Antiviral Properties of Lactoferrin 乳铁蛋白的抗病毒特性
乳铁蛋白-A天然免疫分子的抗病毒特性
意大利罗马Sapienza大学
Antiviral Properties of Lactoferrin-A Natural Immunity Molecule
Lactoferrin, a multifunctional iron binding glycoprotein, plays an important role in immune regulation and defence mechanisms against bacteria, fungi and viruses.
Sapienza University of Rome, Italy
乳铁蛋白是一种多功能的铁结合糖蛋白,在针对细菌,真菌和病毒的免疫调节和防御机制中起着重要作用。乳铁蛋白的铁保留能力与抑制微生物生长以及调节病原菌的运动性,聚集性和生物膜形成有关。
乳铁蛋白与铁的结合能力与它与微生物,病毒和细胞表面相互作用,从而抑制微生物和病毒的粘附并进入宿主细胞有关。乳铁蛋白不仅可以被认为是抵抗粘膜感染的主要防御因素,而且可以被认为是在病毒感染过程中相互作用的多价调节剂。
已证明在感染的早期阶段,对包膜病毒和裸露病毒均具有抗病毒活性,因此可防止病毒进入宿主细胞。通过与硫酸乙酰肝素糖胺聚糖(HSPG)细胞受体或病毒颗粒或两者结合而发挥这种活性。尽管乳铁蛋白具有抗病毒作用,体外研究已得到广泛证实,但很少进行临床试验,相关作用机制仍在争论中。
乳铁蛋白在不同上皮人类细胞中的核定位表明,乳铁蛋白不仅 早期在细胞表面与病毒的相互作用发挥作用,而且在细胞内发挥抗病毒作用。
乳铁蛋白通过与宿主细胞和/或病毒颗粒结合而发挥有效的抗病毒活性的能力,以及其核定位增强了这样的观点,即乳铁蛋白是粘膜壁上的重要砖块,有效抵抗病毒攻击,并且可能有用被用作治疗病毒感染的新策略。(PDF)乳铁蛋白-A天然免疫分子的抗病毒特性
Antiviral Properties of Lactoferrin-A Natural Immunity Molecule
Antiviral Properties of Lactoferrin-A Natural Immunity Molecule
Sapienza University of Rome, Italy
ABSTRACT
Lactoferrin, a multifunctional iron binding glycoprotein, plays an important role in immune regulation and defence mechanisms against bacteria, fungi and viruses. Lactoferrin's iron withholding ability is related to inhibition of microbial growth as well as to modulation of motility, aggregation and biofilm formation of pathogenic bacteria. Independently of iron binding capability, lactoferrin interacts with microbial, viral and cell surfaces thus inhibiting microbial and viral adhesion and entry into host cells. Lactoferrin can be considered not only a primary defense factor against mucosal infections, but also a polyvalent regulator which interacts in viral infectious processes. Its antiviral activity, demonstrated against both enveloped and naked viruses, lies in the early phase of infection, thus preventing entry of virus in the host cell. This activity is exerted by binding to heparan sulphate glycosaminoglycan cell receptors, or viral particles or both. Despite the antiviral effect of lactoferrin, widely demonstrated in vitro studies, few clinical trials have been carried out and the related mechanism of action is still under debate. The nuclear localization of lactoferrin in different epithelial human cells suggests that lactoferrin exerts its antiviral effect not only in the early phase of surface interaction virus-cell, but also intracellularly. The capability of lactoferrin to exert a potent antiviral activity, through its binding to host cells and/or viral particles, and its nuclear localization strengthens the idea that lactoferrin is an important brick in the mucosal wall, effective against viral attacks and it could be usefully applied as novel strategy for treatment of viral infections.(PDF) Antiviral Properties of Lactoferrin-A Natural Immunity Molecule.
Molecules 2011, 166993that lactoferrin is an important brick in the mucosal wall, effective against viral attacks andit could be usefully applied as novel strategy for treatment of viral infections.Keywords: lactoferrin; virus; viral infection1. IntroductionLactoferrin was identified in 1939 in bovine milk [1] and isolated in 1960 from both human [2,3]and bovine milk [4]. Lactoferrin, highly conserved among human, bovine, mouse, and porcine species,is a glycoprotein of about 690 amino acid residues belonging to the transferrin family, able toreversibly chelate two Fe(III) per molecule with high affinity (Kd ~ 10−20 M) retaining ferric iron to pHvalues as low as 3.0, whereas transferrin retains iron to pH of about 5.5 [5,6]. The iron-binding affinityis high enough that, in the presence of lactoferrin or transferrin, the concentration of free iron in bodyfluids cannot exceed 10–18 M, thus preventing the precipitation of this metal as insoluble hydroxides,inhibiting microbial growth and hindering formation of reactive oxygen species. As is apparent fromthree dimensional (3D) structure of human lactoferrin (hLf) [7,8], the molecule is folded into twohomologous lobes (N-lobe residues 1–333 and C-lobe residues 345–691). The two lobes are connected1. Introduction Lactoferrin was identified in 1939 in bovine milk [1] and isolated in 1960 from both human [2,3] and bovine milk [4]. Lactoferrin, highly conserved among human, bovine, mouse, and porcine species, is a glycoprotein of about 690 amino acid residues belonging to the transferrin family, able to reversibly chelate two Fe(III) per molecule with high affinity (Kd ~ 10−20 M) retaining ferric iron to pH values as low as 3.0, whereas transferrin retains iron to pH of about 5.5 [5,6]. The iron-binding affinity is high enough that, in the presence of lactoferrin or transferrin, the concentration of free iron in body fluids cannot exceed 10–18 M, thus preventing the precipitation of this metal as insoluble hydroxides, inhibiting microbial growth and hindering formation of reactive oxygen species. As is apparent from three dimensional (3D) structure of human lactoferrin (hLf) [7,8], the molecule is folded into two homologous lobes (N-lobe residues 1–333 and C-lobe residues 345–691). The two lobes are connected by a peptide (residues 334–344), which forms a 3-turn α-helix, whereas the peptide in transferrin is irregular and flexible. There are non-covalent interactions, mostly hydrophobic, where the two lobes pack together (Figure 1).
Figure 1. Structure of lactoferrin. From Baker and Baker [9].
The amino acid sequence of hLf [10] has a high degree of identity with human transferrin (~60%), and the characteristic twofold internal sequence repeat suggests an ancestral gene duplication. The N and C-terminal halves have ~40% sequence identity.
Molecules 2011, 16 69942. Structure 2.1. Iron-Binding Sites The two lobes of lactoferrin are further divided into two domains (N1 and N2, C1 and C2) and each lobe binds one Fe(III) ion in a deep cleft between two domains (Figure 1). The iron sites are highly conserved in all iron-binding proteins, suggesting a common evolutionary origin [11,12]. The ligands for Fe(III) are the same in both lobes: One aspartic acid, two tyrosines, and one histidine (Asp-60, Tyr-92, Tyr-192, and His-253 in the N-lobe and Asp-395, Tyr-433, Tyr-526, and His-595 in the C-lobe), together with two oxygens from the CO32− anion (Figure 2). Figure 2. Iron binding site in the N-lobe of lactoferrin. From Baker and Baker [9]. Spectroscopic studies and the 3D structure suggest that the CO32− ion binds first, thus neutralizing the positive charge of the arginine residue (Arg-121 in the N-lobe and Arg-465 in the C-lobe) [6,13]. The participation of the CO32− ion in the iron coordination binding appears to be ideal for iron reversible binding [6] since the protonation of CO32− ion is a likely first step in the breakup of the iron site at low pH [14]. 2.2. Conformational Changes Iron binding and release are associated with large conformational changes in which lactoferrin adopts either an open or closed state. The iron-saturated form is closed and much more compact than the apo form [15]. In apo-lactoferrin, the N-lobe is in an open state, while the C-lobe is still closed, thus providing an important clue to the dynamic behaviour of the apo-protein. However, other crystal structures of lactoferrin of different species show some diversity in C-lobe that can adopt open forms, through the same kind of conformational change as was seen for the N-lobe [13].
he comparisons of structural and functional data on lactoferrin and transferrin have suggested the importance of cooperative interactions between the two lobes of the molecule, mediated by the α-helices. Both lactoferrin and transferrin share the property that their bound Fe(III) is spontaneously released in vitro at low pH. For transferrin, the iron release at low pH is considered to be important for iron delivery to cells [16]. The ferri-transferrin is internalized by transferrin receptor-mediated endocytosis, and then iron is released and the receptor is recycled to the cell surface. Although there are indications that the transferrin receptor plays an active part in this process, the ability of transferrin to begin releasing iron at the endosomal pH of about 5.5 is also a critical factor. In contrast, lactoferrin retains Fe(III) to much lower pH, approximately 3.0. The key difference between lactoferrin and transferrin appears to be a cooperative interaction between the two lobes in lactoferrin that does not occur in transferrin. Iron release from the isolated N-lobe of lactoferrin begins at pH 5.0 [17], similarly to transferrin (pH 5.5). It can be supposed that in the absence of the lactoferrin C-lobe, Fe(III)-binding is substantially destabilized. Furthermore, studies on mutant lactoferrin have shown that when Fe(III)-binding in the N-lobe is disabled, Fe(III)-binding in the C-lobe is unaffected, while, when binding in the C-lobe is disabled, Fe(III)-binding in the N-lobe is destabilized, occurring at pH~5.0 [18]. Therefore, there are cooperative interactions between the two lobes of lactoferrin through which Fe(III)-binding in the C-lobe stabilizes Fe(III)-binding in the N-lobe. Conversely, isolated N lobe of transferrin has an iron release at a pH identical to that of the intact protein. Therefore, the lactoferrin structure suggests that the C-terminal helix, which contacts the N-lobe close to the hinge, plays a very important role [19]. As in all lactoferrins, the linker peptide between the two lobes forms an α-helix, whereas in all transferrins has a flexible, extended and irregular structure. It can be hypothesized that the rigidity of the helical linker in lactoferrins allows a stronger interaction between the two lobes that stabilizes Fe(III)-binding in the N-lobe delaying the iron release at low pH [14].
2.3. Binding of other Metals
Lactoferrin is classified as an iron binding protein, but can also bind other metal ions including Cu2+, Mn2+, Zn2+, even if with lower affinity. Metal binding can be assayed by an increase in adsorption at 240–280 nm as consequence of ionization of the tyrosine ligands which bind to the metal ions [13]. The crystal structures of lactoferrin saturated with Mn2+ or Zn2+ have all shown closed forms, thus suggesting that lactoferrin could possess a role in binding other metal ions [9]. Moreover, it has been demonstrated that Mn2+- or Zn2+- saturated forms maintain some physiological functions of lactoferrin, unrelated to its iron binding capability [20] but probably related to its three remarkable concentrations of positive charge: Residues 1–7, 13–30 and inter-lobe region, close to the connecting helix [9]. 2.4. Glycosylation Lactoferrin is a glycosylated protein, possessing different number and location of putative glycosylation sites, according to different species [9]. In particular, hLf possesses three glycosylation sites (Asn-137, Asn-478 and Asn-623) and bovine lactoferrin (bLf) five (Asn-233, Asn-368, Asn-476 and Asn-545). The nature and the location of the glycosylation sites do not influence the polypeptide Molecules 2011, 16 6996folding or iron and other molecules binding properties. Conversely, the loss of carbohydrate or sialic acid increases its sensitivity to proteolysis [9] or influences some physiological functions [20].
3. Human and Bovine Lactoferrin Gene Structure and Regulation
hLf gene maps to human chromosome 3p21.3 [21], while bLf gene is localized to chromosome 22 and syntenic group U12 [22]. The lactoferrin gene is organized into 17 exons. The size of the gene varies from 23 to 35 kb among human [23-25] and bovine species [26]. The signal peptide of lactoferrin consists of 19 amino acids, 11 of which are conserved within these two species. The first five amino acids of bovine protein include two basic amino acids, whereas the human protein begins at glycine and follows with four arginines, which make the hLf unique. The numbers of amino acids encoded by 15 of the 17 exons in these species are identical, and in 12 locations they have identical codon interruptions at the intron-exon splice junctions. Comparing the lactoferrin gene promoters from different species, common and different characteristics are observed. The hLf and bLf promoters contain a non-canonical TATA box, but only hLf has multiple steroid hormone response elements, different species, common and different characteristics are observed. The hLf and bLf promoters contain a non-canonical TATA box, but only hLf has multiple steroid hormone response elements, while none are found in the other species studied, suggesting that the lactoferrin gene is differentially regulated among different species by steroid hormones [27]. The hLf gene expression is upregulated by estrogen with a magnitude of response that is cell-type specific (mammary glands, uterus) and by retinoic acids.
4. Concentrations in Human Body
Lactoferrin is expressed and secreted by glandular epithelial cells and by neutrophils. The highest levels (~7 g/L) is found in human colostrum [28], while it is also present at lower levels in mature milk, in most exocrine secretions (Table 1), and in the secondary granules of mature neutrophils [29,30]. Lactoferrin concentration increases in infection and/or inflammation sites due to the recruitment of neutrophils. 106 neutrophils synthesize 15 μg of lactoferrin
5. Lactoferrin as Human Innate Defence against Infections
Even if lactoferrin and transferrin are similar in many respects, they possess different functions: Transferrin seems to exert a pivotal role in iron uptake by cells, whereas lactoferrin, which is found in many mucosal secretions, can be considered an important brick in the mucosal wall exerting a potent protective function. Unlike transferrin, the capability of lactoferrin to retain iron at acid pH, which characterizes infection and inflammation sites, together with its cationic nature (pI ~ 9) may be responsible for its ability to bind to various microbial and viral negative surface structures [31-33], and to anionic molecules such as DNA [34], heparin [35], glycosaminoglycans [36] could explain the different functions ascribed to this protein (Figure 3). Figure 3. Distribution of surface charge of human lactoferrin. Blue: positive; red: negative. From Baker and Baker [9]. In mucosal secretions, which first have been injured by microorganisms, iron limitation (10–18 M) is considered in the healthy humans a physiological status hindering microbial growth. Conversely, an increase of iron concentration in the secretions, as a consequence of some pathologies, favours microbial virulence [37]. In human mucosa, peptides and proteins, including lactoferrin, symbolize the bricks of natural non-immune defences against microbial infections [38].
