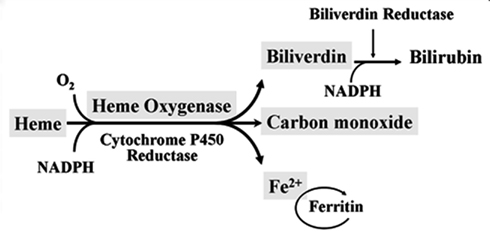
Degradation of Heme and Roles of Heme Oxygenase
Iron and heme metabolism
Distribition of Heme oxygenases:expressed ubiquitously in the body
Inducers;nitric oxide(arginine, nitrate, nitrite in veg), heme, vitamin c and stresses,curcumin and resveratrol,aged garlic extract,ginger extract,gingerols and shogaol-6
conditions: present of adquate oxygen
Funcitions:vasodilation, antioxidant, antiinflammatory, antiapoptotic, antiproliferative,anti-coagulation, and immunomodulatory effects
indications of HO-1/HO-2 inducers:viral/bacterial infections, and gastrointestinal,heart, kidney,lung, liver brain diseases, including fibrotic diseases
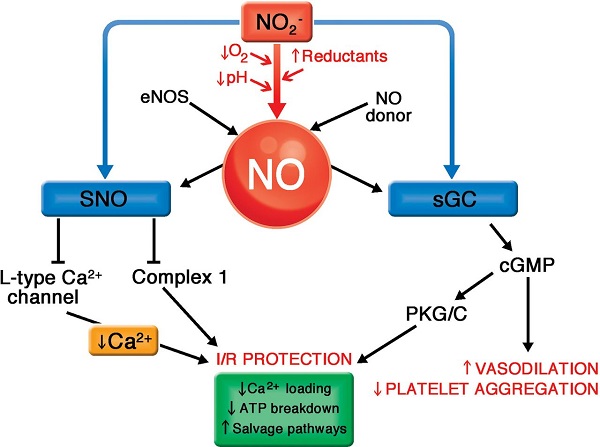
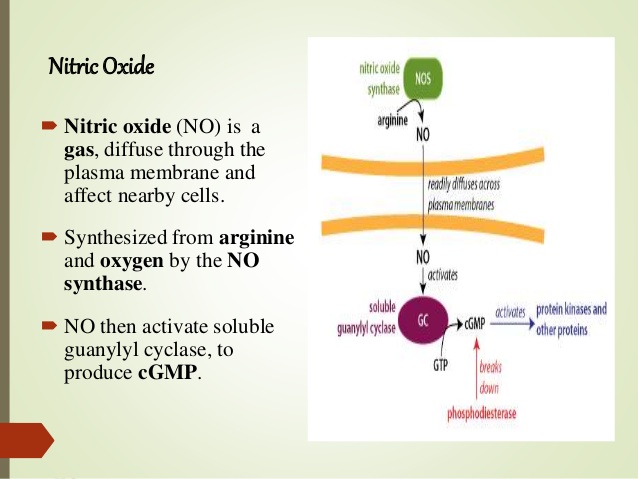
Guanylyl cyclase: 鸟苷酸环化酶
http://content.onlinejacc.org/article.aspx?articleid=1685123
https://www.researchgate.net/figure/The-illustration-of-the-NO-effect-mediated-by-cGMP-GC-guanylyl-cyclase-GC-A-GC-B_fig1_7317265
Arch Biochem Biophys. 2014 Dec 15;564:83-8. doi: 10.1016/j.abb.2014.09.005. Epub 2014 Sep 18.
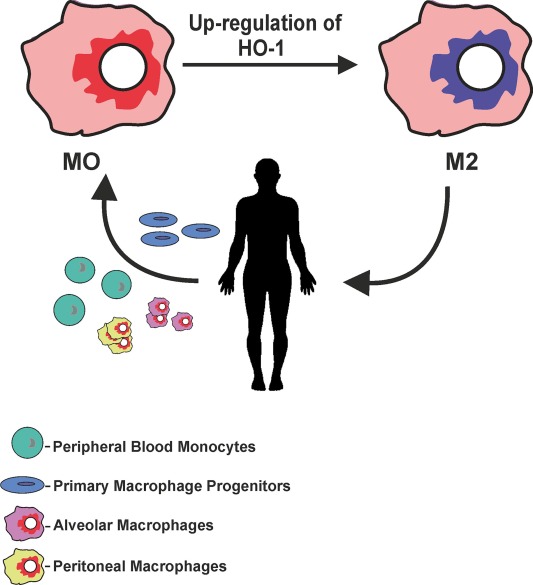
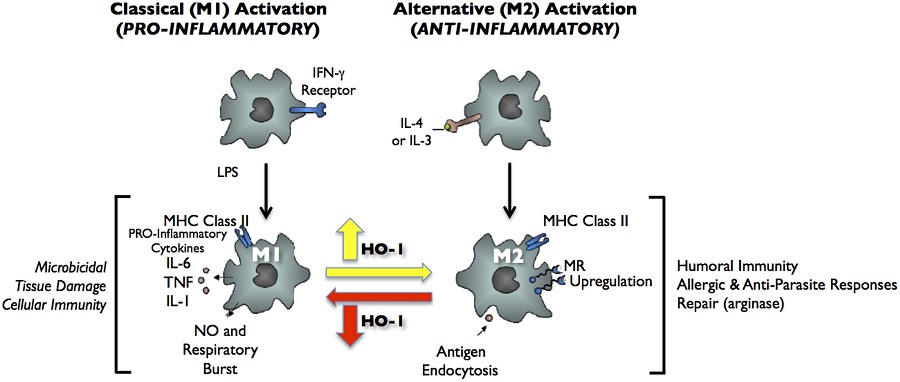
Heme oxygenase-1 and anti-inflammatory M2 macrophages.
Naito Y1, Takagi T2, Higashimura Y3.
Author information
1 Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan. Electronic address: ynaito@koto.kpu-m.ac.jp.
2 Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan.
3 Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan; Department of Food Factor Science, Kyoto Prefectural University of Medicine, Kyoto, Kyoto 602-8566, Japan.
Abstract
Heme oxygenase-1 (HO-1) catalyzes the first and rate-limiting enzymatic step of heme degradation and produces carbon monoxide, free iron, and biliverdin. HO-1, a stress-inducible protein, is induced by various oxidative and inflammatory signals. Consequently, HO-1 expression has been regarded as an adaptive cellular response against inflammatory response and oxidative injury. Although several transcriptional factors and signaling cascades are involved in HO-1 regulation, the two main pathways of Nrf2/Bach1 system and IL-10/HO-1 axis exist in monocyte/macrophage.Macrophages are broadly divisible into two groups: pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages. More recently, several novel macrophage subsets have been identified including Mhem, Mox, and M4 macrophages. Of these, M2 macrophages, Mhem, and Mox are HO-1 highly expressing macrophages. HO-1 has been recognized as having major immunomodulatory and anti-inflammatory properties, which have been demonstrated in HO-1 deficient mice and human cases of genetic HO-1 deficiency. However, the mechanism underlying the immunomodulatory actions of HO-1 remains poorly defined. This review specifically addresses macrophage polarization. The present current evidence indicates that HO-1 induction mediated by multiple pathways can drive the phenotypic shift to M2 macrophages and suggests that HO-1 induction in macrophages is a potential therapeutic approach to immunomodulation in widely diverse human diseases.
Copyright © 2014 Elsevier Inc. All rights reserved.
