Chitin,Chitosan and Chitosan Oligosaccharides(COS)
1.Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides(COS)
2. Protective Effect of Chitosan Oligosaccharides Against Cyclophosphamide-Induced Immunosuppression and Irradiation Injury in Mice.
3. Biological Activity of Chitin and its Derivatives
4.Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides
5.food sources: cuttlebone, crab and shrimp shells and the cell wall of fungi and yeast, chiken feet
6. anti-oxidant,anti‐inflammatory activity of low‐molecular‐weight chitosan oligosaccharide (LCOS) in a porcine small intestinal epithelial cell line (IPEC‐J2).
Chitosan block TNF-alpha pathway...
The results showed that TNF‐α, as inflammation inducer, significantly upregulated the mRNA expression of IL‐8 and MCP‐1. Afterwards, LCOS significantly attenuated mRNA expression of IL‐8 and MCP‐1 induced by TNF‐α in the cells.
7.absorption: LCOS is absorbed and widely distributed in the body
8.cuttlebone composite as bone graft is non-cytotoxic and had no damaging effects in rat hepacytes and muscle tissues.
9.COS exposure was also found to decrease the lipopolysaccharide (LPS)-induced secretion of nitric oxide (NO) in the medium. they examined several pro-inflammatory markers, including neutrophil infiltration in organs and TNF-α and IL-1β in serum, and found levels of these cytokines were significantly reduced in COS-treated animals.
10. cuttlebone stimulates osteoblasts
11. chitosan has a net positive charge
Molecules | Free Full-Text | Chitosan for Gene Delivery and Orthopedic Tissue Engineering Applications | HTML
https://www.mdpi.com/1420-3049/18/5/5611/htm
https://www.slideshare.net/hudaeid/chitosan-55360347
Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications - Green Chemistry (RSC Publishing)
https://pubs.rsc.org/en/content/articlelanding/2017/gc/c6gc02827f#!divAbstractAntitumor activities of D-glucosamine and its ... - CORE
https://core.ac.uk/display/101411733
GlcNH2⋅HCl exhibited antitumor activity against Sarcoma 180 in Kunming mice at dosage of 125~500 mg/kg, dose of 250 mg/kg being the best. GlcNH2⋅HCl at dose of 250 mg/kg could enhance significantly the thymus index, and spleen index and could promote T lymphocyte proliferation induced by ConA.Finally, glucosamine58 (GlcNH2), the deacetylated monomer unit of chitosan, can be advantageously used to decorate nanoparticles for delivery of antibacterial and anticancer drugs.59–60 Indeed, GlcNH2 is known to be toxic to several malignant cell lines like human hepatoma, prostate, leukemia and breast cancer cells.61–64 Hence, GlcNH2 might be a promising target for the treatment of malignant cancer due to its inhibitory effect on transglutaminase 2 (TGase2), which contributes to drug resistance.62 GlcNH2 has also been used as a ligand in a kidneytargeted drug delivery system for delivery of prednisolone leading to an increase in concentration of prednisolone in vivo.65
Chitosan: A Versatile Platform for Pharmaceutical Applications | Sigma-Aldrich
https://www.sigmaaldrich.com/technical-documents/articles/materials-science/chitosan-a-versatile-platform.html
Chitosan Oligosaccharides, Water Soluble Chitosan, Glucosamine, Liquid
http://www.marshallmarine.in/chitosan-oligosacchride.html
Chitosan, for versatile tool for drug and gene delivery
https://pubs.rsc.org/en/content/articlehtml/2016/ra/c6ra05574e
Structure of cuttlebone.
a, Ventral view of the cuttlebone. b, Lateral view of a chamber showing the main constituting elements. ds = dorsal shield, hm = horizontal membrane, ls = last septum, p = pillar, s = septum, s = septum, sz = siphuncular zone, vm = vertical membrane.The cuttlefish Sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor | Scientific Reports
https://www.nature.com/articles/srep11513
J Food Sci. 2018 Feb;83(2):535-542. doi: 10.1111/1750-3841.14048. Epub 2018 Jan 19.
Protective Effect of Chitosan Oligosaccharides Against Cyclophosphamide-Induced Immunosuppression and Irradiation Injury in Mice.
Zhai X1,2,3, Yang X1, Zou P1,2, Shao Y2, Yuan S3, Abd El-Aty AM4, Wang J1,2.
Author information
1
Dept. of Food Sciences and Engineering, School of Chemistry and Chemical Engineering, Harbin Inst. of Technology, 150090 Harbin, PR China.
2
Key Lab. of Agro-Product Quality and Safety, Inst. of Quality Standard and Testing Technology for Agro-Product, Chinese Acad. of Agricultural Sciences, 100081 Beijing, PR China.
3
the Dept. of Pharmacology and Toxicology, Beijing Inst. of Radiation Medicine, 100081 Beijing, PR China.
4
Dept. of Pharmacology, Faculty of Veterinary Medicine, Cairo Univ., 12211 Giza, Egypt.
Abstract
Chitosan oligosaccharides (COS), hydrolyzed products of chitosan, was found to display various biological activities. Herein, we assessed the immunostimulatory activity of COS both in in vitro and in vivo studies. In vitro cytotoxicity studies to murine macrophage RAW264.7 revealed that COS is safe even at the maximum tested concentration of 1000 μg/mL. It also stimulates the production of nitric oxide (NO) and tumor necrosis factor (TNF-α) and enhances the phagocytosis in COS-stimulated RAW264.7. We have shown that the COS could significantly (P < 0.05) restore the reduced immune organs indices, phagocytic index, lymphocyte proliferation, natural killer cell activity, and antioxidant enzyme activities in a cyclophosphamide-induced immunosuppressed mice model. COS can also improve the survival rate in irradiation injury mice and significantly (P < 0.05) increased the spleen indices and up-regulates the CD4+/CD8+ ratio in splenocytes. In sum, the aforementioned results suggest that COS might has the potential to be used as an immunostimulatory agent in patients with immune dysfunctions or be a model for functional food development.
PRACTICAL APPLICATION:
COS might has the potential to be used as an immunostimulatory agent in patients with immune dysfunctions or be a model for functional food development.
© 2018 Institute of Food Technologists®.
KEYWORDS:
chitosan oligosaccharides; immunostimulatory activity; immunosuppression; irradiation injuryProtective Effect of Chitosan Oligosaccharides Against Cyclophosphamide-Induced Immunosuppression and Irradiation Injury in Mice. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/29350748
Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides
by Kazuo Azuma *, Tomohiro Osaki, Saburo Minami and Yoshiharu Okamoto
Department of Veterinary Clinical Medicine, School of Veterinary Medicine, Tottori University, 4-101 Koyama-minami, Tottori 680-8553, Japan
Abstract: Previous reports indicate that N-acetyl-d-glucosamine oligomers (chitin oligosaccharide; NACOS) and d-glucosamine oligomers (chitosan oligosaccharide; COS) have various biological activities, especially against cancer and inflammation. In this review, we have summarized the findings of previous investigations that have focused on anticancer or anti-inflammatory properties of NACOS and COS. Moreover, we have introduced recent evaluation of NACOS and COS as functional foods against cancer and inflammatory disease.
Keywords: oligomers; glucosamine; N-acetyl-d-glucosamine; chitin; chitosan; cancer; anti-inflammatory; inflammatory bowel disease
1. Introduction
Chitin (β-(1-4)-poly-N-acetyl-d-glucosamine) is widely distributed in nature and is the second most abundant polysaccharide after cellulose 1 [1]. Chitin occurs as the major structural component in the exoskeleton of crab and shrimp shells and the cell wall of fungi and yeast [2]. As chitin is not readily dissolved in common solvents, it is often converted to its more deacetylated derivative, chitosan [3,4,5]. Even though chitin and chitosan are known to have important functional activities, their poor solubility makes them difficult to use in food and biomedical applications [6]. In contrast, the hydrolyzed products of chitosan—N-acetyl-d-glucosamine oligomers (chitin oligosaccharide; NACOS) and d-glucosamine oligomers (chitosan oligosaccharide; COS) are readily soluble in water because of their shorter chain lengths [7]. The low viscosity and greater solubility of COS at neutral pH have attracted the interest of many researchers to utilize chitosan in its oligosaccharide form. NACOS and COS are generated by depolymerization of chitin or chitosan by using acid hydrolysis, hydrolysis by physical methods, and enzymatic degradation [8].
Recently, many reports have indicated that NACOS and COS possess numerous biological activities. However, most of these studies were performed either in vitro or via intravenous (i.v.) or intraperitoneal (i.p.) administration. More recently, the anticancer and anti-inflammatory effects of orally administered NACOS or COS have been described. In this review, we focus on these properties of NACOS and COS by first summarizing the findings of previous studies and then discussing the potency of NACOS and COS as functional foods against cancer and inflammation.
2. NACOS, COS, and Their Derivatives as Anti-Cancer Agents
2.1. Anti-Cancer Activities of NACOS and COS
Nam et al. reported chemo-preventive effects of COS in colon cancer cells [9]. The effects were evaluated by measuring the activities of enzymes quinine reductase (QR), ornithine decarboxylase (ODC), and glutathione-S-transferase (GST) as well as glutathione (GSH) levels and cyclooxygenase-2 (COX-2) expression in human colorectal adenocarcinoma cell line, HT-29, treated with COS. These results indicate that COS exerts its chemopreventive effect against colon cancer by increasing QR and GST activities and GSH levels and by inhibiting ODC activity and COX-2 expression in vitro. In another study, Nam et al. also showed that COS pretreatment inhibited pro-inflammatory cytokine-mediated nitric oxide (NO) production, inducible NO synthase (iNOS) expression, and invasiveness of HT-29 cells [10]. Quan et al. have discovered COS to have antiangiogenic activity through an unclear mechanism but hypothesized it to be via inhibition of heparanase [11]. They have also shown that MDA-MB-231 cells treated with COS had a concentration-dependent reduction in matrix metalloproteinase-9 (MMP-9) secretion and activity as well as inhibition of their invasiveness through a matrigel-coated membrane [12].
Shen et al. have investigated the antitumor and antimetastatic potential as well as pathways affected by COS extracted from fungi, in human hepatocellular carcinoma cell line, HepG2 [13]. They discovered that in vitro COS significantly inhibited cell proliferation, reduced the percentage of cells in S-phase, and decreased the rate of DNA synthesis in the cells. Further analysis of expression of cell cycle-related genes revealed that p21 was upregulated, while proliferating cell nuclear antigen (PCNA), cyclin A, and cyclin dependent kinase (CDK)-2 were downregulated. Moreover, they observed that MMP-9, an enzyme associated with metastasis, could be inhibited by COS in Lewis lung carcinoma (LLC) cells. During animal studies, they discovered that intraperitoneal injections of COS inhibited the growth of HepG2 xenografts in severe combined immune deficient (SCID) mice. Furthermore, in an LLC mouse model of primary tumor and metastasis, COS administration was found to inhibit tumor growth, decrease the number of metastatic colonies in lung, and prolong the survival time of the animals.
