HIF-1α and its downstream target gene VEGF levels, providing a theoretical basis for the early diagnosis and treatment of gastric cancer in clinic.Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state - PubMed
https://pubmed.ncbi.nlm.nih.gov/30657557/
Proliferating Cells in Psoriatic Dermis Are Comprised Primarily of T Cells, Endothelial Cells, and Factor XIIIa+ Perivascular Dendritic Cells
1 Department of Dermatology , University of Michigan Medical School and the Ann Arbor Veterans Administration Hospital, Ann Arbor Michigan, U.S.A.
2 Department of Dermatology , The University of California College of Medicine, Irvine, California, U.S.A.Determination of the cell types proliferating in the dermis of patients with psoriasis should identify those cells experiencing activation or responding to growth factors in the psoriatic dermal milieu. Toward that end, sections of formalin-fixed biopsies obtained from 3H-deoxyuridine (3H-dU)-injected skin of eight psoriatic patients were immunostained, followed by autoradiography. Proliferating dermal cells exhibit silver grains from tritium emissions. The identity of the proliferating cells could then be determined by simultaneous visualization with antibodies specific for various cell types. UCHL1+ (CD45RO+) T cells (recall antigen-reactive helper T-cell subset) constituted 36.6 ± 3.1% (mean ± SEM, n = 6) of the proliferating dermal cells in involved skin, whereas Leu 18+ (CD45RA+) T cells (recall antigen naive T-cell subsets) comprised only 8.7 ± 1.5% (n = 6). The Factor XIIIa+ dermal perivascular dendritic cell subset (24.9 ± 1.5% of proliferating dermal cells, n = 6) and Factor VIII+ endothelial cells (23.0 ± 2.3%, n = 6) represented the two other major proliferating populations in lesional psoriatic dermis. Differentiated tissue macrophages, identified by phase microscopy as melanophages or by immunostaining with antibodies to Leu M1 (CD15) or myeloid histiocyte antigen, comprised less than 5% of the proliferating population in either skin type. In addition to calculating the relative proportions of these cells to each other as percent, we also determined the density of cells, in cells/mm2 of tissue. The density of proliferating cells within these populations was increased in involved versus uninvolved skin: UCHL1+, 9.0 ± 1.7 cells/mm2 versus 1.8 ± 0.6 cells/mm2, p < 0.01; Factor XIIIa+, 6.0 ± 0.7 cells/mm2 versus 1.5 ± 0.5 cells/mm2, p < 0.01; Factor VIII+, 5.5 ± 1.4 cells/mm2 versus 0.0 cells/mm2, p < 0.05. The presence of preferential active proliferation of a T-cell subset in lesional dermis suggests that activating signals specific for this subset are contained within the psoriatic dermis in vivo. The activation of recall antigen-reactive T cells may be a driving force behind the dendritic cell and endothelial cell proliferation. Alternatively, the selective proliferation and expansion of these two constitutive cell types (Factor XIIIa+ and Factor VIII+) may result in signals that promote activation of UCHL1+ (CD45RO+) T cells.
Proliferating Cells in Psoriatic Dermis Are Comprised Primarily of T Cells, Endothelial Cells, and Factor XIIIa+ Perivascular Dendritic Cells - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0022202X9190130I
已消退的牛皮癣病变保留了与疾病相关的基因的子集的表达
1
The Laboratory for Investigative Dermatology, The Rockefeller University, New York, New York, USA
2
Center for Clinical and Translational Science, The Rockefeller University, New York, New York, USA
牛皮癣是一种复杂的炎症性疾病,通常可以治愈而无明显疤痕。组织学评估通常提示完全消退,但尚未定义与基因组疾病相关的改变的逆转。基因表达谱分析用于确定依那西普治疗3个月后对治疗有反应的患者牛皮癣基因逆转的程度。我们回顾了炎症基因的组织学,白细胞计数和PCR数据,以比较这些参数的恢复和基因组研究。尽管五个炎性基因的改善没有超过> 75%(IL-12p35,MX1,IL-22,IL-17和IFNγ),但许多细胞标志物的确恢复到接近病灶的水平。牛皮癣相关基因的改善<75%被定义为包含“残留疾病基因组概况”,由248个探针组组成。银屑病组织中未恢复到基线的目标基因包括LYVE-1,WNT5A,RAB31和AQP9。看来,即使牛皮癣中的表皮反应得到完全 消退,但在治疗的病灶中,由关键细胞因子和趋化因子的表达所定义的炎症仍不能完全消退。我们还发现皮肤的结构细胞继续表达分子变化,并且皮肤结构的一些微妙特征(例如淋巴管)并未通过治疗完全正常化。Resolved Psoriasis Lesions Retain Expression of a Subset of Disease-Related Genes - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0022202X15351617
Eur J Immunology. 2013 Apr;
Retinoic acid promotes the development of Arg1-expressing dendritic cells for the regulation of T-cell differentiation
1Laboratory of Immunology and Hematopoiesis, Department of Comparative Pathobiology, College of Veterinary Medicine Purdue Center for Cancer Research, Purdue University, West Lafayette, IN 47907, USA.
Arginase I (Arg1), an enzyme expressed by many cell types including myeloid cells, can regulate immune responses. Expression of Arg1 in myeloid cells is regulated by a number of cytokines and tissue factors that influence cell development and activation. Retinoic acid, produced from vitamin A, regulates the homing and differentiation of lymphocytes and plays important roles in the regulation of immunity and immune tolerance. We report here that optimal expression of Arg1 in DCs requires retinoic acid. Induction of Arg1 by retinoic acid is directly mediated by retinoic acid-responsive elements in the 5' noncoding region of the Arg1 gene. Arg1, produced by DCs in response to retinoic acid, promotes the generation of FoxP3(+) regulatory T (Treg) cells. Importantly, blocking the retinoic acid receptor makes DCs hypo-responsive to known inducers of Arg1 such as IL-4 and GM-CSF in Arg1 expression. We found that intestinal CD103(+) DCs that are known to produce retinoic acid highly express Arg1. Our results establish retinoic acid as a key signal in expression of Arg1 in DCs.
Retinoic acid promotes the development of Arg1-expressing dendritic cells for the regulation of T-cell differentiation - PubMed
https://pubmed.ncbi.nlm.nih.gov/23322377/
Nucleic Acids Res. 2016 Sep 19
Synergistic activation of Arg1 gene by retinoic acid and IL-4 involves chromatin remodeling for transcription initiation and elongation coupling
Department of Pharmacology, Medical School, University of Minnesota, Minneapolis, MN 55455, USA
All-trans Retinoic acid (RA) and its derivatives are potent therapeutics for immunological functions including wound repair. However, the molecular mechanism of RA modulation in innate immunity is poorly understood, especially in macrophages. We found that topical application of RA significantly improves wound healing and that RA and IL-4 synergistically activate Arg1, a critical gene for tissue repair, in M2 polarized macrophages. This involves feed forward regulation of Raldh2, a rate-limiting enzyme for RA biosynthesis, and requires Med25 to coordinate RAR, STAT6 and chromatin remodeler, Brg1 to remodel the +1 nucleosome of Arg1 for transcription initiation. By recruiting elongation factor TFIIS, Med25 also facilitates transcriptional initiation-elongation coupling. This study uncovers synergistic activation of Arg1 by RA and IL-4 in M2 macrophages that involves feed forward regulation of RA synthesis and dual functions of Med25 in nucleosome remodeling and transcription initiation-elongation coupling that underlies robust modulatory activity of RA in innate immunity.Synergistic activation of Arg1 gene by retinoic acid and IL-4 involves chromatin remodeling for transcription initiation and elongation coupling
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5027474/
FEMS Immunology & Medical Microbiology, Volume 47, Issue 3, August 2006,
Effect of all-trans-retinoic acid on the differentiation, maturation and functions of dendritic cells derived from cord blood monocytes
Children's Hospital, Fudan University, Shanghai, China
We investigated the effects of all-trans-retinoic acid on dendritic cells derived from human cord blood monocytes to clarify how vitamin A affects immune function in children. Monocytes were separated from 18 cord blood samples, and dendritic cells were differentiated by culture. The percentage of dendritic cells was markedly lower in all-trans-retinoic acid treated cells than in untreated cells. After exposure to tumour necrosis factor-α for 3 days, all-trans-retinoic acid treated dendritic cells showed a reduced capacity to activate alloreactive T cells compared to untreated cells. In addition, all-trans-retinoic acid-treated dendritic cells could drive T cells towards T-helper cell type 2 responses with decreased secretion of interleukin-12, interferon-γ, and increased production of interleukin-10 and interleukin-4. However, when Ro 41-5253, a selective retinoic acid receptor α antagonist, was add to culture, all the above actions were reversed. Thus, all-trans-retinoic acid may act at the first step of the immune response by inhibiting the differentiation of dendritic cells, maturation and induction of the T-helper cell type-2 response. The actions of all-trans-retinoic acid on dendritic cells were mediated through retinoic acid receptor α.Effect of all-trans-retinoic acid on the differentiation, maturation and functions of dendritic cells derived from cord blood monocytes | Pathogens and Disease | Oxford Academic
https://academic.oup.com/femspd/article/47/3/444/508056
Invest Ophthalmol Vis Sci. 2013 May;
Retinoic Acid Inhibits CD25+ Dendritic Cell Expansion and γδ T-Cell Activation in Experimental Autoimmune Uveitis
1Doheny Eye Institute, Keck School of Medicine of the University of Southern California, Los Angeles, California
Purpose.
