ĪĪ
Circulating Endothelial Progenitor Cells
1. EPC repair damaged EC
2.exercises increase level circulating EPC, by mobilizing release of EPC from bone marrow
3.vitamin D increase circulating EPC.
ĪĪ
ĪĪ
ĪĪ
Recent evidence indicates that circulating endothelial progenitor cells (EPCs), a population of bone marrow-derived cells, have an important role in the process of vascular repair, by promoting re-endothelialization following vascular injury [1]. EPCs are primarily identified by the expression of cell-surface antigenic markers, including CD133, CD34 and vascular endothelial growth factor receptor 2 (VEGFR-2), and have the ability to differentiate into mature cells with an endothelial phenotype [2]. Impairment of EPCs is related to endothelial dysfunction [3,4], coronary artery disease [5,6], heart failure [7] and adverse clinical outcome [8,9].
Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine - Asahara - 2011 - STEM CELLS - Wiley Online Library
https://stemcellsjournals.onlinelibrary.wiley.com/doi/full/10.1002/stem.745Effect of vitamin D on endothelial progenitor cells function https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5435351/
ĪĪ
Published: 27 September 2005
Circulating endothelial progenitor cells
B Garmy-Susini & J A Varner
British Journal of Cancer volume 93, pages855©C858(2005)Cite this article
Abstract
Angiogenesis research investigates the formation of new blood vessels in wound healing, tumour growth and embryonic development. Circulating, bone marrow-derived endothelial progenitor cells (EPCs) were first described 8 years ago, yet the exact nature of these endothelial precursor cells remains unclear. The contributions of circulating EPCs to angiogenesis in tumours, ischaemic injury and other diseases as well as their usefulness in the repair of wounded hearts and limbs remain under intense investigation.
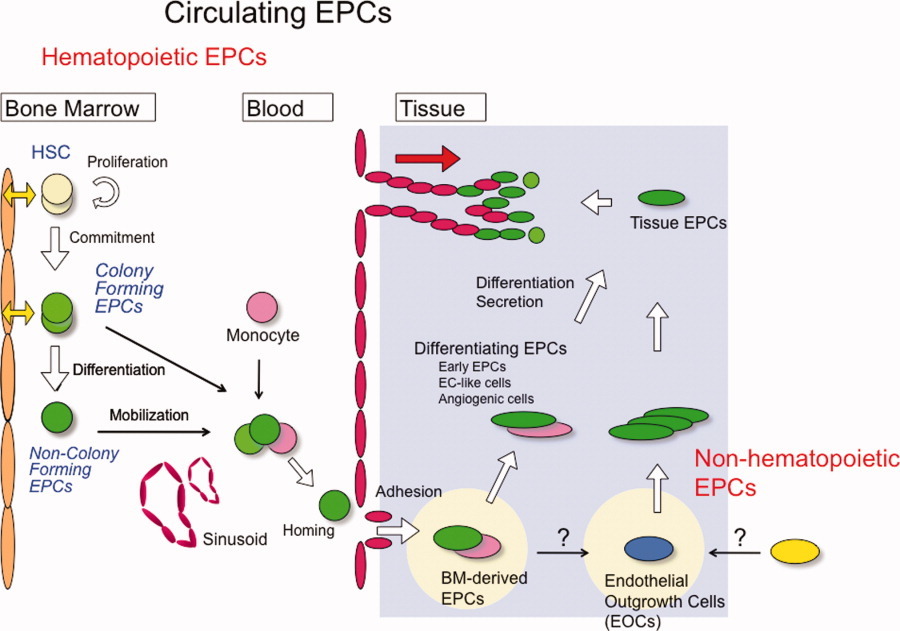
Bone marrow-derived endothelial progenitor cells (EPCs) contribute to tumour angiogenesis. Endothelial progenitor cells (purple circles) arise in the bone marrow as CD34+CD133+VEGFR2+ cells. These cells are induced to leave the bone marrow and enter the vasculature by circulating angiogenic factors such as vascular endothelial growth factor (VEGF). Once in the circulation, these cells can arrest at sites of ischaemia or growth factor release (such as VEGF release), such as in the tumour periphery. These cells then can participate in new vessel formation by differentiating into branching blood vessels.