It has been postulated that the tumor inhibitory effects of NACOS and COS are potentially related to their ability to induce lymphocyte cytokines thorough increased T-cell proliferation. Essentially, the antitumor mechanisms of NACOS and COS are presumably enhanced by acquired immunity via acceleration of T-cell differentiation, which in turn increases cytotoxicity and maintains T-cell activity [14]. Park et al. have examined the effects of molecular weight and degree of deacetylation of chitosan oligosaccharides on their antitumor activity [15]. They fractionated chitosan oligosaccharide (CTS-OS) by gel-filtration chromatography into two major fractions: (1) COS, consisting of glucosamine (GlcN)(n), n = 3–5, with a 100% degree of deacetylation (DDA) and (2) COS, consisting of (GlcN)(5) as the minimum residues and varying number of N-acetylglucosamine (GlcNAc)(n), n = 1–2, with DDA about 87.5% in random order. The cytotoxic potential of these, expressed as EC(50) (the concentration needed for 50% cell death), of CTS-OS, COS, and HOS against cancer cell lines—PC3 (human prostate), A549 (human lung), and HepG2 (human hepatoma), was determined to be 25 μg/mL, 25 μg/mL, and 50 μg/mL, respectively. The high molecular weight chitosan (HMWC) was approximately 50% less effective as compared to both CTS-OS and COS. This data indicate that the molecular weight and DDA of chitosan oligosaccharides are important factors for suppressing cancer cell growth. Table 1 is a summary of the literature on these studies.
Table 1. A summary of anti-cancer activities of NACOS, COS and its derivatives.
Table
2.2. Anti-Cancer Activities of COS Derivatives
The utility of COS derivatives in targeted drug delivery/gene therapy has also been extensively investigated. Huang et al. have studied the derivatives of stearic acid-g-chitosan oligosaccharide (CSO-SA) as potential carriers for intracellular delivery of anticancer agents [16]. They compared the cytotoxicity of podophyllotoxin (PPT) in a free state vs. PPT loaded on CSO-SA micelles (CSO-SA/PPT) against human cancer cell lines, breast carcinoma (MCF-7), lung cancer (A549), and hepatoma (Bel-7402) and discovered better anticancer activity in the micelle-loaded PPT. This higher cytotoxicity observed can be attributed to faster PPT transport into tumor cells mediated by CSO-SA micelles. Hu et al. have evaluated the low-molecular weight polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide (CSOSA-g-PEI) for gene delivery and therapy [17]. The designed CSOSA-g-PEI had notable ion-buffering property and DNA-binding capacity and could form positively-charged, nanosized particles (100–150 nm) with plasmid DNA, and in vitro gene transfection tests demonstrated that CSOSA-g-PEI presented much lower cytotoxicity than and a transfection efficiency comparable to Lipofectamine 2000 in human cancer cell lines, Hela and MCF-7. The transfection efficiency of CSOSA-g-PEI/pDNA could be further enhanced in the presence of serum or by adding arginine during incubation of CSOSA-g-PEI micelles with plasmid DNA. Further biodistribution experiments demonstrated that CSOSA-g-PEI conjugate are highly localized and are increasingly accumulated in the tumor tissue. Efficacy evaluation in vivo showed that CSOSA-g-PEI/plasmid pigment epithelium-derived factor administered intravenously could effectively suppress tumor growth (>60% tumor inhibition) without any systemic toxicity. Termsarasab et al. have tested chitosan oligosaccharide-arachidic acid (CSOAA) conjugate for the development of self-assembled nanoparticles intended for doxorubicin (DOX) delivery [18]. The DOX-loaded CSOAA-based nanoparticles were spherical in shape with mean diameter of 130 nm and were positively charged. Results of in vitro release test revealed that DOX-loaded CSOAA-based nanoparticles had a sustained and pH-dependent drug release profile. In addition, CSOAA showed negligible cytotoxicity in the human head and neck cancer cell line, FaDu and cellular uptake of DOX was higher in the nanoparticle-treated cells in comparison with free DOX-treated cells. Zhu et al. have evaluated the characteristics of galactosylated chitosan oligosaccharide (Gal-CSO) and adenosine triphosphate (ATP) (Gal-CSO/ATP) nanoparticles [19]. They estimated the cytotoxicity of Gal-CSO/ATP nanoparticles in HepG2 cells by using methyl tetrazolium (MTT) assay, and calculated the half maximal inhibitory concentration (IC50) values. Their results showed that the nanoparticles had low cytotoxicity but were taken up by HepG2 cells owing to expression of asialoglycoprotein receptor (ASGP-R) on their surface.
Li et al. have demonstrated targeted delivery of siRNA to the cancer site following conjugation with folic acid-poly (ethylene glycol)-chitosan oligosaccharide lactate (FA-PEG-COL) nanoparticles [20]. In this study, the efficiency of FA-PEG-COL nanoparticles in localizing in tumors was visualized in BALB/c mice bearing OVK18 #2 tumor xenograft by using in vivo imaging, and the researchers discovered that FA-PEG-COL nanoparticles accumulated substantially in tumors as compared to non-targeting COL nanoparticles. Xu et al. have reported a detailed investigation on the oxidation and pH response of ferrocene-modified chitosan oligosaccharide (FcCOS) nanoparticles for 5-fluorouracil (5-FU) delivery [21]. The dispersion of FcCOS nanoparticles depends strongly on pH change and in this study, the researchers showed that 5-FU, the model drug that was efficiently loaded in FcCOS nanoparticles (approximately 238 nm), was released more efficiently with decreasing pH under bubbled N2. Interestingly, the cumulative release of sample under bubbled air and pH of 3.8 was higher at 59.64%, while under bubbled N2 it was 49.02%. These results suggest a synergistic effect of oxidative conditions and low pH in enhancing the disassembly of FcCOS nanoparticles and the release of drug molecules. Table 1 is a summary of the literature on these studies.
3. Anti-Cancer Effects of NACOS and COS Following Oral Administration
In most animal studies that have evaluated the anticancer properties of NACOS and COS, the route of administration has been either i.v. or i.p., and there is not much reported on the beneficial effects of NACOS and COS following oral administration. We recently assessed the anticancer properties of orally administered NACOS and COS in a mouse model of colon cancer using the cell line, colon-26 [22]. We observed that in animals receiving either COS (2% and 4%) or NACOS (2% and 4%), tumor volumes were significantly lower than those in control group (p < 0.05) (Figure 1). Moreover, the active cell proliferation seen in control group was markedly suppressed in the NACOS and COS groups, and instead, necrotic cells were widely observed in the tumors in these animals. Serum levels of interleukin-12p70 and interferon-γ were also considerably increased in the NACOS and COS groups (p < 0.01, Table 2). Collectively, these results indicate that the anticancer effects of NACOS and COS following oral administration could be mediated by enhanced innate immunity. Previous reports have indicated that the inhibitory effect of COS on tumor growth was most likely related to its ability to induce lymphocyte cytokines by increasing T-cell proliferation. Mainly, adaptive immunity is thought to have enhanced the antitumor mechanism of COS by accelerating T-cell differentiation, which in turn increases cytotoxicity and maintains T-cell activity [14]. Studies have demonstrated that the antitumor effects of certain low molecular weight chitosans, such as water-soluble 21- or 46-kDa molecules that form low viscosity solutions, in mice bearing sarcoma (180 tumors) can be attributed to an increase in natural killer (NK) cell activity [23,24]. Another separate report stated that a low molecular weight, water-soluble chitosan and COS could prevent tumor growth by serving as immunomodulator in enhancing cytotoxic activity against tumors [25]. In certain cases of skin disease, low molecular weight, water-soluble chitosan and COS have been shown to activate macrophages via the production of cytokines, interferon (IFN)-γ and interleukin (IL)-12, in intraepithelial lymphocytes [26]. These observations strongly suggest that oral administration of NACOS and COS stimulates the production of IFN-γ and IL-12.
Jfb 06 00033 g001 550 Figure 1. Effect of orally administered NACOS and COS on tumor growth. The effects of orally administered NACOS and COS were evaluated using colon 26 bearing mouse model. Mice were fed 1%, 2% or 4% NACOS or COS contained diet. Data represent the mean ± standard error. n = 8–10 in each groups. ** indicates p < 0.01 and * indicates p < 0.05 as compared to the control group (Tukey-Kramer test). Reprinted with permission. Copyright 2014 Elsevier [22].
Table 2. Effect of orally administered NACOS and COS on serum cytokine levels. Reprinted with permission. Copyright 2014 Elsevier [22].
Table
Anticancer effects of orally administered NACOS and COS have also been evaluated in MyD88 (myeloid differentiation primary response gene 88) knockout mice and were found to be related to MyD88-dependent as well as MyD88-independent pathways [27]. Stimulation of innate immunity is essential for activation of adaptive immunity [27] and in particular, Toll-like receptors (TLR) on the surface of intracellular organelles recognize specific structures on bacteria, viruses, and fungi [28]. In fact, chitin has been known to activate TLR-2 and Myd-88 in a novel IL-17A/IL-17AR-based innate immunity pathway [29] and adapter molecules such as MyD88 and Toll interleukin receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF) play important roles in inducing the production of cytokines via TLRs [30,31]. TLR-4 is also a known stimulator of cytokine production via MyD88 as well as TRIF signaling pathways [31]. In our previous experiments, we have observed that suppression of tumor growth following NACOS and COS treatments, administered orally, was not as robust in MyD88 knockout mice as it was in normal mice. These results suggest that in vivo antitumor effects of NACOS and COS are mediated not only by MyD88 dependent pathways, but also by MyD88 independent pathways.
Kan investigated the therapeutic effect of NACOS, administered through orally route, in patients with cancer [32]. A substantial regression of the cancer was observed in most patients, especially in those with early stage cancer. In addition, patients who were concomitantly treated with chemotherapy and/or surgical operation also showed significant decrease in tumor burden. The anticancer effects observed were regardless of the organ treated. These data reveal a potential for orally administered NACOS to be used in anti-cancer therapy. However, further detailed studies are required in order to successfully evaluate this.