KEYWORDS:
Heme oxygenase; Inflammation; Macrophage; PolarizationHeme oxygenase-1 and anti-inflammatory M2 macrophages. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/25241054
FIGURE 2. Classical activation (M1) and alternative activation (M2) of macrophages. Classical activation is mediated by the priming stimulus IFN-γ, followed by a microbial trigger (lipopolysaccharide, LPS). Alternative activation is mediated by IL- 4 or IL-13. The uptake of apoptotic cells or lysosomal storage of host molecules generates anti-inflammatory responses. Cytokines (IL-10, TGF-β, IFN-α/β) are potent modulators of activation.
Frontiers | Heme oxygenase-1 in pregnancy and cancer: similarities in cellular invasion, cytoprotection, angiogenesis, and immunomodulation | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2014.00295/full
Heme degradation
Functions of heme
Redox chemistry
electron transport: cytochromes in the respiratory chain
enzyme catalysis: cytochrome P450, cyclooxygenase, others
Reversible binding of gases
O2: hemoglobin and myoglobin (80–90% of all heme)
NO: guanylate cyclase
In hemoglobin and myoglobin, the heme iron remains in the ferrous or Fe2+ state throughout the cycle of oxygen binding and release. In redox-active enzymes and in the respiratory chain, heme regularly goes back and forth between the ferrous and the ferric or Fe3+ states, and sometimes also the ferryl or Fe4+ state.
In cytochrome P450 enzymes, the function of heme is to facilitate the formation of reactive oxygen (see slide 19.2). Similarly, formation of reactive oxygen species (ROS) may also occur as a side reaction wherever heme functions in transport of oxygen or of electrons, and protective mechanisms are required to contain damage by ROS. In hemoglobin, molecular oxygen (O2) may abscond with an extra electron and thereby turn itself into superoxide (O2− •), while the heme iron is left one electron short. Hemoglobin that has lost one electron is called methemoglobin; it is unable to carry oxygen. Its iron is reduced to the ferrous form again by an NADH-dependent enzyme, methemoglobin reductase.102
Protective mechanisms that scavenge reactive oxygen species include the enzymes superoxide dismutase and catalase, as well as antioxidants such as tocopherol (vitamin E; see slide 18.7.11) and ascorbic acid (vitamin C). Organisms that lack such protective mechanisms are anaerobic, that is, they survive only in the absence of oxygen. Anaerobic organisms occur among both prokaryotes and eukaryotes.
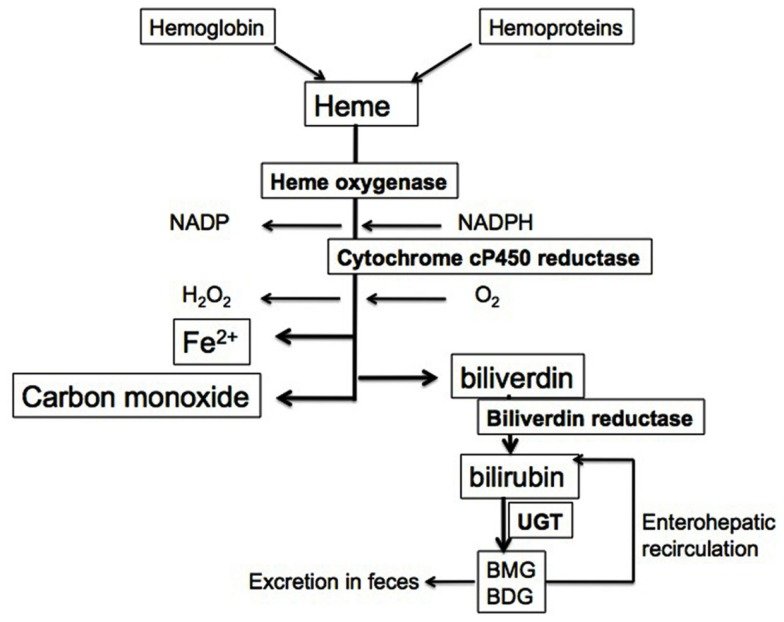
Red blood cells have a regular lifespan of 120 days (although it can be considerably shorter in some diseases). At the end of this lifespan, they are captured and ingested by phagocytes in the spleen and the liver. When the globin protein is proteolytically degraded, heme is released. Heme itself undergoes degradation mostly in the liver. Ring cleavage by heme oxygenase produces biliverdin, which is in turn reduced to bilirubin. Some bilirubin is excreted into the bile as such; however, the greater share is first conjugated with glucuronic acid by UDP-glucuronosyltransferase, form 1A1, and excreted thereafter. The major transport protein responsible for excretion of the diglucuronide is an ABC transporter (ABCC2), the same one that also secretes bile acids (see slide 11.5.3).
In the large intestine, part of the conjugated bilirubin undergoes deconjugation by bacterial β-glucuronidases. In the anaerobic environment that prevails inside the colon, the released bilirubin subsequently undergoes reduction, again by bacterial enzymes, to variously colored pigments that produce the stool color. Another reduction product, urobilinogen, is taken up and excreted with the urine, causing the yellow color of the latter.Is CO a signaling molecule, like NO?
Nitric oxide is an important signaling molecule. As a small molecule, it can easily diffuse out of one cell and into another. Inside the target cell, it binds to soluble guanylate cyclase (sGC). Interestingly, the NO-binding site on sGC is a heme molecule. Binding of NO to one face of the heme releases a histidine side chain on the other, which causes a conformational change and activation of the sGC molecule.
In vitro experiments show that CO can also bind and activate sGC. It has therefore been proposed that heme oxygenase, which produces CO, has a regulatory role in addition to its metabolic one. However, while this idea has been around for awhile, I have not come across solid evidence that supports a significant signaling role of CO in vivo.Iron uptake, transport and storage
uptake in the small intestine: Fe2+—free or bound to heme
transient storage as ferritin inside the intestinal epithelia
transport in the blood: Fe3+—bound to transferrin with very high affinity
cellular uptake: endocytosis of transferrin, release of iron in acidic endosome
storage: intracellular ferritin particles
depletion: scaled-off cells, blood loss, breast milk
Only ferrous iron (Fe2+) can be taken up from the small intestine, so that is what we give to patients. Still, during passage through the intestine, much of the ferrous iron is oxidized to the ferric form (Fe3+), which means that intestinal uptake is not very efficient.
The very high affinity of transferrin to iron means that there is practically no free iron in the blood serum. Bacteria, like human cells, require iron for growth; therefore, keeping free iron very low is an important non-specific immune mechanism. Many pathogenic bacteria produce their own high-affinity iron-binding molecules (siderophores) to acquire iron within this iron-depleted environment.In chronic infections, free iron in the serum is reduced even below the normal low value, presumably in an attempt by the immune system to starve microbial pathogens of iron. The same also happens in tumor patients and non-infectious inflammatory diseases such as rheumatism, since the immune system is not smart enough to tell the difference between these and infections. As a result, an anemia develops in which the blood cells look just like the ones in true iron depletion. However, in contrast to the latter, the level of cellular storage iron will be increased in this case—iron is not lacking, it is just being kept out of circulation. If iron sequestration is observed in a patient, one must search for the underlying disease that causes it.