We determined the mechanism by which all-trans retinoic acid (ATRA) inhibits experimental autoimmune uveitis (EAU) and determined the role of γδ T cells in this autoimmune disease.
Methods.
C57BL/6 (B6) mice were immunized with the uveitogenic, interphotoreceptor retinoid-binding protein1–20 peptide (IRBP1-20) in complete Freund's adjuvant (CFA), with or without a preceding ATRA treatment. Responses and pathogenic activity of Th1- and Th17-autoreactive T cells were compared, and the effects of ATRA on γδ T cells and CD25+ dendritic cell (DC) subset were determined. Interactions among uveitogenic T cells, DC subsets, and γδ T cells were investigated.
Results.
Administration of ATRA to B6 mice in which EAU was induced suppressed the response of Th17 autoreactive T cells, which was associated with decreased generation of the CD25+ DC subset and suppressed activation of γδ T cells. Adoptively transferred γδ T cells isolated from ATRA-treated mice showed a diminished ability to promote the activation of Th17 autoreactive T cells in vitro and in vivo compared to γδ T cells from untreated donors.
Conclusions.
ATRA inhibits the expansion of CD25+ DCs and γδ T-cell activation, thereby restraining the Th17 autoreactive T-cell response.
Keywords: autoimmunity, EAU, interleukin-17, Th17, retinoid acid, uveitis, γδ T cellRetinoic Acid Inhibits CD25+ Dendritic Cell Expansion and γδ T-Cell Activation in Experimental Autoimmune Uveitis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3665306/
Invest Ophthalmol Vis Sci. 2013 May;
Retinoic Acid Inhibits CD25+ Dendritic Cell Expansion and γδ T-Cell Activation in Experimental Autoimmune Uveitis
1Doheny Eye Institute, Keck School of Medicine of the University of Southern California, Los Angeles, California
Purpose.
We determined the mechanism by which all-trans retinoic acid (ATRA) inhibits experimental autoimmune uveitis (EAU) and determined the role of γδ T cells in this autoimmune disease.
Methods.
C57BL/6 (B6) mice were immunized with the uveitogenic, interphotoreceptor retinoid-binding protein1–20 peptide (IRBP1-20) in complete Freund's adjuvant (CFA), with or without a preceding ATRA treatment. Responses and pathogenic activity of Th1- and Th17-autoreactive T cells were compared, and the effects of ATRA on γδ T cells and CD25+ dendritic cell (DC) subset were determined. Interactions among uveitogenic T cells, DC subsets, and γδ T cells were investigated.
Results.
Administration of ATRA to B6 mice in which EAU was induced suppressed the response of Th17 autoreactive T cells, which was associated with decreased generation of the CD25+ DC subset and suppressed activation of γδ T cells. Adoptively transferred γδ T cells isolated from ATRA-treated mice showed a diminished ability to promote the activation of Th17 autoreactive T cells in vitro and in vivo compared to γδ T cells from untreated donors.
Conclusions.
ATRA inhibits the expansion of CD25+ DCs and γδ T-cell activation, thereby restraining the Th17 autoreactive T-cell response.
Keywords: autoimmunity, EAU, interleukin-17, Th17, retinoid acid, uveitis, γδ T cellRetinoic Acid Inhibits CD25+ Dendritic Cell Expansion and γδ T-Cell Activation in Experimental Autoimmune Uveitis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3665306/
J Biomed Science. 2017 Oct 30;
Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions
Background: Cells adapt to hypoxia by transcriptional induction of genes that participate in regulation of angiogenesis, glucose metabolism and cell proliferation. The primary factors mediating cell response to low oxygen tension are hypoxia inducible factors (HIFs), oxygen-dependent transcription activators. The stability and activity of the α subunits of HIFs are controlled by hydroxylation reactions that require ascorbate as a cofactor. Therefore, deficiency of intracellular vitamin C could contribute to HIFs overactivation. In this study, we investigated whether vitamin C content of human thyroid lesions is associated with HIF-1α and HIF-2α protein levels.
Methods: Expression of HIF-1α and HIF-2α as well as vitamin C content was analyzed in thyroid lesions and cultured thyroid carcinoma cell lines (FTC-133 and 8305c) treated with hypoxia-mimetic agent (cobalt chloride) and ascorbic acid. The expression of HIFs and hypoxia-induced glucose transporters were determined by Western blots while quantitative real-time PCR (qRT-PCR) was performed to detect HIFs mRNA levels. Ascorbate and dehydroascorbate levels were measured by HPLC method.
Results: We found an inverse correlation between vitamin C level and HIF-1α but not HIF-2α expression in thyroid lesions. These results agree with our in vitro study showing that vitamin C induced a dose - dependent decrease of HIF-1α but not HIF-2α protein level in thyroid cancer cells FTC-133 and 8305C. The decreased HIF-1α expression was correlated with reduced expression of hypoxia-related glucose transporter 1 (GLUT1) in thyroid cancer cells.
Conclusion: The results demonstrate that HIF-1α activation is associated with vitamin C content in thyroid lesions. Our study suggests that high tumor tissue ascorbate level could limit the expression of HIF-1α and its targets in thyroid lesions.
Keywords: HIF-1α/ HIF-2α/ vitamin C/GLUT1/ thyroid cancer.Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions - PubMed
https://pubmed.ncbi.nlm.nih.gov/29084538/
Cancer Biological Therapy. 2013 Nov;
Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1
The omega-3 polyunsaturated fatty acids (ω3PUFAs) are a class of lipids biologically effective for the treatment of inflammatory disorders, cardiovascular disease and cancer. Patients consuming a high dietary intake of ω3PUFAs have shown a low incidence of metabolic disorders, including cancer. Although the effects of ω3PUFAs intake was shown to be involved in the prevention and treatment of these diseases, the underlying molecular mechanisms involved are not well understood. Here, we show that ω3PUFA, docosahexaenoic acid (DHA) enhanced the tumor suppressor function of LKB1. We observed that when LKB1 expressing cells are treated with DHA, there is an increase in LKB1 activity leading to phosphorylation of AMPK and inhibition of mTOR signaling. Abrogation of LKB1 in MCF-7 cells by siRNA reversed this phenotype. Furthermore, cellular metabolism was altered and ATP levels were reduced in response to DHA treatment, which was further attenuated in cells expressing LKB1. More importantly, in mammary epithelial cells expressing LKB1, the rate of glycolysis was decreased as a result of diminished expression of glycolytic enzymes. Functionally, these events lead to a decrease in the migration potential of these cells. Overall, our discovery shows for the first time that LKB1 function is enhanced in response to ω3PUFA treatment, thereby resulting in the regulation of cell metabolism.Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1 - PubMed
https://pubmed.ncbi.nlm.nih.gov/24025358/
Degradation of HIF-1alpha under Hypoxia Combined with Induction of Hsp90 Polyubiquitination in Cancer Cells by Hypericin: a Unique Cancer Therapy
The perihydroxylated perylene quinone hypericin has been reported to possess potent anti-metastatic and antiangiogenic activities, generated by targeting diverse crossroads of cancer-promoting processes via unique mechanisms. Hypericin is the only known exogenous reagent that can induce forced poly-ubiquitination and accelerated degradation of heat shock protein 90 (Hsp90) in cancer cells. Hsp90 client proteins are thereby destabilized and rapidly degraded. Hsp70 client proteins may potentially be also affected via preventing formation of hsp90-hsp70 intermediate complexes. We show here that hypericin also induces enhanced degradation of hypoxia-inducible factor 1α (HIF-1α) in two human tumor cell lines, U87-MG glioblastoma and RCC-C2VHL−/− renal cell carcinoma and in the non-malignant ARPE19 retinal pigment epithelial cell line. The hypericin-accelerated turnover of HIF-1α, the regulatory precursor of the HIF-1 transcription factor which promotes hypoxic stress and angiogenic responses, overcomes the physiologic HIF-1α protein stabilization which occurs in hypoxic cells. The hypericin effect also eliminates the high HIF-1α levels expressed constitutively in the von-Hippel Lindau protein (pVHL)-deficient RCC-C2VHL−/− renal cell carcinoma cell line. Unlike the normal ubiquitin-proteasome pathway-dependent turnover of HIF-α proteins which occurs in normoxia, the hypericin-induced HIF-1α catabolism can occur independently of cellular oxygen levels or pVHL-promoted ubiquitin ligation of HIF-1α. It is mediated by lysosomal cathepsin-B enzymes with cathepsin-B activity being optimized in the cells through hypericin-mediated reduction in intracellular pH. Our findings suggest that hypericin may potentially be useful in preventing growth of tumors in which HIF-1α plays pivotal roles, and in pVHL ablated tumor cells such as renal cell carcinoma through elimination of elevated HIF-1α contents in these cells, scaling down the excessive angiogenesis which characterizes these tumors.Degradation of HIF-1alpha under Hypoxia Combined with Induction of Hsp90 Polyubiquitination in Cancer Cells by Hypericin: a Unique Cancer Therapy
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0022849
Lasers Med Science. 2018 Aug;
Topically applied hypericin exhibits skin penetrability on nude mice
Hypericin, a powerful natural photosensitizer in photodynamic therapy (PDT), is suitable for treating skin diseases involving excess capillary proliferation. In the present study, we aimed to evaluate the skin penetrability of topically applied hypericin, expecting a reduced risk of prolonged skin photosensitivity, which often occurs after systemic administration. Firstly, the Franz diffusion cell assays were performed to evaluate the penetration effects of different enhancers, including menthol, propylene glycol, camphanone, azone, and carbamide. In view of above evaluation results, we selected menthol as the enhancer in the subsequent in vivo studies. The setting groups were as follows: the blank control group, the light-exposure control group, the gel-base control group, the hypericin gel group, and a hypericin gel-containing menthol group. Except for the blank control, all other animals were irradiated by a LED light. Then, fluorescence microscopy was performed to examine the distribution of hypericin in the skin of nude mouse. Macroscopic and microscopic analyses were also carried out to detect pathological changes in the skin after topical hypericin-PDT treatment. Immunohistochemistry was used to determine the expression change of PECAM-1. As shown in the results, menthol facilitated hypericin penetrate the skin of nude mice most. The results of in vivo assays revealed that hypericin penetrated nude mouse skin, spread to the dermis, and resulted in obvious photosensitivity reaction on the dermal capillaries. Moreover, skin injured by the photosensitive reaction induced by hypericin-PDT treatment was replaced by normal skin within 7 days. We concluded that topical applied hypericin could penetrate nude mouse skin well and has a great potential in PDT treatment of skin diseases.