4. Anti-Inflammatory Activities of COS
Numerous studies have reported the anti-inflammatory properties of COS. In a study conducted by Yoon et al. to investigate the effect of COS on LPS-stimulated RAW 264.7 cells, the researchers discovered that COS exposure led to a dose-dependent attenuation of LPS-induced secretion of TNF-α and IL-6 in the incubation medium [33]. Moreover, a corresponding decrease in TNF-alpha and IL-6 at the mRNA level indicated that COS exposure downregulated the expression of these cytokines at the transcription level. COS exposure was also found to decrease the lipopolysaccharide (LPS)-induced secretion of nitric oxide (NO) in the medium. Interestingly, the addition of external TNF-α to the medium reversed the COS-mediated decrease in IL-6 and NO levels thereby indicating that the anti-inflammatory effect of COS was by modulation of TNF-α pathway Yoon et al. have also investigated the protective effects of COS against glycerol-induced acute renal failure (a model of renal oxidative stress) [34] and their data indicate that COS mitigates the glycerol-induced inflammatory response, improves renal function, and has antioxidant effects in kidney. Fernandes et al. have demonstrated that the anti-inflammatory activity of COS in carrageenan-induced paw edema method was not only dose-dependent but also molecular weight-dependent at higher doses [35]. Quia et al. reported on the protective effect of COS in LPS-induced sepsis [36]. They found that treatment by COS not only attenuated organ dysfunction but also improved survival rate after LPS injection. To further dissect the mechanism, they examined several pro-inflammatory markers, including neutrophil infiltration in organs and TNF-α and IL-1β in serum, and found levels of these cytokines were significantly reduced in COS-treated animals. The redox imbalance in LPS-induced sepsis resulting from depletion of glutathione (GSH) and catalase (CAT) levels and increase in malondialdehyde (MDA) levels was also found to have been reversed by COS exposure. Furthermore, signal pathways activated by LPS, such as c-Jun NH(2)-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), were also found to have been attenuated by COS treatment. These data demonstrate that the protection afforded by COS against LPS challenge in the mouse model could be by virtue of its anti-inflammatory as well as antioxidant properties.
Pangestuti et al. have described the effects of COS in four different molecular weight ranges (<1, 1–3, 3–5, and 5–10 kDa) for their ability to modulate inflammatory mediators in LPS-stimulated BV2 microglial cells [37]. At a concentration of 500 μg/mL, COS was found to attenuate the production of NO and prostaglandin E2 (PGE2) by inhibiting iNOS and COX-2 expression. Furthermore, the expression levels and release of inflammatory cytokines, including TNF-α, IL-6 andIL-1β, were also attenuated by COS. Notably, the inhibitory activity of COS was found to be dependent on its molecular weight, and lower molecular weight COS showed higher activity. In addition, this study confirmed the suppressive effects of COS on phosphorylation of JNK and p38 MAPK. Chung et al. have investigated the effects of COS against allergic reaction and allergy-induced asthma in vivo and in vitro [38]. COS, consisting of glucosamine (GlcN)(n), n = 3–5, was shown to be capable of inhibiting antigen-stimulated degranulation and cytokine generation in rat basophilic leukemia RBL-2H3 cells. This study also examined a protective effect of COS against ovalbumin (OVA)-induced lung inflammation in mouse model of asthma. The researchers discovered that animals receiving a daily oral administration of COS (16 mg/kg body weight/day) had a significant reduction in the mRNA expression and protein levels of IL-4, IL-5, IL-13, and TNF-α in their lung tissue and bronchoalveolar lavage fluid (BALF); protein levels of IL-4, IL-13, and TNF-α in BALF were decreased by 5.8-fold, 3.0-fold, and 9.9-fold, respectively, in comparison with the OVA-sensitized/challenged asthma control group. Choi et al. have demonstrated the effect of COS on body weight gain, adipocyte size, adipokine level, lipid profile, and adipose tissue gene expression profile in high-fat (HF) diet-induced obese mice [39]. Compared with the HF diet mice, mice fed HF diet supplemented with 3% COS had gained 15% less weight but did not display any change in food and energy intake. COS supplementation was also observed to have markedly improved the serum and hepatic lipid profiles. Microarray analysis revealed that dietary COS supplementation modulated adipogenesis-related genes such as matrix metallopeptidases 3, 12, 13, and 14, tissue inhibitor of metalloproteinase 1, and cathepsin K in the adipose tissues. Twenty-five percent of the COS-responsive genes identified are also involved in immune response, including inflammatory response and cytokine production. In a study conducted by Wei et al., it was discovered that pretreatment with COS at 50–200 µg/mL could substantially abrogate NO production through the reduction of iNOS expression in LPS-activated L9 microglial cells [40]. In addition, COS was found to markedly decrease LPS-induced phosphorylation of p38 MAPK and extracellular signal-related protein kinase ½ and could also inhibit activation of NF-κB and activator protein-1 (AP-1) In a rat model of autoimmune anterior uveitis, Fang et al. discovered that COS treatment markedly attenuated the clinical scores and infiltration of leukocytes in the iris/ciliary body (ICB) in a dose-dependent manner [41]. The expression of inflammatory mediators such as TNF-α, iNOS, MCP-1 (Monocyte Chemotactic Protein-1), RANTES (CCL-5; regulated on activation normal T cell expressed and secreted), fractalkine, and intercellular adhesion molecule (ICAM)-1 was also substantially decreased in animals treated with COS. Moreover, in the ICB, COS decreased the degradation of IKB and levels of p65 thereby resulting in inhibition of DNA-binding by NF-KB. Under in vitro conditions, sensitized lymphocytes derived from the spleens of COS-treated animals had a reduced chemotactic mobility towards the aqueous humor and the levels of the previously mentioned inflammatory mediators in culture media was found to be reduced.
Li et al. have reported a mechanism by which COS attenuates inflammation in endothelial cells [42]. Regardless of the endothelial cell type, COS was found to be instrumental in suppressing the LPS-induced nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB)-dependent inflammatory gene expression, and this was associated with reduced nuclear translocation of NF-κB. LPS enhances O-GlcNAc modification of NF-κB/p65 and activates NF-κB pathway, and this could be prevented either by siRNA knockdown of O-GlcNAc transferase (OGT) or pretreatment with COS. Inhibition of MAPK or superoxide generation is also known to abolish LPS-induced NF-κB O-GlcNAcylation. Consistent with these observations, aortic tissue from LPS-treated mice showed enhanced NF-κB/p65 O-GlcNAcylation, and this was absent in tissues from mice that were pretreated with COS. Hence, COS-mediated attenuation of inflammatory response in vascular endothelial cells is most likely through decreased OGT-dependent O-GlcNAcylation of NF-κB. In a separate report, Li et al. stated that in porcine iliac artery endothelial cells (PIECs) treated with COS, the LPS-induced mRNA expression of E-selectin and ICAM-1 was reduced through the inhibition of p38 MAPK/ERK1/2 and NF-κB signal cascade. Inhibition of p38 MAPK and ERK1/2, also resulted in suppression of LPS-induced nuclear translocation of NF-κB p65. Both these effects were dose-dependent and ultimately inhibited adhesion of U973 cells to PIECs. Based on these results, it can be concluded that inhibition of MAPK phosphorylation and NF-κB activation in LPS-treated PIECs by COS results in decrease in expression of E-selectin and ICAM-1. Table 3 is a summary of the literature on these studies.
Table 3. A summary of anti-inflammatory activities of COS.
Table
5. Anti-Inflammatory Effects of COS for Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) includes ulcerative colitis (UC), and Crohn’s disease, and is characterized by chronic inflammation of the gut [44]. Over the past 40 years, the incidence of IBD has steadily increased in some areas of the world [45], possibly due to changes in dietary habits (particularly consumption of diets low in fiber content) in these regions [46]. Yousef et al. have reported that in human colonic epithelial cell line, T84, subjected to LPS or TNF-α-stimulation, COS treatment prevented LPS binding to cells, NF-κB activation, production of TNF-α and IL-6, loss of epithelial barrier integrity, and TNF-α and oxidative stress-induced apoptosis [47]. They also discovered that in a mouse model of acute colitis, oral administration of COS protected against mortality and intestinal inflammation. In addition, NF-κB activation, and levels of TNF-α and IL-6 in colonic tissues were suppressed in mice that received COS. Importantly, COS administration after colitis induction was effective in ameliorating intestinal inflammation in acute [induced by 5% dextran sulfate sodium (DSS)] as well as chronic (induced by cyclic administration of 2.5% DSS) colitis models. These results suggest that COS may be effective in the treatment of IBD through inhibition of NF-κB signaling and apoptosis of intestinal epithelial cells.
Our group has also evaluated the anti-inflammatory effects of orally administered COS in a mouse model [48] and discovered that COS improved shortening of colon length and tissue injury (as assessed by histology) (Figure 2). In addition, COS inhibited myeloperoxidase activation in inflammatory cells as well as activation of NF-κB, COX-2, and iNOS thereby preventing inflammation of colonic mucosa (Figure 3 and Figure 4).
NF-κB occupies a pivotal position in several signaling pathways involved in innate immunity. It stimulates expression of COX-2, PGE2, and pro-inflammatory cytokines (IL-6, TNF-α, and MCP-1) [49] and is the critical transcription factor needed to express genes associated with pro-inflammatory responses [50]. Cyclooxygenases are the enzymes responsible for biosynthesis of prostaglandins (from arachidonic acid) and these influence many biological processes, including homeostasis and inflammation [51]. In fact, COX-2 expression is increased mainly during inflammatory processes and cell transformation [52]. It has become increasingly clear that nitric oxide (NO) over-production by iNOS is deleterious to intestinal function [53,54], and iNOS levels are considered to be important determinants of colonic damage [55]. Hence, sustained overproduction of NO mediated by iNOS may have a role in the pathogenesis of IBD and induction of experimental colitis in the colon [54]. Oral administration of COS has been shown to reduce serum levels of pro-inflammatory cytokines (TNF-α and IL-6). Pro-inflammatory cytokines (IL-6, TNF-α, MCP-1) are known to trigger leukocyte activation and accumulation in tissues and play a significant role in inflammatory conditions, such as [56].
Jfb 06 00033 g002 550 Figure 2. Effect of orally administered COS on colon injury in experimental IBD model. (A) Sections of colon tissue were stained with hematoxylin and eosin. Data are for one mouse per group from the NT, DSS, COS, and GlcN groups. Bar = 200 μm. (B) Data are the mean ± S.E. of 30 fields/100× magnification field in each group (Steel-Dwass test). ** p < 0.01. Reprinted with permission. Copyright 2015 Elsevier [48].