Loss of iron occurs with cells being scaled off from the skin and intestinal epithelia, with blood loss (menstruation, blood donations), with diaplacental transfer to a growing fetus, and with breast milk. Mothers who have many children within a relatively short period of time have a good chance to incur iron depletion, so this is something to watch out for as a physician.Iron and heme metabolism
http://watcut.uwaterloo.ca/webnotes/Metabolism/Iron.html
Toxicology 2005
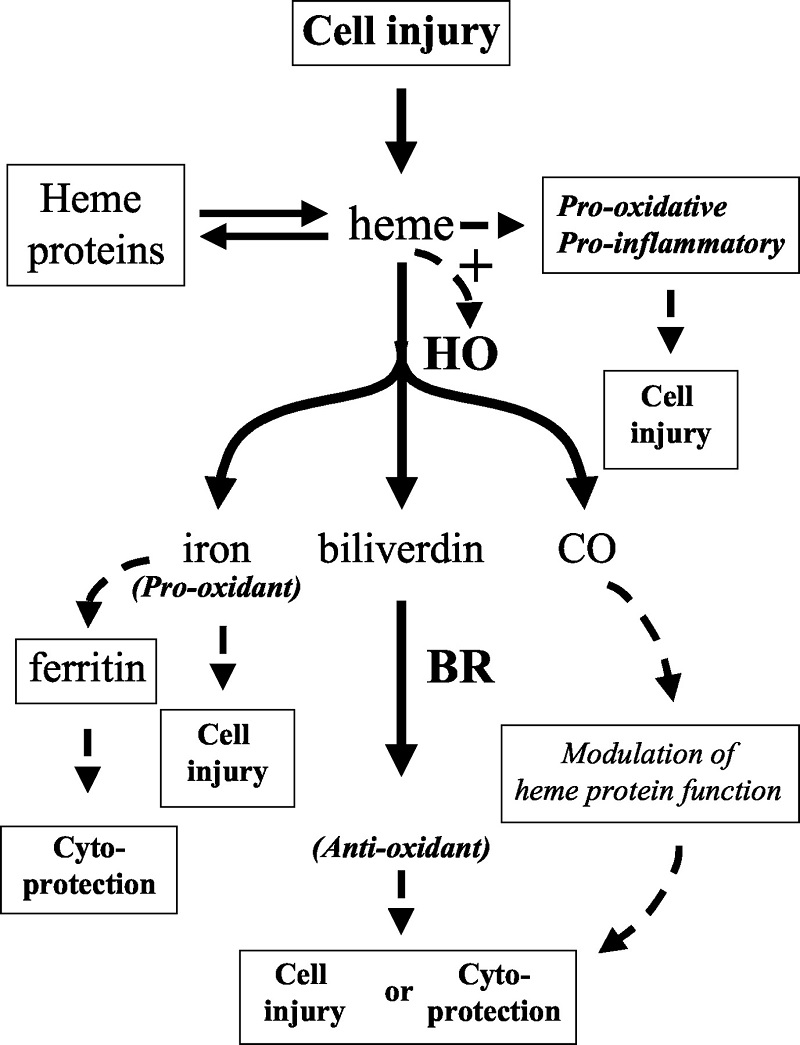
Free heme toxicity and its detoxification systems in human
Author links open overlay panelSanjayKumarUdayBandyopadhyay
Division of Drug Target Discovery and Development, Central Drug Research Institute, Chatter Manzil Palace, Mahatma Gandhi Marg, Lucknow 226001, Uttar Pradesh, India
Abstract
Severe hemolysis or myolysis occurring during pathological states, such as sickle cell disease, ischemia reperfusion, and malaria results in high levels of free heme, causing undesirable toxicity leading to organ, tissue, and cellular injury.Free heme catalyzes the oxidation, covalent cross-linking and aggregate formation of protein and its degradation to small peptides. It also catalyzes the formation of cytotoxic lipid peroxide via lipid peroxidation and damages DNA through oxidative stress. Heme being a lipophilic molecule intercalates in the membrane and impairs lipid bilayers and organelles, such as mitochondria and nuclei, and destabilizes the cytoskeleton. Heme is a potent hemolytic agent and alters the conformation of cytoskeletal protein in red cells. Free heme causes endothelial cell injury, leading to vascular inflammatory disorders and stimulates the expression of intracellular adhesion molecules. Heme acts as a pro-inflammatory molecule and heme-induced inflammation is involved in the pathology of diverse conditions; such as renal failure, arteriosclerosis, and complications after artificial blood transfusion, peritoneal endometriosis, and heart transplant failure. Heme offers severe toxic effects to kidney, liver, central nervous system and cardiac tissue. Although heme oxygenase is primarily responsible to detoxify free heme but other extra heme oxygenase systems also play a significant role to detoxify heme. A brief account of free heme toxicity and its detoxification systems along with mechanistic details are presented.
Free heme toxicity and its detoxification systems in human - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0378427405000883
Vascular Endothelium Survives (and Dies) in an Iron-Rich Environment
József Balla, Gregory M. Vercellotti, Viktória Jeney, Akihiro Yachie, Zsuzsa Varga, Harry S. Jacob, John W. Eaton, and György Balla
Published Online:2 Nov 2007https://doi.org/10.1089/ars.2007.1787
Abstract
Iron-derived reactive oxygen species are involved in the pathogenesis of numerous vascular disorders. One abundant source of redox active iron is heme, which is inherently dangerous when it escapes from its physiologic sites. Here, we present a review of the nature of heme-mediated cytotoxicity and of the strategies by which endothelium manages to protect itself from this clear and present danger.Of all sites in the body, the endothelium may be at greatest risk of exposure to heme. Heme greatly potentiates endothelial cell killing mediated by leukocytes and other sources of reactive oxygen. Heme also promotes the conversion of low-density lipoprotein to cytotoxic oxidized products. Hemoglobin in plasma, when oxidized, transfers heme to endothelium and lipoprotein, thereby enhancing susceptibility to oxidant-mediated injury. As a defense against such stress, endothelial cells upregulate heme oxygenase-1 and ferritin.
Heme oxygenase opens the porphyrin ring, producing biliverdin, carbon monoxide, and a most dangerous product—redox active iron. The latter can be effectively controlled by ferritin via sequestration and ferroxidase activity. These homeostatic adjustments have been shown to be effective in the protection of endothelium against the damaging effects of heme and oxidants; lack of adaptation in an iron-rich environment led to extensive endothelial damage in humans.
Heme, Heme Oxygenase, and Ferritin: How the Vascular Endothelium Survives (and Dies) in an Iron-Rich Environment | Antioxidants & Redox Signaling
https://www.liebertpub.com/doi/10.1089/ars.2007.1787
Front. Pharmacol., 19 July 2012 | https://doi.org/10.3389/fphar.2012.00119
Heme oxygenase-1, oxidation, inflammation, and atherosclerosis
Jesus A. Araujo*, Min Zhang and Fen Yin
Division of Cardiology, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA, USA
Atherosclerosis is an inflammatory process of the vascular wall characterized by the infiltration of lipids and inflammatory cells. Oxidative modifications of infiltrating low-density lipoproteins and induction of oxidative stress play a major role in lipid retention in the vascular wall, uptake by macrophages and generation of foam cells, a hallmark of this disorder. The vasculature has a plethora of protective resources against oxidation and inflammation, many of them regulated by the Nrf2 transcription factor. Heme oxygenase-1 (HO-1) is a Nrf2-regulated gene that plays a critical role in the prevention of vascular inflammation. It is the inducible isoform of HO, responsible for the oxidative cleavage of heme groups leading to the generation of biliverdin, carbon monoxide, and release of ferrous iron. HO-1 has important antioxidant, antiinflammatory, antiapoptotic, antiproliferative, and immunomodulatory effects in vascular cells, most of which play a significant role in the protection against atherogenesis. HO-1 may also be an important feature in macrophage differentiation and polarization to certain subtypes. The biological effects of HO-1 are largely attributable to its enzymatic activity, which can be conceived as a system with three arms of action, corresponding to its three enzymatic byproducts. HO-1 mediated vascular protection may be due to a combination of systemic and vascular local effects. It is usually expressed at low levels but can be highly upregulated in the presence of several proatherogenic stimuli. The HO-1 system is amenable for use in the development of new therapies, some of them currently under experimental and clinical trials. Interestingly, in contrast to the HO-1 antiatherogenic actions, the expression of its transcriptional regulator Nrf2 leads to proatherogenic effects instead. This suggests that a potential intervention on HO-1 or its byproducts may need to take into account any potential alteration in the status of Nrf2 activation. This article reviews the available evidence that supports the antiatherogenic role of HO-1 as well as the potential pathways and mechanisms mediating vascular protection.