6. Antibacterial Activity of Lactoferrin Related and Unrelated to Its Iron Withholding Ability
The first function attributed to lactoferrin was antibacterial activity depending on its ability to sequester iron necessary for bacterial survival and growth [39]. This action of lactoferrin was considered bacteriostatic, as reversible by the addition of ferric iron [40]. However, bacterial pathogens are able to overcome iron limitation by means of two principal systems. The first is represented by the synthesis of small chelators, siderophores, which bind ferric iron with high affinity and transport it into bacteria through a specific receptor [41,42]. In addition to the synthesis of siderophores, some highly host-adapted bacterial species acquire iron directly through surface receptors able to specifically bind lactoferrin, and transport it across the outer membrane. The iron is bound by a periplasmic iron-binding protein, FbpA, and transported into the cell via an inner membrane complex comprised of FbpB and FbpC [43]. A lactoferrin bactericidal iron independent effect was also described [44]. A direct interaction between lactoferrin and lipopolysaccharide (LPS) of Gram-negative or lipoteichoic acid of Gram positive bacteria is required for the lethal effect [45-47]. Furthermore, it has been demonstrated that lactoferrin binds to the lipid A of LPS [48,49], inducing a release of LPS. This bactericidal activity of lactoferrin appears to be located in the N-terminal region as its derivative cationic peptide, called lactoferricin (Lfcin), is several fold more active than the intact protein [50-52]. However, the release of LPS can be annulled by high calcium concentration in the culture media [53]. As lactoferrin is also able to bind Ca(II) through the carboxylate groups of the sialic acid residues, present on two glycan chains, it cannot be ruled out that the release of LPS from Gram-negative bacteria can be also due to this additional binding property of lactoferrin [53].
7. Inhibition of Viral Infections by Lactoferrin
The antiviral activity of hLf was first demonstrated in mice infected with the polycythemia inducing strain of the Friend virus complex (FVC-P) [54]. Since 1994, a potent antiviral activity of both hLf and bLf against enveloped and naked viruses has been shown [55]. In most of these studies, when lactoferrin was tested both in apo- and in metal-saturated forms, no striking differences in the antiviral effect between the different forms were reported. Both lactoferrins act in the early phase of the viral infection thus preventing entry of virus into the host cell, either by blocking cellular receptors or by direct binding to virus particles [20]. bLf is often reported to exhibit higher antiviral activity than hLf [56]. Concerning lactoferricin, a pepsin-digested lactoferrin derivative, the antiviral activity of this highly positively charged loop domain of lactoferrin, was demonstrated for the first time by Andersen and co-workers [57] against human cytomegalovirus (HCMV) infection in vitro.
7.1. Herpesvirus
The in vitro activity of hLf and bLf against human cytomegalovirus (HCMV) infection has been described in 1994 [55]. Successively, other studies showed that both lactoferrin and cyclic lactoferricin prevented HCMV entrance into the host cells [58]. It has been reported that when negatively charged groups were added to lactoferrin by succinylation, the antiviral potency was mostly decreased, whereas the addition of positive charges through amination of the protein resulted in an increased anti-HCMV activity [57]. Successively other authors confirmed that lactoferrin inhibit the early steps of cytomegalovirus infection and that the antiviral effect is due to its cationic properties [59]. hLf as well as bLf, independently of iron-saturation or the presence of sialic acid, inhibited infection and replication of HSV-1 in human embryo lung cells [55]. Both hLf and bLf were found to prevent HSV-1 and HSV-2 cytopathic effect and yield in Vero cells [60,61].
The effectiveness of apo-lactoferrin on HSV-1 and HSV-2 infection was compared with that of metal ion saturated forms [Fe(III)-, Mn(II)-, Zn(II)-lactoferrin] and results of this study showed that the antiviral effect of the differently saturated bLf towards both viruses was mainly exerted during the initial viral adsorption phase [61]. In the attempt to identify the regions of lactoferrin responsible for the anti-HSV-1 activity, the inhibiting effect of peptide fragments, derived from the tryptic digestion of bLf, were also analyzed [62]. Among high molecular weight peptides, one fragment corresponding to the C-lobe was ten-fold more effective than another one corresponding to a large portion of the N-lobe. On the other hand, this last one was still six-fold less active than native bLf [62,63]. Two negatively charged small peptides deriving from N-lobe, previously shown effective on HSV-1 infection, have been further studied and results of this research demonstrated that the net negative charge of these peptides was not responsible for the antiviral activity [64]. It is well known that the initial attachment of HSV to cells occurs through binding of the viral glycoprotein(s) gC or gB to heparan sulfate of host cells. In the absence of HS, virus can bind to chondroitin sulfate proteoglycans, although with lower efficiency [65]. Marchetti and co-workers [66] demonstrated that bLf was a strong inhibitor of HSV-1 infection in cells expressing either heparan sulfate or chondroitin sulfate or both, but was ineffective or less efficient in glycosaminoglycan deficient cells or in cells treated with glycosaminoglycan-degrading enzymes, suggesting that the anti-HSV-1 activity of lactoferrin is dependent on its interaction with cell surface glycosaminoglycan chains of heparan sulfate and chondroitin sulfate [65]
The mechanism of inhibiting activity of both hLf and bLf against HSV-2 has been further investigated [67]. The antiviral effect of these proteins towards HSV-2 strain and its glycoprotein C (gC)-truncated derivative HSV-2 gC-neg1 has been tested in monkey kidney cells. The results indicated that the antiviral activity of bLf does not involve gCeHS interaction as there was no difference in its effectiveness towards wild type and mutant virus. As regards hLf, the mutant virus HSV-2 gC-neg1 was more sensitive compared with the wild type, suggesting that the human protein might interact with some viral structures that in wild-type viruses are masked by gC. When the modulation of HSV-2 infection by bLf and hLf was investigated under different experimental conditions, the bovine protein proved more effective than the human protein. Moreover, differently from what observed with HSV-1, bLf inhibited HSV-2 plaque-forming activity also in cells devoid of GAG expression, thus suggesting that bLf may block a virus receptor of non-GAG nature [67]. This observation adds new information on the anti herpes virus activity of this protein, confirming it as an outstanding candidate for the treatment of herpetic infections. Concerning hLf, in addition to inhibiting the adsorption and post-attachment events of HSV-1 infection, hLf is also able to neutralize HSV-1 and that the inhibition of cell-to-cell spread involves viral gD [68]. Andersen and co-workers [69] demonstrated that bovine Lfcin inhibited HSV-1 and HSV-2 infection probably by blocking the entry of the virus and that the human homolog (amino acids 18–42), which shares 36% sequence similarity with Lfcin (amino acids 17–41), displayed much lower antiviral activity. The same authors [70] demonstrated that also Lfcin was dependent on the presence of heparan sulfate at the cell surface to exert its antiviral activity. Other studies demonstrated that lactoferrin and several of the Lfcin derivatives exhibited similar affinity for heparan sulfate, but the lactoferrin proteins were more active compared with the smaller peptides [71]. The antiviral activity has also been reported for human Lfcin [72]. The anti-HSV activity of Lfcin seems to involve viral interaction with the cell surface glycosaminoglycan heparan sulfate, thereby blocking viral entry. Lfcin inhibited cell-to-cell spread of both HSV-1 and HSV-2. Inhibition of cell to-cell spread by bovine Lfcin involved cell surface chondroitin sulfate. Based on transmission electron microscopy studies, human Lfcin, like bovine Lfcin, was randomly distributed intracellularly, thus differences in their antiviral activity could not be explained by differences in their distribution. In
Molecules 2011, 16 7000contrast, the cellular localization of iron-saturated (holo)-lactoferrin appeared to differ from that of apo-lactoferrin, indicating that holo- and apo- lactoferrin may exhibit different antiviral mechanisms [72]. Lactoferrin and Lfcin agaist HSV-1 cellular uptake and intracellular trafficking were further studied by immunofluorescence microscopy. In comparison to the untreated infected control cells, both the bLf- and bovine Lfcin-treated cells showed a significant reduction in HSV-1 cellular uptake. The few virus particles that were internalized appeared to have a delayed intracellular trafficking. Thus, in addition to their interference with the uptake of the virus into host cells, lactoferrin and Lfcin also exert their antiviral effect intracellularly [73,74]. Concerning in vivo studies against CMV, experiments in BALB/c mice showed that the administration of bLf, before murine CMV infection, completely protected mice from death [75]. Successively, other authors also analyzed the anti-cytomegalovirus activity of lactoferrin in vivo in rat models with and without immune suppression, demonstrating that treatment with lactoferrin (intravenously) was helpful when infection was initiated with cell-free virus, but not with virus infected leukocytes and that lactoferrin exerted its effects via inhibition of cell entry rather than via stimulation of the immune system [59]. In in vivo studies on HSV-1, it has been demonstrated that topical administration of 1% bLf, prior to the virus inoculation, suppressed HSV-1 infection in the mouse cornea but not viral propagation [76]. The influence of bLf feeding on the HSV-1 cutaneous infection of mice has been evaluated and results of this study, in which mice were infected with HSV-1 ten days after lactoferrin administration, showed that lactoferrin inhibited the appearance of skin lesions [77]. Recently, it has been also reported that cervicovaginal lavage differently inhibited HSV infection by a mean value of approximately 57% during the follicular or luteal phase, but only by 36% in hormonal contraceptive users [78]. Being lactoferrin synthesis under steroid control, its influence on the antiviral activity of cervical fluids cannot be ruled out. Concerning animal herpes virus, it has been reported that exposure of susceptible cells to bLf prior or during viral adsorption strongly inhibited feline herpes virus 1 (FHV-1) replication [79]. Other studies demonstrated that both the apo- and holo-lactoferrin inhibited canine herpes virus multiplication in Madin-Darby canine kidney (MDCK) cells [80].
7.2. Human Immunodeficiency Virus (HIV)
It has been demonstrated that both bLf and hLf were able to inhibit the HIV-1-induced cytopathic effect. Addition of negatively charged groups to lactoferrin by succinylation resulted in a strong antiviral effect on HIV-1 and HIV-2, while the addition of positive charges to lactoferrin through amination resulted in a loss of anti-HIV activity [58,81,82]. Both HIV-1 replication and syncytium formation were efficiently inhibited, in a dose-dependent manner, by apo- or holo-, Mn(II) and Zn(II)- lactoferrin [83]. Other studies demonstrated that bLf strongly inhibited viral reverse transcriptase but only slightly inhibited HIV-1 protease and integrase [84]. Studies on bLf-resistant HIV-1 variants showed that the viral envelope protein, which contains two mutations that are associated with an altered virus-host interaction and a modified receptor-co-receptor interaction, mediated the bLf resistance phenotype demonstrating that bLf targeted the HIV-1 entry process [85]. Recently, when proteins from milk and serum were tested for their ability to block dendritic cell-mediated HIV-1
Molecules 2011, 16 7001transmission, bLf turned out to be the most potent inhibitor [86]. Finally, a synergy between lactoferrin in combination with Zidovudine against HIV-1 replication in vitro, has been reported [87]. It has been reported that oral administration of bLf suppressed oral inflammation in feline immunodeficiency virus FIV-infected cats with intractable stomatitis infection. This result suggests that bLf therapy may have a potential application to improve and protect functions of overactivated lymphocytes by modulating the cell proliferation, cell cycle and cytokines expression as shown in cats in terminal stage of FIV infection [88].
7.3. Friend Virus Complex (FVC)
The effects of lactoferrin treatment on the development of erythroleukemia in the spleen of mice infected with FVC were studied [89]. The treatment was started at days 7 and 14 before viral infection and days 0, 1, 3, 7, and 11 after viral infection, and in the spleens were analyzed 14 days after infection. In mice whose treatment was initiated at days 0 and 1 few leukemic cells were present in the spleen whereas in mice whose treatment was initiated at day 3 leukemic cells began to spread out in the red pulp and encroached upon the white pulp and in mice whose treatment was initiated at days 7 and 11 many leukemic cells were present in the red pulp. The morphologic features of the spleen in animals, whose treatment was initiated at day 7 or 14 before viral infection, were similar to those of untreated control groups [89]. Results of another in vivo study suggested that the protective effect of holo lactoferrin was probably due to an action on cells responding to the FVC or to an action on cells which influence the cells responding to the FVC or which influence the virus [54]. Finally, holo-lactoferrin and recombinant murine (rmu) interferon γ (IFNγ), alone or in combination, were used to influence disease progression in mice infected with the polycythemia-inducing strain of the Friend virus complex (FVC-P). Results of this study showed that spleen focus forming virus (SFFV) titers and levels of SFFV mRNA and genomic DNA dramatically decreased in mice treated with the combination of lactoferrin and rmu-IFNγ. Moreover, the combined treatment also enhanced the survival rates of FVC P-infected mice, suggesting a synergistic suppressive effect of lactoferrin with rmu-IFNγ on disease progression in FVC-P-infected mice [90].
7.4. Human Hepatitis C Virus (HCV)
Both bLf and hLf effectively prevented human hepatitis C virus (HCV) infection in cultured human hepatocytes (PH5CH8), bLf being the most active. In this study, a direct interaction between lactoferrins and E1 and E2 HCV envelope proteins has been reported [91]. It has been also demonstrated that pre-incubation of HCV with bLf inhibits viral infection, while cell pretreatment with bLf was ineffective [92]. Further studies demonstrated that bLf inhibited HCV entry into the cells by interacting with viral particles immediately after mixing of bLf and HCV inoculum [93]. Nozaki and co-workers [94] better characterized the binding activity of lactoferrin to hepatitis C virus E2 envelope protein and determined the region of lactoferrin important for this activity. Results from this study provided the first identification of a natural protein-derived peptide that, specifically binding HCV E2 protein, prevented HCV infection [94].