Jfb 06 00033 g003 550 Figure 3. Effects of orally administered COS on NF-κB activation in colon in an experimental IBD model. (A) Areas stained positive for NF-κB are shown by arrows. Data are for one mouse per group from NT, DSS, COS, and GlcN groups. Bar = 100 μm. (B) Data are the mean ± S.E. of 30 fields/100× magnification field in each group (Steel-Dwass test). ** p < 0.01. Reprinted with permission. Copyright 2015 Elsevier [48].
Jfb 06 00033 g004 550 Figure 4. Effects of orally administered COS on iNOS activation in colon in an experimental IBD model. The immunohistochemistry of iNOS in the colon is shown. Areas stained positive for iNOS are shown by arrows and arrowheads. Data are for one mouse per group from NT, DSS, COS and GlcN groups. Bar = 100 μm. Reprinted with permission. Copyright 2015 Elsevier [48].
Our results suggest that a possible mechanism for the anti-inflammatory effects of orally administered COS is by suppression of inflammatory processes, including expression of NF-κB, COX-2, iNOS, and pro-inflammatory cytokines. Moreover, COS was found to prolong survival time in mice in an experimental model.
6. Next Step to Use NACOS, COS and Its Derivatives for Patient
A schema of this review is shown in Figure 5. An irrefutable amount of evidence has already established the anticancer and anti-inflammatory properties of NACOS and COS in experimental models. More recently, it has been shown that the beneficial traits are retained when NACOS and COS are administered by the oral route. To our knowledge, one article reported the safety of oral administration of COS by short-term study [57].
However, the exact mechanisms behind the actions of NACOS and COS are not yet fully dissected, and further mechanistic studies will be required to harness the benefits of NACOS and COS in therapeutics. More recently, beneficial effects of nanomaterials based on chitin and chitosan are also reporting [2,58,59,60,61,62,63]. Effective usage including combination of nanomaterials from chitin and chitosan with NACOS and COS is must be researched.
JFB | Free Full-Text | Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides | HTML
https://www.mdpi.com/2079-4983/6/1/33/htm
Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications
Simone S. Silva, ORCID logo *ab João F. Manoab and Rui L. Reisab
3B's Research Group – Biomaterials, Biodegradables, and Biomimetics, University of Minho, Headquarters of the European Institute of Excellence on Tissue Engineering and Regenerative Medicine, Avepark - Parque de Ciência e Tecnologia, Zona Industrial da Gandra, Barco GMR, Portugal
Abstract
Ionic liquids (ILs) have huge potential to provide advances in many areas such as energy, pharmaceutical formulations, biomedical sciences, and technology. In the biomedical field, ILs have been intensively investigated for use as potential solvents for some polysaccharides to overcome their lack of solubility and processability. This review focuses on the application of ILs as solvents and reaction media to develop chitin- and chitosan-based materials. Dissolution of chitin and chitosan in ILs such as 1-butyl-imidazolium acetate (BMIMAc) and 1-ethyl-3-methylimidazolium chloride (BMIMCl) has been used to create materials including sponges, films, microspheres, and aerogels. Moreover, ILs have a key role in chemical reactions, hydrolysis, acetylation, deacetylation and graft copolymerization of chitin/chitosan, promoting homogeneous media and thus enhancing the efficiency of the reactions. The resulting materials can be applied in wound healing, tissue regeneration, gene delivery, and drug delivery systems. In particular, they have been designed to support tissue regeneration and to act as hemostatic and antibacterial agents and/or delivery vehicles for drugs. Although IL platforms offer new ways for the sustainable processing of chitin and chitosan to a variety of matrices, studies involving their in vivo biocompatibility are scarce, and this has prevented these advances being turned into clinical solutions.Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications - Green Chemistry (RSC Publishing)
https://pubs.rsc.org/en/content/articlelanding/2017/gc/c6gc02827f#!divAbstract
Front Bioeng Biotechnol. 2019; 7: 243.
Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides
Michal Benedykt Kaczmarek,1,2 Katarzyna Struszczyk-Swita,1 Xingkang Li,2 Miroslawa Szczęsna-Antczak,1 and Maurycy Daroch2,*
Author information Article notes Copyright and License information Disclaimer
1Institute of Technical Biochemistry, Lodz University of Technology, Łódź, Poland
2School of Environment and Energy, Peking University Shenzhen Graduate School, Shenzhen, China
Edited by: Inder Mohan Saxena, University of Texas at Austin, United States
Abstract
Chitin and its N-deacetylated derivative chitosan are two biological polymers that have found numerous applications in recent years, but their further deployment suffers from limitations in obtaining a defined structure of the polymers using traditional conversion methods. The disadvantages of the currently used industrial methods of chitosan manufacturing and the increasing demand for a broad range of novel chitosan oligosaccharides (COS) with a fully defined architecture increase interest in chitin and chitosan-modifying enzymes.Enzymes such as chitinases, chitosanases, chitin deacetylases, and recently discovered lytic polysaccharide monooxygenases had attracted considerable interest in recent years.
These proteins are already useful tools toward the biotechnological transformation of chitin into chitosan and chitooligosaccharides, especially when a controlled non-degradative and well-defined process is required.
This review describes traditional and novel enzymatic methods of modification of chitin and its derivatives. Recent advances in chitin processing, discovery of increasing number of new, well-characterized enzymes and development of genetic engineering methods result in rapid expansion of the field. Enzymatic modification of chitin and chitosan may soon become competitive to conventional conversion methods.
Chitin Production Methods
The primary source of raw materials to produce chitin and its N-deacetylated derivatives are wastes of the fishing industry. Exoskeletons of marine organisms, including shrimp, crab, crayfish, krill, squid, are widely used for this purpose (Abdou et al., 2008). Nowadays, chitin and chitosans are obtained by two types of extraction methods: chemical and biotechnological. Chemical processes which involve the use of strong acids and bases are currently the most widely used methods in both laboratory and industrial-scale production.
The process of chitin extraction and its transformation into chitosan includes three major steps: demineralization, deproteinization, and deacetylation. Additionally, decolorization process using various organic and inorganic solvents such as glacial acetone (Soon et al., 2018), sodium hypochlorite (Srinivasan et al., 2018) can be employed to eliminate pigments. Demineralization step is performed to remove the calcium carbonate and calcium chloride, which are the main inorganic constituents of the exoskeletons of crustaceans. For this, inorganic acids such as HCl, HNO3, and H2SO4 (Kumar Gadgey and Bahekar, 2017), and strong organic acids HCOOH and CH3COOH (Regis et al., 2015) are used. The most common acid used in the production of chitin is hydrochloric acid, due to its high efficiency in the removal of the minerals. The next major step in chitin extraction is deproteinization of raw materials. This step is performed using alkali solution to remove proteins. A wide range of chemical reagents have been tested for protein removal including NaOH, Na2CO3, NaHCO3, KOH, K2CO3, Ca(OH)2, Na2SO4, NaHSO4, CaHSO4, Na3PO4, and Na2S (Younes and Rinaudo, 2015). However, the most commonly used is NaOH solution. The chemical extraction of chitin involves large amounts of hazardous alkaline and acid wastes which are dangerous for the environment.Chitosan Production Methods
Currently, there are two well-known methods of chitosan preparation. The first approach is to extract chitosan directly from cell walls of molds. The second approach utilizes thermo-chemical or enzymatic methods of chitin deacetylation to remove the N-acetyl groups from chitin.
In principle, chitin can be deacetylated using either acids or alkalis. Since glycosidic bonds are very susceptible to acid hydrolysis; the alkali-catalyzed deacetylation is used more frequently to avoid unwanted chain termination (Younes and Rinaudo, 2015). For this purpose, 50% NaOH solution is most often used at high temperature (Soon et al., 2018; Srinivasan et al., 2018).