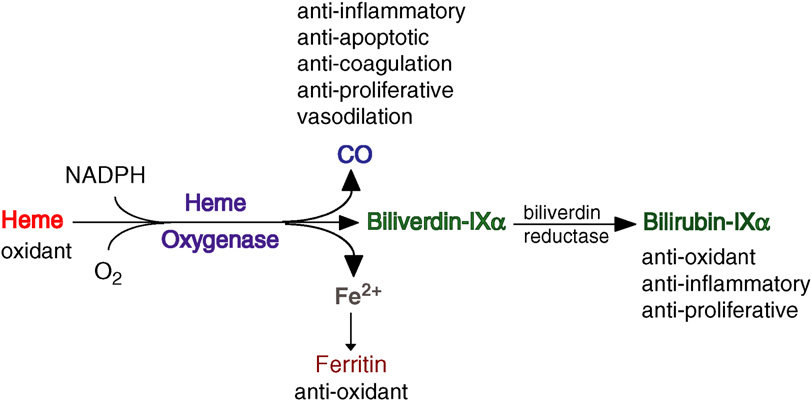
Heme Oxygenase-1 and Vascular Inflammation: Importance of Basal Levels
Heme oxygenase (HO) is a rate-limiting enzyme in the catabolism of heme. In association with cytochrome P450 reductase and in the presence of NADPH and three molecules of molecular oxygen (O2) per heme molecule, it catalyzes the oxidative cleavage of heme (Fe-protoporphyrin-IX) to render equimolar amounts of biliverdin, ferrous iron (Fe2+), and carbon monoxide (CO; Maines, 1997; Figure 1). Biliverdin can then be converted to bilirubin by the cytosolic enzyme biliverdin reductase (BVR). There are three HO isoforms (HO-1, HO-2, HO-3) that have been described (Siow et al., 1999), although HO-3 may be a pseudogene derived from HO-2 transcripts (Hayashi et al., 2004). While HO-2 is constitutively expressed, HO-1 is normally expressed at low levels in most tissues but it is highly inducible by a variety of stimuli. Indeed, HO-1 may be among the most critical cytoprotective mechanisms that are activated during times of cellular stress such as inflammation, ischemia, hypoxia, hyperoxia, hyperthermia, or radiation (Choi and Alam, 1996). It is thought to play a key role in maintaining antioxidant/oxidant homeostasis and in the prevention against vascular injury (Abraham and Kappas, 2008).Macrophages, Dendritic Cells, and Lymphocytes
Macrophages are the main inflammatory cells that infiltrate the vascular wall in atherogenesis, involved in the initiation as well as progression of atherosclerotic lesions. Upon activation of endothelial cells, blood monocytes are recruited to the site of injury and/or EC activation where cytokines and/or chemokines are released, adhere to the endothelium and transmigrate to the subendothelial space where they further differentiate into macrophages. HO-1 is highly upregulated in macrophages in atherosclerotic lesions and several lines of evidence support its protective antiatherogenic role in these cells. First, HO-1 has been shown to exert antioxidant and antiinflammatory effects in macrophages (Lee et al., 2003; Philippidis et al., 2004; Levonen et al., 2007; Orozco et al., 2007). Indeed, we have reported that decreased or absent HO-1 expression in peritoneal macrophages results in enhanced ROS formation and increased inflammatory cytokines such as MCP-1, interleukin 6 (IL-6), and the murine interleukin 8 homolog (KC; Orozco et al., 2007; Figure 5A). Second, HO-1 expression influences lipid loading and foam cell formation as both partial and total HO-1 deficiency results in enhanced lipid loading and foam cell formation in peritoneal macrophages treated with oxLDL, at least partially mediated by increased expression of the scavenger receptor A (SR-A; Orozco et al., 2007). Third, lack of HO-1 expression in bone marrow-derived cells resulted in atherosclerotic plaques with a larger inflammatory component, suggestive of greater vulnerability for rupture (Orozco et al., 2007). Irradiated LDL-R−/− mice with their bone marrow reconstituted with HO-1−/− donor cells resulted in plaques with increased macrophage area as compared with control mice reconstituted with WT bone marrow (Orozco et al., 2007). Fourth, HO-1 expression may play a role in macrophage differentiation and polarization (Figure 5B).
Figure 5. Heme oxygenase-1 inhibits macrophage proinflammatory activity. (A) Peritoneal macrophages from HO-1+/+, HO-1+/−, and HO-1+/+ mice were cultured in the presence or absence of oxLDL 50 μg/ml for 6 h. IL-6 and MCP-1 were determined by ELISA. *p < 0.001 as compared with controls. Data taken from Orozco et al. (2007). (B) HO-1 expression varies in different subtypes of macrophages. Upon monocyte differentiation into macrophages, they can polarize into one of the five proposed subtypes, M1, M2, M4, Mox, and Mhem. Each subtype is induced by specific stimuli as shown along the arrows that derive from the macrophage Mø atop, and characterized by a set of phenotypic markers and/or gene expression shown below each one of them. While some of macrophage subtypes are proposed to simulate atherogenesis such as M1 and M4, others are proposed to inhibit lesion development such as M2 and Mhem. Mox macrophages, generated after treatment with oxPAPC, are dependent on Nrf2 with a role in atherogenesis still to be determined.
Heme Oxygenase System, Enzymatic Byproducts, and Protection Against Atherosclerosis
Heme oxygenase-1 is not only expressed ubiquitously in the body but it is also located in several compartments within the cell. It is a 32-kDa protein most abundantly located in the microsomal fraction, with a short transmembrane segment of ∼ 2 kDa in the endoplasmic reticulum membrane and ∼30 kDa cytosolic portion. However, HO-1 can also be found in the plasma membrane, associated with caveolae (Kim et al., 2004), in the mitochondria (Converso et al., 2006) and in the nucleus (Lin et al., 2007, 2008).
Summary and Perspectives
The overall importance of HO is underlined by its large degree of evolutionary conservation in all kingdoms of life as it is expressed in prokaryotic and eukaryotic cells, bacteria, yeasts, protozoa, plants, invertebrate and vertebrate animals. In all these various living organisms, HO contributes to the catabolic processing of hemoproteins and avoidance of the potential toxicity that could be associated to the release of Fe atoms present on them.
Heme oxygenase appears to exert most of its actions thanks to its enzymatic activity and the generation of various enzymatic byproducts that act on a multiplicity of pathways. Therefore, HO activity can be visualized as a HO system with three branches or arms of action that consist in: (1) generation of Biliverdin and subsequent reduction to bilirubin, (2) release of CO, and (3) release and safe disposal of Fe2+. These arms of action modulate various pathways with a high degree of overlap and/or cross-over, which may help to ensure the induction of the desired biological effects. HO-1 is most abundant in the cytosol, attached to the smooth endoplasmic reticulum (SER) membrane. However, its presence in other subcellular localizations such as the plasma membrane, mitochondria, or nucleus raises the question whether HO could exert some protein-protein or protein-DNA interactions that could play other roles, such as transcriptional regulation, which requires further exploration. HO-1 and the HO system in general are thought to play a critical role in cellular homeostasis where the presence of complementarity or overlap between the arms of action could result in some degree of redundancy that can help to ensure the induction of HO mediated critical effects. These principles are also seen in the protection against vascular inflammation and atherosclerosis. Thus, HO three arms of action have various degrees of antioxidant, antiinflammatory, antiapoptotic, antiproliferative, and immunomodulatory effects, most of which may play a significant role in the protection against atherosclerotic lesion formation.