Molecules 2011, 16 7002Successively, 33 amino acid residues (termed C-s3-33; amino acid 600-632) from hLf were found to be primarily responsible for the binding activity to the HCV E2 envelope protein and for the inhibiting activity against HCV infection. If this sequence was repeated two or three times, two or three C-s3-33 repeated sequences possessed a stronger antiviral activity than of C-s3-33, thus suggesting that tandem repeats of lactoferrin-derived anti-HCV peptide are useful as anti-HCV reagents [95]. Other two helical peptides deriving from lactoferrin were found to bind hepatitis C virus envelope protein E2 [96]. Concerning in vivo studies, following the first pilot study of Tanaka and co-workers [97], a trial was designed to evaluate the relationship between the dose of bLf and its effect on serum alanine aminotransaminase and HCV RNA levels in forty-five patients with chronic hepatitis C [98]. The excellent tolerance and potential anti-HCV activity of bLf shown in this trial suggested that further trials using a large number of patients were obligatory. In a successive study the effects of long-term oral administration of bLf on serum parameters in patients with chronic hepatitis C have been analyzed and results obtained suggested that oral administration of lactoferrin induced a Th1-cytokine dominant environment in the peripheral blood so favouring the eradication of HCV by a combined interferon therapy [99]. It has also been demonstrated that an elevated percentage of HCV infected patients were endotoxemic [100]. These patients were poor responders to the IFNα/ribavirin treatment and exhibited high serum levels of lactoferrin antibodies that affected the antiviral activity of lactoferrin and abrogated the lactoferrin binding to lipopolysaccharides. This interaction inhibited the binding of lipopolysaccharide to lipopolysaccharide-binding protein, thus preventing its fixation to CD14 (+) cells and leading to a reduced release of pro-inflammatory cytokines [101]. Concerning the effectiveness of oral bLf mono therapy, in a clinical trial patients with chronic hepatitis C randomly received either oral bLf at a dose of 1.8 g daily for 12 weeks, or an oral placebo. There was no significant difference in viral response rates between the two groups, indicating any significant bLf efficacy in patients with chronic hepatitis C [102]. Different results have been obtained by comparing the viral response to bLf mono therapy at higher doses (daily dose of 3.6 g instead of 1.8 g) for 8 weeks followed by bLf, interferon and ribavirin combined therapy for 24 weeks. The results showed that the decrease in HCV RNA titer by lactoferrin mono therapy contributes to the effectiveness of the combined therapy of interferon and ribavirin in patients with chronic hepatitis C [103]. An interesting observational study has been reported on Egyptian patients feed with camel milk which contains lactoferrin. In in vitro model, purified camel lactoferrin interacts with HCV, thus leading to a complete virus entry inhibition [104].
7.5. Human Hepatitis B Virus (HBV)
Lactoferrin also prevents HBV infection in cultured cells and, differently to HCV, cell pretreatment with lactoferrin was required to inhibit HBV infection. As pre-incubation of HBV with bLf had no inhibitory effect on viral infection, these results suggested that bLf interaction with susceptible cells was important for its anti-HBV effect [105]. However, it was unclear whether bLf could inhibit HBV amplification in HBV-infected cells.
Molecules 2011, 16 7003Recently, Li et al. reported that bLf, and its iron-, and zinc-saturated forms significantly inhibited the amplification of HBV-DNA in a dose-dependent manner in HBV-infected HepG2 cells, while bLf hydrolysate were ineffective [106]. Mother-to-child transmission of HBV is among the most important causes of chronic HBV infection and is the commonest mode of transmission worldwide. WHO postulates that chronic HBV infection of the mother could not be an argument against breastfeeding. Even if breast milk provides a number of bioactive including lactoferrin, there have not been sufficient studies that can even partially explain the possible effect of breastfeeding on eventual prevention of mother-to-child transmission of HBV [107].7.6. Respiratory Syncytial Virus (RSV) and Parainfluenza Virus (PIV)
It has been demonstrated that lactoferrin inhibited both RSV absorption and growth in vitro nevertheless its antiviral activity was low when added to an infant formula [108]. Successively, Sano and co-workers [109] showed that RSV-induced IL-8 secretion from HEp-2 cells was down regulated by lactoferrin and that both RSV infectivity and uptake were decreased by lactoferrin treatment. To clarify the mechanism of this effect, the interaction of lactoferrin with RSV F protein, the most important surface glycoprotein for viral penetration, was examined and results obtained showed that lactoferrin directly interacted with the F (1) subunit, which involved antigenic sites of F protein [109]. Concerning PIV, Lf exhibits antiviral activity against hPIV-2 by inhibiting virus adsorption to the surface of the cells thus preventing viral infection and replication [110].
7.7. Alphavirus
The mechanism of hLf antiviral activity was also investigated by utilizing alphaviruses (Sindbis virus and Semliki Forest virus) adapted or non-adapted to interaction with heparan sulfate [111]. Results obtained demonstrated that lactoferrin was able to prevent in vitro infection only by heparin sulfate-adapted viral strains suggesting that hLf inhibited infection of heparan sulfate-adapted alphavirus by interfering with virus-receptor interaction [111].
7.8. Hantavirus
Hantaviral foci number, in cultured cells infected with SR-11, was reduced with bLf treatment [112]. Mechanisms of anti-hantaviral activities of bLf and ribavirin (Rbv) were also investigated. Preincubation of cells with bLf before infection inhibited hantavirus focus formation of 85% whereas post infection treatment with Rbv inhibited the focus formation of 97.5%. Conversely, other in vitro experiments showed that Hantaan hantavirus, the prototype hantavirus, is insensitive to several antiviral salivary proteins, and is partly resistant to the antiviral effect of saliva [113]. It has been found that combined bLf and Rbv treatment completely prevented focus formation [114]. Consequently, in in vivo studies, bLf pre- and Rbv post-treatment were evaluated in suckling mice infected with hantavirus, of which 7% survived. Lactoferrin administered before viral challenge improved survival rates to up to 70% for single administration and up to 94% for double administration. Rbv gave survival rates up to 81%. These results suggested that both lactoferrin and Rbv were efficacious in the treatment of hantavirus infection in vivo [114].
Molecules 2011, 16 7004
7.9. Human Papillomavirus (HPV)
Results of studies carried out utilizing HPV 16-like particles and cultured cells demonstrated that lactoferrin acted early in the HPV uptake process with a dose-dependent relationship and that bLf was a more potent inhibitor of HPV entry than hLf [115]. Differently, bLf and hLf were found both potent inhibitors of HPV-5 and -16 infections [ex115ora 116]. Moreover, bovine Lfcin 17–42 and human Lfcin 1–49 had an antiviral effect and this efficacy differed depending on size, charge and structures of the Lfcin [116,117].
7.10. Rotavirus
The anti-rotavirus effect of bLf was tested in cultured human intestinal cells (HT-29 cells), expressing the differentiation phenotype of mature enterocytes, the in vivo target of rotavirus infection [118]. Results obtained showed that bLf prevented either virus attachment to intestinal cell receptors or an unknown post adsorption step. Although the antiviral activity was mediated by the N-lobe [119], it was hypothesized that bLf prevention of viral attachment was not related to a competition for common binding sites on HT-29 cells, since the rotavirus strain utilized in this study binds to glycidic residues different from glycosaminoglycans [120], and flow cytometry assays demonstrated a specific interaction of lactoferrin with viral particles. The bLf inhibition in the post adsorption step could be attributed to the withholding of calcium, which is important for the morphogenesis of the virus. Other studies investigated the role of metal binding, sialic acid and tryptic fragments of bLf in the activity towards rotavirus infection [119], Results obtained demonstrated that the effect of differently metal saturated lactoferrin was exerted during and after the viral attachment step, the removal of sialic acid enhanced the anti-rotavirus activity of lactoferrin, and that a large fragment (86–258) and a small peptide (324–329: YLTTLK) obtained by tryptic digestion of bLf were able to inhibit rotavirus even if at lower extent than undigested protein [119]. The effect of whey protein concentrate supplemented with or without lactoferrin on a rotavirus infection model in suckling rats has been also investigated, focusing on the diarrhoea process and gut and systemic host immune function. Whey protein concentrate supplemented with or without lactoferrin reduces the severity of rotavirus-induced acute gastroenteritis and modulates the immune response against the pathogen [121].
7.11. Feline Calicivirus (FCV)
Incubation of bLf cultured cells either before or together with FCV inoculation substantially reduced FCV infection. Lactoferrin was detected on the surface of cells by immunofluorescence, suggesting that the interference of viral infection may be attributed to lactoferrin binding to susceptible cells, thereby preventing the attachment of the virus particles [122]. Lfcin also reduced FCV infection [122]. It has been further found that increasing the net negative charges of lactoferrin by acylation eliminated its antiviral effects against feline calicivirus [123].
7.12. Adenovirus
Both bLf and hLf prevented adenovirus infection in vitro in a dose-dependent manner [124] and, as already reported for other viruses, bLf showed the highest antiviral activity. Differently from that observed with poliovirus [125] and in agreement with data reported for many other virus models, metal-saturation of bLf did not significantly influence its activity against adenovirus infection. bLf inhibited the early step of viral infection, preventing adenovirus antigen synthesis only when pre incubated with epithelial cells or when added during the attachment step. bLf activity was mediated by the N-lobe, whereas the C-lobe lacked of any effect [126]. Moreover, bovine Lfcin is able to prevent adenovirus infection [127]. This antiviral activity of bLf occurs through bLf interaction with adenovirus particles and in particular, with the adenovirus penton base (polypeptide III), the protein responsible for viral attachment to the integrin cell receptors [128]. However, the interaction of bLf with host cell receptors cannot be excluded. The primary receptor described for infection of most adenovirus species (A, C, D, E and F) was the coxsakievirus-adenovirus receptor (CAR) [129-131]. The infection of adenovirus serotype 5 (species C), a common human pathogen exploited as a viral vector for gene therapy and vaccination, involves binding of the viral fiber knob to CAR on the target cells [132], followed by an interaction between the viral penton base with integrins on the cell surface [133]. It has been reported that adenoviral infection was prominently enhanced by bLf but not hLf, and was not prominently enhanced using blood monocyte-derived macrophages, suggesting that the relevant receptor is expressed on monocyte-derived dendritic cells [134]. These data are conflicting with those showing an antiviral effect against adenovirus by both hLf and bLf [124,126]. Concerning adenovirus serotypes 19 and 37 shown to be etiological agent of epidemic keratoconjunctivitis [135], it is important to underline that the tears contain lactoferrin, produced in the acinar cells of the lacrimal gland, at a concentration of around 2.2 mg/mL [136]. In tears, due to the high concentration of free lactoferrin, it is most likely that lactoferrin provides a protective role against viral adhesion and pathogenesis [137]. Conversely, commercial hLf (Sigma-Aldrich) was found to promote adenoviral infection of corneal epithelial cells [138] as well as hLf or bLf from milk or recombinant hLf from rice to enhance adenoviral infection of some myeloid cells [139] This paradoxical enhancement of adenoviral infection by lactoferrin should be explained by the high degree of degradation of commercial hLf showed by Johansson et al. [138], and by different glycosilation sites of rhLf, as well as by the degrees of purity or of iron saturation not reported by Adams et al. [139].
7.13. Picornavirus
Studies on poliovirus type 1 infection in Vero cells demonstrated that both bLf and hLf inhibited viral cytopathic effect with a dose-dependent relationship by interfering with an early step of viral infection [125]. Other researchers have studied the ability of bLf fully saturated with ferric, zinc and manganese ions to prevent poliovirus infection. Results obtained demonstrate that only Zn(II)-lactoferrin was capable of inhibiting infection when added after the viral adsorption step. As the inhibition was proportional to the different degrees of lactoferrin metal saturation, the possibility that
Molecules 2011, 16 7006this phenomenon could be mediated by the intracellular delivery of metal ions, already demonstrated for iron saturated lactoferrin [140], has been investigated. This hypothesis was confirmed by the dose34 dependent inhibition obtained with the addition of different zinc sulfate concentrations to infected monolayers [125]. It is likely that zinc could interfere with viral protein maturation as it has been previously demonstrated that the inclusion of zinc, at a concentration that inhibits the proteolytic post translational processing of poliovirus polyprotein, resulted in impaired poliovirus infection [141]. Moreover, the incubation of poliovirus in a Zn(II) containing buffer resulted in marked structural alterations of the capsid and an increase in the permeability to RNase, so that the infectivity of the virus was significantly affected [142]. hLf and bLf were also assayed in vitro to assess their inhibiting capacity on the cytopathic effect of enterovirus 71 (EV71) on human embryonic rhabdomyosarcoma cells [143]. Both proteins were found to be potent inhibitors of EV71 infection, with bLf being the most active. Results from kinetic experiments suggested that lactoferrin probably exerted its effect on viral adsorption. An interesting study has been carried out in a transgenic mouse model for demonstrating the protective effects of recombinant lactoferrin against EV71 infection. Transgenic mice carrying alpha lactalbumin-porcine lactoferrin and BALB/c wild-type mice were infected with EV71. Following EV71 inoculation on the 4th day of life, pups ingesting transgenic milk showed the significantly higher survival rate and heavier body weight compared with wild-type mice. RT-PCR analysis for EV71 viral RNA showed that the recombinant porcine lactoferrin had a blocking effect on EV71 infection. Our data suggest that oral intake of porcine lactoferrin-enriched milk exhibited the ability to prevent EV71 infection [144]. The effect of lactoferrin on echovirus 6 infection in vitro was also investigated [145]. Results of this study showed that echovirus 6 infected cells die as a result of apoptosis and that programmed cell death is inhibited by bLf treatment. This was the first report in which the prevention of viral-induced apoptosis by lactoferrin was demonstrated [145]. The same authors have successively investigated the mechanism of bLf anti-echoviral effect demonstrating that echovirus infects susceptible cells by an endocytic pathway and that lactoferrin treatment is able to prevent viral genome delivery into the cytoplasm. It is likely that lactoferrin interaction with echovirus capsid proteins induces alterations that stabilize the conformation of the virion making it resistant to uncoating. Taken together the results of these studies, the inhibition of echovirus 6 infectivity by lactoferrin seems to be dependent on its interaction not only with cell surface glycosaminoglycan chains but also with viral structural proteins, demonstrating that this glycoprotein targets the virus entry process [146]. On the other hand, the lactoferrin efficacy could be based on its positive charge, because the increasing of net negative charges by acylation eliminated antiviral effects [123].7.14. Rhinovirus
Lactoferrin did not inhibit rhinoviruses, while human milk decreased the growth of some of the rhinoviruses [147].
7.15. Influenza A
Virus Influenza is one of the main plagues worldwide. The statistical likelihood of a new pandemic outbreak, together with the alarming emergence of influenza virus strains that are resistant to available antiviral medications, highlights the need for new antiviral drugs. It has been found in in vitro model that cell cultures died as a result of apoptosis following to H3N2 influenza A virus infection. Similarly to that first demonstrated in echovirus 6, bLf treatment inhibited programmed cell death by interfering with function of caspase 3, a major virus induced apoptosis effector, as well as blocked nuclear export of viral ribonucleoproteins so preventing viral assembly [148]. Since 2003, H5N1 avian influenza A virus was detected and identified in South East Asia. Both the native and esterified bLf seem to be the most active antiviral proteins among the tested samples, followed by b-lactoglobulin. a-Lactalbumin had less antiviral activity even after esterification [149].
7.16. Japanese Encephalitis Virus
It has been hypothesized that bLf could be effective against japanese encephalitis virus (JEV) through its ability to bind to glycosaminoglycan, one possible receptor for JEV. The results showed that bLf inhibited the early events essential to initiate JEV infection, which includes blocking virus attachment to cell membranes and reducing viral penetration. Even if these results support the premise that the interaction of bLf with cell surface expressed glycosaminoglycans, plays an essential role in the antiviral activity, bLf was functional in inhibiting viral entry into heparin sulfate-deficient cells. This finding provided evidence to suggest that also cell surface-expressed low-density lipoprotein receptor LDLR may play a role in JEV infection [150].
