氰化物中毒is vitamin C a antidote for cyanide poisoining?
is methylene blue a antidote?
CN-
methemoglobin,
http://io9.com/this-is-a-very-strange-cure-for-cyanide-poisoning-1727160459
http://www.spring8.or.jp/en/news_publications/press_release/2010/100413/
http://www.youtube.com/watch?v=VfW64-vT7F8
ArticlePDF Available
Reversal of cyanide inhibition of cytochrome C oxidase by the auxiliary substrate nitric oxide: An endogenous antidote to cyanide poisoning?
January 2004Journal of Biological Chemistry 278(52):52139-45Nitric oxide (NO) is shown to overcome the cyanide inhibition of cytochrome c oxidase in the presence of excess ferrocytochrome c and oxygen. Addition of NO to the partially reduced cyanide-inhibited form of the bovine enzyme is shown by electron paramagnetic resonance spectroscopy to result in substitution of cyanide at ferriheme a3 by NO with reduction of the heme. The resulting nitrosylferroheme a3 is a 5-coordinate structure, the proximal bond to histidine having been broken. NO does not simply act as a reversibly bound competitive inhibitor but is an auxiliary substrate consumed in a catalytic cycle along with ferrocytochrome c and oxygen. The implications of this observation with regard to estimates of steady-state NO levels in vivo is discussed. Given the multiple sources of NO available to mitochondria, the present results appear to explain in part some of the curious biomedical observations reported by other laboratories; for example, the kidneys of cyanide poisoning victims surprisingly exhibit no significant irreversible damage, and lethal doses of potassium cyanide are able to inhibit cytochrome c oxidase activity by only approximately 50% in brain mitochondria.
用辅助底物一氧化氮逆转细胞色素C氧化酶的氰化物抑制:一种内源性氰化物中毒的解毒剂?
2004年1月生物化学杂志278(52):52139-45
在过量的细胞色素c和氧气存在下,一氧化氮(NO)被证明可以克服细胞色素c氧化酶的氰化物抑制。电子顺磁共振光谱显示,在部分还原的氰化物抑制形式的牛酶中加入NO,可以使铁血红素a3上的氰化物被NO取代,同时血红素也被还原。由此产生的亚硝基二茂铁a3是一个5坐标结构,靠近组氨酸的键已经被破坏。NO不仅仅作为可逆结合的竞争性抑制剂,而是与细胞色素c和氧一起在催化循环中消耗的辅助底物。