Heme oxygenase-1 has been shown to be a critical gene in the cellular response against prooxidative stimuli such as oxidized phospholipids. When considering the cellular response as a whole, HO-1 acts as a hub of gene-gene interactions that modulate the triggering and activation of several protective molecular pathways. This has been demonstrated in endothelial cells and is likely to be the case in other vascular cells such as monoctyes/macrophages. In addition, HO-1 may be a protective gene against proatherogenic effects caused by environmental factors such as cigarette smoking or exposure to air pollution.
Basal HO-1 mRNA expression levels in response to injurious stimuli appear to be more important than the degree of upregulation or fold induction for the protection against those stimuli in the vasculature. In some circumstances, the levels of HO-1 protein and HO functional activity may not be concordant which could be due to posttranslational modifications. Therefore, a better understanding of their kinetics in vascular processes such as atherosclerosis is required to shed light on the reasons why very high levels of upregulated HO-1 in vascular cells may be overwhelmed by the pathogenic process and fail to abrogate the progression of atherosclerotic lesions. Importantly, HO-1 can be targeted pharmacologically by interventions that seek its upregulation or enhancement of its various arms of action, via: (1) induction of HO-1 expression with drugs such as heme arginate, (2) administration of CO by inhalation or use of CO releasing molecules (CORM), and (3) administration of biliverdin/bilirubin or use of inhibitors of bilirubin conjugation that would result in increased bilirubin levels.
One potential caveat, however, is that modulation of HO-1 could result in alteration of the activation status of its transcription factor Nrf2, which has been shown to exert proatherosclerotic effects. The discordant effects between Nrf2 and HO-1 underline the high degree of complexity that therapeutic modulation of vascular oxidative stress must address. This is in addition to studies that have shown that while some oxidation products promote inflammation in the vascular wall, others could have antiinflammatory activity instead. Indeed, electrophilic nitro-fatty acids which are formed via nitric oxide or nitrite-dependent redox reactions have been shown to exert antiinflammatory actions (Khoo and Freeman, 2010). Therefore, any pharmacological modulation of HO-1 should take into consideration any potential alteration of the Nrf2 activation status.
Frontiers | Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis | Pharmacology
https://www.frontiersin.org/articles/10.3389/fphar.2012.00119/full
Free Radic Biol Med. Author manuscript; available in PMC 2011 Apr 17.
Heme Oxygenase-1, a Critical Arbitrator of Cell Death Pathways in Lung Injury and Disease
Danielle Morse,1 Ling Lin,2 Augustine M. K. Choi,1 and Stefan W. Ryter1
Author information Copyright and License information Disclaimer
1Division of Pulmonary and Critical Care Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115.
2Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA 15213.
Increases in cell death by programmed (ie., apoptosis, autophagy) or non-programmed mechanisms (ie., necrosis) occur during tissue injury, and may contribute to the etiology of several pulmonary or vascular disease states.The low molecular weight stress protein heme oxygenase-1 (HO-1) confers cytoprotection against cell death in various models of lung and vascular injury by inhibiting apoptosis, inflammation, and cell proliferation.
HO-1 serves a vital metabolic function as the rate-limiting step in the heme degradation pathway and in the maintenance of iron homeostasis.
The transcriptional induction of HO-1 occurs in response to multiple forms of chemical and physical cellular stress. The cytoprotective functions of HO-1 may be attributed to heme turnover, as well as to beneficial properties of its enzymatic reaction products: biliverdin-IXα, iron, and carbon monoxide (CO).
Recent studies have demonstrated that HO-1 or CO inhibits stress-induced extrinsic and intrinsic apoptotic pathways in vitro. A variety of signaling molecules have been implicated in the cytoprotection conferred by HO-1/CO, including autophagic proteins, p38 mitogen activated protein kinase, signal transducer and activator of transcription proteins, nuclear factor-κB, phosphatydylinositol-3-kinase/Akt, and others.
Enhanced HO-1 expression or the pharmacological application of HO end-products affords protection in preclinical models of tissue injury, including experimental and transplant-associated ischemia/reperfusion injury, promising potential future therapeutic applications.
Heme Oxygenase-1, a Critical Arbitrator of Cell Death Pathways in Lung Injury and Disease
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3078523/
Curr Pharm Des. 2017;23(26):3884-3898. doi: 10.2174/1381612823666170413122439.
Therapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury.
Cheng Y1, Rong J2.
Author information
1 School of Chinese Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, 10 Sassoon Road, Pokfulam, Hong Kong. China.
2 School of Chinese Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, P.O. Box: 0000-000, Hong Kong. China.
Abstract
BACKGROUND:
Ischemia-reperfusion (I/R) injury causes the dysfunctions of different major organs, leading to morbidity and mortality on the global scale. Among a battery of therapeutic targets, the heme oxygenase- 1 (HO-1)/carbon monoxide (CO) system has been evaluated for the development of new therapies against I/R injury. The enzyme HO-1 catalyzes the degradation of heme into three biologically active end products, namely biliverdin/bilirubin, CO and ferrous ion. Interestingly, CO is one of a few bioactive gaseous molecules with the capability of regulating inflammation, cell survival and growth. In fact, several CO-releasing compounds have been developed for directly reprogramming the intracellular apoptotic, inflammatory and proliferative signaling networks. In parallel, chemical and genetic approaches have also been evaluated for up-regulating HO-1 expression as an endogenous mechanism to ameliorate I/R injury and heal wounds.
METHODS:
In this review, we discussed the recent studies on the therapeutic potential of HO-1/CO system in the treatment of I/R injury in the heart, brain, liver, kidney, lung, intestine and retina. We focused on the activities and underlying mechanisms of various therapeutic strategies to regulate HO-1/CO system against I/R injury.
RESULTS:
A large number of studies have demonstrated that HO-1/CO system exhibits potent anti-oxidative, antiapoptotic, anti-inflammatory and cytoprotective activities against I/R injury. The regulation of HO-1/CO expression has been achieved either by genetic overexpression of HO-1 cDNA or pharmacological induction with drugs including curcumin and resveratrol.
CONCLUSION:
The HO-1/CO system is a potential target for treating I/R injury. Further studies should be directed to in vivo efficacy and clinical application of HO-1/CO system in the therapy of I/R injury.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
KEYWORDS:
Ischemia-reperfusion injury; anti-apoptotic; anti-inflammatory; anti-oxidative; hemo oxygenase-1/carbon monoxide; pharmacological inductionTherapeutic Potential of Heme Oxygenase-1/carbon Monoxide System Against Ischemia-Reperfusion Injury. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/28412905
PLoS One. 2010; 5(12): e15358.
Garlic Accelerates Red Blood Cell Turnover and Splenic Erythropoietic Gene Expression in Mice: Evidence for Erythropoietin-Independent Erythropoiesis
Bünyamin Akgül, 1 , 2 Kai-Wei Lin, 1 Hui-Mei Ou Yang, 1 , 3 Yen-Hui Chen, 1 , 4 Tzu-Huan Lu, 1 , 3 Chien-Hsiun Chen, 1 , 3 Tateki Kikuchi, 1 , 4 Yuan-Tsong Chen, 1 , 3 and Chen-Pei D. Tu 1 , 5
1 Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan Authority,
2 Department of Molecular Biology and Genetics, Izmir Institute of Technology, Urla, Turkey,
3 National Genotyping Center, Academia Sinica, Taipei, Taiwan Authority,
4 Taiwan Mouse Clinic, National Phenotyping Center, Academia Sinica, Taipei, Taiwan Authority,
5 Department of Biochemistry and Molecular Biology, The Pennsylvania State University, University Park, Pennsylvania, United States of America,
University of Louisville, United States of America
Abstract
Garlic (Allium sativum) has been valued in many cultures both for its health effects and as a culinary flavor enhancer. Garlic's chemical complexity is widely thought to be the source of its many health benefits, which include, but are not limited to, anti-platelet, procirculatory, anti-inflammatory, anti-apoptotic, neuro-protective, and anti-cancer effects.While a growing body of scientific evidence strongly upholds the herb's broad and potent capacity to influence health, the common mechanisms underlying these diverse effects remain disjointed and relatively poorly understood.