7.17. Tomato Yellow Leaf Curl Virus
The antiviral activity of native and esterified whey protein fractions including lactoferrin was studied to inhibit tomato yellow leaf curl virus (TYLCV) on infected tomato plants. Whey proteins fractions and their esterified derivatives were sprayed into TYLCV-infected plants. Samples were collected from infected leaves before treatment, 7 and 15 days after treatment for DNA and molecular hybridization analysis. Native and esterified lactoferrin showed complete inhibition after 7 days [151].
8. Conclusions
The protective effect of lactoferrin towards microbial infections has been widely demonstrated in a large number of in vitro studies. Its high cationic feature favors the binding to microbial and viral surface components as well as to heparansulfate proteoglycans (HSPG), cell receptors for bacterial adhesion and enveloped viral particle early interactions. The capability of lactoferrin to exert antiviral activity, through its binding to host cells or viral particles or both, strengthens the idea that this glycoprotein is an important brick in the mucosal wall, effective against viral attacks. During viral infection, the epithelium can be injured, with the consequence of loss of integrity and protection. As a matter of fact, the mucosa plays an important role as a protective physical and functional barrier between the external environment and underlying tissues, while the components of its secretions, especially lactoferrin are central elements in the initiation and regulation of innate and adaptive immune responses.
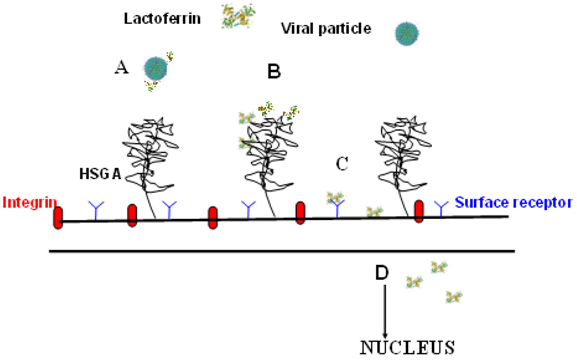
图4显示了乳铁蛋白(Lactoferrin)预防病毒感染的不同机制:与病毒颗粒(Viral particle)的结合(A),与硫酸乙酰肝素糖胺聚糖(HSGA)的结合(B),与病毒受体的结合(C)和细胞内定位(D) ,涉及凋亡或炎症途径。
Figure 4 shows the different mechanisms of lactoferrin in preventing viral infection: The binding to viral particles (A), the binding to heparan sulfate glycosaminoglycans (HSGA) (B),the binding to viral receptors (C), and intracellular localization (D), involving apoptosis or inflammatory pathways.
It is believed that the magnitude of inflammation is a major contributing factor to viral disease severity [152]. Epidemiological evidence and clinical observations of natural infections in humans suggest that different viruses may be associated with different inflammatory responses. Whether or not these differences can be attributed to the viruses themselves or to hosts that are susceptible to severe infection or prone to produce high levels of inflammation with a given virus is unknown. In this context it should be important to consider the role of lactoferrin in in vivo modulating the type or magnitude of the inflammatory response during viral infections. Unfortunately, few clinical trials on lactoferrin efficacy against viral infections have been carried out. The scarcity of clinical trials hinders to compare the inflammatory response and the lactoferrin efficacy in different animal models infected with different viruses. However, the antiviral activity of lactoferrin detected in cultured cell monolayers infected by enveloped and naked viruses, has been found to be not related to the degrees of lactoferrin iron saturation, while Zn- and Mn-saturated lactoferrin exerted a potent antiviral capacity against HSV, HIV and poliovirus infection [61,83,125]. Conversely, lactoferrin antiviral activity is strongly related to its binding to viral particles or to host cells or both. Lactoferrin antiviral activity is also associated to the prevention of Echovirus 6- and H3N2 influenza virus-induced apoptosis [145,148]. Figure 4 shows the different mechanisms of lactoferrin in preventing viral infection: The binding to viral particles (A), the binding to heparan sulfate glycosaminoglycans (HSGA) (B),the binding to viral receptors (C), and intracellular localization (D), involving apoptosis or inflammatory pathways. Figure 4. Different mechanisms of lactoferrin in preventing viral infection.
乳铁蛋白能够有效抑制冠状病毒进入细胞 中国医学科学院、清华大学和协和医学院和美国明尼苏达大学的一项联合研究的发现
乳铁蛋白抑制冠状病毒进入细胞的机理
新冠状病毒进入细胞的通道-ACE2受体-主要分布在肺泡、肠上皮细胞、心脏和肾小管研究证明,新冠状病毒与SARS病毒具有96%以上的同源性,基因相似度为80%,都是通过细胞表面的ACE2受体进入细胞。
冠状病毒上的突刺蛋白(S)首先会接触细胞膜上突出的HSPG(硫酸肝素蛋白聚糖)。通过HSPS接近细胞膜上的ACE2受体进入细胞。
由于HSPG是带负电的,而乳铁蛋白(LF)是带正电的,所以互相吸引。
因此,高浓度的乳铁蛋白可以屏蔽HSPG跟病毒的接触,阻止病毒靠近和通过细胞膜上的ACE2受体进入细胞。
日本富山医科大学:乳铁蛋白能减轻流感病毒性肺炎
在这个研究中,研究人员首先让小鼠感染流感病毒前1天分别喂食乳铁蛋白和开水(对照组作为效果对照),在感染6天后,服用乳铁蛋白/乳过氧化物酶组小鼠的肺实变积分(炎症程度)、支气管肺泡灌洗液(BALF)中回收的浸润白细胞数量明显低于对照组,同时,血清中的炎症因子IL-6明显低于对照组。研究发现,乳铁蛋白通过抑制炎症细胞的浸润而减轻流感病毒性肺炎。
口服牛乳铁蛋白和乳过氧化物酶对流感病毒感染的影响...Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection...
J Infect Chemother. 2014 Nov;20(11):666-71. doi: 10.1016/j.jiac.2014.08.003. Epub 2014 Aug 30.
Lactoferrin for prevention of common viral infections.
Wakabayashi H1, Oda H2, Yamauchi K2, Abe F2.
Author information
1
Food Science & Technology Institute, Morinaga Milk Industry Co., Ltd., Japan. Electronic address: h_wakaby@morinagamilk.co.jp.
2
Food Science & Technology Institute, Morinaga Milk Industry Co., Ltd., Japan.
Abstract
Although lactoferrin has many biological functions, the host-protective effects against pathogenic microorganisms including bacteria, fungi, and viruses are regarded as one of the most important.Here, we review research on the protective role of lactoferrin administration against common viral infections. Many studies have shown the in vitro antiviral activity of lactoferrin against viral pathogens that cause common infections such as the common cold, influenza, gastroenteritis, summer cold, and herpes, where lactoferrin inhibits mainly viral attachment to the target cells.
Recently, studies indicating the in vivo protective effects of lactoferrin by oral administration against common viral infections have been increasing. For instance, norovirus is an extremely important emerging human pathogen that causes a majority of gastroenteritis outbreaks worldwide that may be a target candidate for lactoferrin. Lactoferrin consumption reduced the incidence of noroviral gastroenteritis in children and a similar effect was observed in a wide range of ages in a preliminary survey. A recent in vitro study reported that lactoferrin inhibits both cellular attachment of the murine norovirus, a virus closely-related to the human norovirus, and viral replication in the cells by inducing antiviral cytokines interferon (IFN)-α/β.
Lactoferrin administration also enhances NK cell activity and Th1 cytokine responses, which lead to protection against viral infections.
In conclusion, lactoferrin consumption may protect the host from viral infections through inhibiting the attachment of a virus to the cells, replication of the virus in the cells, and enhancement of systemic immune functions.
Copyright © 2014 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
KEYWORDS:
Gastroenteritis; Interferon; Lactoferrin; NK; Norovirus; VirusLactoferrin for prevention of common viral infections. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/25182867
Journal of Dairy Science
Volume 96, Issue 4, April 2013, Pages 2095-2106
Journal of Dairy Science
Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor
Author links open overlay panelYu-TangTung*1Hsiao-LingChen†1Chih-ChingYen*‡1Po-YingLee§Hsin-ChungTsai*#Ming-FongLin*#Chuan-MuChen*
Show more
https://doi.org/10.3168/jds.2012-6153Get rights and content
Open Archive in partnership with American Dairy Science Association (ADSA)
Under an Elsevier user licenseopen archive
Abstract
Lung cancers are among the most common cancers in the world, and the search for effective and safe drugs for the chemoprevention and therapy of pulmonary cancer has become important. In this study, bovine lactoferrin (bLF) was used in both in vitro and in vivo approaches to investigate its activity against lung cancer. A human lung cancer cell line, A549, which expresses a high level of vascular endothelial growth factor (VEGF) under hypoxia, was used as an in vitro system for bLF treatment. A strain of transgenic mice carrying the human VEGF-A165 (hVEGF-A165) gene, which induces pulmonary tumors, was used as an in vivo lung cancer therapy model. We found that bLF significantly decreased proliferation of A549 cells by decreasing the expression of VEGF protein in a dose-dependent manner. Furthermore, oral administration of bLF at 300 mg/kg of body weight 3 times a week for 1.5 mo to the transgenic mice overexpressing hVEGF-A165 significantly eliminated expression of hVEGF-A165 and suppressed the formation of tumors. Additionally, treatment with bLF significantly decreased the levels of proinflammatory cytokines, such as tumor necrosis factor-α, and antiinflammatory cytokines, such as IL-4 and IL-10. Levels of IL-6, which is both a proinflammatory and an antiinflammatory cytokine, were also reduced. Treatment with bLF decreased levels of tumor necrosis factor-α, IL-4, IL-6, and IL-10 cytokines, resulting in limited inflammation, which then restricted growth of the lung cancer. Our results revealed that bLF is an inhibitor of angiogenesis and blocks lung cell inflammation; as such, it has considerable potential for therapeutic use in the treatment of lung cancer.
Previous article in issueNext article in issue
Key words
bovine lactoferrinpulmonary cancervascular endothelial growth factor (VEGF)transgenic mice
Introduction
For several decades, lung cancer has been the most common cancer worldwide (Li et al., 2010). Most new cases of lung cancer now occur in developing countries (55%) and it is still the most common type of cancer in men (1.1 million cases, 16.5% of the total), with high rates in central, eastern, and southern Europe, North America, and East Asia (Ferlay et al., 2010). Although the treatment of lung cancer has improved, the mortality rate of lung cancer patients remains high. To reduce these high rates of mortality, many researchers have focused on methods for tumor prevention in addition to more effective treatments (Lin et al., 2009). Recently, researchers have found natural food components or products of digestion that could mediate the process of angiogenesis and metastasis (Singh et al., 2006, Yang and Wang, 2010). Researchers have shown the anticancer potential of dietary proteins, peptides, and amino acids, which may be natural products of fermentation and enzymatic hydrolysis or products of gastrointestinal digestion (de Mejia and Dia, 2010). These compounds mediate apoptosis and angiogenesis, which are vital steps in controlling tumor metastasis.
Bovine lactoferrin (bLF), a siderophilic protein with 2 iron-binding sites, has a wide range of biological activities, including anticancer effects, antimicrobial effects, and improvement of immunomodulatory functions (de Mejia and Dia, 2010). Chemopreventive and cell growth inhibitory activities of bLF have been demonstrated in esophageal (Ushida et al., 1999), lung (Li et al., 2011), colon (Tsuda et al., 1998), bladder (Masuda et al., 2000), mammary (Yamada et al., 2008; Duarte et al., 2011), stomach (Xu et al., 2010), and tongue (Tanaka et al., 2000) cancers. The anticancer functions of bLF are thought to be exerted through its innate ability to bind iron (González-Chávez et al., 2009). The iron could accelerate oxidation, thereby disrupting nucleic acid structure. Other potential mechanisms of anticancer functions include induction of programmed cell death and regulation of cell cycle protein expression (Lönnerdal and Iyer, 1995; Rodrigues et al., 2009). However, the antiangiogenesis effects of bLF during tumor growth are poorly understood.
In the early stages of cancer, the unregulated proliferation of cancer cells leads to a deficiency of both nutrients and oxygen, causing significant cell death. Cell death triggers an inflammatory response, activates hypoxia-inducible factor 1α (HIF-1α), promotes the expression of the vascular endothelial growth factor (VEGF)-A mRNA, and causes angiogenesis. Of the VEGF family, VEGF-A is the one of most interest in human medicine for specialists and medical teams. Four isoforms of VEGF-A are known, including VEGF-A121, VEGF-A165, VEGF-A189, and VEGF-A206; VEGF-A165, the common type, primarily functions to promote angiogenesis. Secreted VEGF-A165 binds to the receptor VEGFR2 and activates a downstream signal that induces vasculogenesis (Ferrara, 2002). When cancer cells secrete a large amount of VEGF-A165, vasculogenesis is induced to provide sufficient nutrients and oxygen to the tumor, thus increasing the tumor growth rate. Expression of VEGF-A165 is positively related to the growth and spread of cancer cells (Coussens and Werb, 2002). Therefore, the development of medicines that target VEGF-A165 is an important topic of study.
In this study, we used both in vitro and in vivo approaches to investigate the effects of VEGF expression in lung cancers treated with bLF, which was purified from bovine milk. Human lung cancer cells and an animal model of human (h)VEGF-A165-induced lung tumor transgenic mice were applied to examine the protection mechanisms of bLF on human and mouse lung carcinomas.Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S0022030213001434
Curr Pharm Des. Author manuscript; available in PMC 2010 Aug 4.
Published in final edited form as:
Curr Pharm Des. 2009; 15(17): 1956–1973.
PMCID: PMC2915836
NIHMSID: NIHMS222034
PMID: 19519436
Lactoferrin as a Natural Immune Modulator
Jeffrey K. Actor,1,* Shen-An Hwang,1 and Marian L. Kruzel2
Author information Copyright and License information Disclaimer
1 Department of Pathology and Laboratory Medicine, University of Texas Health Science Center at Houston, TX 77030
2 Department of Integrative Biology and Pharmacology, University of Texas Health Science Center at Houston, TX 77030, USA
* Address correspondence to this author at the UT-Houston Medical School, University of Texas, Health Science Center at Houston, Department of Pathology and Laboratory Medicine, MSB 2.214, 6431 Fannin Street; Houston, TX 77030, USA; Tel: 713-500-5344; Fax: 713-500-0730; ude.cmt.htu@rotcA.K.yerffeJ
The publisher's final edited version of this article is available at Curr Pharm Des
See other articles in PMC that cite the published article.