Biological Activity of Chitin and its Derivatives
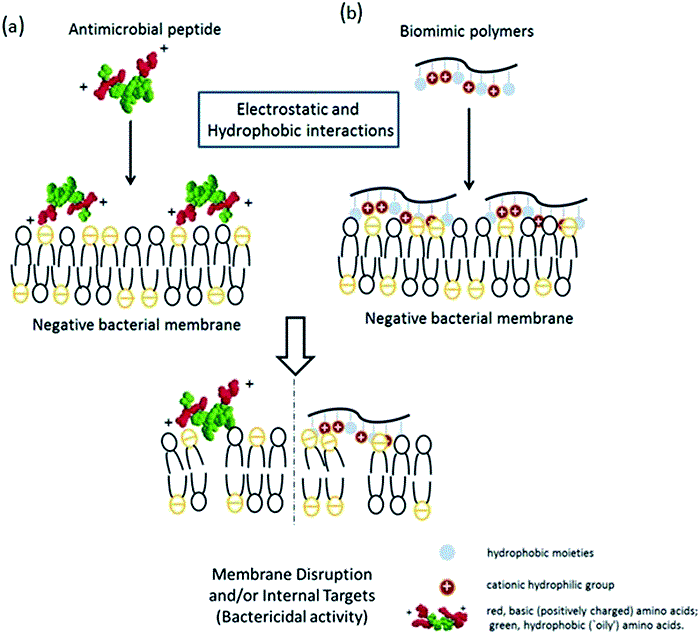
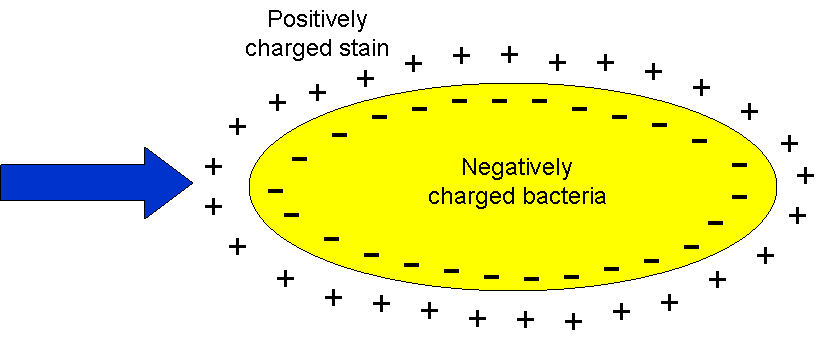
The specific properties of chitin provide numerous potential applications of this biopolymer. Unfortunately, the use of chitin is significantly limited due to the low reactivity and lack of solubility in water and common organic solvents. The most useful chitin derivative is chitosan, which is beneficial for biomedical applications due to biocompatibility, biodegradability and low toxicity. The most important biological activities of chitosan and its degradation products (COS) include antimicrobial, antiviral, antitumor, and antioxidant activities. The spectrum of antimicrobial activity of chitosan and COS includes bacteria, filamentous fungi, and yeast. Chitosan, however, shows its antimicrobial activity only in an acidic medium because of its poor solubility above pH 6.5. Thus, water-soluble COS may be good candidates as a polycationic biocide. The mechanism of their antimicrobial activity has not yet been clearly explained. According to Liaqat and Eltem (2018) contradictions in the proposed mechanisms may be the result of the use of various microorganisms and methods in research, as well as the quality, purity and characteristics of the COS being analyzed. One of the theories explaining this mechanism says that the inhibitory effect of chitosan and COS on bacterial growth is related to their polycationic nature, resulting from the presence of free -NH2 groups in units of D-glucosamine forming the chains of these compounds. This enables them to bind strongly to carboxyl groups with negative charge of compounds building external cell membranes of microorganisms (Kittur et al., 2003; Vishu Kumar et al., 2005, 2007). Chitosan and its oligomers can reduce the permeability of the cell membrane, forming a coating on its surface and thereby blocking cell access to external nutrients, which leads to its death (Vishu Kumar et al., 2007). It is generally recognized that the number of -NH2 groups and also the antibacterial activity often increases with the simultaneous increase of their DP value(degree of polymerization) (Vishu Kumar et al., 2005). The higher activity of chitosan degradation products in relation to the high molecular biopolymer is explained by the possibility of the former penetrating the cells, where they block RNA transcription as a result of adsorption with bacterial DNA (Kim et al., 2003; Mei et al., 2015). The mechanism of interaction of chitosan and its degradation products with bacterial cells depends to a large extent on the structure of the cell wall of a given microorganism. In the case of gram-positive bacteria having a cytoplasmic membrane covered with a cell wall formed of several dozen layers of peptidoglycan containing negative GlcNAc, N-acetylmuramic acid, numerous amino acids, or teichoic acids, primarily for strong binding characterized by the opposite charge COS and LMWCh. This causes deformation of the bacterial cell wall, which in turn is associated with the exposure of the cytoplasmic membrane to osmotic shock, the burst of the cytoplasm and ultimately the death of bacteria. In contrast, the gram-negative bacterial cell contains an outer membrane consisting, among others from lipopolysaccharides (LPS) and proteins; a cell wall with only 1–3 layers of peptidoglycan and a cytoplasmic membrane. Negatively charged O-specific side polysaccharide chains form an ionic type combination with COS or LMWCh amine groups. In the case of COS, cell access to external nutrients is blocked. Due to the strong binding of LPS side chains to the outer membrane of the cell, its destruction does not occur—as was the case with the gram-positive group of bacteria. The smaller the DP of chitosan degradation products and the higher the electronegative charge of bacteria, the easier the associated and aggregation of these compounds occurs, and thus the blockade of the supply of external nutrients and the final cell death (Vishu Kumar et al., 2005). On the other hand, the charge of oligomers with a higher DP, i.e., LMWCh, is large enough to remove the LPS associated with them from the cell membrane and subsequently to cell lysis (mechanism as in the case of gram-positive bacteria) (Vishu Kumar et al., 2007).
The antimicrobial properties of chitosan and its degradation products depend on many factors, including their source and concentration, molecular weight and deacetylation degree, and the strain of the microorganism on which they were tested (Kyoon et al., 2003; Liu et al., 2006; Li et al., 2014; Laokuldilok et al., 2017; Bonilla et al., 2019; Shi et al., 2019). It was found that in the case of COS, their DP with a value of not less than five is essential for antibacterial activity of fully deacetylated COS (Li et al., 2014). Jeon et al. (2001) indicated that COS exhibits antimicrobial activity against Gram-positive and Gram-negative bacteria. However, high-molecular-weight COSs (5 000–10 000 Da) exhibited higher antimicrobial activity than low-molecular-weight COSs. It has been proven that positively charged COSs interact with negatively charged bacterial cell walls, resulting in suppression of the metabolic activity of bacteria by reducing nutrient permeation through the cell wall. Therefore, the death rate of bacterial cells increases upon an increase in the DD of COSs (Tsai et al., 2002). On the other hand, reports are confirming that acetylated sequences in COS structure are essential for their antimicrobial activities, and COS comprising more number of acetylated sequences (less number of free amino groups) have shown higher antimicrobial activities (Sánchez et al., 2017). Further work is needed to determine the mechanism of antimicrobial activity of chitosans and COS and to affect their activity primarily DD and DP. Examples of antimicrobial activities of chitosan and chitooligosaccharides are summarized in Table 8. The antifungal activity of chitosan is commonly used in agriculture for the reduction of mycelial growth, sporangial production, release of zoospores, germination of cysts and the induction of local and systemic resistance (Atia et al., 2005) Additionally, results reported by Mei et al. (2015) proved the potential of COS for clinical application. Enzymatically produced, well-characterized chitooligosaccharides exhibited excellent antifungal properties against dermatophyte fungus Trichophyton rubrum in a guinea pig model.
Table 8
The antimicrobial activities of chitosan and its degradation products.
Chitosan/COS Activity against References
MW [kDa]/DP DD [%]
MW 1–10 75 Vibrio parahaemolyticus Park et al., 2004
MW 8; 66; 197 85 E. coli,
S. aureus,
Candida albicans,
C. tropicaliss Zhang et al., 2019
DP 2–12 – Alternaria alternate, Rhizopus stolo
Botrytis cinereanifera Oliveira et al., 2008
MW 49.5; 138 and 142 91 E. coli,
S. aureus,
C. albicans Pan et al., 2019
MW3 0–10; 10–5; <5 84 E. coli,
Listeria monocytogenes Sánchez et al., 2017
MW 5.1; 14.3 and 41.1 99 E. coli,
Salmonella typhimurium, Salmonella enteritidis Laokuldilok et al., 2017
MW 194 Staphylococcus aureu
MW 28 89 S. typhimurium Jeon et al., 2001
There are several reports on the antiviral properties of chitosan and COS, but the mechanism of their activity has also not yet been clearly explained. Chitosan, as well as its degradation products, most likely inhibit viral infections by reducing virus infectivity and inducing the resistance of plant and animal organisms. Suppression of infectivity may also be associated with preventing the absorption of viral particles into the cell membrane. The sulphated COS with MW in the range of 3–5 kDa is an effective compound to stop replication of HIV-1 virus by blocking viral entry and virus-cell fusion probably via disrupting the binding of HIV-1 to CD4 cell surface receptor (Artan et al., 2009). The study of antiviral activity of chitosan oligomers with MW from 17 to 2 kDa and DD 98.5, 83, and 75% were tested against the tobacco mosaic virus by Davydova et al. (2011). The obtained results confirmed that these samples inhibited the formation of local necrosis induced by the virus by 50–90%.
Chitosan and COS-like chitosans can be considered as potential anticancer agents because of their anti-tumor activities. Unfortunately, the mechanism of their action on tumor cells has not been elucidated to date. Huang et al. (2006) proposed a hypothesis according to which COS as a negatively charged polysaccharides that can adsorb on a cancer cell. The electrostatic interactions between cancer cells and polycationic polymer significantly change the permeability of cancer cells. Mattaveewong et al. (2016) suggest that tumor cells are not killed directly by COS. These small oligosaccharides suppress the NF-κB and mechanistic target of rapamycin (mTOR) by AMP-Activated Protein Kinase (AMPK) activation. Recent research revealed the potential of COS as an immunostimulatory agent which may be used in anticancer therapies related to immunomodulation (Zheng et al., 2016; Xing et al., 2017). The molecular weight of COS has an essential effect on anticancer activity. It has been reported that chitohexanoses are the most promising oligomers to manifest the anticancer effect (Xiong et al., 2009; Li et al., 2011). Wang et al. (2007) published the results of studies confirming the influence of the degree of COS acetylation on anticancer activity. The antiangiogenic activity of acetylated COSs was significantly stronger than the parent oligosaccharide. Other research indicated that antiangiogenic activity of COS is also dependent on FA and DP of oligomers and that the FA is more critical of the two parameters (Wu et al., 2012). Chitosan and its derivatives were used as transporters of anti-cancer drugs. It has been investigated that anticancer agents conjugated with chitosan can execute anticancer effects with a decrease of side effects and gradual release of free drug in the cancer tissues (De Campos et al., 2001; Janes et al., 2001). Liposome-chitosan nanoparticles were used to obtain dose-dependent tumor-weight inhibition drug release system, which showed promising results in in vivo studies (Li et al., 2009). Yin et al. (2017) reported that the COS (MW 2,000–5,000 Da) tethered on the liposomes through disulphide linkers (-SS-) to cholesterol may be an excellent platform for cytoplasmic delivery of anticancer drugs. An amphiphilic all-trans-retinoic acid (ATRA) conjugated COS nanoparticles also revealed the promising potential as drug carriers for co-delivery of ATRA, paclitaxel, and other hydrophobic therapeutic agents (Zhang J. et al., 2015).
In recent years, the possibility of using chitosan and COS as free radical scavengers are also of significant interest. It is known that the mechanism of their antioxidant activity is associated with the presence of free amino group in the glucopyranose rings, which by reacting with free radicals form stable forms of macro-radicals. In addition, the -NH2 groups exhibit chelating properties concerning many metal ions, including Fe2+, which are activators in the formation of hydroxyl radicals—the most dangerous for the human body. Antioxidant activities of chitosan and COS are affected by DD and MW (Park et al., 2004; Zhao et al., 2013). Studies by Park et al. (2004) suggested that the scavenging activity of chitosan depended on its DD and chitosan with a higher DD exhibited better scavenging activity. In contrast, chitosan oligosaccharides (MW 5 kDa, DD 97%) and its derivatives tested by Zhao et al. (2013) showed a higher scavenging effect than chitosan used to obtain them (MW 120 kDa, DD 97%). Like other properties of chitooligosaccharides, their antioxidant activity is also dependent on the physicochemical properties of COS. Studies attempted to determine the relationship between antioxidant activity of COS and their MW indicated that that low MW (5,000 Da) COS had shown the highest antioxidant capabilities. Additionally, it has been found that the antioxidant activity of COS can be predicted based on the composition of oligomers expressed as the ratio of acetylated vs. deacetylated units (Mengíbar et al., 2013). Antioxidant activity of COS is another promising characteristic which can be used to produce value-added products for food preservation and functional food. Studies conducted by Yang et al. (2017) play an active part in the prevention of beer flavor deterioration by inhibiting the formation of staling compounds and increasing radical scavenging activity. The activity of COS was dependent on the molecular weight of oligomers. Additionally, COS showed radical scavenging activity in the finished beer, which is expected to improve the shelf life stability during beer storage.