1782年,舍勒是第一个分离出氰化氢的人。后来,他意外地成为了氰化氢的受害者,显示出了氰化氢的毒性。亚历山德拉看起来……
2006年4月19日
亚历山德拉•林赛。
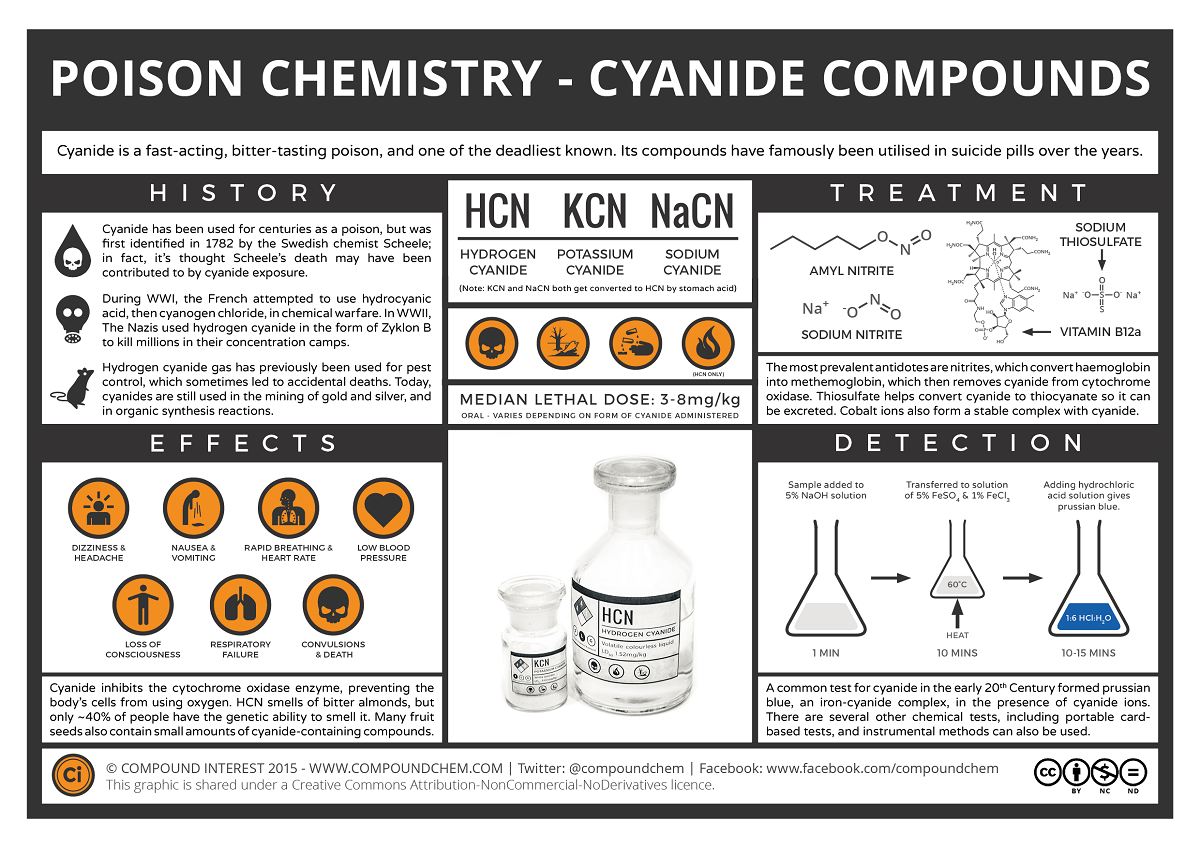
谋杀、恐怖主义和自杀……这些都是与氰化物中毒有关的情况。人们希望这些是罕见的事件,但氰化物中毒的发生率是非常普遍和不断增加的。原因在于,聚丙烯腈、尼龙和三聚氰胺等人造聚合物在燃烧时会产生氰化氢气体。
这些物质被用在衣服和家具上,所以HCN气体可以在火灾中产生,任何靠近火源的人都可能暴露在它之下。氰化氢在1782年由舍勒首次分离出来。后来,他意外地成为了它的受害者,用实际行动证明了它的毒性。它常与苦杏仁的气味联系在一起,但事实上只有40%的人能闻到它。
氰化物自然存在于每个人的血液中,含量非常少,吸烟的人血液中氰化物的含量往往比不吸烟的人多。
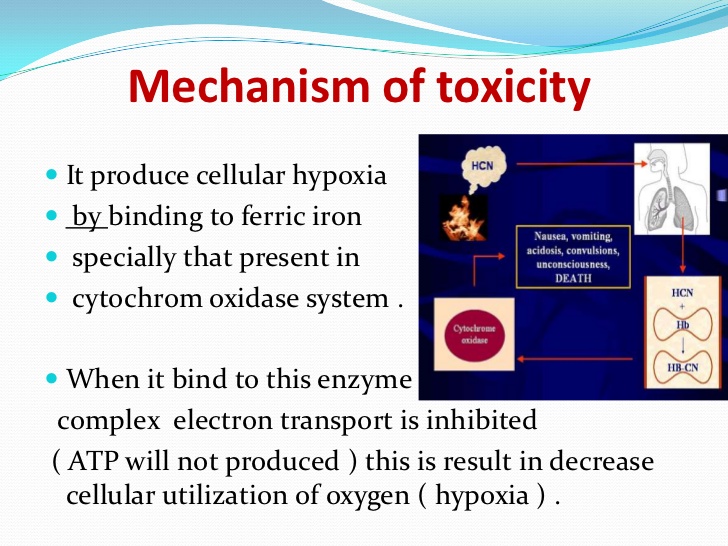
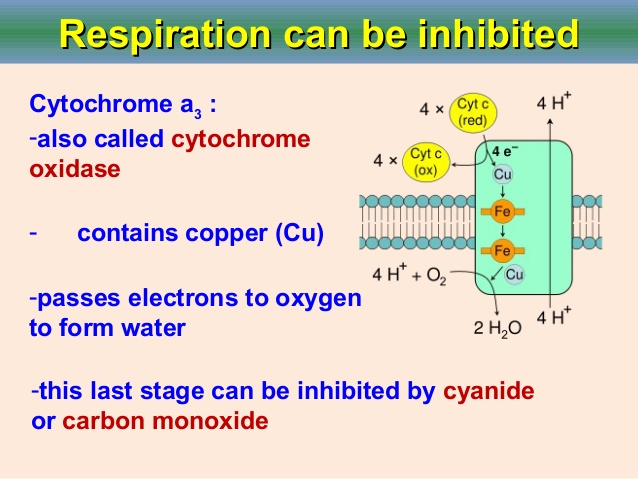
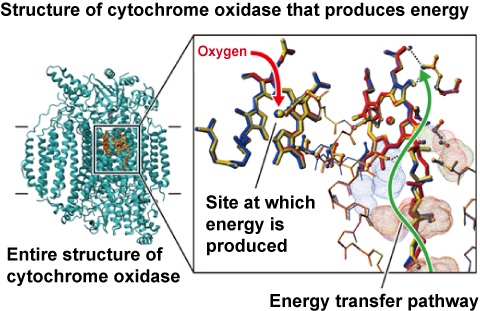
现在是定义“氰化物”的好时机。氰化物是带负电荷的离子,CN,但在生理pH 7.4时,它以氰化氢的形式存在,HCN。氰化物一旦进入血液,大多数(92-99%)会与红细胞中的血红蛋白结合。从那里它被带到身体的组织,在那里它与一种叫做细胞色素氧化酶的酶结合,阻止细胞使用氧气。氰化物中毒的症状和体征包括头痛、呼吸困难和呕吐、昏迷和死亡。氰化物中毒后康复的人通常不会受到任何长期影响。
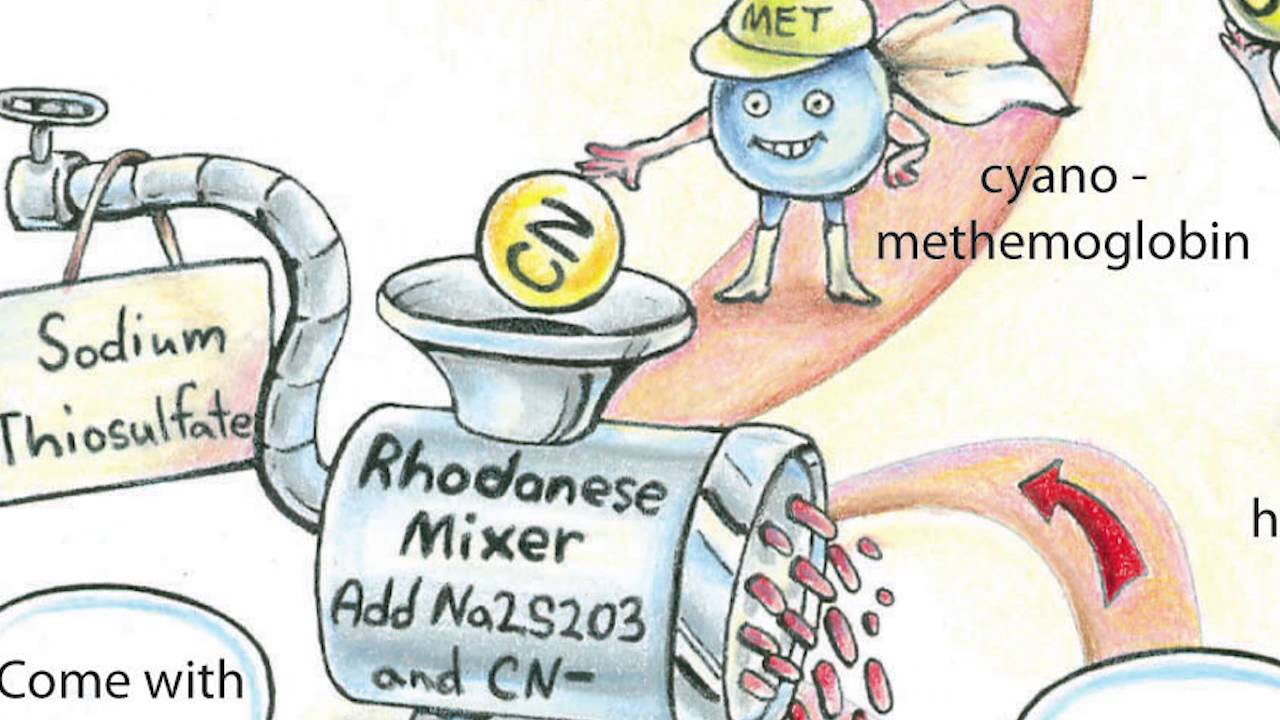
氰化物代谢迅速,通常被一种叫做rhodanese的酶转化为硫氰酸盐。硫氰酸盐的毒性比氰化物要小得多,人体可以将其排出体外。但是(必须有一个但是!)这种酶需要另一种化学物质,硫代硫酸盐,才能做到这一点,而硫代硫酸盐很快就会被消耗掉。
氰化物和含氰化物的化合物被广泛应用于电镀、化学合成和熏蒸等工业过程中。有些食物含有氰化物,可在人体内转化为氰化物;其中包括木薯根、青豆和竹笋。此外,硝基普钠(有时用于治疗高血压)和Laetrile(一种抗癌物质)等药物也会向血液循环中释放氰化物。