We adopted a phenotype-driven approach to investigate the effects of garlic in a mouse model. We examined RBC indices and morphologies, spleen histochemistry, RBC half-lives and gene expression profiles, followed up by qPCR and immunoblot validation. The RBCs of garlic-fed mice register shorter half-lives than the control. But they have normal blood chemistry and RBC indices. Their spleens manifest increased heme oxygenase 1, higher levels of iron and bilirubin, and presumably higher CO, a pleiotropic gasotransmitter. Heat shock genes and those critical for erythropoiesis are elevated in spleens but not in bone marrow. The garlic-fed mice have lower plasma erythropoietin than the controls, however. Chronic exposure to CO of mice on garlic-free diet was sufficient to cause increased RBC indices but again with a lower plasma erythropoietin level than air-treated controls. Furthermore, dietary garlic supplementation and CO treatment showed additive effects on reducing plasma erythropoietin levels in mice. Thus, garlic consumption not only causes increased energy demand from the faster RBC turnover but also increases the production of CO, which in turn stimulates splenic erythropoiesis by an erythropoietin-independent mechanism, thus completing the sequence of feedback regulation for RBC metabolism. Being a pleiotropic gasotransmitter, CO may be a second messenger for garlic's other physiological effects.
Garlic Accelerates Red Blood Cell Turnover and Splenic Erythropoietic Gene Expression in Mice: Evidence for Erythropoietin-Independent Erythropoiesis
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3012072/
Curcumin-induced heme oxygenase-1 expression plays a ...
https://www.ncbi.nlm.nih.gov/pubmed/22771723
In vivo, curcumin induced heme oxygenase-1 protein expression in the lung tissue of murine lung metastasis tumor model and in the bladder tissue of murine orthotopic bladder tumor model. Taken together, our data suggest that curcumin-induced heme oxygenase-1 attenuates the anti-invasive effect of curcumin in cancer therapy, and co-treatment by heme oxygenase-1 inhibitor enhances the anti …
Cited by: 9
Publish Year: 2012
Author: Szu Yuan Wu, Ying Ray Lee, Chin Chin Huang, Yi
Upregulation of heme oxygenase-1 expression by curcumin ...
https://cellandbioscience.biomedcentral.com/articles/10.1186/s13578-017-0146-6
Apr 21, 2017 · Exposure of cells with curcumin caused a dose-dependent induction of heme oxygenase-1 (HO-1) protein expression. Curcumin also decreased the cleaved caspase-3 (CC3) protein expression level and increased the Bcl-2/Bax ratio in H2O2-stimulated H9c2 cells. ZnPP-IX, a HO-1 inhibitor, partly reversed the anti-apoptotic effect of curcumin.
Cited by: 8
Publish Year: 2017
Author: Xiaobo Yang, Hong Jiang, Yao Shi
Curcumin blocks fibrosis in anti-Thy 1 glomerulonephritis ...
https://www.sciencedirect.com/science/article/pii/S0085253815511010
Induction of heme oxygenase 1 (HO-1) protein expression in cultured rat mesangial cells by curcumin. Quiescent cells were treated with curcumin as shown and HO-1 expression was analyzed using Western blot. The blots show a representative of three to four experiments.
Cited by: 77
Publish Year: 2005
Author: Jens Gaedeke, Jens Gaedeke, Nancy A. Noble, Nancy A. Noble, Wayne A. Border, Wayne A. Border
Curcumin, an antioxidant and anti-inflammatory agent ...
https://www.sciencedirect.com/science/article/pii/S089158490000294X
Effect of curcumin on heme oxygenase activity and HO-1 protein expression in vascular endothelial cells. (A) Heme oxygenase activity was measured in endothelial cells 18 h after exposure to various concentrations of curcumin (0–30 μM).
Cited by: 861
Publish Year: 2000
Author: Roberto Motterlini, Roberta Foresti, Rekha Bassi, Co
Free Radical Biology and Medicine
Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress
RobertoMotterliniaRobertaForestiaRekhaBassiaColin JGreena
a Vascular Biology Unit, Department of Surgical Research, Northwick Park Institute for Medical Research, Harrow, UK
Abstract
Curcumin, a widely used spice and coloring agent in food, has been shown to possess potent antioxidant, antitumor promoting and anti-inflammatory properties in vitro and in vivo.The mechanism(s) of such pleiotropic action by this yellow pigment is unknown; whether induction of distinct antioxidant genes contributes to the beneficial activities mediated by curcumin remains to be investigated.
In the present study we examined the effect of curcumin on endothelial heme oxygenase-1 (HO-1 or HSP32), an inducible stress protein that degrades heme to the vasoactive molecule carbon monoxide and the antioxidant biliverdin. Exposure of bovine aortic endothelial cells to curcumin (5–15 μM) resulted in both a concentration- and time-dependent increase in HO-1 mRNA, protein expression and heme oxygenase activity.
Hypoxia (18 h) also caused a significant (P < 0.05) increase in heme oxygenase activity which was markedly potentiated by the presence of low concentrations of curcumin (5 μM).
Interestingly, prolonged incubation (18 h) with curcumin in normoxic or hypoxic conditions resulted in enhanced cellular resistance to oxidative damage; this cytoprotective effect was considerably attenuated by tin protoporphyrin IX, an inhibitor of heme oxygenase activity.
In contrast, exposure of cells to curcumin for a period of time insufficient to up-regulate HO-1 (1.5 h) did not prevent oxidant-mediated injury. These data indicate that curcumin is a potent inducer of HO-1 in vascular endothelial cells and that increased heme oxygenase activity is an important component in curcumin-mediated cytoprotection against oxidative stress.
Keywords
Antioxidantheme oxygenase-1cytoprotectionbilirubincarbon monoxidehypoxiapharmacological preconditioningFree radicalsCurcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S089158490000294X
Kidney International
Volume 68, Issue 5, November 2005, Pages 2042-2049
Curcumin blocks fibrosis in anti-Thy 1 glomerulonephritis through up-regulation of heme oxygenase 1
Author links open overlay panelJensGaedekeNancy A.NobleWayne A.Border
Fibrosis Research Laboratory, University of Utah, Salt Lake City, Utah
Med. Klinik m.S. Nephrologie, Charité, Campus Mitte, Humboldt Universität, Berlin, Germany
Received 5 August 2004, Accepted 20 June 2005, Available online 16 December 2015.
Background
Induction of heme oxygenase 1 (HO-1) has been shown to be beneficial in a variety of pathologic settings. Curcumin, a polyphenolic compound, has antifibrotic effects in lung models of fibrosis, and is known to induce HO-1 in renal tubular cells. In this study, we determined whether curcumin has antifibrotic properties in glomerular fibrosis and if these effects are mediated by induction of HO-1.
METHODS
Curcumin effects on HO-1 expression in cultured mesangial cells and in glomeruli in vivo were analyzed by Northern and Western blotting. The dose-dependent effect of curcumin on glomerular fibrosis was tested in the anti-Thy 1 glomerulonephritis model. Curcumin was applied at doses of 10 to 200 mg/kg body weight by intraperitoneal injection from days 3 to 5 after induction of disease. On day 6, glomeruli were harvested and markers of fibrosis [plasminogen activator inhibitor-1 (PAI-1), transforming growth factor-β (TGF-β), fibronectin, periodic acid-Schiff (PAS) staining] were analyzed. The effect of HO-1 inhibition was tested in a second experiment were nephritic rats were treated with curcumin (100 mg/kg body weight) or the combination of curcumin and the HO-1 inhibitor zinc protoporphyrin (100 μg/kg).