Keywords: Hypericin; Photodynamic therapy; Photosensitizer; Skin penetrability.Topically applied hypericin exhibits skin penetrability on nude mice - PubMed
https://pubmed.ncbi.nlm.nih.gov/29915976/
Degradation of HIF-1alpha under Hypoxia Combined with Induction of Hsp90 Polyubiquitination in Cancer Cells by Hypericin: a Unique Cancer Therapy
The perihydroxylated perylene quinone hypericin has been reported to possess potent anti-metastatic and antiangiogenic activities, generated by targeting diverse crossroads of cancer-promoting processes via unique mechanisms. Hypericin is the only known exogenous reagent that can induce forced poly-ubiquitination and accelerated degradation of heat shock protein 90 (Hsp90) in cancer cells. Hsp90 client proteins are thereby destabilized and rapidly degraded. Hsp70 client proteins may potentially be also affected via preventing formation of hsp90-hsp70 intermediate complexes. We show here that hypericin also induces enhanced degradation of hypoxia-inducible factor 1α (HIF-1α) in two human tumor cell lines, U87-MG glioblastoma and RCC-C2VHL−/− renal cell carcinoma and in the non-malignant ARPE19 retinal pigment epithelial cell line. The hypericin-accelerated turnover of HIF-1α, the regulatory precursor of the HIF-1 transcription factor which promotes hypoxic stress and angiogenic responses, overcomes the physiologic HIF-1α protein stabilization which occurs in hypoxic cells. The hypericin effect also eliminates the high HIF-1α levels expressed constitutively in the von-Hippel Lindau protein (pVHL)-deficient RCC-C2VHL−/− renal cell carcinoma cell line. Unlike the normal ubiquitin-proteasome pathway-dependent turnover of HIF-α proteins which occurs in normoxia, the hypericin-induced HIF-1α catabolism can occur independently of cellular oxygen levels or pVHL-promoted ubiquitin ligation of HIF-1α. It is mediated by lysosomal cathepsin-B enzymes with cathepsin-B activity being optimized in the cells through hypericin-mediated reduction in intracellular pH. Our findings suggest that hypericin may potentially be useful in preventing growth of tumors in which HIF-1α plays pivotal roles, and in pVHL ablated tumor cells such as renal cell carcinoma through elimination of elevated HIF-1α contents in these cells, scaling down the excessive angiogenesis which characterizes these tumors.Degradation of HIF-1alpha under Hypoxia Combined with Induction of Hsp90 Polyubiquitination in Cancer Cells by Hypericin: a Unique Cancer Therapy
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0022849
Study on HIF-1α Gene Translation in Psoriatic Epidermis with the Topical Treatment of Capsaicin Ointment
Objective. To investigate the mechanism of capsaicin in treating active psoriasis vulgaris. Methods. HIF-1α gene translation in active psoriatic lesions before and after 21-day treatment with capsaicin ointment was detected by in situ hybridization. Results. There was positive staining of HIF-1α gene in all the layers of psoriatic epidermis (100.0%) before the treatment with capsaicin ointment, but the dyeing in epidermis were reduced obviously (22.2%) after the treatment for 21 days. Conclusion. HIF-1α gene translation in psoriatic epidermis was downregulated after capsaicin treatment for 21 days.
1. Introduction
Psoriasis is a common skin disease characterized by hyperplastic regenerative epidermal growth and infiltration of immunocytes. The etiology of psoriasis is unknown although several genetic and cellular factors have been elucidated. Capsaicin is a naturally occurring substance derived from plants of the Solanaceae family (red peppers) with a kind of major pharmacologic effects on the peripheral part of the sensory nervous system, particularly on the primary afferent neurons of C-fiber type [1]. Hypoxia-inducible factor-1α (HIF-1α) is a more important factor in psoriatic epidermal proliferation. In order to find a new therapeutic method for psoriasis, we detected the translation of HIF-1α gene in psoriatic epidermis by in situ hybridization technique before and after 21 days treatment with capsaicin ointment.
After the treatment with capsaicin in psoriatic lesions, it downregulates the translation of HIF-1α mRNA, which induces the transcription of HIF-1α mRNA in all the layers of psoriatic epidermis and plays a role in inhibiting proliferation of keratinocytes. It has been shown that the expressions of HIF-1α protein were very weak in the control skin but very strong in psoriatic lesions. HIF-1α have high expression in psoriasis and might play an important role in the genesis and development of psoriasis
Study on HIF-1α Gene Translation in Psoriatic Epidermis with the Topical Treatment of Capsaicin Ointment
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3263732/
HIF-1α in Epidermis: Oxygen Sensing, Cutaneous Angiogenesis, Cancer, and Non-Cancer Disorders
Besides lung, postnatal human epidermis is the only epithelium in direct contact with atmospheric oxygen. Skin epidermal oxygenation occurs mostly through atmospheric oxygen rather than tissue vasculature, resulting in a mildly hypoxic microenvironment that favors increased expression of hypoxia-inducible factor-1α (HIF-1α). Considering the wide spectrum of biological processes, such as angiogenesis, inflammation, bioenergetics, proliferation, motility, and apoptosis, that are regulated by this transcription factor, its high expression level in the epidermis might be important to HIF-1α in skin physiology and pathophysiology. Here, we review the role of HIF-1α in cutaneous angiogenesis, skin tumorigenesis, and several skin disorders.HIF-1α is known to activate many angiogenic factors (growth factors, chemokines, and cytokines) at the transcriptional level, including VEGF, placental growth factor, angiopoietins 1 and 2, platelet-derived growth factor-B, stromal cell-derived factor-1, transforming growth factor-β, and stem cell factor within various cells involved in wound healing
Whereas rapidly produced cytoplasmic ROS downregulate HIF-1α expression, delayed mitochondrial ROS production results in its upregulation
It is likely that spatiotemporal repression and activation of HIF-1α has a substantial influence on the regulation of keratinocyte responses to UVB irradiation. In fact, downregulation of HIF-1α protein expression immediately after UVB irradiation has been found to be regulated at the translational level and to be important to release keratinocytes from UVB-induced cell cycle arrest (Cho et al., 2009). Late HIF-1α upregulation, which is regulated by mitogen-activated protein kinase (Rezvani et al., 2007; Nys et al., 2010), phosphatidylinositol 3-kinase/AKT (Wunderlich et al., 2008), and/or ATF3 (Turchi et al., 2008), has a proapoptotic effect in UVB-irradiated keratinocytes (Rezvani et al., 2007; Turchi et al., 2008; Nys et al., 2010) through upregulation of proapoptotic genes (such as Noxa, BCL2/adenovirus E1B 19-kDa-interacting protein (BNIP3), or tumor necrosis factor-related apoptosis-inducing ligand (Turchi et al., 2008; Nys et al., 2010) and interaction with p53 (Rezvani et al., 2007). Affecting DNA repair efficiency is the other means by which HIF-1α can modulate keratinocyte responses to UVB (Rezvani et al., 2010a). Biphasic variation of HIF-1α upon exposure of keratinocytes to UVB was also found to regulate the removal rate of 6–4 photoproducts and cyclobutane pyrimidine dimers, the most frequent types of UVB-induced lesions primarily removed by nuclear excision repair. This study showed that the effect of HIF-1α on the nuclear excision repair machinery relies on the transcriptional regulation of XPC, XPD, XPB, XPG, and Cockayne syndrome A and B expression by direct HIF-1α binding to the hypoxia-response elements of these genes in their promoter region (Rezvani et al., 2010a).