氰化物盐在历史上被用于自杀和谋杀。现在公众不容易买到它们,所以它们现在不常用于这方面。尽管如此,最近还是发生了一些案件,例如1982年芝加哥有7人被加入氰化物盐的泰诺片(止痛药)杀害。
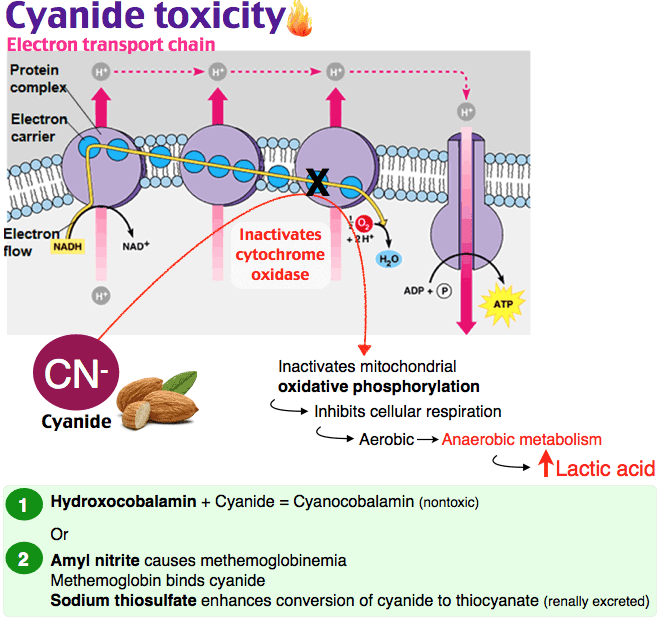
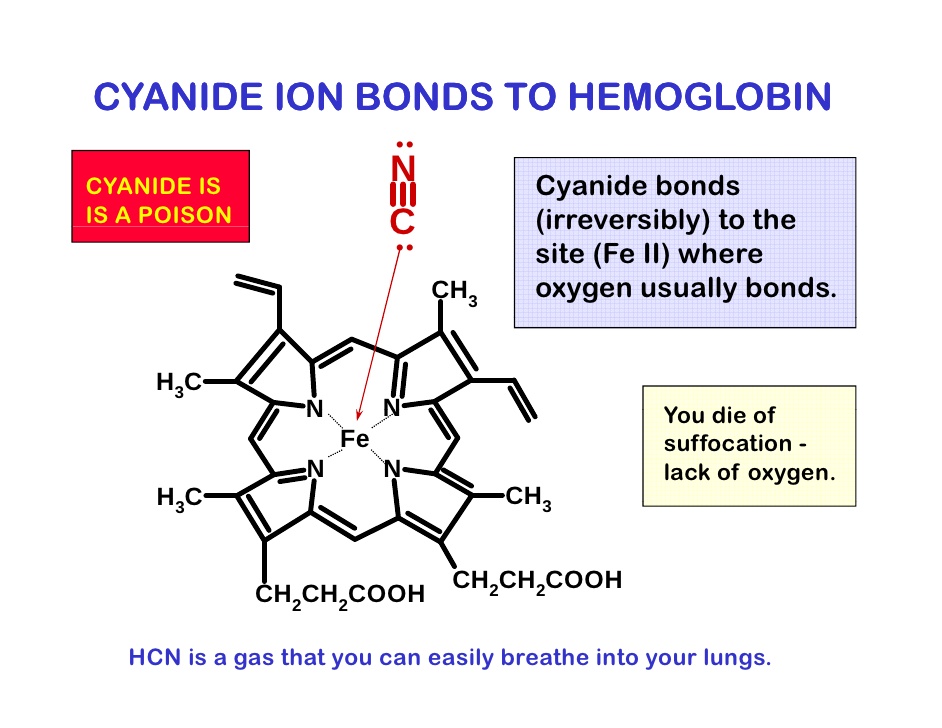
图1:氰化物在体内的影响。氰化氢气体(HCN)被吸入并锁在血红蛋白上,血红蛋白是红细胞中携带氧气的分子(右下)。然后,它通过血液输送到全身的细胞,并与一种叫做细胞色素氧化酶(左下)的重要代谢酶结合,阻止细胞利用氧气产生能量。在这种情况下,氰化物有效地使人体化学窒息。
图1:氰化物在体内的影响。氰化氢气体(HCN)被吸入并锁在血红蛋白上,血红蛋白是红细胞中携带氧气的分子(右下)。然后,它通过血液输送到全身的细胞,并与一种叫做细胞色素氧化酶(左下)的重要代谢酶结合,阻止细胞利用氧气产生能量。在这种情况下,氰化物有效地使人体化学窒息。
有解毒剂可用于氰化物中毒,但它们可能有不良的副作用或本身有毒。例如,一些药物可以将正常的血红蛋白转变为另一种形式的高铁血红蛋白,因为氰化物与这种类型的血红蛋白有很高的亲和性,可以与氰化物结合,阻止它到达组织。
不幸的是,氧化血红蛋白不能与氧气结合,所以很明显,我们不希望有太多这种形式的血红蛋白。还可以给予额外的硫代硫酸盐,以便代谢更多的氰化物,这经常用于高铁血红蛋白形成者。钴(II)化合物形成氰化物,这些被使用,但它们是剧毒的。羟钴胺(维生素B12a)形成氰钴胺(维生素B12),毒性较低,但尚未广泛使用。由于这些药物的毒性,它们的剂量必须与体内氰化物的含量有关。
所以,另一个原因是要确保你已经检查了烟雾报警器的电池工作!
Cyanide Poisoning
Scheele was the first to isolate Hydrogen cyanide in 1782 then went on to show its toxic effects by accidentally becoming its victim. Alexandra looks...
19 April 2006
By ALEXANDRA LINDSAY.
alexandralindsay1_toxic.gif
Murder, terrorism and suicide...These are the scenarios generally associated with cyanide poisoning. One would hope these are rare occurrences but incidences of cyanide poisoning are all too common and increasing.Toxic_icon The reason for this is that when man-made polymers such as polyacrylonitrile, nylon and melamine are burned they produce hydrogen cyanide (HCN) gas.
These substances are used in clothes and furnishings and so HCN gas can be produced during a fire and anyone near the fire can is exposed to it. Hydrogen cyanide was first isolated in 1782 by Scheele. He later went on to provide a practical demonstration of its toxic effects by accidentally becoming its victim. It is often associated with the smell of bitter almonds but in fact only about 40% of people can smell it.
Cyanide is naturally present in everyone's blood in very small amounts, and people who smoke tend to have more in their blood than people who don't.