Go to:
Abstract
Lactoferrin, an iron-binding glycoprotein, is a cell-secreted mediator that bridges innate and adaptive immune function in mammals. It is a pleiotropic molecule that directly assists in the influence of presenting cells for the development of T-helper cell polarization. The aim of this review is to provide an overview of research regarding the role of lactoferrin in maintaining immune homeostasis, in particular as a mediator of immune responses to infectious assault, trauma and injury. These findings are critically relevant in the development of both prophylactic and therapeutic interventions in humans. Understanding these particular effects of lactoferrin will provide a logical framework for determining its role in health and disease.
Keywords: Lactoferrin, innate immunity, adaptive immunity, immunomodulation, inflammation, oxidative stress, adjuvant
Go to:
INTRODUCTION
Immune responses are designed to interact with the environment to protect the host against pathogenic invaders, conferring a state of health through effective elimination of infectious agents (bacteria, viruses, fungi, and parasites) and modulation of systemic responses comprising host immune surveillance. Recent research has identified lactoferrin, a member of the transferrin family of iron binding glycoproteins, as a critical component in mediation of immune response, especially for coordinated interactions between innate and adaptive components and associated responses. Engagement of innate components leads to triggering of signal pathways to promote inflammation, ensuring that invading pathogens remain in check while the specific immune response is either generated or upregulated. Lactoferrin is a key molecule involved in these processes.
The immune system protects the body from potentially harmful environmental stimuli through recognition and responding with multiple immunological reactions. Myeloid cells, including the highly phagocytic, motile polymorphonuclear neutrophils, macrophages and dendritic cells, provide a first line of defense against most pathogens. There is emerging evidence that many mediators originating from the myeloid lineage revive immune homeostasis in most insult-induced metabolic disparity [1,2]. Thus, the utility of such immune mediators represents a novel therapeutic approach that depends on immunopotentiation, immunosuppression, or induction of immunological tolerance. Lactoferrin is one of these mediators that naturally bridge the innate and adaptive immune functions by regulating target cell response, including those involved in oxidative stress and systemic inflammatory responses. It is also recognized as a significant contributor in regulation of antigen presentation and development of productive T helper cell response.
Lactoferrin is a well conserved, monomeric 80-kDa single polypeptide chain glycoprotein of about 690 amino acid residues [3,4]. While lactoferrin is found primarily in mucosal secretions, synthesized by epithelial cells, it is also present in neutrophilic granules [5]. Lactoferrin is considered a first-line defense protein involved in protection against a multitude of microbial infections [6–8] and prevention of systemic inflammation [9–11]. While the goal of this review is to examine immune modulating activity of lactoferrin, it is important to consider that lactoferrin also exhibits direct effects on pathogens [12–15]. These include bacteriostatic and bactericidal effects, the former being a result of iron sequestration by lactoferrin and the latter dealing with lactoferrin capabilities to bind lipopolysaccharide (LPS) [16–23]. The ability of lactoferrin to bind large quantities of iron also provides protection against pathogens and their metabolites by enhancing phagocytosis and cell adherence and controlling the release of proinflammatory cytokines [24,25]. Other direct effects of lactoferrin include anti-viral [21,26–32], anti-parasitic [33–35], and anti-fugal [36–41] activities. Additionally, lactoferrin possesses indirect activity, often through prevention of pathogen invasion by blocking interaction with receptors used for entry on host cells [42–44].
While suppressing microbial growth, lactoferrin also exerts direct first-line defense activity through its significant impact on the development of adaptive immune responses. Sequestration of iron by lactoferrin reduces insult-induced oxidative stress, thus altering the magnitude and specific production of cytokines [45]. Lactoferrin has a profound modulatory action on the adaptive immune system [46–48] by promoting the maturation of T-cell precursors into competent helper cells and by the differentiation of immature B-cells into efficient antigen presenting cells [49]. In addition, lactoferrin augments the delayed type hypersensitivity (DTH) response to antigens, leading to a strong induction of cell-mediated immunity in mice [50,51]. Each of these topics will be discussed in detail.LACTOFERRIN AS A MEDIATOR OF OXIDATIVE STRESS-INDUCED RESPONSES
Lactoferrin is a primary constituent of immune homeostasis, functioning to reduce oxidative stress at the molecular level, and thus controlling excess inflammatory response. Oxidative stress occurs when the production of potentially destructive reactive oxygen species (ROS) exceeds the body’s own natural antioxidant defenses and results in cellular damage. A cell is able to overcome small perturbations and regain its original state; but, severe oxidative stress can cause cell death. While moderate oxidation can trigger apoptosis, more intense stresses can cause necrosis within tissue [52–54]. There is substantial evidence that oxidative stress is a causative factor in the pathogenesis of major neurodegenerative diseases, including Parkinson’s disease [55], Alzheimer’s disease [56], and amyotrophic lateral sclerosis [57,58], and is involved in cases of stroke, trauma, seizures [59,60], rheumatoid arthritis, fatigue, or cancer [61,62]. Also, there is ample evidence that allergic disorders, such as asthma, rhinitis, and atopic dermatitis, are mediated by oxidative stress [63]. In fact, the oxidative stress-induced immune hypersensitivity indicates a shift in immunostasis towards the Th2 responses. The Th1/Th2 balance is responsible for coordinating the immune system and become very important during aging processes, including the development of autoimmune, neurodegenerative and immune hypersensitivity disorders.
Transitional metals may be considered as key factors in the oxidative stress. In particular, traces of iron can be detrimental to physiological processes under reactive oxygen conditions. Iron is crucial in modulating production of reactive oxygen species (ROS), by virtue of its ability to catalyze a two step process known as the Haber-Weiss reaction [64]. In normal physiological conditions, the production and neutralization of these reactive oxygen species largely depends on the efficiency of key enzymes, including superoxide dismutase, catalase and glutathione peroxidase. Inefficiency of these enzymes leads to overexpression of hydroxyl radical via iron-dependent Haber-Weiss reaction, and subsequent increase in lipid peroxidation. It is hypothesized that endogenous lactoferrin can prevent lipid peroxidation by virtue of iron sequestration. This may have a significant systemic implication as lipid peroxidation products, namely hydroxyalkenals, can randomly inactivate or modify functional proteins and affect vital metabolic pathways. There is substantial evidence that acute overproduction of ROS can result in pathological damage due to severe injury or death of cells in affected tissues [52].
Go to:
ANTI-OXIDANT PROPERTIES OF LACTOFERRIN
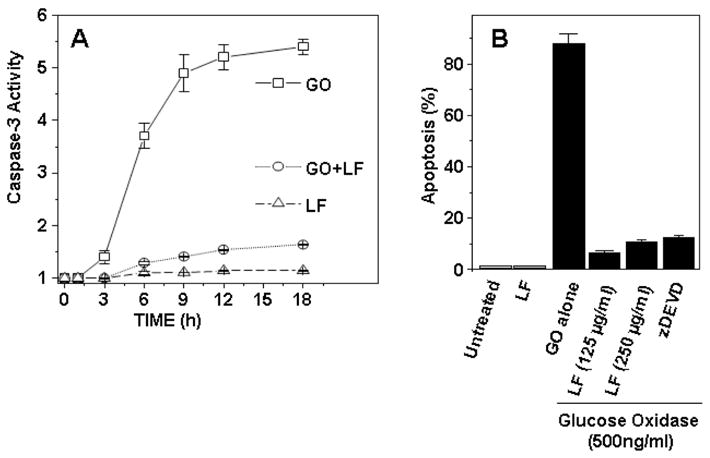
Preliminary experiments in our laboratory demonstrated that lactoferrin can function as an anti-oxidant, reducing intracellular levels of ROS induced by glucose oxidize (GO). Based on this initial study it was hypothesized that lactoferrin could function to reduce oxidative stress-induced apoptosis. Apoptosis is a programmed cells death, characterized by activation of caspases. The human monocytic cell line (U937) was used to measure caspase-3 activation by lactoferrin. Cells were pre-treated with lactoferrin (125 or 250 μg/mL) or N-acetyl-L-cysteine (as control; 10 mM), and then exposed to GO (500 ng/mL; predetermined to killed cells via apoptosis in preliminary studies). Caspase-3 activity was determined by measuring the change in absorbance at 405 nm. Indeed, lactoferrin was able to limit caspase-3 activation in apoptotic U937 cells (Fig. 1). To further test inhibition of apoptosis by lactoferrin, Annexin V-FITC staining was employed. U937 cells were treated with lactoferrin, GO, or in combination. Comparisons were made to z-DEVD-fmk (N-benzyloxycarbonyl-Asp(OMe)-Glu(OMe) -Val-Asp(OMe)-fluoro-methylketone), a caspase–3 inhibitor control. Again, lactoferrin effectively reduced glucose oxidase-induced apoptosis (Fig. 1). There is also published confirmation that daily oral administration of lactoferrin can support the immune system response through antioxidative mechanisms [65].
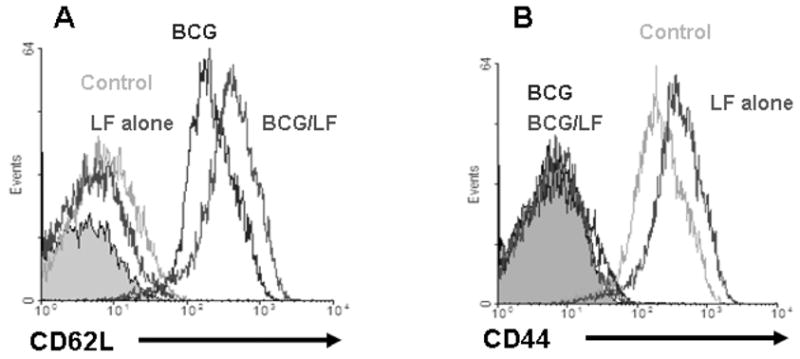
Fig. 1
LF inhibits oxidative stress-induced apoptosis
Lactoferrin was assessed for activity to limit apoptosis as defined by activation of caspase-3 (A) or Annexin V presentation (B). U937 cell pellets were examined post-treatment with glucose oxidase (GO, 500 ng/mL), with or without lactoferrin (LF). Enzymatic reactions were performed with appropriate caspase substrate [45]. Caspase activity was determined by measuring the change in absorbance at 405 nm (A). Alternatively, cells were treated with GO alone or in the presence of lactoferrin (125 or 250 μg/mL) or with z-DEVD-fmk (N-benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoro-methylketone) a caspase–3 inhibitor of GO-induced Annexin V binding (B). Cells were then stained with Annexin V-FITC as described [204]. The mean fluorescence for 12,000 cells from three or more independent experiments are shown, expressed as ±SEM.
LACTOFERRIN AS A MEDIATOR OF SYSTEMIC INFLAMMATORY RESPONSE SYNDROME (SIRS)
Early host defenses during septicemia and endotoxemia include a rapid rise in serum lactoferrin concentration [66]. The significance of this response is well established, although the mechanisms are not clearly understood. Systemic inflammatory response syndrome (SIRS), induced either by endotoxin or live bacteria, represents the earliest manifestation of immune function in which molecular aspects of increased ROS leads to exacerbated inflammation. There is growing evidence showing that progression of systemic inflammatory response syndrome into sepsis is due to the cellular damage and death induced by acute inflammatory responses. The cell death depends in part upon mitochondrial dysfunction, which is often characterized by increased production of reactive oxygen species (ROS), increased membrane permeability and eventual release of cell death mediators from mitochondria [67]. Extensive mitochondrial damage leads to loss of cellular ATP pools, which can be linked to necrotic cell death, to further inflammatory responses. Consequently, mitochondrial dysfunction contributes to a wide range of human pathologies including SIRS and sepsis. Lactoferrin is a critical component involved in mediation of this response, so as to allow controlled regulation of inflammation without rapid induction of pathological damage. The mechanism of action for lactoferrin contains multiple components for differential regulation of cellular immune responses during the development of SIRS. Both endotoxemia and bacteremia are manifested by severe clinical syndromes characterized by proinflammatory cytokine release, increased expression of adhesion molecules, and massive release of reactive oxygen species [68–70]. Vascular inflammation occurs within minutes of Gram negative bacterial infection and coincides with a burst of proinflammatory cytokines derived from activated monocytes-macrophages. There is increasing evidence that bacteremia and endotoxemia stimulate the immune system into a self-perpetuating, generalized state of hyperactivity. In particular, the systemic inflammatory response to bacterial LPS induces gut-associated lymphoid tissue to produce and liberate proinflammatory cytokines which in turn affects gut mucosal permeability. This contributes to enteric bacterial translocation to distant sites [71–74]. Recently, the LPS-induced oxidative burst was found to be of mitochondrial origin, and release of reactive oxygen species (ROS) was localized to the respiratory complex III. Importantly, lactoferrin nearly abolished LPS-induced increases in mitochondrial ROS generation and accumulation of oxidative damage to DNA. In vivo, pretreatment of experimental animals with LF significantly lowered LPS-induced mitochondrial dysfunction shown by decreased release of H2O2 and reduced DNA damage in the mitochondria (personal communication, S. Boldogh). The potential use of lactoferrin for amelioration of clinical sepsis has been an active area of research in our laboratory.