HIF-1α in psoriasis
Several lines of evidence suggest that HIF-1α could have an important role in psoriasis, a chronic skin disease characterized by keratinocyte hyperproliferation, epidermal inflammation, and angiogenesis. In fact, pivotal angiogenic genes such as VEGF and its receptors are upregulated in psoriasis (Detmar et al., 1994). Transgenic mice with VEGF upregulation in keratinocytes show inflammation and all the hallmarks of psoriasis, suggesting a causative role of VEGF in this disease (Xia et al., 2003). On the other hand, transgenic delivery to the skin of inflammatory mediators, such as tumor necrosis factor-α or keratinocyte growth factors like transforming growth factor-α, did not completely reproduce the psoriatic phenotype (Vassar and Fuchs, 1991; Cheng et al., 1992; Guo et al., 1993; Carroll et al., 1997; Schon, 1999), suggesting that VEGF upregulation is an early step in the pathophysiology of this disease (Detmar, 2004). HIF-1α has been found to be upregulated in psoriatic epidermis, in an expression pattern similar to VEGF mRNA expression (Rosenberger et al., 2007; Tovar-Castillo et al., 2007; Ioannou et al., 2009), thereby suggesting a possible application of HIF-1α inhibitors in the therapy of psoriasis.hypoxia inducible factor scoreranged from 1 to 9 with a mean of 4.55±2.43. Skin localization of HIF-1 inpsoriatic cases was supra basal in 26 cases (65.0%), prickle in 38 cases (95%),papillary in 16 cases (40%) and supra papillary in 38 cases (95.0%). Thus, themajor concentration was found in prickle and supra-papillary areas and theleast at the papillary area (table 1).
HIF-1α in Epidermis: Oxygen Sensing, Cutaneous Angiogenesis, Cancer, and Non-Cancer Disorders - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0022202X15354038
Hypoxia inducible factor-1 (HIF-1) alpha in Psoriatic Patients
September 2012
hypoxia inducible factor score ranged from 1 to 9 with a mean of 4.55±2.43. Skin localization of HIF-1 in psoriatic cases was supra basal in 26 cases (65.0%), prickle in 38 cases (95%), papillary in 16 cases (40%) and supra papillary in 38 cases (95.0%). Thus, the major concentration was found in prickle and supra-papillary areas and the least at the papillary area (table 1).Conclusion: The present study demonstrated a marked increase of HIF- 1 alpha immunoreactivity in psoriatic skin. In addition, our results indicated that the immunohistochemical pattern of this overexpression could be used as a diagnostic adjunct in distinguishing psoriasis from its histopathologic mimics.
HIF-1α in Epidermis: Oxygen Sensing, Cutaneous Angiogenesis, Cancer, and Non-Cancer Disorders - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0022202X15354038
phosphodiesterase type 4 (PDE-4) inhibitor
New Potential Advances In Treating Psoriasis: A Skin Condition That Affects 125+ Million People
https://www.forbes.com/sites/saibala/2020/07/23/new-potential-advances-in-treating-psoriasis-a-skin-condition-that-affects-125-million-people/?sh=7b7b8ff653f2
Ascorbic acid is a dose-dependent inhibitor of adipocyte differentiation, probably by reducing cAMP pool
Ascorbic acid (AA) is the active component of vitamin C and antioxidant activity was long considered to be the primary molecular mechanism underlying the physiological actions of AA. We recently demonstrated that AA is a competitive inhibitor of adenylate cyclase, acting as a global regulator of intracellular cyclic adenosine monophosphate (cAMP) levels. Our study, therefore, aimed to determine new targets of AA that would account for its potential effect on signal transduction, particularly during cell differentiation. We demonstrated that AA is an inhibitor of pre-adipocyte cell line differentiation, with a dose-dependent effect. Additionally, we describe the impact of AA on the expression of genes involved in adipogenesis and/or the adipocyte phenotype. Moreover, our data suggest that treatment with AA partially reverses lipid accumulation in mature adipocytes. These properties likely reflect the function of AA as a global regulator of the cAMP pool, since an analog of AA without any antioxidant properties elicited the same effect. Additionally, we demonstrated that AA inhibits adipogenesis in OP9 mesenchymal cell line and drives the differentiation of this line toward osteogenesis. Finally, our data suggest that the intracellular transporter SVCT2 is involved in these processes and may act as a receptor for AA.
Frontiers | Ascorbic acid is a dose-dependent inhibitor of adipocyte differentiation, probably by reducing cAMP pool | Cell and Developmental Biology
https://www.frontiersin.org/articles/10.3389/fcell.2014.00029/full
Proc Natl Acad Sci U S A. 1990 May;
Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation
Transforming growth factor beta 1 (TGF-beta 1) is a potent growth inhibitor for many cell types, including most epithelial cells. However, the mechanism of growth inhibition is unknown. In skin keratinocytes, TGF-beta 1 has been shown to inhibit growth and to rapidly reduce c-myc expression. It has been demonstrated that protein synthesis is required for TGF-beta 1 regulation of c-myc in keratinocytes. Here we present evidence that treatment of mouse BALB/MK keratinocyte cells with either antisense c-myc oligonucleotides or TGF-beta 1 inhibited cell entry into S phase. These results suggest that TGF-beta inhibition of c-myc expression may be essential for growth inhibition by TGF-beta 1. The block in c-myc expression by TGF-beta 1 occurred at the level of transcriptional initiation. Studies with a series of 5' deletion c-myc/chloramphenicol acetyltransferase constructs indicated that a cis regulatory element(s), which resides between positions -100 and +71 relative to P1 transcription start site, is responsible for the TGF-beta 1 responsiveness. Based on these data, it is proposed that the mechanism of TGF-beta 1 growth inhibition involves synthesis or modification of a protein that may interact with a specific element(s) in the 5' regulatory region of the c-myc gene, resulting in inhibition of transcriptional initiation.Transforming growth factor beta 1 suppression of c-myc gene transcription: role in inhibition of keratinocyte proliferation - PubMed
https://pubmed.ncbi.nlm.nih.gov/2187192/#:~:text=Transforming%20growth%20factor%20beta%201%20%28TGF-beta%201%29%20is,inhibit%20growth%20and%20to%20rapidly%20reduce%20c-myc%20expression.
Lactic Acid Inhibits NF-κB Activation by Lipopolysaccharide in Rat Intestinal Mucosa Microvascular Endothelial Cells
Lactic Acid Inhibits NF-κB Activation by Lipopolysaccharide in To investigate whether lactic acid could inhibit the LPS-activation of NF-κB p65 in rat intestinal mucosa microvascular endothelial cells (RIMMVECs), RIMMVECs, cultured in vitro, were pretreated with different concentrations of lactic acid and then exposed to lipopolysaccharide (LPS). Cells and cell culture media were then collected at different time intervals. Production of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) was examined at the protein level by enzyme-linked immunosorbent assay. The influence of lactic acid on the LPS-activation of NF-κB was examined at mRNA and protein levels by real-time quantitative PCR and Western blot analysis, respectively. TNF-α and IL-6 protein levels were significantly decreased after pretreatment with lactic acid compared with cells exposed to LPS only. After pretreatment with 7.5, 5.0, and 2.5 μL mL−1 lactic acid, NF-κB mRNA levels were increased by 1.51-, 2.62- and 3.00-fold, respectively, compared with levels in control cells without LPS treatment. Western blot analysis indicated that the level of NF-κB p65 in the lactic acid-pretreated group was significantly lower than that in the group treated with LPS only (positive control) and was unchanged compared with the group without LPS treatment (blank control). These results suggest that lactic acid may inhibit LPS-activation of NF-κB, leading to the down-regulation of TNF-α and IL-6.Lactic Acid Inhibits NF-κB Activation by Lipopolysaccharide in Rat Intestinal Mucosa Microvascular Endothelial Cells
https://www.researchgate.net/publication/232414737_Lactic_Acid_Inhibits_NF-kB_Activation_by_Lipopolysaccharide_in_Rat_Intestinal_Mucosa_Microvascular_Endothelial_Cells
... In particular, LA can inhibit the inflammatory response by decreasing interleukin-6 (IL-6) and tumour necrosis factor-a (TNF-a) messenger ribonucleic acid (mRNA) levels. Also LA prevented the activation of nuclear factor NF-Kappa B (NF-jB) in these experiments (Jiang et al. 2013; Liu et al. 2011a, b; Xu et al. 2013). However, thus far, the effect of J. sellowii as an antiinflammatory herb as indicated by its use in Brazilian traditional medicine has not been investigated. ...