Circulating endothelial progenitor cells | British Journal of Cancer
https://www.nature.com/articles/6602808ĪĪ
Cell Trafficking of Endothelial Progenitor Cells in Tumor Progression
Pilar de la Puente, Barbara Muz, Feda Azab and Abdel Kareem Azab
DOI: 10.1158/1078-0432.CCR-13-0462 Published July 2013
Abstract
Blood vessel formation plays an essential role in many physiologic and pathologic processes, including normal tissue growth and healing, as well as tumor progression. Endothelial progenitor cells (EPC) are a subtype of stem cells with high proliferative potential that are capable of differentiating into mature endothelial cells, thus contributing to neovascularization in tumors. In response to tumor-secreted cytokines, EPCs mobilize from the bone marrow to the peripheral blood, home to the tumor site, and differentiate to mature endothelial cells and secrete proangiogenic factors to facilitate vascularization of tumors.In this review, we summarize the expression of surface markers, cytokines, receptors, adhesion molecules, proteases, and cell signaling mechanisms involved in the different steps (mobilization, homing, and differentiation) of EPC trafficking from the bone marrow to the tumor site. Understanding the biologic mechanisms of EPC cell trafficking opens a window for new therapeutic targets in cancer. Clin Cancer Res; 19(13); 3360©C8. ©2013 AACR.
ĪĪ
Cell Trafficking of Endothelial Progenitor Cells in Tumor Progression | Clinical Cancer Research
https://clincancerres.aacrjournals.org/content/19/13/3360ĪĪ
Copyright ©2012 Baishideng Publishing Group Co., Limited. All rights reserved.
World J Cardiol. Dec 26, 2012; 4(12): 312-326
Published online Dec 26, 2012. doi: 10.4330/wjc.v4.i12.312
Circulating endothelial and progenitor cells: Evidence from acute and long-term exercise effects
Matina Koutroumpi, Stavros Dimopoulos, Katherini Psarra, Theodoros Kyprianou, Serafim Nanas
Matina Koutroumpi, Stavros Dimopoulos, Serafim Nanas, Cardiopulmonary Exercise Testing and Rehabilitation Laboratory, 1st Critical Care Medicine Department, Evangelismos Hospital, National and Kapodistrian University of Athens, 10676 Athens, Greece
Katherini Psarra, Department of Immunology, Evangelismos Hospital, 10676 Athens, Greece
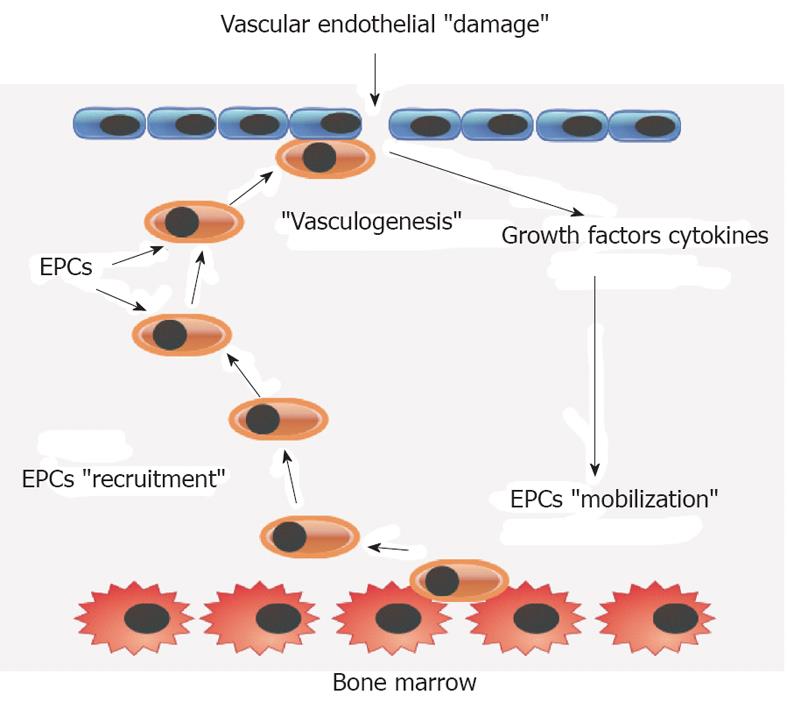
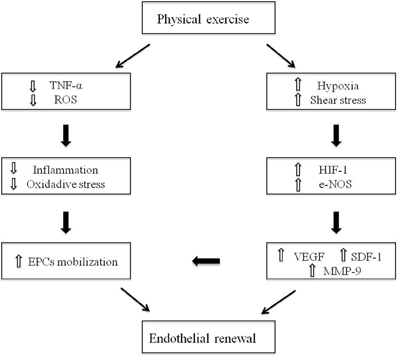
Theodoros Kyprianou, Critical Care Department, Nicosia General Hospital, 2029 Nikosia, CyprusCirculating bone-marrow-derived cells, named endothelial progenitor cells (EPCs), are capable of maintaining, generating, and replacing terminally differentiated cells within their own specific tissue as a consequence of physiological cell turnover or tissue damage due to injury.