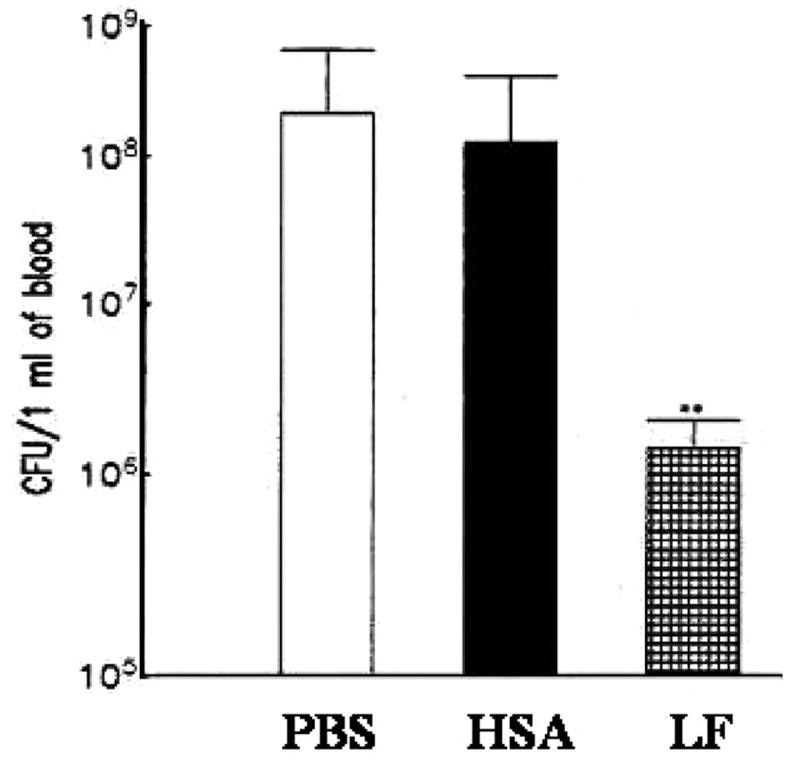
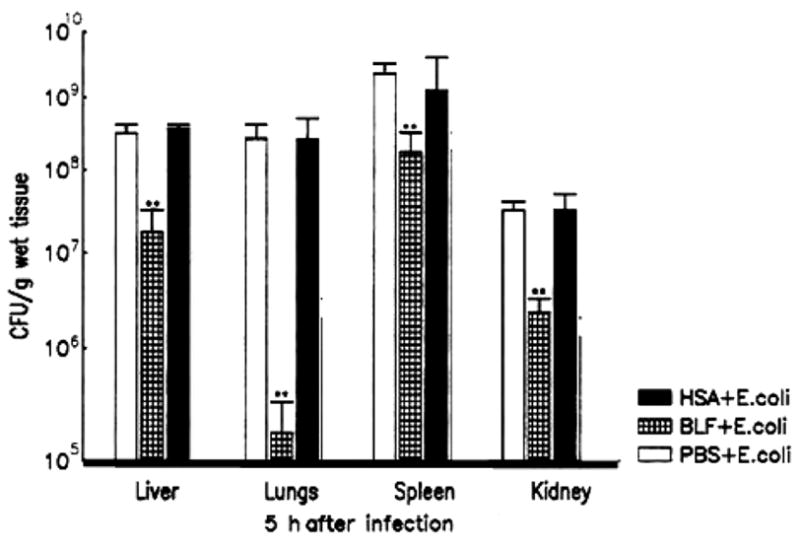
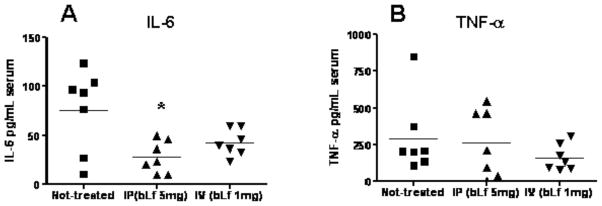
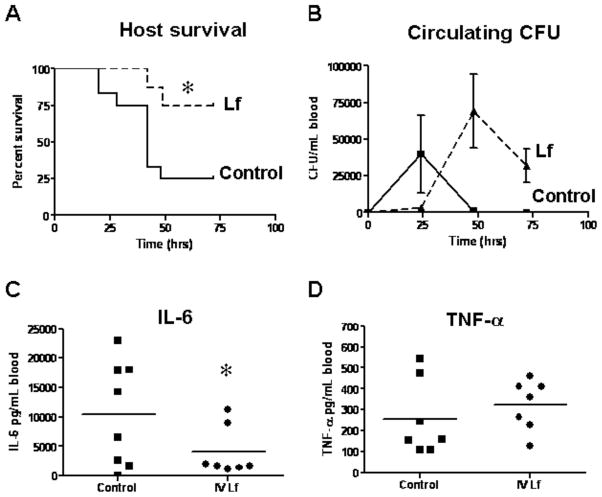
Injection of LPS into animals virtually reproduces the pathophysiologic changes induced by live bacteria, and it is considered a standard model for sepsis. Using this model, it was demonstrated that treatment of mice with lactoferrin reduced or eliminated many of the biological effects normally seen upon administration of endotoxin [75–79] (summarized in Table 1). A single dose of lactoferrin administered 1 h before LPS injection significantly increased survival of mice when compared with the saline-treated controls. Overall, the mortality rate was 16.7% in the lactoferrin-treated mice and 83.3% in the saline control group [61].
Table 1
The Role of Lactoferrin in Moderation of Development of LPS-Induced Endotoxemia in MiceTable 1
The Role of Lactoferrin in Moderation of Development of LPS-Induced Endotoxemia in Mice
Biological Effect LPS-Induced Endotoxemia Lactoferrin Pretreatment/LPS-Induced Endotoxemia
Behavioral Activity Severely Reduced (Lethargy) Normal Body Temperature Severe Hyperthermia Moderate Hyperthermia Intestinal Structure Severe Injury Moderate Injury
Survival 83.3% Mortality
P<0.0516.7% Mortality
P<0.05
Inflammatory Mediators: IL-6 at 2 h Increase Reduction (P<0.05) TNF-α at 2 h Increase Reduction (P<0.05) Nitric Oxide at 6 h and 18 h Increase Reduction (P<0.05)
The protective effect of lactoferrin against bacteremia has also been demonstrated in mice [14,80]. We further investigated this observation in CFW mice for ability to clear bacteria from serum and tissue when treated with lactoferrin. Mice were administered a bolus dose of lactoferrin (10 mg) 1 h prior to challenge with enterotoxigenic Escherichia coli (E. coli 844 078K 80/B) at a lethal dose (2×108 organisms, intravenous injection). 80% of mice treated with lactoferrin survived through 30 days, compared to 100% death by three days in mice given either saline or human serum albumin (HSA) control injections (data not shown). Levels of organisms were quantitated post injection of enterotoxigenic bacteria. Mice pretreated with lactoferrin demonstrated greater than 100-fold reduction in organisms in circulation compared to controls (Fig. 2). E. coli was also measured in lungs, spleens, livers and kidneys. The effect was most dramatic when examining bacterial load remaining within lung tissue (Fig. 3), where there was over 1000-fold reduction in number of organisms. All tissues demonstrated decreased bacterial loads (at least 10-fold reduction in spleen, liver and kidney), when compared with phosphate buffered saline and/or HSA controls. This indicates increased killing, and not merely a difference in sequestration of organisms. Taken together, these data show that lactoferrin will protect against progression of insult-induced systemic inflammatory responses in models of SIRS and sepsis by reducing immune mediators and oxidative stress that contribute to tissue damage.
Fig. 2
Effect of lactoferrin on clearance of E. coli from circulation
Mice were challenged with a lethal dose of enterogenic bacteria (2×108 organisms, intravenous injection). The level of organisms within whole blood was measured 5 h post infection. Treatment of mice with LF (10 mg/mouse) 24 h prior to E. coli challenge (Lethal Dose, LD100) resulted in 100 fold faster clearing of bacteria from circulation, when compared with controls. E. coli + PBS (phosphate buffered saline, open); HSA (human serum albumin, closed); LF (lactoferrin, hatched). **p<0.05
Fig. 3
Effect of lactoferrin on the clearance of E. coli from various organs
Mice were challenged with a lethal dose of enterogenic bacteria (2×108 organisms, intravenous injection). Number of organisms was measured in lungs, spleens, livers and kidneys 5 h post infection. Treatment with LF (10 mg/mouse) 24 h prior to E. coli challenge (LD100) decreased the number of organisms recovered in spleens, livers and kidneys over 10 fold, and in lungs over 1000 fold, when compared with PBS and/or HSA controls. **p<0.05.
In other murine bacteremia model using E. coli (strain SM105) [81], mice were intravenous injected with approximately 1.5 × 109 CFU/mouse, similar to models previously described [82]. At this dose, mice survived the infection, with high levels of circulating proinflammatory mediators and large bacterial load in tissues. Addition of lactoferrin prior to infectious challenge led to non-significant reduction in organ bacterial load in all tissues examined (data not shown). Serum cytokines were measured in the above mice just prior to sacrifice. In all cases cytokines were reduced due to pretreatment with lactoferrin. The greatest reductions were noticed in IL-6, which persisted through 4 days post infection. It was of interest to note that no concurrent changes were observed in TNF-α at that time, although it is speculated that decreases may have occurred if earlier times were examined (Fig. 4). A significant decrease of serum IL-12 and IL-10 cytokine levels was also observed (data not shown). Changes in intestinal tissue (jejunal section) were also examined (Fig. 5). At 4 days post infection, control mice demonstrated disruption to microvilli, with large sub-mucosal accumulation of activated cells. The pretreatment with lactoferrin led to nearly full protection of gut associated tissue in all mice examined.
Fig. 4
Bovine lactoferrin pre-treatment leads to decreased proinflammatory responses from mice infected with E.coli
BALB/c mice were pre-treated with bovine lactoferrin at 1 mg/mouse (intravenous) or 5 mg/mouse (intraperitoneal) or remained untreated controls. After 4 h, mice were infected with a sub-lethal dose (~5×108 CFU/mouse) of E.coli SM105 (intravenous). At day 4 post-infection, blood was collected for analysis of cytokine production of IL-6 (A) and TNF-α (B) by ELISA. * p<0.05 compared to the control group not treated with lactoferrin.
Fig. 5
Lactoferrin pre-treatment protects host gut integrity against sub-lethal challenge with E. coli
BALB/c mice challenged with a sub-lethal dose of E.coli SM105 demonstrate severe jejunal tissue destruction at 4 days post infection (A). Parenchyma from mice pretreated with lactoferrin (1 mg/mouse, intravenous, 4 hours prior to challenge) remained intact (B), with no demonstration of tissue damage Jejunal tissue was collected and fixed with 10% formalin, paraffin embedded, sectioned, and stained with H&E. Visualized at 100×.
LACTOFERRIN AS A POTENTIAL MODULATOR OF EVENTS DURING MRSA CHALLENGE
Overwhelming immune responses due to Gram-positive bacterial infections can also elicit similar cascades as demonstrated above with the Gram-negative E. coli. For example, Staphylococcus aureus initiates a cytokine storm, in large part due to superantigens that trigger non-specific lymphocyte activation [83–85]. Indeed, with the advent of multi-drug resistant organisms, it is critical to develop current therapeutic approaches that include anti-inflammatory and antimicrobial strategies. In particular a new threat comes from the methicillin-resistant Staphylococcus aureus (MRSA) infection, which is responsible for more deaths in the United States each year than AIDS. It is also a major cause of sepsis in patients who are disease or drug-immunosuppressed.
Pilot experiments were completed examining MRSA in a peritonitis murine model to investigate the potential for lactoferrin to mediate inflammatory responses. Lactoferrin was given prior to challenge with a lethal dose of MRSA, and mice were monitored for survival, bacteremia, and proinflammatory cytokines. Mice pre-treated with lactoferrin significantly increased host survival (75%) compared to the control non-treated group (25%) (Fig. 6A). Serum bacteremia showed a delay in translocation of MRSA from the injection site, peritoneal cavity, to the circulation. Of high interest, circulating cytokine levels post challenge from mice pre-treated with lactoferrin exhibited a marked decrease in IL-6, with no changes in TNF-α (Fig. 6C,D), similar to that observed in the above E. coli peritonitis experiments. These experiments point to a potential for lactoferrin to function as a therapeutic against both Gram-positive and Gram-negative organisms, by inducing moderation of proinflammatory mediators during bacterial infection.
Fig. 6
Effect of lactoferrin on host protection against MRSA infection
BALB/c mice were pre-treated with bovine lactoferrin (1 mg/mouse, intravenous) or remain untreated. After 2 h, mice were infected with MRSA (strain Mu50, American Type Tissue Culture, intaperitoneal, 1×109 CFU/mouse). Mice were monitored for survival (A). At various time points, blood was collected for analysis of bacterial load (B) and cytokine production (C,D) by ELISA. * p<0.05 compared to the no lactoferrin control group.
LACTOFERRIN AS A MEDIATOR BRIDGING THE INNATE AND ADAPTIVE IMMUNE RESPONSES
The role that lactoferrin plays in regulating innate immune responses confirms its importance as a first line host defense mechanism against invading pathogens. Indeed, multiple laboratories are actively investigating the role of lactoferrin to modulate both acute and chronic inflammation [11,45,86]. Most intriguing is the ability of lactoferrin to induce mediators from innate immune cells that subsequently impact adaptive immune cell function.
By virtue of high affinity to iron, lactoferrin is considered an important component of nonspecific host defense system against various pathogens in humans. A high level of lactoferrin in plasma has been suggested to be a predictive indicator of sepsis-related morbidity and mortality [10]. Lactoferrin is also known to exert changes on leukocytes of the innate immune system, through increasing natural killer (NK) cell activity [87,88], promoting function of neutrophils by enhancing phagocytic activity and modifying production of reactive oxygen species [89–91], and activating macrophages through increasing cytokine and NO production and limiting intracellular pathogen proliferation [50,92–96]. The degranulation of neutrophils in response to inflammatory signals introduces lactoferrin into an environment that is populated with a mix of both innate leukocytes (macrophages, dendritic cells, NK cells) and adaptive immune cells (T-cells and B-cells). The discovery of lactoferrin receptors on a wide variety of immune cells, and their demonstrated capability to bind lactoferrin, [97–102], confirms the potential for this molecule to function in a manner to modulate and affect responses of both the innate and adaptive immune system.
Modulation of cytokine production from leukocyte populations by lactoferrin has been profoundly examined. Proinflammatory cytokines, TNF-α, IL-6, and IL-1β, can be modulated by lactoferrin, either to increase [94,103,104] or decrease [11,76,105,106] production dependent upon the type of insult recognized by the immune system in concert with local environmental conditions. In addition to changes in the above mentioned proinflammatory cytokines, lactoferrin can also increase in vivo and in vitro production of IL-12 [107–111], a cytokine produced by antigen presenting cells (APCs) upon encountering pathogens. IL-12, a heterodimer comprised of two subunits p40 and p35, functions to enhance production of IFN-γ, increase proliferation, and augment cytotoxic activity of lymphocytes of the innate (NK cells) and adaptive (CD4+ and CD8+ T-cells) immune responses. IL-12 is a main factor in driving development of T-cell helper type 1 (TH1) immunity [112,113]. These regulatory effects of lactoferrin on proinflammatory cytokines and IL-12 clearly illustrate its function as a bridge between the innate and adaptive immune responses.
Mediation of Antigen Presentation Cell Function by Lactoferrin
The modulation of APCs by lactoferrin has the potential to affect T-cell activity associated with specific antigenic recognition [114]. Professional antigen presenting cells, macrophages, dendritic cells and B cells present antigens to CD4+ T-cells via major histocompatibility complex II (MHC II), referred to as human leukocyte antigen (HLA) DR, DP, and DQ in humans. While macrophages and dendritic cells play a distinct role in maintaining, augmenting, and generating antigen specific T-cell functions and activities, the primary function of B-cells is to utilize specific surface receptors (antibodies) to capture foreign antigens and present associate epitopes to T cells to allow further maturation of the antibody response and isotype switching. The effects of lactoferrin on APCs are as varied as those observed for T-cell populations (described below), with results often dependent upon experimental parameters.