The biodegradability of chitin and chitosan was principally attributed to their susceptibility to enzymatic hydrolysis by lysozyme, that exists in all human body tissues. It has been demonstrated that chitosan can also be metabolized in animal and human tissues by the combined action of lipase and chitosanases (Poshina et al., 2018). Thus, chitosan and its derivatives have been considered as promising vehicles for oral prolonged-release drugs and as a matrix in drug release systems in the form of beads and granules. Physical hydrogels of chitosan which are usually used for this purpose can be formed by various reversible links such as ionic interactions (crosslinked hydrogels) and polyelectrolyte complexes (PEC), or secondary interactions (chitosan/poly(vinyl alcohol) complexed hydrogels), grafted chitosan hydrogels, and entangled hydrogels (Berger et al., 2004). PECs of chitosan with polyanions of natural origin like pectin, alginate, carboxymethyl cellulose, or with synthetic ones like poly (acrylic acid) have been discovered as matrices for controlled-release systems (Berger et al., 2004). Chandy et al. (2002) reported that chitosan-polyethene glycol-alginate microspheres are suitable materials for the delivery of low molecular weight (LMW) heparin with antithrombotic properties. Chitosan and its derivatives can be used to form products with haemostatic properties. It has been found that in the initial phase of chitosan/blood interactions, plasma proteins absorb on chitosan-based systems. In the next step, the adhesion and activation of platelets occur, which leads to the formation of a thrombus (Yeh and Lin, 2008). It was claimed that chitosan was hypocholesterolemic and hypolipidemic (Domard and Domard, 2002). Pan et al. (2016) investigated that functional food based on the chitosan and its derivatives effectively improve liver lipids metabolism and protect the liver from the oxidized trauma by enhancing hepatic function. Biocompatible, natural and synthetic carriers are commonly used in tissue engineering techniques as a support for initial cell attachment and subsequent tissue formation. Chitosan shows a similar spatial structure as glycosaminoglycans (GAGs) found in the extracellular matrix of several human tissues. The physical and chemical properties of chitosan facilitate the adhesion of the cells and maintenance of the differentiating functions (Croisier and Jérôme, 2013). Gelatin-chitosan hydrogels were successfully used as a culture substratum for respiratory epithelial cells. However, two-dimensional gel conformation was not sufficient to induce very high ciliogenesis and mucus secretion (Risbud et al., 2001). A three-dimensional biodegradable hydroxyapatite/chitosan-gelatin network was used as a biomimetic scaffold for bone cells growth and proliferation. The obtained cell/scaffold constructs had good biomineralization effect after 3 weeks in culture (Zhao et al., 2002). As a polycationic biopolymer, chitosan and its derivatives can form complexes with nucleic acids. This property was utilized in gene transfection experiments, in which chitosan with DD around 80–90% has proved useful as a gene carrier for in vitro and in vivo processes (Köping-Höggård et al., 2001; Mao et al., 2001; Kwon et al., 2013). It has been demonstrated that the reduction of chitosan DD results in a reduction of DNA binding efficiency and consequently in a decreased expression of transfected genes (Kiang et al., 2004; Huang et al., 2005). Furthermore, complexes formed with higher molecular weight chitosan are more stable and demonstrate higher transfection efficiency (Bordi et al., 2014). In addition to the indicated examples of chitosan applications, this biopolymer has been used in many other industries, e.g., as adsorbents for dye removal from water and wastewater (Vakili et al., 2014), as ingredients of cosmetic that increases the water-resistance of emulsions protecting against sun irradiation and consequently enhances its film-forming ability (Aranaz et al., 2018), as a food ingredient (Shahidi et al., 1999), as a carrier for enzyme immobilization (Biró et al., 2008; Hou et al., 2019).
As it was mentioned, conventional methods of chitosan and chitooligosaccharides preparation are difficult to control and often lead to a mixture of products with different properties. The indicated examples clearly show that the biological activities of COS are significantly affected by the DA, DP, MW, FA, and PA; therefore it is crucial to develop fully controlled production methods of chitosan and chitooligosaccharides—application of appropriate enzymes (biocatalysts) can be very helpful in achieving this goal.
Keywords: chitin, chitosan, chitooligosaccharides, enzymatic modifications, lytic polysaccharide monooxygenase, chitin deacetylase, chitinase, chitosanaseEnzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6776590/
Mar Drugs. 2019 Aug 1;17(8). pii: E452. doi: 10.3390/md17080452.
Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects.
Schmitz C1, Auza LG2, Koberidze D2, Rasche S2,3, Fischer R2,4, Bortesi L2.
Author information
1
Aachen-Maastricht Institute for Biobased Materials, Maastricht University, Brightlands Chemelot Campus, Urmonderbaan 22, 6167 RD Geleen, The Netherlands. christian.schmitz@maastrichtuniversity.nl.
2
Aachen-Maastricht Institute for Biobased Materials, Maastricht University, Brightlands Chemelot Campus, Urmonderbaan 22, 6167 RD Geleen, The Netherlands.
3
Department Plant Biotechnology, Fraunhofer Institute for Molecular Biology and Applied Ecology IME, Forckenbeckstraße 6, 52074 Aachen, Germany.
4
Indiana Bioscience Research Institute, 1345 W 16th St #300, Indianapolis, IN 46202, USA.
Abstract
Chitin is an abundant polysaccharide primarily produced as an industrial waste stream during the processing of crustaceans. Despite the limited applications of chitin, there is interest from the medical, agrochemical, food and cosmetic industries because it can be converted into chitosan and partially acetylated chitosan oligomers (COS). These molecules have various useful properties, including antimicrobial and anti-inflammatory activities.The chemical production of COS is environmentally hazardous and it is difficult to control the degree of polymerization and acetylation. These issues can be addressed by using specific enzymes, particularly chitinases, chitosanases and chitin deacetylases, which yield better-defined chitosan and COS mixtures. In this review, we summarize recent chemical and enzymatic approaches for the production of chitosan and COS. We also discuss a design-of-experiments approach for process optimization that could help to enhance enzymatic processes in terms of product yield and product characteristics. This may allow the production of novel COS structures with unique functional properties to further expand the applications of these diverse bioactive molecules.
KEYWORDS:
chitin; chitosan; chitosan oligomers; deacetylation; depolymerization; design of experiments; enzymatic conversion
CHITOSAN OLIGOSACCHARIDES(COS)
The hydrolyzed products of chitosan are called as chitosan oligosaccharides (COSs) due to its shorter chain length and presence of amino groups in D-glucosamine, which is readily soluble in water. The low viscosity and greater solubility of COS at neutral pH serves application in versatile areas. COS produced by acid or enzymatic hydrolysis method, in which enzymatic hydrolysis is preferred due to its safe and ease of control. Due to its cationic character, chitosan presents a wide variety of physicochemical and biological properties, including antimicrobial, antioxidant and antihypertensive properties. Its cationic character, chitosan presents a wide variety of physicochemical and biological properties, including antimicrobial, antioxidant and antihypertensive properties.
With an excellent absorption due to its high solubility, it has been found as high functional bioactive material with a wide range of immune enhancement, antitumor effect, antibacterial function, and calcium absorption acceleration effect. Chitosan oligosaccharide is a saccharine which is combined with glucosamine hexaose from two to ten with a structure of Chitosan. COS in food and nutrition arenas have emphasized their ability to improve food quality and human health progression.The health benefits of COS including lowering of blood cholesterol, lowering of high blood pressure, protective effects against infections , controlling arthritis and enhancing antitumor properties.
Chitosan Oligosaccharides, Water Soluble Chitosan, Glucosamine, Liquid
http://www.marshallmarine.in/chitosan-oligosacchride.html
Molecular Mechanisms of Chitosan Interactions with Fungi and Plants
by Federico Lopez-Moya *OrcID, Marta Suarez-FernandezOrcID and Luis Vicente Lopez-Llorca
Department of Marine Sciences and Applied Biology, Laboratory of Plant Pathology, Multidisciplinary Institute for Environmental Studies (MIES) Ramon Margalef, University of Alicante, 03080 Alicante, Spain
*
Int. J. Mol. Sci. 2019, 20(2), 332; https://doi.org/10.3390/ijms20020332
Abstract
Chitosan is a versatile compound with multiple biotechnological applications. This polymer inhibits clinically important human fungal pathogens under the same carbon and nitrogen status as in blood.Chitosan permeabilises their high-fluidity plasma membrane and increases production of intracellular oxygen species (ROS).
Conversely, chitosan is compatible with mammalian cell lines as well as with biocontrol fungi (BCF). BCF resistant to chitosan have low-fluidity membranes and high glucan/chitin ratios in their cell walls. Recent studies illustrate molecular and physiological basis of chitosan-root interactions. Chitosan induces auxin accumulation in Arabidopsis roots. This polymer causes overexpression of tryptophan-dependent auxin biosynthesis pathway. It also blocks auxin translocation in roots. Chitosan is a plant defense modulator. Endophytes and fungal pathogens evade plant immunity converting chitin into chitosan. LysM effectors shield chitin and protect fungal cell walls from plant chitinases. These enzymes together with fungal chitin deacetylases, chitosanases and effectors play determinant roles during fungal colonization of plants. This review describes chitosan mode of action (cell and gene targets) in fungi and plants. This knowledge will help to develop chitosan for agrobiotechnological and medical applications. View Full-Text
Keywords: chitosan; antimicrobial compounds; auxin; effectors; LysM motifs; plant immunity; ROSIJMS | Free Full-Text | Molecular Mechanisms of Chitosan Interactions with Fungi and Plants
https://www.mdpi.com/1422-0067/20/2/332
J Food Sci. 2018 Feb;83(2):535-542. doi: 10.1111/1750-3841.14048. Epub 2018 Jan 19.
Protective Effect of Chitosan Oligosaccharides Against Cyclophosphamide-Induced Immunosuppression and Irradiation Injury in Mice.
Zhai X1,2,3, Yang X1, Zou P1,2, Shao Y2, Yuan S3, Abd El-Aty AM4, Wang J1,2.
Author information
1
Dept. of Food Sciences and Engineering, School of Chemistry and Chemical Engineering, Harbin Inst. of Technology, 150090 Harbin, PR China.
2
Key Lab. of Agro-Product Quality and Safety, Inst. of Quality Standard and Testing Technology for Agro-Product, Chinese Acad. of Agricultural Sciences, 100081 Beijing, PR China.