Endothelium maintenance and restoration of normal endothelial cell function is guaranteed by a complex physiological procedure in which EPCs play a significant role.
Decreased number of peripheral blood EPCs has been associated with endothelial dysfunction and high cardiovascular risk.
In this review, we initially report current knowledge with regard to the role of EPCs in healthy subjects and the clinical value of EPCs in different disease populations such as arterial hypertension, obstructive sleep-apnea syndrome, obesity, diabetes mellitus, peripheral arterial disease, coronary artery disease, pulmonary hypertension, and heart failure.
Recent studies have introduced the novel concept that physical activity, either performed as a single exercise session or performed as part of an exercise training program, results in a significant increase of circulating EPCs.
In the second part of this review we provide preliminary evidence from recent studies investigating the effects of acute and long-term exercise in healthy subjects and athletes as well as in disease populations.
Key Words: Circulating endothelial cells, Circulating progenitor cells, Exercise, Cardiovascular disease
Circulating endothelial and progenitor cells: Evidence from acute and long-term exercise effects
https://www.wjgnet.com/1949-8462/full/v4/i12/312.htmĪĪ
Front. Physiol., 20 February 2012 | https://doi.org/10.3389/fphys.2012.00030
Endothelial progenitor cells enter the aging arena
K. Williamson, S. E. Stringer and M. Y. Alexander*
Cardiovascular Research Group, School of Biomedicine, University of Manchester, Manchester, UK
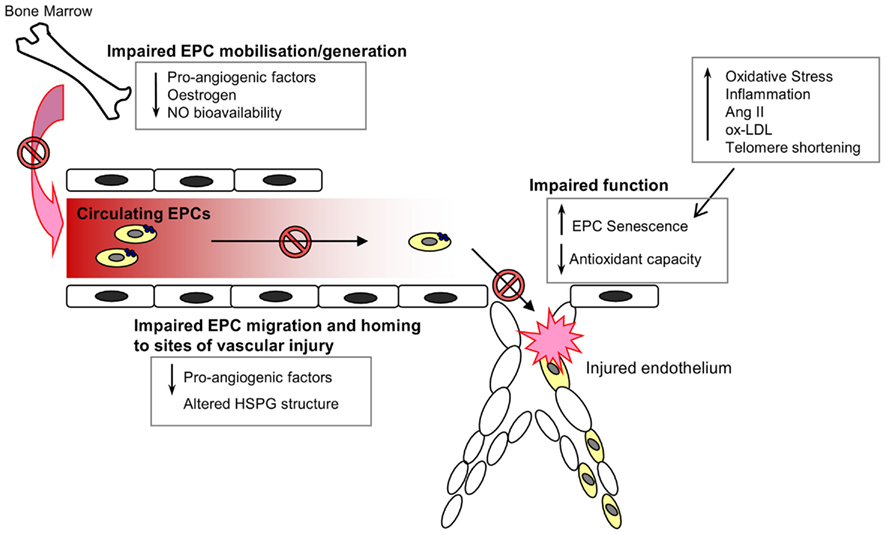
Age is a significant risk factor for the development of vascular diseases, such as atherosclerosis. Although pharmacological treatments, including statins and anti-hypertensive drugs, have improved the prognosis for patients with cardiovascular disease, it remains a leading cause of mortality in those aged 65 years and over. Furthermore, given the increased life expectancy of the population in developed countries, there is a clear need for alternative treatment strategies. Consequently, the relationship between aging and progenitor cell-mediated repair is of great interest. Endothelial progenitor cells (EPCs) play an integral role in the cellular repair mechanisms for endothelial regeneration and maintenance. However, EPCs are subject to age-associated changes that diminish their number in circulation and function, thereby enhancing vascular disease risk. A great deal of research is aimed at developing strategies to harness the regenerative capacity of these cells.In this review, we discuss the current understanding of the cells termed Ī░EPCs,Ī▒ examine the impact of age on EPC-mediated repair and identify therapeutic targets with potential for attenuating the age-related decline in vascular health via beneficial actions on EPCs.