Xiao-ming REN's research works | Technical Institute of Physics and Chemistry, Beijing (IPC) and other places
https://www.researchgate.net/scientific-contributions/Xiao-ming-REN-2001606499
Lactic Acid Reduces LPS-Induced TNF-α and IL-6 mRNA Levels Through Decreasing IKBα Phosphorylation
June 2013Journal of Integrative Agriculture 12(6):1073–1078
This study explored the effects over time of lactic acid (LA) on I κBα phosphorylation and nuclear factor-kappa B (NF-κB) p65 protein expression, and on tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) mRNA levels in rat intestinal mucosa microvascular endothelial cells (RIMMVECs) stimulated by lipopolysaccharide (LPS). IκBα, phosphorylated IκBα (ρ-I κBα) and p65 protein levels were monitored by Western blot analysis, and TNF-α and IL-6 mRNA levels were analyzed using real-time PCR. LA treatment reduced TNF-α and IL-6 mRNA levels in LPS-stimulated RIMMVECs, with the greatest effect being after 3 h. The highest inhibitory effect of LA on I κBα phosphorylation to prevent activation of NF- κB was after 6 h. These results suggest that LA reduces TNF-α and IL-6 mRNA levels through decreasing IκBα phosphorylation and blocking the dissociation of IKK complex, which prevents activation of NF- κB.Lactic Acid Reduces LPS-Induced TNF-α and IL-6 mRNA Levels Through Decreasing IKBα Phosphorylation
https://www.researchgate.net/publication/257737962_Lactic_Acid_Reduces_LPS-Induced_TNF-a_and_IL-6_mRNA_Levels_Through_Decreasing_IKBa_Phosphorylation
Lactic Acid Reduces LPS-Induced TNF-α and IL-6 mRNA Levels Through Decreasing IKBα Phosphorylation
Xiao-ming REN's research works | Technical Institute of Physics and Chemistry, Beijing (IPC) and other places
https://www.researchgate.net/scientific-contributions/Xiao-ming-REN-2001606499
J Pathology. 2017 Feb;
Proinflammatory effect of high-mobility group protein B1 on keratinocytes: an autocrine mechanism underlying psoriasis development
1Department of Dermatology, Xijing Hospital, Fourth Military Medical University, Xi'an, Shannxi, PR China.
Psoriasis is an autoimmune skin disease, in which keratinocytes play a crucial pathogenic role. High-mobility group protein B1 (HMGB1) is an inflammatory factor that can be released from keratinocyte nuclei in psoriatic lesions. We aimed to investigate the proinflammatory effect of HMGB1 on keratinocytes and the contribution of HMGB1 to psoriasis development. Normal human keratinocytes were treated with recombinant human HMGB1, and the production of inflammatory factors and the intermediary signalling pathways were examined. Furthermore, the imiquimod-induced psoriasis-like mouse model was used to investigate the role of HMGB1 in psoriasis development in vivo. A total of 11 inflammatory factors were shown to be upregulated by HMGB1 in keratinocytes, among which interleukin (IL)-18 showed the greatest change. We then found that activation of the nuclear factor-κB signalling pathway and inflammasomes accounted for HMGB1-induced IL-18 expression and secretion. Moreover, HMGB1 and downstream IL-18 contributed to the development of psoriasiform dermatitis in the imiquimod-treated mice. In addition, T-helper 17 immune response in the psoriasis-like mouse model could be inhibited by both HMGB1 and IL-18 blockade. Our findings indicate that HMGB1 secreted from keratinocytes can facilitate the production and secretion of inflammatory factors such as IL-18 in keratinocytes in an autocrine way, thus promoting the development of psoriasis. Blocking the proinflammatory function of the HMGB1-IL-18 axis may be useful for psoriasis treatment in the future.
高流动性组蛋白B1对角质形成细胞的促炎作用:牛皮癣发展的自分泌机制
银屑病是一种自身免疫性皮肤病,角质形成细胞在其发病中起重要作用。高流动性组蛋白B1 (HMGB1)是一种炎性因子,可从银屑病病灶角化细胞核中释放出来。我们的目的是研究HMGB1对角质形成细胞的促炎作用以及HMGB1在银屑病发展中的作用。用重组人HMGB1处理正常人角质形成细胞,并检测炎症因子的产生和中间信号通路。此外,采用米喹莫诱导的银屑病样小鼠模型,研究HMGB1在体内银屑病发展过程中的作用。在角质形成细胞中,HMGB1共上调了11种炎症因子,其中以白介素-18变化最大。然后我们发现核因子-κB信号通路的激活和inflammasomes占HMGB1-induced地震-表达和分泌。此外,HMGB1和下游IL-18促进了咪喹莫治疗小鼠银屑病样皮炎的发生。此外,HMGB1和IL-18阻断均能抑制银屑病样小鼠T-helper 17免疫应答。我们的研究结果表明,角质形成细胞分泌的HMGB1可促进角质形成细胞自分泌IL-18等炎症因子,从而促进银屑病的发生发展。阻断HMGB1-IL-18轴的促炎功能可能有助于今后银屑病的治疗。Proinflammatory effect of high-mobility group protein B1 on keratinocytes: an autocrine mechanism underlying psoriasis development - PubMed
https://pubmed.ncbi.nlm.nih.gov/27859256/
J Dermatology. 2017 May;
Involvement of high mobility group box-1 in imiquimod-induced psoriasis-like mice model
1Department of Dermatovenereology, Chengdu Second People's Hospital, Chengdu, China.
2Department of Dermatovenereology, West China Hospital of Sichuan University, Chengdu, China.
3Department of Dermatovenereology, Chengdu Qingbaijiang Distinct People's Hospital, Chengdu, China.
In the previous work, we have indicated that HMGB1, a pro-inflammatory cytokine, is closely associated with the pathogenesis of psoriasis. To further clarify the role of HMGB1 in the pathogenesis of psoriasis, we investigated the direct function of HMGB1 application and HMGB1 blockade in imiquimod (IMQ)-induced psoriatic mouse model in this study. Mice were treated with imiquimod (IMQ) to induce psoriasis-like inflammation, and consecutively injected with recombinant HMGB1 or phosphate-buffered saline (PBS) i.d. Abundant cytoplasmic expression of HMGB1 was observed in lesional skin from IMQ-treated skin. The injection of HMGB1 into the IMQ-treated skin further aggravated the psoriasis-like disease, enhanced the infiltration of CD3+ T cells, myeloperoxidase+ neutrophils and CD11c+ dendritic cells, increased the number of γδ T cells, and upregulated the mRNA expression of interleukin (IL)-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ and IL-17 compared with the PBS injection. Finally, by using anti-HMGB1 monoclonal antibody or HMGB1 inhibitor glycyrrhizin, we indicated that HMGB1 blockade reduced the number of γδ T cells, suppressed the mRNA expression of IL-6, TNF-α, IFN-γ and IL-17, and moderated clinical and histological evolvement in the IMQ-treated skin. Our data suggest that HMGB1 may act as a pro-inflammatory cytokine, and contribute to the development of IMQ-induced psoriasis-like inflammation. HMGB1 blockade may represent a new direction in the suppression of psoriasis.高活动性组box-1参与咪喹莫诱导的类银屑病小鼠模型
在之前的工作中,我们发现HMGB1是一种促炎细胞因子,与银屑病的发病机制密切相关。为了进一步阐明HMGB1在银屑病发病机制中的作用,本研究探讨了HMGB1的应用和HMGB1阻断在IMQ诱导的银屑病小鼠模型中的直接作用。小鼠经咪喹莫特(IMQ)诱导银屑病样炎症,并连续注射重组HMGB1或磷酸盐缓冲生理盐水(PBS) i.d。小鼠经咪喹莫特(IMQ)处理后,受损皮肤细胞质中大量表达HMGB1。HMGB1的注入到IMQ-treated皮肤进一步加剧psoriasis-like疾病,增强CD3 + T细胞的渗透,髓过氧物酶+中性粒细胞和CD11c +树突细胞,增加的数量γδT细胞和调节的mRNA表达白介素(IL) 6、肿瘤坏死因子(TNF) -α干扰素(IFN) -γ和IL-17比PBS注入。最后,通过使用抗HMGB1单克隆抗体或HMGB1抑制剂甘草酸,我们发现,HMGB1阻断降低了γδ T细胞数量,抑制了IL-6、TNF- TNF-、IFN- IL-17 mRNA的表达,并调节了imq处理皮肤的临床和组织学进展。我们的数据表明,HMGB1可能作为一种促炎细胞因子,参与了imq诱导的银屑病样炎症的发展。HMGB1阻断可能是抑制银屑病的一个新方向。Involvement of high mobility group box-1 in imiquimod-induced psoriasis-like mice model - PubMed
https://pubmed.ncbi.nlm.nih.gov/27943400/
Effects of essential fatty acids on mediators of mast cells in culture
The objective of this study was to investigate the effects of α-linolenic acid (18:3n-3) and linoleic acid (18:2n-6) on the fatty acid composition and the activity and release of mast cell mediators in the canine mastocytoma cell line C2. Cells were cultured in Dulbecco's modified Eagle's medium mixed with 50% Ham's F12 (containing linoleic acid 0.14 μM). The basic medium (DEH) was supplemented with 0.14 μM α-linolenic acid. 14.0 μM α-linolenic acid (DEH-n-3) or 14.0 μM linoleic acid (DEH-n-6) was added. Eight days after culturing of C2 in DEH-n-3 we measured elevated levels of n-3 fatty acids up to 22:3. The tryptase activity and the stimulated PGE2 production and histamine release were reduced. In contrast, after culturing of C2 in DEH-n-6 we determined elevated levels of n-6 fatty acids up to 20:3, increased tryptase activity and stimulated histamine release. Thus 18:3n-3 has anti-inflammatory effects in cultured canine mastocytoma cells.