3
the Dept. of Pharmacology and Toxicology, Beijing Inst. of Radiation Medicine, 100081 Beijing, PR China.
4
Dept. of Pharmacology, Faculty of Veterinary Medicine, Cairo Univ., 12211 Giza, Egypt.
Abstract
Chitosan oligosaccharides (COS), hydrolyzed products of chitosan, was found to display various biological activities. Herein, we assessed the immunostimulatory activity of COS both in in vitro and in vivo studies. In vitro cytotoxicity studies to murine macrophage RAW264.7 revealed that COS is safe even at the maximum tested concentration of 1000 μg/mL. It also stimulates the production of nitric oxide (NO) and tumor necrosis factor (TNF-α) and enhances the phagocytosis in COS-stimulated RAW264.7. We have shown that the COS could significantly (P < 0.05) restore the reduced immune organs indices, phagocytic index, lymphocyte proliferation, natural killer cell activity, and antioxidant enzyme activities in a cyclophosphamide-induced immunosuppressed mice model. COS can also improve the survival rate in irradiation injury mice and significantly (P < 0.05) increased the spleen indices and up-regulates the CD4+/CD8+ ratio in splenocytes. In sum, the aforementioned results suggest that COS might has the potential to be used as an immunostimulatory agent in patients with immune dysfunctions or be a model for functional food development.
PRACTICAL APPLICATION:
COS might has the potential to be used as an immunostimulatory agent in patients with immune dysfunctions or be a model for functional food development.
© 2018 Institute of Food Technologists®.
KEYWORDS:
chitosan oligosaccharides; immunostimulatory activity; immunosuppression; irradiation injuryProtective Effect of Chitosan Oligosaccharides Against Cyclophosphamide-Induced Immunosuppression and Irradiation Injury in Mice. - PubMed - NCBI
https://www.ncbi.nlm.nih.gov/pubmed/29350748
Published: 20 February 2017
Cuttlebone-like V2O5 Nanofibre Scaffolds – Advances in Structuring Cellular Solids
Andrea Knöller, Tomče Runčevski, Robert E. Dinnebier, Joachim Bill & Zaklina Burghard
The synthesis of ceramic materials combining high porosity and permeability with good mechanical stability is challenging, as optimising the latter requires compromises regarding the first two properties. Nonetheless, significant progress can be made in this direction by taking advantage of the structural design principles evolved by nature. Natural cellular solids achieve good mechanical stability via a defined hierarchical organisation of the building blocks they are composed of. Here, we report the first synthetic, ceramic-based scaffold whose architecture closely mimics that of cuttlebone –a structural biomaterial whose porosity exceeds that of most other natural cellular solids, whilst preserving an excellent mechanical strength. The nanostructured, single-component scaffold, obtained by ice-templated assembly of V2O5 nanofibres, features a highly sophisticated and elaborate architecture of equally spaced lamellas, which are regularly connected by pillars as lamella support. It displays an unprecedented porosity of 99.8 %, complemented by an enhanced mechanical stability. This novel bioinspired, functional material not only displays mechanical characteristics similar to natural cuttlebone, but the multifunctionality of the V2O5 nanofibres also renders possible applications, including catalysts, sensors and electrodes for energy storage.
Introduction
Progress in energy storage and conversion, sensing, filtering, gas distribution and catalysis depends on the availability of functional materials that combine high surface area and permeability into high open porosity, coupled with good mechanical stability1,2. Porous ceramic materials that fulfil these criteria are accessible through mimicking structuring concepts found in biomaterials3,4. With a remarkable porosity of 93 %, natural cuttlebone5 outperforms most cellular biomaterials, including bone6,7 (<79 %) and wood8 (<70 %). This rigid and ultralight aragonite-based scaffold, found in cuttlefish (Sepia Officinalis L.), features a high porosity paired with an excellent mechanical stability, and hence represents an ideal model for the design of advanced, bioinspired functional materials. Like other structural biomaterials, cuttlebone accomplishes its excellent mechanical stability via a hierarchically organized structure from the nano- to the micrometre scale. Aragonite fibres of different length, size and orientation9, complemented by about 4.5 wt.% of organic phase5, form regularly stacked cavities in the form of micrometre-thick lamellas. These lamellas are separated and supported by numerous, evenly distributed, micrometre-thick pillars10, resulting in a highly complex porous architecture that is able to resist external pressures of about 1 MPa5.Cuttlebone-like V 2 O 5 Nanofibre Scaffolds – Advances in Structuring Cellular Solids | Scientific Reports
https://www.nature.com/articles/srep42951
Communication
Published: 18 December 2009
Anti-inflammatory effect of chitosan oligosaccharides in RAW 264.7 cells
Eun-Jin Yang, Jong-Gwan Kim, Ji-Young Kim, Seong Chul Kim, Nam Ho Lee & Chang-Gu Hyun
Central European Journal of Biology volume 5, pages95–102(2010)Cite this article
Abstract
We examined the effects of chitosan oligosaccharides (COSs) with different molecular weights (COS-A, 10 kDa < MW < 20 kDa; COS-C, 1 kDa < MW < 3 kDa) on the lipopolysaccharide (LPS)-induced production of prostaglandin E2 and nitric oxide and on the expression of cyclooxygenase-2 and inducible nitric oxide synthase in RAW264.7 macrophages.COS-A (0.4%) and COS-C (0.2%) significantly inhibited PGE2 production in LPS-stimulated macrophages without cytotoxicity. The effect of COS-A and COS-C on COX-2 expression in activated macrophages was also investigated by immunoblotting. The inhibition of PGE2 by COS-A and COS-C can be attributed to the blocking of COX-2 protein expression. COS-A (0.4%) and COS-C (0.2%) also markedly inhibited the LPS-induced NO production of RAW 264.7 cells by 50.2% and 44.1%, respectively. The inhibition of NO by COSs was consistent with decreases in inducible nitric oxide synthase (iNOS) protein expression.
To test the inhibitory effects of COS-A and COS-C on other cytokines, we also performed ELISA assays for IL-1β in LPS-stimulated RAW 264.7 macrophage cells, but only a dose-dependent decrease in the IL-1β production exerted by COS-A was observed. In order to test for irritation and the potential sensitization of COS-A and COS-C for use as cosmetic materials, human skin primary irritation tests were performed on 32 volunteers; no adverse reactions of COSs usage were observed. Based on these results, we suggest that COS-A and COS-C be considered possible anti-inflammatory candidates for topical application.
Anti-inflammatory effect of chitosan oligosaccharides in RAW 264.7 cells | SpringerLink
https://link.springer.com/article/10.2478/s11535-009-0066-5
Involvement of PKA signalling in anti‐inflammatory effects of chitosan oligosaccharides in IPEC‐J2 porcine epithelial cells
J. W. Yang G. Tian D. W. Chen Y. Yao J. He P. Zheng X. B. Mao J. Yu Z. Q. Huang B. Yu
First published: 16 March 2017 https://doi.org/10.1111/jpn.12686 Citations: 8
Summary
Weaning is characterized by intestinal inflammation, which is a big challenge in pig industry. Control of intestinal inflammation is important for improvement of growth performance and health. Therefore, the study was focused on the anti‐inflammatory activity of low‐molecular‐weight chitosan oligosaccharide (LCOS) in a porcine small intestinal epithelial cell line (IPEC‐J2).The results showed that TNF‐α, as inflammation inducer, significantly upregulated the mRNA expression of IL‐8 and MCP‐1. Afterwards, LCOS significantly attenuated mRNA expression of IL‐8 and MCP‐1 induced by TNF‐α in the cells.
Mannose (MAN), as ligand of mannose receptor, had no effect on the anti‐inflammatory activity of LCOS, which suggested that mannose receptor may not involve in the anti‐inflammatory activity of LCOS in IPEC‐J2 cells. Interestingly, N‐[2‐(p‐bromocinnamylamino)ethyl]‐5‐isoquinolinesulfonamide 2HCl hydrate (H89), as PKA (protein kinase A)‐specific inhibitor, reversed the mRNA expression of IL‐8 when co‐cultured with LCOS. Furthermore, LCOS concentration dependent downregulated the mRNA expression of claudin‐1 compared with TNF‐α treatment. However, the trans‐epithelial electric resistance (TEER) was not affected by LCOS when co‐cultured with TNF‐α in 3 hr. In conclusion, LCOS have a potent anti‐inflammatory activity, and as a feed additives, may be useful for the inhibition of inflammatory process in weaning period of pigs with intestinal inflammation occurring.
Involvement of PKA signalling in anti‐inflammatory effects of chitosan oligosaccharides in IPEC‐J2 porcine epithelial cells - Yang - 2018 - Journal of Animal Physiology and Animal Nutrition - Wiley Online Library
https://onlinelibrary.wiley.com/doi/10.1111/jpn.12686
Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of actionInflammatory bowel disease (IBD) results from intestinal epithelial barrier defect and dysregulated mucosal immune response. This study aimed to evaluate the therapeutic potential of chitosan oligosaccharide (COS), a biodegradation product of dietary fiber chitosan, in the treatment of IBD and to elucidate its possible mechanisms of action. Oral administration of COS protected against mortality and intestinal inflammation in a mouse model of acute colitis induced by 5% dextran sulfate sodium (DSS). The most effective dose range of COS was 10-20 mg/kg/day. In addition, nuclear factor kappa B (NF-κB) activation, and levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in colonic tissues were suppressed in mice receiving COS. Similar protective effect of COS against mortality and intestinal inflammation was observed in another mouse model of acute colitis induced by rectal instillation of 4% acetic acid. Importantly, COS administration after colitis induction was effective in ameliorating intestinal inflammation in both acute colitis models induced by 5% DSS and chronic colitis models induced by cycles of 2.5% DSS. In human colonic epithelial cells (T84 cells), COS treatment prevented NF-κB activation, production of TNF-α and IL-6, and loss of epithelial barrier integrity under both lipopolysaccharide (LPS) and TNF-α-stimulated conditions. Furthermore, binding of LPS to T84 cells, and TNF-α and oxidative stress-induced apoptosis of T84 cells were prevented by treatment with COS. These results suggest that COS may be effective in the treatment of IBD through inhibition of NF-κB signaling and apoptosis of intestinal epithelial cells.
Chitosan oligosaccharide as potential therapy of ...
https://www.researchgate.net/publication/223957612_Chitosan_oligosaccharide_as...Although the anti-inflammatory activity of chitosan oligosaccharides is well reported [40][41][42] [43], most of the works have been performed with COS mixtures not fully characterized in terms of ...