This would be a good moment to define 'cyanide'. Cyanide is the negatively charged ion, CN- but at physiological pH 7.4, when unbound, this is in the form of hydrogen cyanide, HCN. Once cyanide is taken into the blood stream the majority (92-99%) is found bound to hemoglobin (Hb) in red blood cells. From there it is taken to the body's tissues where it binds to an enzyme called cytochrome oxidase and stops cells from being able to use oxygen. The signs and symptoms of cyanide poisoning range from headache, difficulty in breathing and vomiting to unconsciousness and death. People who have recovered from cyanide poisoning do not usually suffer any long-term effects.
Cyanide can be metabolized rapidly and is generally converted to thiocyanate by an enzyme called rhodanese. Thiocyanate is much less toxic than cyanide and the body can then get rid of this. But (there had to be a but!) the enzyme needs another chemical, thiosulfate, to be able to do this and this can be used up quite quickly.
Cyanide and cyanide containing compounds are used in lots of industrial processes such as electroplating, chemical synthesis and fumigation. Some food types contain compounds called cyanogenic glycosides which can be converted to cyanide in the body; these include cassava roots, lima beans and bamboo shoots. In addition drugs such as sodium nitroprusside, sometimes used for the treatment of hypertension and Laetrile, an anti-cancer agent, also release cyanide into the circulation.
Cyanide salts are the forms which historically have been used for suicide and murder. They can't be bought as easily now by the public and so they aren't used much for this now. Nevertheless there are recent cases, for example in 1982 seven people in Chicago were killed by Tylenol tablets (painkillers) which were spiked with cyanide salts.
Figure 1: The effects of cyanide within the body. Hydrogen cyanide gas (HCN) is inhaled and locks onto haemoglobin, the oxygen-carrying molecule in red blood cells (bottom right). It is then distributed via the bloodstream to cells throughout the body where it binds to an important metabolic enzyme called cytochrome oxidase (bottom left), preventing cells from using oxygen to produce energy. In this way cyanide effectively chemically asphyxiates the body.
Figure 1: The effects of cyanide within the body. Hydrogen cyanide gas (HCN) is inhaled and locks onto haemoglobin, the oxygen-carrying molecule in red blood cells (bottom right). It is then distributed via the bloodstream to cells throughout the body where it binds to an important metabolic enzyme called cytochrome oxidase (bottom left), preventing cells from using oxygen to produce energy. In this way cyanide effectively chemically asphyxiates the body.
There are antidotes available for cyanide poisoning but they can have bad side effects or be toxic themselves. For example some drugs act to change normal hemoglobin to another form called methemoglobin, as cyanide has a high affinity for this type of hemoglobin, which binds the cyanide and stops it from reaching the tissues.
Unfortunately oxidized hemoglobin does not bind oxygen and so obviously it is not desirable to have too much of this form present. Extra thiosulfate can also be given so that more cyanide can be metabolized, this is often used with methemoglobin formers. Cobalt (II) compounds form cyanide complexes and these are used but they are highly toxic. Hydroxycobalamin (Vitamin B12a) which forms cyanocobalamin (Vitamin B12) has lower toxicity but it is not widely available. Due to the toxicity of these drugs it is important they are given in doses related to the amount of cyanide in the body.
So, yet another reason to make sure you've checked the batteries work in your smoke alarm!