Effects of essential fatty acids on mediators of mast cells in culture - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S095232780300022X
Arch Dermatological Research, 2010 Sep
Involvement of IL-17F via the induction of IL-6 in psoriasis
Recently, the important role of T helper 17 (Th17) cells in psoriasis has been clarified; however, the role of IL-17F produced by Th17 cells is still not fully understood. IL-6 exhibits multiple biologic functions, such as regulation of immunological responses including those in psoriatic reactions. Therefore, we examined the production of IL-6 protein in normal human epidermal keratinocytes (NHEKs) stimulated by IL-17F, TNF-alpha, IL-17A, and IL-17A in combination with TNF-alpha, and PBS control. We then examined the expression of IL-6 mRNA in mouse skin after intradermal injection of IL-17F. Finally, IL-17F expression in skin biopsy specimens from psoriasis patients was examined by immunohistochemistry. The results showed that IL-17F induced production of IL-6 in NHEKs in a time-dependent manner. This could be attenuated by chimeric inhibitor blocking the IL-17 receptor. The amounts of IL-6 stimulated by IL-17F were much higher than those stimulated by TNF-alpha or IL-17A. IL-6 was also significantly upregulated via synergistic stimulation with IL-17A plus TNF-alpha. The expression of IL-6 mRNA 24 h after IL-17F injection in the mouse skin was 3.2-fold higher than that in the control group. Immunohistochemistry of inflammatory cells in the dermis demonstrated a large number of CD4(+) T cells showing IL-17F positivity in psoriatic skin lesions, but few or none in non-lesional psoriatic skin. Our results indicate that IL-17F produced by CD4(+) T cells causes the inflammation in psoriasis partly through induction of IL-6 in keratinocytes.IL-17F通过IL-6诱导参与银屑病
近年来,T辅助体17 (Th17)细胞在银屑病中的重要作用已被阐明;然而,Th17细胞产生的IL-17F的作用仍未完全了解。IL-6具有多种生物学功能,如调节免疫反应,包括银屑病反应。因此,我们检测了IL-17F、tnf -α、IL-17A和IL-17A联合tnf -α和PBS对照下正常人表皮角质形成细胞(NHEKs)中IL-6蛋白的产生。然后我们检测了IL-17F皮内注射后小鼠皮肤中IL-6 mRNA的表达。最后用免疫组化方法检测银屑病患者皮肤活检标本中IL-17F的表达。结果表明,IL-17F诱导NHEKs细胞产生IL-6,并呈时间依赖性。这可以通过阻断IL-17受体的嵌合抑制剂来减弱。IL-17F刺激的IL-6含量明显高于tnf -α和IL-17A刺激的IL-6含量。IL-6也通过IL-17A和tnf -α的协同刺激显著上调。IL-17F注射后24 h小鼠皮肤组织中IL-6 mRNA表达量比对照组高3.2倍。真皮炎症细胞免疫组化显示,银屑病皮损中CD4(+) T细胞大量,IL-17F呈阳性,非皮损皮损中IL-17F呈阴性或阴性。我们的研究结果表明,CD4(+) T细胞产生的IL-17F在一定程度上是通过诱导角质形成细胞中的IL-6而引起银屑病炎症的。Involvement of IL-17F via the induction of IL-6 in psoriasis - PubMed
https://pubmed.ncbi.nlm.nih.gov/20148256/
Clin Exp Immunology. 2020 Aug;
IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa
1School of Biochemistry and Immunology, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin, Ireland.
2School of Medicine, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin, Ireland.
Abstract
The skin is one of the most important organs in the body, providing integrity and acting as a barrier to exclude microbes, allergens and chemicals. However, chronic skin inflammation can result when barrier function is defective and immune responses are dysregulated or misdirected against harmless or self-antigens. During the last 15 years interleukin (IL)-17 cytokines have emerged as key players in multiple inflammatory disorders, and they appear to be especially prominent in skin inflammation. IL-17 cytokines produced by T cells and other cell types potently activate keratinocytes to promote inflammation in a feed-forward loop. Given this key pathogenic role of the IL-17 pathway in autoimmune and inflammatory disease, it has been the focus of intense efforts to target therapeutically. The inflammatory effects of IL-17 can be targeted directly by blocking the cytokine or its receptor, or indirectly by blocking cytokines upstream of IL-17-producing cells. Psoriasis has been the major success story for anti-IL-17 drugs, where they have proven more effective than in other indications. Hidradenitis suppurativa (HS) is another inflammatory skin disease which, despite carrying a higher burden than psoriasis, is poorly recognized and under-diagnosed, and current treatment options are inadequate. Recently, a key role for the IL-17 pathway in the pathogenesis of HS has emerged, prompting clinical trials with a variety of IL-17 inhibitors. In this review, we discuss the roles of IL-17A, IL-17F and IL-17C in psoriasis and HS and the strategies taken to target the IL-17 pathway therapeutically.
Keywords: IL-17; hidradenitis suppurativa; psoriasis.
© 2020 British Society for Immunology.IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa - PubMed
https://pubmed.ncbi.nlm.nih.gov/32379344/
IL-6 Signaling in Myelomonocytic Cells Is Not Crucial for the Development of IMQ-Induced Psoriasis
1 Institute for Molecular Medicine, University Medical Center of the Johannes-Gutenberg University of Mainz, Mainz, Germany
2. Max Planck Institute for Metabolism Research, Cologne, Germany
Psoriasis is an autoimmune skin disease that is associated with aberrant activity of immune cells and keratinocytes. In mice, topical application of TLR7/8 agonist IMQ leads to a skin disorder resembling human psoriasis. Recently, it was shown that the IL-23/ IL-17 axis plays a deciding role in the pathogenesis of human psoriasis, as well as in the mouse model of IMQ-induced psoriasis-like skin disease. A consequence of IL-17A production in the skin includes increased expression and production of IL-6, resulting in the recruitment of neutrophils and other myelomonocytic cells to the site of inflammation. To further investigate and characterize the exact role of IL-6 signaling in myelomonocytic cells during experimental psoriasis, we generated mice lacking the IL-6 receptor alpha specifically in myelomonocytic cells (IL-6RαΔmyel). Surprisingly, disease susceptibility of these mice was not affected in this model. Our study shows that classical IL-6 signaling in myelomonocytic cells does not play an essential role for disease development of IMQ-induced psoriasis-like skin disease.IL-6 Signaling in Myelomonocytic Cells Is Not Crucial for the Development of IMQ-Induced Psoriasis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4801375/
Dendritic cells and atopic eczema/dermatitis syndrome
Bubnoff, Dagmar von; Koch, Susanne; Bieber, ThomasAuthor Information
Current Opinion in Allergy and Clinical Immunology: October 2003 -
Abstract
Purpose of review
The manifestation of atopic eczema/dermatitis syndrome is believed to result from a complex interrelationship of environmental factors, pharmacological abnormalities, skin barrier defects, and immunological phenomena. Although we are only beginning to understand the molecular basis of this disease, much progress has been made in defining key events leading to the manifestation of allergic inflammation. Here, we review recent findings that underscore the importance of dendritic cells as being central to shape these proinflammatory responses.
Recent findings
Evidence for a differential regulation of the high affinity receptor for IgE, FcεRI, on the surface of atopic dendritic cells compared with non-atopic dendritic cells became apparent. In atopic donors, in contrast to non-atopic donors, the intracellular expression of the γ-chains of FcεRI is sufficient, thus leading to the assembly with the α-chain and surface expression of the receptor. This finding is of considerable interest for an understanding of the pathophysiology of IgE-mediated dendritic cell functions in atopic eczema/dermatitis syndrome. In addition, it has been shown that keratinocytes from the epidermal skin of individuals with atopic eczema/dermatitis syndrome express human thymic stromal lymphopoietin, which activates dendritic cells to attract T helper type 2 cells into the skin. Furthermore, these activated dendritic cells prime naïve T cells into T helper type 2 cells.
Summary
The past few years have seen a remarkable process of refocusing in atopy. Dendritic cells in particular have been at the centre of this process. It has become unequivocally clear that these cells have the power of shaping the allergic response.Dendritic cells and atopic eczema/dermatitis syndrome : Current Opinion in Allergy and Clinical Immunology
https://journals.lww.com/co-allergy/Abstract/2003/10000/Dendritic_cells_and_atopic_eczema_dermatitis.6.aspx
Lecithin Is A Pretty Powerful Psoriasis Cure And Fatty ...
Lecithin was known to help in some cases of psoriasis decades ago. Here is a paper “LECITHIN FEEDING IN THE SYNDROME OF PSORIASIS” from 1942. Prevalence of fatty liver in psoriatics Those who still don’t get it and omit the liver function as one of the major causes of psoriasis should read the next lines really carefully.
https://www.psoriasisdietplan.com/2017/11/lecithin...
Lecithin Treatment for Psoriasis
https://www.earthclinic.com/lecithin-treatment-for-psoriasis.html
Jan 04, 2021 · I had psoriasis. (for decades) and the only thing that would clear it up was lecithin. When I first learned of it, I found gel caps that I was taking (1gram) 3x a day, it did start going away, and I looked for other sources, and found lecithin granules which I started taking 1tbl …
Effect of all-trans-retinoic acid on the differentiation, maturation and functions of dendritic cells derived from cord blood monocytes
Mature dendritic cells exhibit reduced phagocytic activity and increased expression of major histocompatibility complex (MHC) and costimulatory molecules, and they secrete large amounts of immune cytokines to stimulate a primary cell-mediated immune response. Moreover, dendritic cells are critical in shaping the emerging response, thereby controlling the course of infection (Moll, 2003). Dendritic cells can also tune the immune response by modulating either the amplitude or the class of the response.