Macrophages
Macrophages are professional APC that function in the innate immune response through phagocytosis of foreign bodies followed by subsequent release of proinflammatory mediators. They also interact with the adaptive immune system to stimulate antigen-specific T-cells. Receptors for lactoferrin, both bovine and human forms, are present on macrophages, as assessed by binding studies [115]. Numerous studies have focused on lactoferrin’s suppressive effects towards proinflammatory cytokines and type I interferon (IFN-α/β) production, and it’s promotion in generating reactive oxygen species (such as inducible nitric oxide) [96,116]. For example, lactoferrin can decrease TNF-α, IL-6, IL-1β produced from BCG (bacille Calmette-Guerin strain of Mycobacterium bovis) -infected bone marrow derived macrophages (Fig. 7). Lactoferrin can also increase phagocytic activity from unstimulated, LPS stimulated, or infected (vesicular stomatitis virus, Candida albicans, or BCG) macrophages [111,117].
Fig. 7
Lactoferrin decreases proinflammatory cytokines from BCG infected macrophages
Bone marrow derived macrophages were infected with BCG (MOI 10: 1) without (white) or with (black) bovine lactoferrin (100 μg/mL). At 72 h, supernatants were collected and analyzed by ELISA for TNF-α, IL-6, and IL-1β. * p<0.05, *** p<0.001.
In addition to the modulation of these innate activities of macrophages, lactoferrin also affects the contribution of macrophages to function as presenters of antigen to activate antigen specific CD4+ T-cells of the adaptive immune system. This process induces a series of events that are essential to host control of intracellular pathogens, including macrophage migration to sites of inflammation and/or infection. Lactoferrin injected into mice sensitized to SRBC suppressed the inhibition of macrophage migration by migration inhibition factor [118]. At the site of inflammation/infection, stimulation of antigen specific CD4+ T-cells requires presentation of antigenic peptides by macrophages via MHC II and co-stimulatory signals CD80, CD86, and CD40. BCG infected bone marrow derived macrophages that were stimulated with IFN-γ, demonstrated suppressed expression of MHC II which was partially rescued by bovine lactoferrin [119]. Lactoferrin increased surface expression of CD40 on murine macrophage cell line (RAW 264.7) and peritoneal macrophages, with levels comparable to LPS treatment [120]. The expression of CD80 and CD86 is also modulated differentially by lactoferrin. CD80 expression is significantly suppressed in bone marrow derived macrophages treated with BCG in the presence of lactoferrin, with no changes in CD86 even upon activation with IFN-γ [119]. The increase in the ratio of CD86: CD80 suggests that lactoferrin treated BCG infected bone marrow derived macrophages are more capable of stimulating T-cell activation during antigen presentation [121,122]. Indeed, bone marrow derived macrophages cultured with BCG in the presence of lactoferrin stimulated higher production of IFN-γ from BCG sensitized CD3+ and CD4+ splenocytes compared to similarly treated cells without lactoferrin [119].
One of the major cytokines produced by macrophages is IL-12, an important modulator of the TH1 cytokine, IFN-γ. Macrophages, especially at the site of infection, are a main source of IL-12 [123,124], which acts as a co-stimulator to maximize secretion of IFN-γ from differentiated TH1 cells and memory T-cells [125,126]. Peritoneal macrophages isolated from mice after intraperitoneal injection of lactoferrin demonstrated increased production of IL-12 [50]. LPS stimulated macrophages (bone marrow derived, as well as J774A.1 and Raw 264.7 cell lines) infected with BCG increased ratio of IL-12 compared to IL-10, a main negative regulator of IL-12 [50,110,111]. Similar results were found in vivo; the expression of message in the small intestines of mice fed lactoferrin exhibited a similar increase in IL-12p40 with a concurrent decrease of IL-10 [108]. In addition, it is of interest to note that culturing of peritoneal macrophages with lactoferrin elevated levels of IL-18, a cytokine that has shown to act in concert with IL-12 to increase IFN-γ production, and caspase-1, an enzyme that modifies IL-18 into the active form [127–129].
Dendritic Cells
The dendritic cell APCs are comprised of a group of functionally related professional phagocytes that can manipulate naïve T-cell differentiation [130] and redirect memory T-cell functions [131,132]. The ability of lactoferrin to promote antigen specific DTH responses and BCG specific T-cell activation indicates a role in the initiation of T-cell activation, perhaps through modulation of dendritic cell function. Similar to macrophages, dendritic cells possess lactoferrin receptors as both bovine and human lactoferrin can bind to the surface of peripheral blood derived dendritic cells [102,133].
Majority of studies on lactoferrin effecting dendritic cell functions focus on Langerhan cells (LC), epidermal dendritic cells that induce and regulate skin/epidermal immune responses, such as demonstrated in LCs migration in allergen-induced models. Mice given recombinant lactoferrin intradermally in the ear pinnae 2 hours prior to injection of the chemical allergen inhibited LCs from migrating into the lymph node [134]. Prior application (2 or 4 h) of recombinant human lactoferrin on volunteers exposed topically to the contact allergen DPC (diphenylcyclopropenone) also prevented LC migration from the skin [135–137]. Both mouse and human recombinant lactoferrin decrease LC migration induced by IL-1β, but not TNF-α; administration of lactoferrin suppressed the production of de novo TNF-α stimulated by application of IL-1β [134,135]. This effect of lactoferrin to decrease stimulation induced production of cytokines is well documented, as mentioned previously. Even dendritic cells derived from murine bone marrow, when stimulated with LPS, decreased production of IL-12 and IL-1β when cultured with lactoferrin [138].
The ability of dendritic cells to migrate upon stimulation or capture of antigen is important in its function to promote development of antigen specific immune responses, as is its ability to mature, defined by a decrease in antigen uptake and increased expression of MHC II, CD86, CD40 and stimulation of T-cells. It was noted that recombinant lactoferrin can marginally increase expression of CD86 in dendritic cells stimulated with nickel sulphate [139]. Recently, evidence suggests that recombinant lactoferrin may act as a maturation factor for human monocyte derived dendritic cells. Immature dendritic cells cultured with lactoferrin upregulate surface expression of a series of maturation markers, including HLA II (human leukocyte antigen II), CD80, CD86, and CD83, all of which are involved in antigen presentation and activation of CD4+ T-cells. Chemokine receptors associated with migration and homing to draining lymph nodes were similarly elevated from immature DCs cultured with lactoferrin. More importantly, lactoferrin matured dendritic cells increased production of TH1 modulating cytokine, IL-12, with concurrent decrease of the negative regulator IL-10 and a TH2 modulating cytokine IL-6. Lastly, lactoferrin cultured immature DCs exhibit other maturation functions, exhibited by decreased antigen uptake and increased T-cell stimulation [140].
Our studies on the effect of lactoferrin on murine bone marrow derived dendritic cells (BMDCs) are in good agreement with the above mentioned findings. While lactoferrin does not promote maturation of immature murine BMDCs during BCG infection, the addition of lactoferrin does maintain expression of the dendritic cell marker, CD11c (personal communication; S-A. Hwang). Visually, addition of lactoferrin to uninfected or BCG infected BMDCs appears to promote dendritic cell morphological changes, as evident by elongated cellular bodies with extending pseudopodia. Whereas, BMDCs cultured without lactoferrin possess a more rounded cellular appearance (data not shown). In addition, murine BMDCs treated with BCG and lactoferrin increased expression of the migration marker CD62L, a marker that traffics cells to the draining lymph node. Concurrently, CD44 expression in non-infected BMDCs decreased, lowering non-specific migration events at sites of inflammation (Fig. 8). This is a novel finding, with the suggestion that lactoferrin may have differential effects on BMDCs compared to epidermal DCs (LC), relative to migration events. Taken together, these studies point to the possibility that lactoferrin overcomes the natural downregulation of BCG on dendritic cell function. Overall, lactoferrin is a strong mediator of dendritic cell function. These observations, combined with the effects detailed above on macrophages, suggest that lactoferrin exerts its effect on cells involved in primary engagement of pathogens (antigens) that have potential to direct development of subsequent adaptive immunity.
Fig. 8
BCG infected BMDCs increase CD62L and decrease CD44 when cultured with bovine lactoferrin
Bone marrow derived dendritic cells (>90% CD11c+) (5×105 cells/mL) were cultured with BCG (MOI 10: 1) with or without bovine lactoferrin (100 mg/mL) or remained uninfected. After 72 h, cells were collected and stained for expression of CD62L-FITC (A) or CD44-FITC (B).
Lactoferrin Modulates Antigen Specific Adaptive Immune Responses
The above effects of lactoferrin on APC activation, maturation, migration, and antigen presentation suggest that lactoferrin has a unique ability to bridge innate and adaptive cell function, for both T cells as well as B cell responses.
T lymphocytes
The link between cellular and humoral responses is strong, and many cytokines and mediators have been identified as early mediators of effector T cell development [141]. The adaptive immune response is dominated by T-cell (CD3+) activity, which encompasses differentially functional subsets. In the context of lactoferrin, most studies have focused mainly on CD4+ T-cells and its two well-studied differentiation generated subsets, T-cell helper type 1 (TH1) and type 2 (TH1). TH1 cells “help” stimulate and activate macrophages, resulting in intracellular killing events to eliminate intracellular pathogens often through production of reactive oxygen mediates. The TH2 subtype stimulates and activates B-cells, enabling isotype switching and promoting production of antigen specific antibodies [142,143]. A third subset, the more recently defined TH17 subset through to be associated with clinical disease progression, has not yet been examined for affect by lactoferrin [141].
Expression of lactoferrin receptors have been reported on all T-cell subsets, including δγ T-cells [98]. Both bovine and human lactoferrin are capable of binding to surface receptors on the human T-cell line (Jurkat) [144,145], the binding of which may be involved in receptor-mediated endocytosis [146]. T-cells are affected by lactoferrin in a variety of ways, often depending on their current state of maturation, differentiation, and/or activation. Human milk-derived lactoferrin is able to mature CD4−CD8− murine T-cells, with a preference towards expression of CD4 [49], primarily through induced intracellular MAP kinase pathways via Erk2 and a requirement for p56lck (kinase specific to lymphocytes) [147]. Expression of human T-cell ζ-chain, part of the T-cell receptor (TCR) complex and involved in receptor signaling, is increased by human lactoferrin [148]. An adhesion molecule, leukocyte function associated antigen (LFA-1), present on both CD4+ and CD8+ T-cells and involved in cell-to-cell contact is similarly upregulated in human peripheral blood mononuclear cells cultured with human lactoferrin [46]. These associated changes in surface molecules that regulate T-cell function suggest that lactoferrin is capable of modulating T-cell activity and reactivity to local antigens.
As previously discussed, lactoferrin exerts anti-inflammatory and stimulatory effects; affects on T-cells also exhibit this duality in response [149]. Addition of lactoferrin to mitogen activated T-cells decreases overall cytokine production [150]. ConA stimulated murine splenocytes (non-adherent) cultured with bovine or human milk lactoferrin demonstrate decreased production of IFN-γ and IL-2 (Fig. 9). In a nickel induced T-cell activation model, a recombinant lactoferrin decreased T-cell proliferation, cytokine production (IL-5), and chemokine receptor expression (CCR4) [151]. Conversely, in vivo examination of delayed type hypersensitivity (DTH), a T-cell mediated response, found that introduction of lactoferrin enhanced the DTH response as measured by footpad swelling to a variety of antigens, including ovalbumin, sheep red blood cells, and BCG [50,152,153]. Indeed, administered lactoferrin can potentiate the restoration of humoral immune response in immunocompromised hosts [154,155], suggesting a mechanism for stimulating adaptive cell reconstitution through proliferative pathways. In a murine model of immune suppression induced by an immune suppressive agent (cyclophosphamide) or stress, lactoferrin delivered either orally or intraperitoneally restored the host DTH response, in part by increasing circulating T-cell populations [154,156,157]. Mice orally fed lactoferrin increased circulating total leukocytes, CD3+CD4+, CD3+TCRγδ+, and granulocyte populations [108]. In mice implanted with tumor cells, oral delivery of lactoferrin elevated the lymphoid and intestinal resident CD4+ and CD8+ T-cells [158].
Fig. 9
Lactoferrin decreases T-cell cytokines from Con A stimulated splenocytes
Non-adherent splenocytes (2×106 cells/mL) were isolated from C57BL/6 mice. Production of IFN-γ (A) and IL-2 (B) were measured by ELISA after 72 h stimulation with Con A (2 μg/mL) in the presence of increasing concentration of bovine or human lactoferrin (1, 10, 100 μg/mL). * p<0.05
The effect of lactoferrin on T-cell populations can be further delineated into specific targeting of cellular subset responses. A well studied phenomenon is the ability of lactoferrin to modulate and direct changes in the balance of TH1 and TH2 immunity often defined by the T-cell cytokines IFN-γ and IL-4 and IL-5 respectively. As with the overall T-cell response, lactoferrin is capable of affecting both TH1 and TH2 cellular subsets. The effects appear to be dependent on activation events, relative to complexity of stimulus or antigen. A comparison in delivery of lactoferrin reported that mitogen stimulated (ConA) splenocytes from mice orally given lactoferrin increased TH2 cytokines whereas injection with lactoferrin intramuscularly increased IL-2 (a TH1 associated T-cell cytokine) [159]. Lactoferrin suppressed IFN-γ production from anti-CD3/CD28 activated human CD4+ T-cells [160]. In a Toxoplasma gondii infection model, oral lactoferrin favored a TH2 response in the gut mucosa, observed by a decrease in IFN-γ and elevation in IL-10 (a TH2 associated cytokine) [161]. Lactoferrin inhibited proliferation of a TH1 cell line but not a TH2 cell line. This inhibition correlated to changes in receptors for autocrine growth factors, IL-2 and IL-4 [162]. Lactoferrin reduced surface expression of IL-2R on TH1 cells, whereas there were no changes in expression of IL-4R on TH2 cells during antigen stimulation [163].
In contrast, orally administered lactoferrin given to multiple sclerosis patients, naive mice, or tumor-carrying mice increased IFN-γ levels [157,158,164], indicating an increase in TH1 T-cell response. Similarly, Staphylococcus aureus infection of transgenic mice expressing human lactoferrin saw a shift of host responses towards TH1, as observed by increased levels of IFN-γ and TNF-α and decreased IL-5 and IL-10 [165]. This polarization of host immunity towards TH1 by lactoferrin can remain even after the initial T-cell activation subsided. In one specific example of polarization, recall responses in mice vaccinated with BCG/lactoferrin exhibited higher production of IFN-γ compared to mice immunized with BCG only [166,167]. The ability of lactoferrin to modulate antigen-specific T-cell response that can last several weeks after the initial sensitization suggest that lactoferrin affects events during priming of naïve T-cells to specific antigens.