Frontiers | Endothelial Progenitor Cells Enter the Aging Arena | Physiology
https://www.frontiersin.org/articles/10.3389/fphys.2012.00030/fullĪĪ
Published: 19 December 2017
Effects of resistance exercise on endothelial progenitor cell mobilization in women
Fernando Ribeiro, Ilda P. Ribeiro, Ana C. Gonçalves, Alberto J. Alves, Elsa Melo, Raquel Fernandes, Rui Costa, Ana B. Sarmento-Ribeiro, Jos©” A. Duarte, Isabel M. Carreira, Sarah Witkowski & Jos©” Oliveira
Scientific Reports volume 7, Article number: 17880 (2017) Cite this article
Abstract
This study aimed to determine the effect of a single bout of resistance exercise at different intensities on the mobilization of circulating EPCs over 24 hours in women. In addition, the angiogenic factors stromal cell-derived factor 1 (SDF-1”┴), vascular endothelial growth factor (VEGF), hypoxia-inducible factor 1-alpha (HIF-1”┴) and erythropoietin (EPO) were measured as potential mechanisms for exercise-induced EPCs mobilization. Thirty-eight women performed a resistance exercise session at an intensity of 60% (n = 13), 70% (n = 12) or 80% (n = 13) of one repetition maximum. Each session was comprised of three sets of 12 repetitions of four exercises: bench press, dumbbell curl, dumbbell squat, and standing dumbbell upright row. Blood was sampled at baseline and immediately, 6 hours, and 24 hours post-exercise.Circulating EPC and levels of VEGF, HIF-1”┴ and EPO were significantly higher after exercise (P < 0.05). The change in EPCs from baseline was greatest in the 80% group (P < 0.05), reaching the highest at 6 hours post-exercise. The change in EPCs from baseline to 6 hours post-exercise was correlated with the change in VEGF (r = 0.492, P = 0.002) and HIF-1”┴ (r = 0.388, P = 0.016). In general, a dose-response relationship was observed, with the highest exercise intensities promoting the highest increases in EPCs and angiogenic factors.
Effects of resistance exercise on endothelial progenitor cell mobilization in women | Scientific Reports
https://www.nature.com/articles/s41598-017-18156-6ĪĪ
Front. Physiol., 03 February 2014 | https://doi.org/10.3389/fphys.2013.00414
Effects of physical activity on endothelial progenitor cells (EPCs)
Chiara De Biase, Roberta De Rosa, Rossella Luciano, Stefania De Luca, Ernesto Capuano, Bruno Trimarco and Gennaro Galasso*
Department of Advanced Biomedical Sciences, Ī░Federico IIĪ▒ University of Naples, Naples, Italy
Physical activity has a therapeutic role in cardiovascular disease (CVD), through its beneficial effects on endothelial function and cardiovascular system. Circulating endothelial progenitor cells (EPCs) are bone marrow (BM) derived cells that represent a novel therapeutic target in CVD patients, because of their ability to home to sites of ischemic injury and repair the damaged vessels.Several studies show that physical activity results in a significant increase in circulating EPCs, and, in particular, there are some evidence of the beneficial exercise-induced effects on EPCs activity in CVD settings, including coronary artery disease (CAD), heart failure (HF), and peripheral artery disease (PAD).
The aim of this paper is to review the current evidence about the beneficial effects of physical exercise on endothelial function and EPCs levels and activity in both healthy subjects and patients with CVD.
ĪĪ
Frontiers | Effects of physical activity on endothelial progenitor cells (EPCs) | Physiology
https://www.frontiersin.org/articles/10.3389/fphys.2013.00414/fullĪĪ
Nature
Published: 20 January 2016
Angiocrine functions of organ-specific endothelial cells
Shahin Rafii, Jason M. Butler & Bi-Sen Ding
Nature volume 529, pages316©C325(2016)Cite this articleDepartment of Medicine, Division of Regenerative Medicine, Ansary Stem Cell Institute, Weill Cornell Medical College, 1300 York Avenue, New York, 10065, New York, USA
Abstract
Endothelial cells that line capillaries are not just passive conduits for delivering blood. Tissue-specific endothelium establishes specialized vascular niches that deploy sets of growth factors, known as angiocrine factors. These cues participate actively in the induction, specification, patterning and guidance of organ regeneration, as well as in the maintainance of homeostasis and metabolism.When upregulated following injury, they orchestrate self-renewal and differentiation of tissue-specific resident stem and progenitor cells into functional organs. Uncovering the mechanisms by which organotypic endothelium distributes physiological levels of angiocrine factors both spatially and temporally will lay the foundation for clinical trials that promote organ repair without scarring.
Angiocrine functions of organ-specific endothelial cells | Nature
https://www.nature.com/articles/nature17040ĪĪ