Dendritic cells are the most potent of the antigen-presenting cells, and their interaction with T cells is a key event in the early stages of a primary immune response. Because dendritic cells have the unique ability to activate naive T cells and are required for inducing a primary response (Liu, 2001), they are important regulators of the immune functions in newborns. Dendritic cells can now be differentiated from peripheral blood monocytes by in vitro culture with GM-CSF and IL-4. These cultured dendritic cells show functional and phenotypic characteristics typical of the immature stage of differentiation and can be further differentiated in vitro into mature dendritic cells with TNF-α, lipopolysaccharide, IL-1, or CD40L (Chapuis et al., 1997).
Because immature dendritic cells are efficient in antigen capture and can use several pathways, such as macropinocytosis or phagocytosis of viruses, bacteria, apoptotic and necrotic cell fragments, as well as intracellular parasites, their immature status may contribute to antigen capture of anti-infection immunity. However, the evidence shows that dendritic cells not only initiate T-cell responses but are also involved in silencing T-cell immune responses. The functional activities of dendritic cells depend mainly on their state of activation and differentiation; that is, terminally differentiated mature dendritic cells can efficiently induce the development of T-effecter cells, whereas immature dendritic cells are involved in maintaining peripheral tolerance (Mahnke et al., 2002).
The interaction of dendritic cells with T cells is important in directing T-helper cell differentiation (Kalinski et al., 1999) and in determining the resulting cytokine production profile and effecter function. The production of IL-12 by dendritic cells directs Th1 differentiation, whereas IL-4 mediates Th2 cell differentiation. Moreover, IL-10 was recently shown to skew T-cell responses toward T regulatory cells that produce high levels of IL-10 and inhibit antigen-specific T-cell responses (Corinti et al., 2001). The present study indicates that atRA can decrease IL-12 synthesis by dendritic cells in mRNA and protein levels; this is followed by a decrease in IFN-γ mRNA and protein, but an increase in IL-10 production and IL-4 gene transcription. Thus, atRA-treated dendritic cells bias the immune response in a Th2 direction, a fact that supports robust antibody-mediated