RESULTS
Curcumin potently induced mesangial cell HO-1 expression in vitro and up-regulated glomerular HO-1 expression in nephritic animals in vivo. Curcumin treatment led to a significant, dose-dependent reduction of markers of fibrosis and proteinuria, with maximal inhibition at doses of 50 to 100 mg/kg. Beneficial effects of curcumin on markers of fibrosis and proteinuria were lost after HO-1 inhibition.
Conclusion
Curcumin has antifibrotic effects in glomerular disease, which are mediated through an induction of HO-1.
Keywords
TGF-betacurcuminheme oxygenase 1renal fibrosisglomerulonephritisCurcumin blocks fibrosis in anti-Thy 1 glomerulonephritis through up-regulation of heme oxygenase 1 - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0085253815511010
Toxicol Appl Pharmacol. 2012 Dec 1;265(2):241-52. doi:
L-ascorbate attenuates methamphetamine neurotoxicity through enhancing the induction of endogenous heme oxygenase-1.
Huang YN1, Wang JY, Lee CT, Lin CH, Lai CC, Wang JY.
Department of Nursing, Hsin Sheng College of Medical Care and Management, Taoyuan, Taiwan.
Abstract
Methamphetamine (冰毒 METH) is a drug of abuse which causes neurotoxicity and increased risk of developing neurodegenerative diseases. We previously found that METH induces heme oxygenase (HO)-1 expression in neurons and glial cells, and this offers partial protection against METH toxicity.In this study, we investigated the effects of l-ascorbate (vitamin C, Vit. C) on METH toxicity and HO-1 expression in neuronal/glial cocultures. Cell viability and damage were evaluated by 3-(4,5-dimethylthianol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) reduction and lactate dehydrogenase (LDH) release, respectively. Neuronal and glial localization of HO-1 were identified by double immunofluorescence staining. Reactive oxygen species (ROS) production was measured using the fluorochrome 2',7'-dichlorofluorescin diacetate. HO-1 mRNA and protein expression were examined by RT-qPCR and Western blotting, respectively.
Results show that Vit. C induced HO-1 mRNA and protein expressions in time- and concentration-dependent manners. Inhibition of p38 mitogen-activated protein kinase (MAPK) but not extracellular signal-regulated kinase (ERK) significantly blocked induction of HO-1 by Vit. C. HO-1 mRNA and protein expressions were significantly elevated by a combination of Vit. C and METH, compared to either Vit. C or METH alone. Pretreatment with Vit. C enhanced METH-induced HO-1 expression and attenuated METH-induced ROS production and neurotoxicity. Pharmacological inhibition of HO activity abolished suppressive effects of Vit. C on METH-induced ROS production and attenuated neurotoxicity.
We conclude that induction of HO-1 expression contributes to the attenuation of METH-induced ROS production and neurotoxicity by Vit. C. We suggest that HO-1 induction by Vit. C may serve as a strategy to alleviate METH neurotoxicity.
L-ascorbate attenuates methamphetamine neurotoxicity through enhancing the induction of endogenous heme oxygenase-1. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/23022510
J Clin Biochem Nutr. 2009 Jul; 45(1): 9–13.
The Expression of Heme Oxygenase-1 Induced by Lansoprazole
Tomohisa Takagi, Yuji Naito,* and Toshikazu Yoshikawa
Molecular Gastroenterology and Hepatology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto 602-8566, Japan
Abstract
Our previous studies have demonstrated that lansoprazole inhibits acute inflammatory reactions as well as intestinal mucosal injuries induced by ischemia-reperfusion or indomethacin administration in rats. Thus, proton pump inhibitors such as lansoprazole have been demonstrated to prevent gastrointestinal mucosal injury by mechanisms independent of acid inhibition. In our in vitro study, lansoprazole induced the expression of heme oxygenase-1 (HO-1) on rat gastric epithelial cells (RGM-1 cells), and exerted anti-inflammatory effect on the dependent of HO-1 expression. Furthermore, NF-E2-related factor-2 (Nrf2) played an important role in HO-1 expression induced by lansoprazole. In this review, we focused on lansoprazole-induced HO-1 expression, its anti-inflammatory action, and the role of Nrf2 in its expression.
Keywords: Lansoprazole, Heme Oxygenase-1 (HO-1), NF-E2-related factor-2 (Nrf2)
The Expression of Heme Oxygenase-1 Induced by Lansoprazole
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2704331/PLoS One. 2014; 9(6): e99134.
Pharmacological Preconditioning with Vitamin C Attenuates Intestinal Injury via the Induction of Heme Oxygenase-1 after Hemorrhagic Shock in Rats
Bing Zhao,# 1 Jian Fei,# 2 Ying Chen, 1 Yi-Lin Ying, 1 Li Ma, 3 Xiao-Qin Song, 1 Lu Wang, 1 Er-Zhen Chen, 1 , * and En-Qiang Mao 1 , *
Prasun K. Datta, Editor
1 Department of Emergency Intensive Care Unit, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China,
2 Department of Surgery, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China,
3 Department of Emergency Intensive Care Unit, the Third People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China,
Temple University, United States of America,
Abstract
Pre-induction of heme oxygenase (HO)-1, which is regarded as an effective method of “organ preconditioning”, exerts beneficial effects during hemorrhagic shock (HS). However, the available HO-1 inducers exhibit disadvantages such as toxicity or complex technical requirements. Therefore, a safe and convenient HO-1 inducer would be promising and could be exploited in the treatment of foreseeable hemorrhaging, such as prior to major surgery.Here we investigated the effect of vitamin C (VitC), a common antioxidant, on intestinal HO-1 expression and examined whether VitC pretreatment prevented HS related intestinal tissue injuries after HO-1 induction.
First, we conducted an in vitro study and found that HO-1 expression in rat intestinal epithelial cells (IEC-6) was induced by non-toxic VitC in a time and concentration dependent manner, and the mechanism was related to the activation of extracellular signal-regulated kinase 1/2 (ERK1/2).
Next, we conducted an in vivo study and found that VitC induced intestinal HO-1 protein expression (mainly observed in the intestinal epithelial cells) and HO-1 activity in normal SD rats, and that these HO-1 levels were further enhanced by VitC in a rat model of HS.
The HS related intestinal injuries, including histological damage, pro-inflammatory cytokine levels (tumor necrosis factor and interleukin-6), neutrophil infiltration and apoptosis decreased after VitC pretreatment, and this alleviating of organ injuries was abrogated after the inhibition of HO-1 activity by zinc protoporphyrin-IX. It was of note that VitC did little histological damage to the intestine of the sham rats. These data suggested that VitC might be applied as a safe inducer of intestinal HO-1 and that VitC pretreatment attenuated HS related intestinal injuries via the induction of HO-1.
Pharmacological Preconditioning with Vitamin C Attenuates Intestinal Injury via the Induction of Heme Oxygenase-1 after Hemorrhagic Shock in Rats
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4057195/
J Immunol. 2014 Sep 15;193(6):3013-22. doi: 10.4049/jimmunol.1401075. Epub 2014 Aug 8.
Heme oxygenase-1 dysregulates macrophage polarization and the immune response to Helicobacter pylori.
Gobert AP1, Verriere T2, Asim M2, Barry DP2, Piazuelo MB2, de Sablet T2, Delgado AG2, Bravo LE3, Correa P2, Peek RM Jr4, Chaturvedi R2, Wilson KT5.