Cyanide Poisoning | Science Features | Naked Scientistshttps://www.thenakedscientists.com/articles/science-features/cyanide-poisoning
物理应用(1985)
。2018年5月1日,124(5):1164 - 1176。doi: 10.1152 / japplphysiol.00967.2017。Epub 2018年2月8日
亚甲蓝对抗氰化物心脏毒性:细胞机制
摘要
在成年左心室小鼠心肌细胞中,暴露于葡萄糖剂量依赖性的氰化钠(NaCN)可降低收缩幅度,在100时达到约80%的最大抑制作用。NaCN暴露10min显著降低收缩和细胞内Ca2+浓度([Ca2+]i)的瞬时振幅,收缩期而非舒张期[Ca2+]i,以及最大l型Ca2+电流(ICa)振幅,提示急性改变[Ca2+]i稳态主要是观察到的兴奋-收缩异常的原因。此外,NaCN去极化静息膜电位(Em)、降低动作电位(AP)振幅、AP持续时间在50% (APD50)和90%复极化(APD90)延长,去极化激活的K+电流被抑制,但对Na+-Ca2+交换电流(INaCa)无影响。NaCN并不影响细胞三磷酸腺苷水平但线粒体膜电位去极化的(ΔΨm)和增加超氧化物(O2·)的水平。亚甲蓝(MB;20µg / ml)添加3分钟后NaCN恢复收缩和[Ca2 +]我瞬时振幅,收缩期[Ca2 +]i,AP振幅,APD50, APD90, ICa, depolarization-activated K +电流,ΔΨm和O2·——对正常水平。
我们的结论是,MB通过保持细胞内Ca2+稳态和兴奋-收缩耦合(ICa),最小化心律失常的风险(Em、AP配置和去极化激活的K+电流),以及降低O2·-水平,逆转了nacn诱导的心脏毒性。新的&值得注意的氰化物中毒由于工业接触,烟雾吸入和生物恐怖主义表现为心源性休克,需要迅速有效的解毒剂。在氰化物暴露的早期阶段,三磷酸腺苷水平正常,但心肌细胞收缩力降低,这主要是由于离子通道氧化还原环境的改变导致Ca2+稳态的改变。
亚甲基蓝,美国食品和药物管理局批准的药物,通过恢复氧化还原状态和Ca2+通道功能改善氰化物毒性。
关键词:钙;氰化物毒性;离子通道;线粒体;活性氧物种。J Appl Physiol (1985)
. 2018 May 1;124(5):1164-1176. doi: 10.1152/japplphysiol.00967.2017. Epub 2018 Feb 8.
Methylene blue counteracts cyanide cardiotoxicity: cellular mechanisms
Abstract
In adult left ventricular mouse myocytes, exposure to sodium cyanide (NaCN) in the presence of glucose dose-dependently reduced contraction amplitude, with ~80% of maximal inhibitory effect attained at 100 µM. NaCN (100 µM) exposure for 10 min significantly decreased contraction and intracellular Ca2+ concentration ([Ca2+]i) transient amplitudes, systolic but not diastolic [Ca2+]i, and maximal L-type Ca2+ current ( ICa) amplitude, indicating acute alteration of [Ca2+]i homeostasis largely accounted for the observed excitation-contraction abnormalities. In addition, NaCN depolarized resting membrane potential ( Em), reduced action potential (AP) amplitude, prolonged AP duration at 50% (APD50) and 90% repolarization (APD90), and suppressed depolarization-activated K+ currents but had no effect on Na+-Ca2+ exchange current ( INaCa). NaCN did not affect cellular adenosine triphosphate levels but depolarized mitochondrial membrane potential (ΔΨm) and increased superoxide (O2·-) levels.Methylene blue (MB; 20 µg/ml) added 3 min after NaCN restored contraction and [Ca2+]i transient amplitudes, systolic [Ca2+]i, Em, AP amplitude, APD50, APD90, ICa, depolarization-activated K+ currents, ΔΨm, and O2·- levels toward normal.
We conclude that MB reversed NaCN-induced cardiotoxicity by preserving intracellular Ca2+ homeostasis and excitation-contraction coupling ( ICa), minimizing risks of arrhythmias ( Em, AP configuration, and depolarization-activated K+ currents), and reducing O2·- levels. NEW & NOTEWORTHY Cyanide poisoning due to industrial exposure, smoke inhalation, and bioterrorism manifests as cardiogenic shock and requires rapidly effective antidote.
In the early stage of cyanide exposure, adenosine triphosphate levels are normal but myocyte contractility is reduced, largely due to alterations in Ca2+ homeostasis because of changes in oxidation-reduction environment of ion channels. Methylene blue, a drug approved by the U.S. Food and Drug Administration, ameliorates cyanide toxicity by normalizing oxidation-reduction state and Ca2+ channel function.
Keywords: calcium; cyanide cardiotoxicity; ion channels; mitochondria; reactive oxygen species.Methylene blue counteracts cyanide cardiotoxicity: cellular mechanisms - PubMed
https://pubmed.ncbi.nlm.nih.gov/29420146/