immunity.Vitamin A deficiency increases the mortality of infants with severe infectious disease (Fitch & Neville, 2002). The main reason may be that vitamin A deficiency impairs innate immunity by impeding normal regeneration of mucosal barriers damaged by infection and by diminishing the function of neutrophils, macrophages and natural killer cells. In particular, vitamin A deficiency diminishes antibody-mediated responses directed by Th2 cells, although some aspects of Th1-mediated immunity are also diminished (Stephensen, 2001). Those changes can be restored with vitamin A supplementation. Our study indicates that vitamin A has a direct function on dendritic cells and provides another explanation for the benefit of physiological concentrations of vitamin A on anti-infectious immune responses.
In conclusion, cord blood dendritic cells appear to be targets for atRA, as indicated by the effects of atRA on their differentiation, maturation and function. In addition, atRA-treated dendritic cells can inhibit IL-12 while enhancing IL-10 production, therefore driving T-helper responses toward Th2. Thus, the mechanism of atRA-regulated immune functions is not limited to acting on immune effector cells and T and B lymphocytes but also acts on antigen-presenting cells. All-trans-retinoic acid may act at the very first step of the neonate immune response by modulating dendritic cells. Retinoic acid receptor α may be important in regulating atRA effects on dendritic cells.
Effect of all-trans-retinoic acid on the differentiation, maturation and functions of dendritic cells derived from cord blood monocytes | Pathogens and Disease | Oxford Academic
https://academic.oup.com/femspd/article/47/3/444/508056
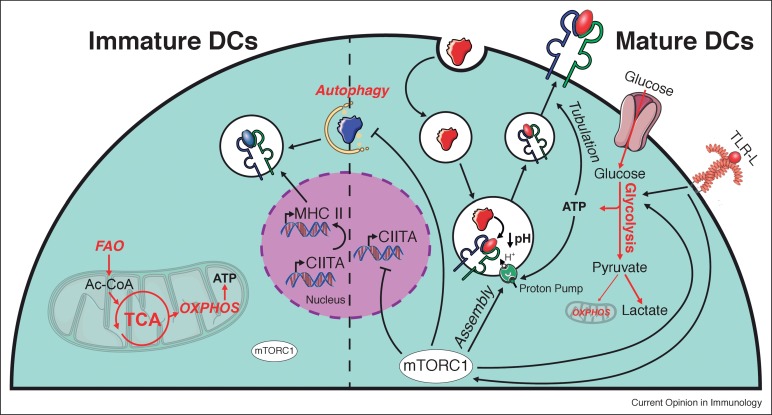
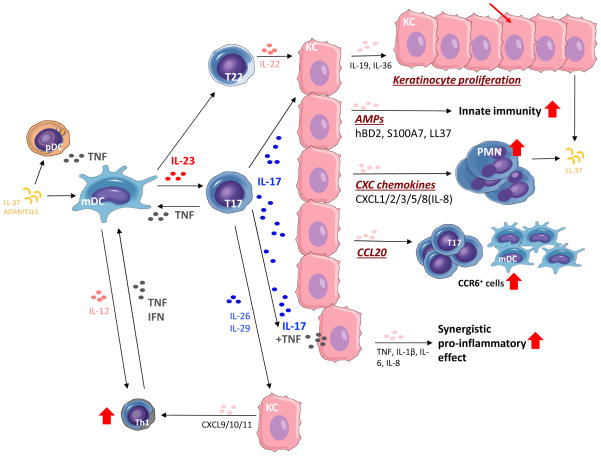
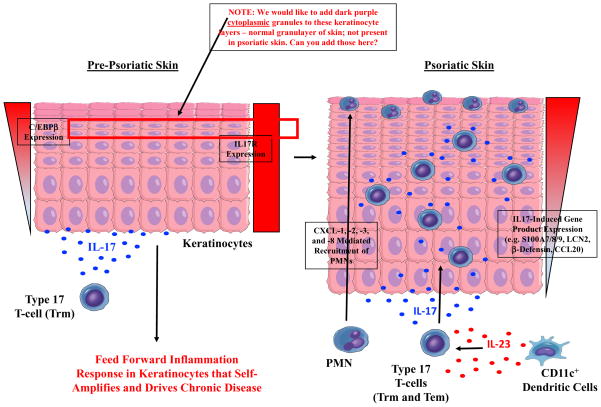



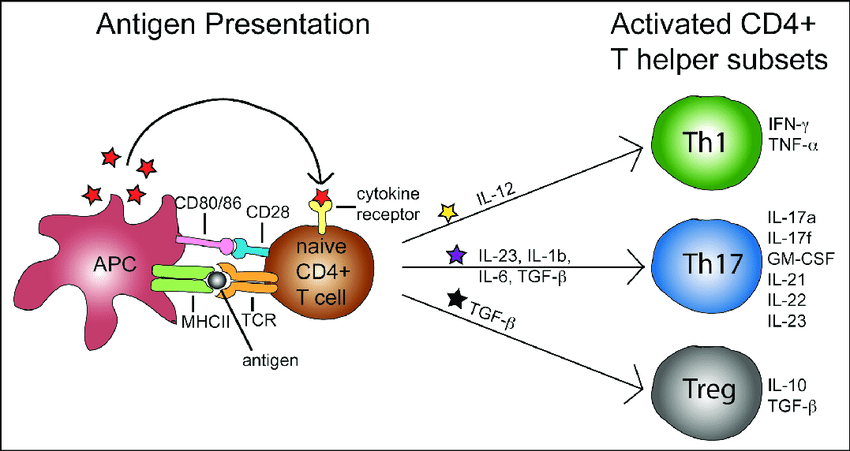
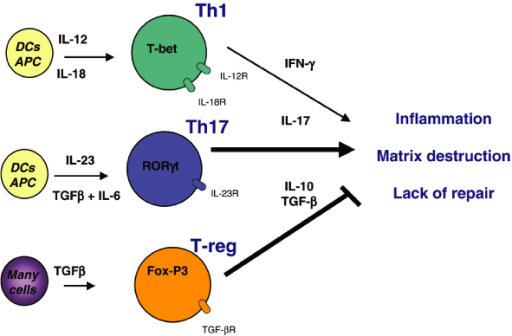
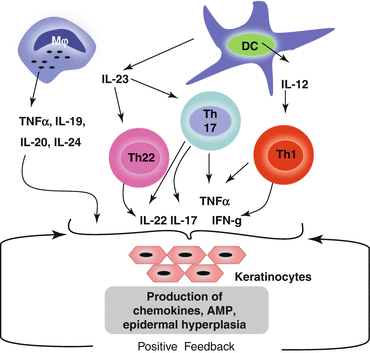
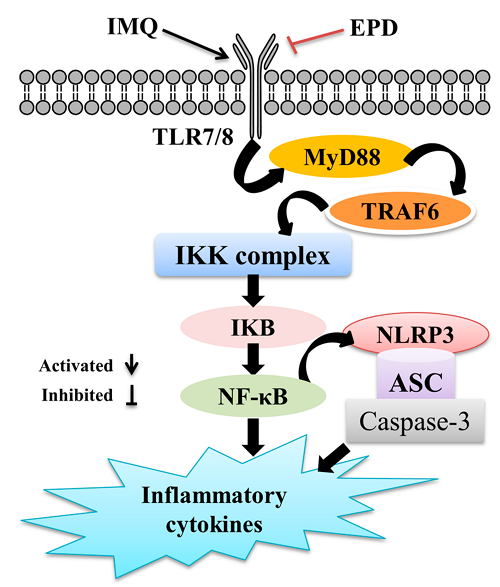
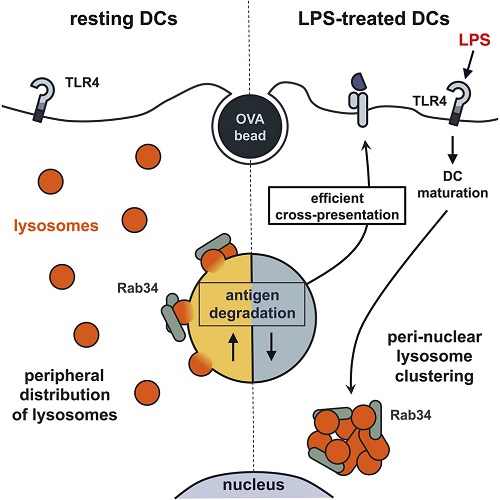
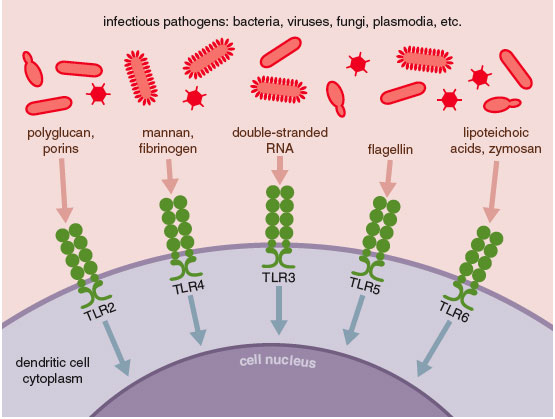
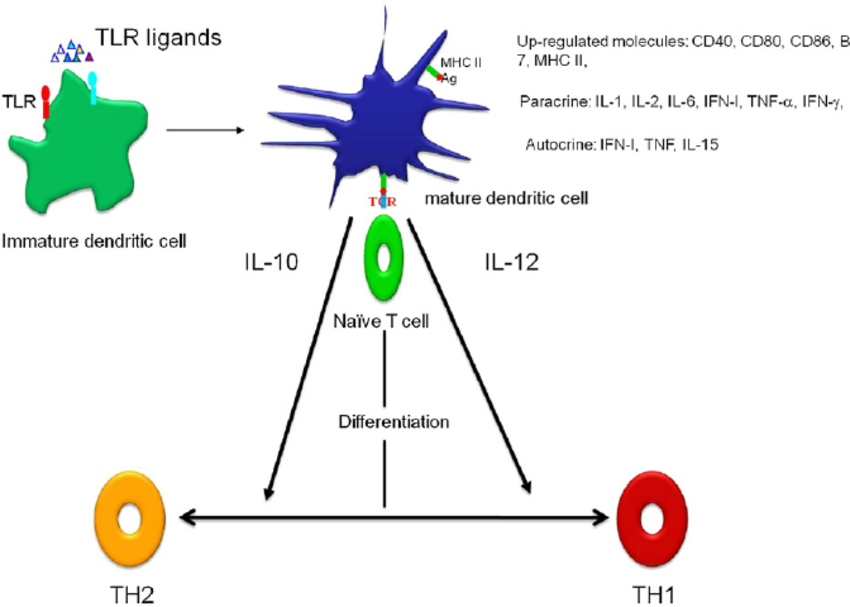
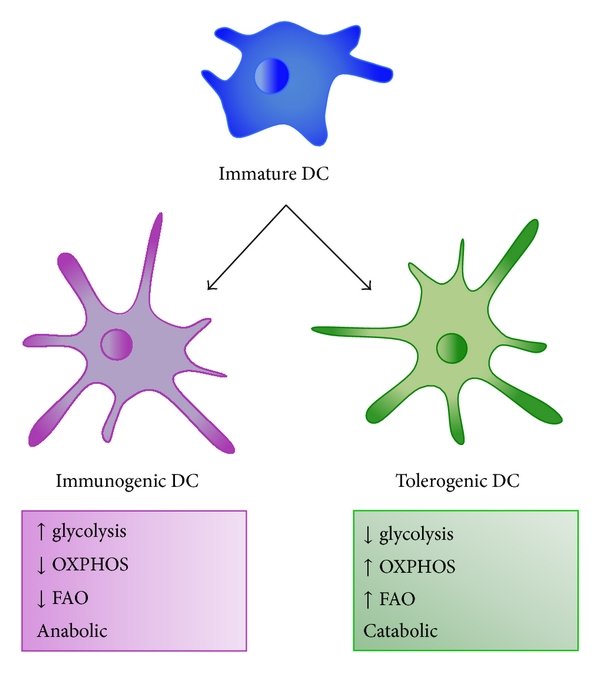
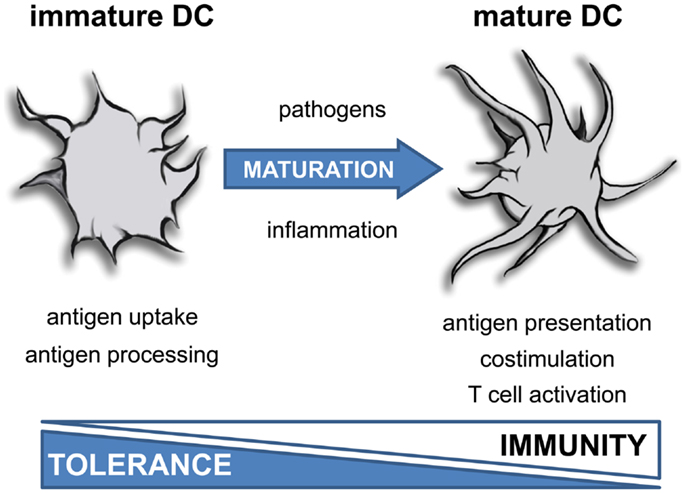
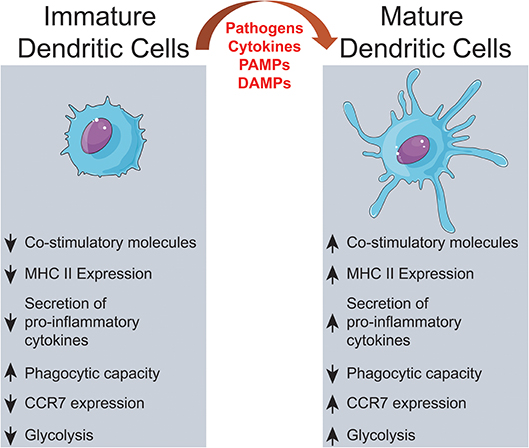
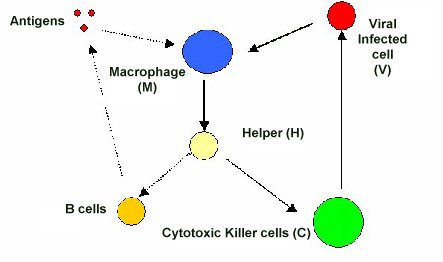

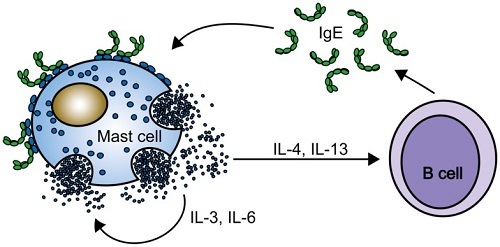
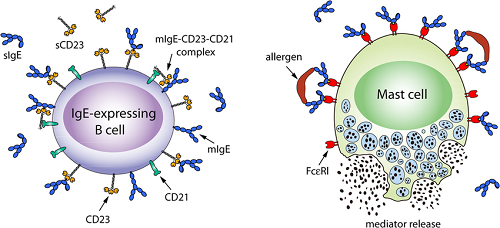


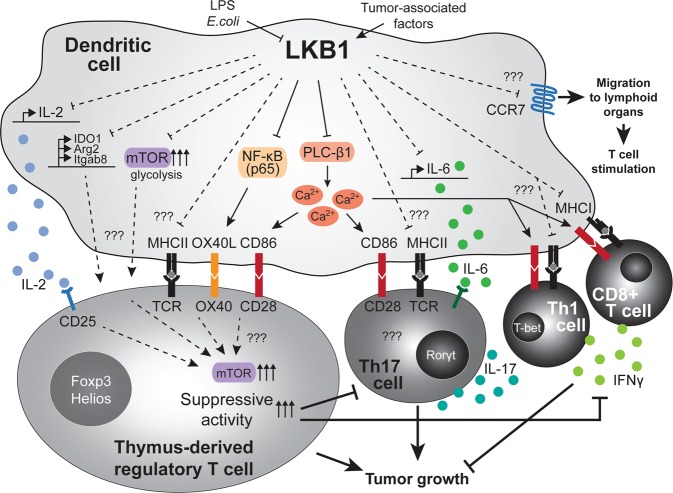
.jpg)