Author information
1
Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232; Institut National de la Recherche Agronomique, Unité de Recherche Microbiologie (UR454), 63122 Saint-Genès-Champanelle, France;
2
Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232;
3
Departamento de Patología, Escuela de Medicina, Universidad del Valle, Cali, Colombia;
4
Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232; Department of Cancer Biology, Vanderbilt University School of Medicine, Nashville, TN 37232; Veterans Affairs Tennessee Valley Healthcare System, Nashville, TN 37212; and.
5
Division of Gastroenterology, Hepatology, and Nutrition, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232; Department of Cancer Biology, Vanderbilt University School of Medicine, Nashville, TN 37232; Veterans Affairs Tennessee Valley Healthcare System, Nashville, TN 37212; and Department of Pathology, Microbiology and Immunology, Vanderbilt University School of Medicine, Nashville, TN 37232 keith.wilson@vanderbilt.edu.
Abstract
Helicobacter pylori incites a futile inflammatory response, which is the key feature of its immunopathogenesis. This leads to the ability of this bacterial pathogen to survive in the stomach and cause peptic ulcers and gastric cancer.Myeloid cells recruited to the gastric mucosa during H. pylori infection have been directly implicated in the modulation of host defense against the bacterium and gastric inflammation.
Heme oxygenase-1 (HO-1) is an inducible enzyme that exhibits anti-inflammatory functions. Our aim was to analyze the induction and role of HO-1 in macrophages during H. pylori infection.
We now show that phosphorylation of the H. pylori virulence factor cytotoxin-associated gene A (CagA) in macrophages results in expression of hmox-1, the gene encoding HO-1, through p38/NF (erythroid-derived 2)-like 2 signaling. Blocking phagocytosis prevented CagA phosphorylation and HO-1 induction. The expression of HO-1 was also increased in gastric mononuclear cells of human patients and macrophages of mice infected with cagA(+) H. pylori strains.
Genetic ablation of hmox-1 in H. pylori-infected mice increased histologic gastritis, which was associated with enhanced M1/Th1/Th17 responses, decreased regulatory macrophage (Mreg) response, and reduced H. pylori colonization.
Gastric macrophages of H. pylori-infected mice and macrophages infected in vitro with this bacterium showed an M1/Mreg mixed polarization type; deletion of hmox-1 or inhibition of HO-1 in macrophages caused an increased M1 and a decrease of Mreg phenotype.
These data highlight a mechanism by which H. pylori impairs the immune response and favors its own survival via activation of macrophage HO-1.
Copyright © 2014 by The American Association of Immunologists, Inc.
PMID: 25108023 PMCID: PMC4171064 DOI: 10.4049/jimmunol.1401075
[Indexed for MEDLINE] Free PMC ArticleHeme oxygenase-1 dysregulates macrophage polarization and the immune response to Helicobacter pylori. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/25108023
PLoS One. 2014; 9(6): e99134.
Pharmacological Preconditioning with Vitamin C Attenuates Intestinal Injury via the Induction of Heme Oxygenase-1 after Hemorrhagic Shock in Rats
Bing Zhao,# 1 Jian Fei,# 2 Ying Chen, 1 Yi-Lin Ying, 1 Li Ma, 3 Xiao-Qin Song, 1 Lu Wang, 1 Er-Zhen Chen, 1 , * and En-Qiang Mao 1 , *
Prasun K. Datta, Editor
1 Department of Emergency Intensive Care Unit, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China,
2 Department of Surgery, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China,
3 Department of Emergency Intensive Care Unit, the Third People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China,
Temple University, United States of America,
Abstract
Pre-induction of heme oxygenase (HO)-1, which is regarded as an effective method of “organ preconditioning”, exerts beneficial effects during hemorrhagic shock (HS). However, the available HO-1 inducers exhibit disadvantages such as toxicity or complex technical requirements. Therefore, a safe and convenient HO-1 inducer would be promising and could be exploited in the treatment of foreseeable hemorrhaging, such as prior to major surgery.Here we investigated the effect of vitamin C (VitC), a common antioxidant, on intestinal HO-1 expression and examined whether VitC pretreatment prevented HS related intestinal tissue injuries after HO-1 induction.
First, we conducted an in vitro study and found that HO-1 expression in rat intestinal epithelial cells (IEC-6) was induced by non-toxic VitC in a time and concentration dependent manner, and the mechanism was related to the activation of extracellular signal-regulated kinase 1/2 (ERK1/2).
Next, we conducted an in vivo study and found that VitC induced intestinal HO-1 protein expression (mainly observed in the intestinal epithelial cells) and HO-1 activity in normal SD rats, and that these HO-1 levels were further enhanced by VitC in a rat model of HS.
The HS related intestinal injuries, including histological damage, pro-inflammatory cytokine levels (tumor necrosis factor and interleukin-6), neutrophil infiltration and apoptosis decreased after VitC pretreatment, and this alleviating of organ injuries was abrogated after the inhibition of HO-1 activity by zinc protoporphyrin-IX. It was of note that VitC did little histological damage to the intestine of the sham rats. These data suggested that VitC might be applied as a safe inducer of intestinal HO-1 and that VitC pretreatment attenuated HS related intestinal injuries via the induction of HO-1.
Pharmacological Preconditioning with Vitamin C Attenuates Intestinal Injury via the Induction of Heme Oxygenase-1 after Hemorrhagic Shock in Rats
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4057195/
Nitric Oxide Induces Heme Oxygenase-1 Gene Expression and Carbon Monoxide Production in Vascular Smooth Muscle Cells
William Durante , Michael H. Kroll , Nick Christodoulides , Kelly J. Peyton , and Andrew I. Schafer
Houston VA Medical Center (W.D., M.H.K., N.C., K.J.P., A.I.S.) and the Departments of Medicine (W.D., M.H.K., N.C., A.I.S.) and Pharmacology (W.D.), Baylor College of Medicine, Houston, Tex.
Abstract
Since recent studies demonstrate that vascular smooth muscle cells synthesize two distinct guanylate cyclase–stimulatory gases, NO and CO, we examined possible regulatory interactions between these two signaling molecules. Treatment of rat aortic smooth muscle cells with the NO donors, sodium nitroprusside, S-nitroso-N-acetyl-penicillamine, or 3-morpholinosydnonimine, increased heme oxygenase-1 (HO-1) mRNA and protein levels in a concentration- and time-dependent manner.Both actinomycin D and cycloheximide blocked NO-stimulated HO-1 mRNA and protein expression. Nuclear run-on experiments demonstrated that NO donors increased HO-1 gene transcription between 3- and 6-fold. In contrast, NO donors had no effect on the stability of HO-1 mRNA.
Incubation of vascular smooth muscle cells with the membrane-permeable cGMP analogues, dibutyryl cGMP and 8-bromo-cGMP, failed to induce HO-1 gene expression. Treatment of vascular smooth muscle cells with NO donors also stimulated the production and release of CO, as demonstrated by the CO-dependent increase in intracellular cGMP levels in coincubated platelets.
Finally, incubating vascular smooth muscle cells with interleukin-1β and tumor necrosis factor-α induced NO synthesis and also significantly increased the level of HO-1 protein.
The cytokine-stimulated production of both NO and HO-1 protein in smooth muscle cells was blocked by the NO synthase inhibitor methyl-l-arginine.
These results demonstrate that exogenously administered or endogenously released NO stimulates HO-1 gene expression and CO production in vascular smooth muscle cells. The ability of NO to induce HO-catalyzed CO release from vascular smooth muscle cells provides a novel mechanism by which NO might modulate soluble guanylate cyclase and, thereby, vascular smooth muscle cell and platelet function.
Nitric Oxide Induces Heme Oxygenase-1 Gene Expression and Carbon Monoxide Production in Vascular Smooth Muscle Cells | Circulation Research
https://www.ahajournals.org/doi/full/10.1161/01.RES.80.4.557


.jpg)