B lymphocytes
In addition to lactoferrin’s role as a modulator of excessive response, an eloquent series of experiments demonstrated that lactoferrin can also function to enhance depleted immunoreactivity. This is most evident in the role of lactoferrin to affect B lymphocytes. Lactoferrin has the ability to promote maturation of murine immature B-cells, evident by increasing complement 3 receptor (C3R) expression and promoting acquisition of surface IgD. Immature B-cells cultured with lactoferrin increased their ability to promote antigen-specific T-cell proliferation, indirectly indicating enhanced B-cell antigen presentation [48]. B-cell presented antigens specifically stimulated T-cells to induce cytokines required for isotype switching events. Oral delivery of lactoferrin increased secretion of overall IgA and IgG from murine Peyer’s patches [168] with specific antibody titers remaining elevated in both serum and intestinal secretions [159]. In a chemotherapy-induced immune suppression murine model, lactoferrin given intraperitoneally prior to a sub-lethal dose of cyclophosphamide lessened the suppression of antibody forming cells. Lactoferrin was also able to facilitate restoration of the immune response [156]. In another series of experiments, lactoferrin specifically exhibited the ability to overcome the suppressive action of methotrexate in the secondary, humoral immune response to sheep erythrocytes in mice [169]. Furthermore, the biological activity of different glycoforms was tested in vitro, revealing the importance of N-acetylneuraminic (sialic) acid as a terminal sugar in propagation of the reconstituted immune responses [170]. Consequentially, this suggests that lactoferrin can act on B cells, a known antigen presenter, to allow subsequent T cells interactions that favor elevation of the antibody response.
ADJUVANT ACTIVITY OF LACTOFERRIN
Because of the above stated effects of lactoferrin on cells involved in antigen presentation, as well as on its direct mediation of lymphocyte function, it is an excellent candidate for use as an adjuvant. Lactoferrin has clearly demonstrated ability to promote T-cell mediated delayed-type hypersensitivity against a variety of antigens, including experimental antigens ovalbumin and sheep red blood cells, as well as antigens of critical therapeutic importance, such as BCG [50,153]. Indeed, the potential for use of lactoferrin adjuvant even extends to other species, including fish [171,172].
A promising utility for lactoferrin as an adjuvant is for enhancing the efficacy of the tuberculosis vaccine. Our laboratory demonstrated that lactoferrin add-mixed into the immunization formulation with BCG is able to augment efficacy of the BCG vaccine to confer additional protection in the lung and spleen against aerosol MTB challenge, with generation of increased cytokines critical for protective DTH response of a TH1 profile [50,110,111,166,167]. One remarkable finding was that addition of lactoferrin to the BCG vaccine led to reduced pathological damage upon subsequent infection with virulent MTB. Positive results were demonstrated when admixed in oil-based vehicle (incomplete Freund’s adjuvant) or when given with BCG in saline in a single dose or with a booster at 2 weeks [50,167] with decreased organ bacterial load compared to non-immunized controls [166,167] and reduced lung histopathology (Fig. 10). The tuberculosis induced granulomatous response from mice immunized with BCG/lactoferrin had an apparent reduction in lung inflammatory responses, showing normal lung parenchyma surrounding granulomas that were visually highly lymphocytic in composition. Similar findings hold true using a novel recombinant human lactoferrin (personal communication, J. Actor).
Fig. 10
Mice immunized with BCG and lactoferrin demonstrate diminished inflammation and destructive pulmonary histopathology upon challenge with virulent MTB
C57BL/6 mice (6 per group) demonstrated reduced histological manifestation of disease in the BCG/lactoferrin immunized groups at day 65 post challenge. The top panels depict histopathology from a non-immunized control and is compared to mice immunized and boosted with BCG vaccine (middle panels). The lactoferrin adjuvant immunized mice (bottom panels) revealed striking reduction in granulomas with evidence of lymphocytic clusters and contained focal pockets of inflamed monocytes. Staining with H&E (20× or 100× magnification).
While lactoferrin itself is an effective adjuvant for development of DTH, others have tried to improve efficacy using a linked monophosphoryl lipid A complex to stimulate humoral and cellular immune response to ovalbumin and sheep red blood cells [173]. The same group also demonstrated enhanced immunity against Plesiomonas shigelloides using the lactoferrin-monophosphoryl lipid A complex [174]. Finally, numerous trials have examined lactoferrin to modulate responses to cancer antigens, for use in prophylactic or therapeutic treatment [175–179].
RECEPTORS FOR LACTOFERRIN ON IMMUNE SYSTEM CELLS
The effort to identify lactoferrin receptor(s) that are responsible for the immune modulatory activities observed proves to be challenging. Recent and significant discoveries have expanded the identification of receptor(s) responsible for lactoferrin effects on leukocytes. Lactoferrin was first identified as an important defense component of colostrum and mature milk, thereby promoting the hypothesis that its function involves protecting neonatal gut barriers. In concurrence with this hypothesis, lactoferrin receptor (LfR) was first discovered in the small intestinal through examination of iron delivery to the duodenal mucosa [180]. A recent study of LfR in mouse small intestine suggests that lactoferrin may act as the main source of iron during early stages of life [181]. Subsequent binding affinity studies identified LfR on B-cell, T-cells, and monocytes [97,182–184], as well as on platelets [185], and intestinal cells [180]. The low-density lipoprotein receptor-related protein-1 and -2 (LRP1 and LRP2), which are multi-ligand receptors, are considered primary lactoferrin receptors. Although members of the LRP family are generally considered endocytic receptors, it has been suggested that LRP1 also functions as a signaling receptor [186].
The receptors for lactoferrin can be subdivided according to potential immune function. The LfR is described as a ~105kDa surface protein expressed on activated human lymphocytes [187]. Human Jurkat T-cells internalize and release human lactoferrin, with 30–40% of lactoferrin degraded each cycle [146]. The specificity of LfR can cross species barriers. Human monocytes bind human lactoferrin [188], with similar interactions observed in B-cells [97,189]. Likewise, murine peritoneal macrophages uptake human lactoferrin [190]. Additionally, other cell types possess LfR and are capable of internalizing lactoferrin, including murine and bovine brain endothelial capillary cells [191,192], rat hepatocytes [193], and human placental cytotrophoblast cell line [194]. The mechanism of LfR endocytosis appears to be clathrin mediated [97,194].
Studies on lactoferrin binding affinities to target cells identify low affinity binding sites, believed to be mediated by sulfated chains of proteoglycans [183]. Both bovine and human lactoferrin bind to the surface of THP-1 cells, a human monocytic cell line. Binding can be reduced by inhibiting sulphation on the cell surface [115, 133, 195]. Both human and bovine lactoferrin bind purified heparin sulfate chains, and to a lesser extent, chondroitin sulfate chains. The decrease in ability of lactoferrin to prevent viral infectivity of heparin deficient or heparin and chondroitin deficient murine L fibroblasts demonstrates the specificity of lactoferrin to glycosaminoglycans [196].
The anti-inflammatory effects of lactoferrin on leukocytes stimulated with LPS are well known. These anti-inflammatory effects of lactoferrin directly relates to interaction with proteins involved in LPS signaling. Lipopolysaccharide-binding protein (LBP) is a secreted protein that facilitates LPS binding to CD14, a co-receptor for toll-like receptor 4 (TLR-4), to enhance LPS stimulation of the target cell. Milk-derived lactoferrin competes with LPS for LPB and subsequently, can decrease LPS binding to CD14 [23,197]. Similarly, human lactoferrin is capable of interacting with soluble CD14 to prevent CD14-LPS induction of adhesion molecule expression [198]. However, there are no reports of direct interaction of lactoferrin with the signaling molecule, TLR-4. Indirectly, TLR-4 plays no role in bovine lactoferrin induced IL-6 production from murine peritoneal cells, but TLR-4 expression is required for optimal lactoferrin stimulated expression of CD40 [120]. Another study showed that TLR-4 is required for lactoferrin to activate the anti-viral state in host cells against vesicular stomatitis virus, but TLR-4 is dispensable in lactoferrin stimulation of TNF-α production [96]. Recently, the anti-inflammatory effect of lactoferrin on B-cells is attributed to its ability to bind CpG-containing oligonucleotides [199,200], promoting the possibility that lactoferrin may interact with TLR-9.
IMPORTANCE OF SUGAR RESIDUES ON LACTOFERRIN FOR IMMUNE ACTIVATION
The key to understanding the molecular basis of lactoferrin various activities is thought to reside in part according to patterns of glycosylation [201]. There are three possible N-linked glycosylation sites in human lactoferrin, one at Asn138, a second site at Asn479, and a third site at Asn624; differential utilization of these sites results in distinct glycosylation variants. Human lactoferrin glycans are the N-acetyllactosaminic type, α1,3-fucosylated on the N-acetyl-glucosamine residue linked to the peptide chain. Unlike the milk-derived lactoferrin, the neutrophilic form however is not fucosylated, and the difference in molecular structure and function of the two forms is not fully understood. Furthermore there is antigenic variation between human and bovine lactoferrins, mostly due to approximately 70–77% amino acid sequence homology but also due to distinct glycosylation patterns. There are at least 5 glycosylation sites present on bovine lactoferrin; Asn-233, Asn-2281, Asn-368, Asn-476 and Asn-545. In addition the glycans present on at least two of these sites (Asn-233 and Asn-545) are of the high mannose type [202]. The others are complex-type glycans that likely contain mannose moieties (Table 2).
Table 2
The Sugar Content of Bovine and Human Lactoferrins
Monosaccharide [%] of the Total MW Bovine Lactoferrin Human Lactoferrin Fucose 0.21 - Galactose 0.45 1.08 Mannose 4.84 1.35 N-Acetylglucosamine 4.25 2.48 N-Acetylgalactosamine 0.85 - Acetylneuraminic Acid 0.6 1.0 Total 11.2 5.9
A recent study highlighted the importance of these glycans, in particular the residual sugars, regarding the biological activity of the recombinant human lactoferrin glycoforms glycoengineered in Pichia pastoris. The importance of N-acetylneuraminic (sialic) acid as a terminal sugar in propagation of lactoferrin mediated immune response was demonstrated in a study designed to overcome the suppressive action of methotrexate in the secondary, humoral immune response in mice [170]. Another indirect method linking lactoferrin to mechanisms that overcome suppression may include IL-8, a chemokine that binds proteoglycans. Lactoferrin may participate in the formation of chemokine gradients at sites of inflammation. Specifically, human lactoferrin effectively competes with IL-8 for proteoglycan binding sites, and may serve as an explanation of the anti-inflammatory effects of lactoferrin observed in in vivo sepsis models [203].
As lactoferrin contains multiple sites for glycosylation, it is recognized by the group of C-type lectin receptors, which includes mannose receptor and DC-SIGN (specific ICAM-3-grabbing non-integrin). Indeed, dendritic cells pre-treated with bovine lactoferrin inhibit infection with HIV-1, resulting from lactoferrin binding to DC-SIGN to block its interaction with gp-120 (HIV envelope protein) and prevent virus transmission [102]. Glycosylation is also required for lactoferrin’s adjuvant activities; increased generation of delayed type hypersensitive response to ovalbumin can be abolished by addition of methyl-α-D-mannopyranoside [47,152]. Briefly, the adjuvant activity of lactoferrin in the generation of DTH to antigen can be inhibited by bovine serum albumin bearing alpha-D-mannopyranosyl residues. Inhibition of the effector DTH response could be specifically reversed by methyl-alpha-D-mannopyranoside (MMan) but not by D-galactose (Gal). Comparative studies between bovine and human lactoferrins (BLF and HLF) revealed that the adjuvant effect of BLF was stronger than that of HLF; nevertheless, both effects were inhibited by MMan. Thus, it is postulated that the mannose receptor (MR) is a primary receptor for lactoferrin in mediation of its immunotropic activities. However, there are no studies that report evidence of direct binding of lactoferrin to the mannose receptor.
Identifying sugar residues on lactoferrin that mediate interactions with receptors that bind and/or participate in the various immune regulatory functions of lactoferrin remains an ongoing research goal. While many receptors have been identified, most of the known receptors function in the endocytic process or as adhesion molecules but have limited intracellular signaling activities. The challenge in understanding the mechanism of lactoferrin in modulating functions of leukocytes is in sorting through the various potential binding possibilities to identify specific signaling receptors.
CONCLUSIONS
Lactoferrin is a key element to combat excessive inflammation and direct host immune function to protect against overaggressive microbial insults. Found in exocrine secretions, lactoferrin serves as a natural regulator of host defense. It is one of the first factors released by neutrophils upon encounter with pathogens and contributes to innate activation by directing development of adaptive responses. In combination with its historical role in limiting microbial proliferation and functioning as a direct microbicidal agent, lactoferrin plays a central role in host immunity. Lactoferrin has the ability to modulate cytokine production from monocytes, as well as from lymphocytes, during activation from foreign stimuli or mitogens. In addition, along with co-stimulatory mediators, lactoferrin can modulate chemokine recognition and lymphocyte migratory potential. This, coupled with the ability to affect production and activity of reactive oxygen species, allows lactoferrin to serve as a unique regulator to a wide array of responses, including those involved in septic shock (e.g. systemic inflammatory response syndrome), inflammation, and subsequent development of disease related pathologies.Lactoferrin as a Natural Immune Modulator
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2915836/
Molecules 2011, 166993that lactoferrin is an important brick in the mucosal wall, effective against viral attacks andit could be usefully applied as novel strategy for treatment of viral infections.Keywords: lactoferrin; virus; viral infection1. IntroductionLactoferrin was identified in 1939 in bovine milk [1] and isolated in 1960 from both human [2,3]and bovine milk [4]. Lactoferrin, highly conserved among human, bovine, mouse, and porcine species,is a glycoprotein of about 690 amino acid residues belonging to the transferrin family, able toreversibly chelate two Fe(III) per molecule with high affinity (Kd ~ 10−20 M) retaining ferric iron to pHvalues as low as 3.0, whereas transferrin retains iron to pH of about 5.5 [5,6]. The iron-binding affinityis high enough that, in the presence of lactoferrin or transferrin, the concentration of free iron in bodyfluids cannot exceed 10–18 M, thus preventing the precipitation of this metal as insoluble hydroxides,inhibiting microbial growth and hindering formation of reactive oxygen species. As is apparent fromthree dimensional (3D) structure of human lactoferrin (hLf) [7,8], the molecule is folded into twohomologous lobes (N-lobe residues 1–333 and C-lobe residues 345–691). The two lobes are connected