RSC Advances,2016
Chitosan-coated liposomes encapsulating curcumin: study of lipid–polysaccharide interactions and nanovesicle behavior
M. Hasan,a G. Ben Messaoud,a F. Michaux,a A. Tamayol,bcd C. J. F. Kahn,e N. Belhaj,f M. Lindera and E. Arab-Tehrany*a
Author affiliations
* Corresponding authors
a Université de Lorraine, LIBio, ENSAIA, 2 avenue de la Forêt de Haye, F-54505 Vandoeuvre-lès-Nancy, France
E-mail: elmira.arab-tehrany@univ-lorraine.fr
b Center for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02139, USA
c Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
d Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, MA, USA
e Aix-Marseille Université, IFSTTAR, LBA UMR_T24, F-13016 Marseille, France
f Lucasmeyer Cosmetics, ZA les Belles Fontaines, 99 route de Versailles, 91160 Champlan, France
Abstract
Despite various spectacular therapeutic properties, curcumin has low bioavailability mainly due to its poor solubility in water. In this paper, we encapsulated curcumin by nanoliposomes prepared from salmon purified phospholipid and coated with chitosan.Various techniques were used in order to study the interactions among phospholipid, chitosan and curcumin. FTIR results showed both electrostatic and hydrophobic interactions as well as hydrogen bonding between chitosan and phospholipid, while hydrophobic forces and hydrogen bonding dominated the interactions between curcumin and phospholipid as well as between curcumin and chitosan.
Shear viscosity measurements demonstrated a flow behavior change from Newtonian to shear thinning after liposome coating. The increase/decrease stress ramp showed that the addition of chitosan layer decreased significantly the hysteresis loop area (thixotropic behavior) and therefore increased significantly the liposomal dispersion stability. The viscoelastic properties investigated by small-amplitude oscillatory shear rheology demonstrated improvement of mechanical stability after chitosan addition. Small-angle X-ray scattering experiments revealed that the liposome membrane structure was not affected by the chitosan layer or the encapsulated curcumin.
Chitosan-coated liposomes encapsulating curcumin: study of lipid–polysaccharide interactions and nanovesicle behavior - RSC Advances (RSC Publishing)
https://pubs.rsc.org/en/content/articlelanding/2016/ra/c6ra05574e/unauth#!divAbstract
Can glucosamine supplements protect my knee cartilage from osteoarthritis?
Answer From Brent A. Bauer, M.D.
Study results on this question have been mixed, with some suggesting possible benefit and some showing no benefit on cartilage protection.
Glucosamine is one of the substances in your body that is used to build cartilage. Osteoarthritis is the most common type of arthritis. When you have osteoarthritis, the slick cartilage that covers the ends of your bones and helps joints move smoothly wears away.
Study results have been varied, partly because not all the studies have used the same type of glucosamine. And not all the studies included a placebo comparison, or ensured that neither the patient nor the researchers knew which pill was being administered.
That said, glucosamine is relatively inexpensive and safe. If other treatments aren't helping, you might want to talk to your doctor about whether a trial of glucosamine is right for you. It may take several months before you see any pain relief.Glucosamine: Does it protect cartilage in osteoarthritis? - Mayo Clinic
https://www.mayoclinic.org/diseases-conditions/osteoarthritis/expert-answers/glucosamine/faq-20058430
Do glucosamine or chondroitin cause regeneration of cartilage in osteoarthritis?
J Fam Pract. 2003 March;52(3):229-239
By David Priebe, MD Todd McDiarmid, MD Leslie Mackler, MLS
Author and Disclosure Information
EVIDENCE-BASED ANSWER
No direct evidence suggests glucosamine or chrondroitin cause regeneration of cartilage in osteoarthritis. Use of glucosamine sulfate in knee osteoarthritis prevents joint space narrowing on radiographs (strength of recommendation [SOR]: B, based on 1 randomized controlled trial).
Intramuscular chondroitin polysulfate prevents radiographic progression of finger osteoarthritis (SOR: B, based on 1 randomized controlled trial).
Both chondroitin sulfate and glucosamine sulfate stimulate chondrocyte growth in vitro and in animal models (SOR: D, based on several bench research studies).
Evidence summary
A systematic review of glucosamine sulfate use for osteoarthritis, based on early research (1956–1991), found that it has anti-inflammatory properties and rebuilds damaged cartilage.1 These studies evaluated chondrocytes grown in culture and animal models.1,2 Chondroitin sulfate also stimulates chondrocyte biosynthesis in both animal and in vitro studies. There is insufficient evidence to demonstrate glucosamine sulfate or chondroitin sulfate stimulates chrondrocyte growth in humans with osteoarthritis.2,3
Joint space narrowing on radiographs suggests progression of osteoarthritis. This narrowing is thought to imply cartilage destruction or loss due to osteoarthritis. A double-blinded randomized controlled trial studied the effect of glucosamine sulfate on tibial-femoral compartment joint space narrowing in 212 patients older than 50 with mild to moderate knee osteoarthritis.4 Patients took either 1500 mg/day of glucosamine sulfate or placebo over 3 years. Knee radiographs in a standing anterior-posterior view, using visual and digital analysis, were used to assess joint space narrowing.5 The average mean joint space loss was 0.31 mm in the placebo group and 0.07 mm in the treatment group (P<.05; 95% confidence interval, 0.13–0.48).
The clinical relevance of knee joint space narrowing is undetermined. Radiographic evaluation of a weight-bearing joint space may not be an accurate or reproducible technique. A study of 15 patients with mild to moderate knee osteoarthritis used standing and semi-flexed radiographic views after an analgesic and nonsteroid anti-inflammatory drug washout period, and 1 to 12 weeks after resumption of analgesic therapy (mean 6.0 weeks).6 Knee pain significantly decreased radiographic joint space in the standing anteriorposterior position, but not in the semiflexed position. Using the standing anterior-posterior method may confound accurate interpretation of joint space narrowing and changes in articular cartilage since glucosamine may have an anti-inflammatory effect.6
One double-blinded randomized controlled trial, comparing chondroitin sulfate with placebo, evaluated joint space in patients with symptomatic hand osteoarthritis.7 One hundred sixty-five Caucasian patients, aged 40 to 70 years, were randomized to receive either a 50-mg intramuscular injection of chondroitin polysulfate, twice weekly, for 8 weeks, every 4 months, versus placebo, or 400 mg of oral chondroitin sulfate, 3 times a day, versus placebo.
Osteoarthritis progression in the metacarpalphalangeal and interphalangeal joints was assessed with radiographs over 3 years. Evaluators used the Anatomic Lesion Progression Scale to assess the development of osteophytes and joint space narrowing, with or without subchondral bone changes, to determine osteoarthritis progression. This scale makes it very difficult to determine whether improvements are clinically significant.
Chondroitin sulfate and polysulfate did not prevent osteoarthritis from occurring in previously normal joints. In joints already affected, intramuscular chondroitin polysulfate significantly reduced progression of distal interphalangeal, proximal interpharangeal, and metacarpophalangeal joint space narrowing (P<.013), using the progression scale. Oral chondroitin sulfate did not prevent progression.7
Recommendations from others
The American College of Rheumatology stated in 2000 that recommending glucosamine sulfate or chondroitin sulfate for osteoarthritis might be premature due to the methodology, lack of standardization, and insufficient information on study designs. More research was recommended.8
These products are sold as supplements in the United States. Their purity is often questionable and thus may affect study results. When studying glucosamine, the National Institutes of Health was forced to manufacture the drug itself due to lack of a reliable amount present in commercial products.9Do glucosamine or chondroitin cause regeneration of cartilage in osteoarthritis? | MDedge Family Medicine
https://www.mdedge.com/familymedicine/article/59438/rheumatology/do-glucosamine-or-chondroitin-cause-regeneration-cartilage
Glucosamine/Chondroitin as an Anti-inflammatory
By Chris Centeno, MD / March 27, 2015Most people think of Glucosamine and it’s cousin Chondroitin as something to take for arthritis. In fact, most of the research to date on these two have been focused on their ability to help provide nutrition for cartilage. However, most people don’t know that the Glucosamine/Chondroitin anti-inflammatory properties make them powerful anti-inflammatories in their own right.
Glucosamine and Chondroitin are basic cartilage components that are frequently taken by arthritis sufferers. The research seems to be a back and forth battle between academia who doesn’t like that a supplement that competes with drugs could help protect cartilage, and a bevy of studies showing cartilage protection. Having said that, outside of slowing cartilage degeneration in arthritis, if these supplements were potent anti-inflammatories without the horrible cardiac side effects of common NSIAD drugs like Motrin, that would be a new avenue for research and would also explain why they seem to work so well for many arthritis patients.
I’ve blogged before on a study showing that a Glucosamine/Chondroitin (GC) combination therapy was as powerful an anti-inflammatory as Celebrex. So we have some idea that these two pack an anti-inflammatory punch. This new double blinded study looked solely at blood levels of a common inflammatory marker known as CRP (c-reactive protein) in a small group of overweight patients. Half the patients received GC and half took a placebo for four weeks and then the groups switched (crossed over), where the half that was taking GC took a placebo and vice versa. When the patients were taking GC, their CRP levels were 23% lower, meaning these was a measurable anti-inflammatory effect. Other measurements such as one measuring cell to cell signals were also impacted by GC.
The upshot? The evidence seems to be mounting that GC is a potent anti-inflammatory outside of it’s ability to protect cartilage. So why take NSAIDs with nasty side effects when you can take a natural supplement with no known side effects?Glucosamine/Chondroitin Anti-inflammatory Properties - Regenexx©
https://regenexx.com/blog/glucosamine-chondroitin-anti-inflammatory/
Carbohydrate Polymers
Volume 71, Issue 3, 8 February 2008, Pages 435-440
Carbohydrate Polymers
Absorption and distribution of chitosan in mice after oral administrationLintaoZengabCaiqinQinaWeiWangbWeilinChibWeiLia
aa
Laboratory for Natural Polysaccharides, Xiaogan University, Xiaogan 432000, China
b
Department of Chemistry, Central China Normal University, Wuhan 430079, Chin
Abstract
Four chitosan samples with different molecular weight Mw and the degree of deacetylation DD (HCS 7.60 × 105 and 85.5%, MCS 3.27 × 104 and 85.2%, COS 0.99 × 103 and 85.7%, WSC 3.91 × 104 and 52.6%) were prepared, and labeled by fluorescein isothiocyanate. These labeled samples were used to investigate the absorption and distribution in mice after oral administration. The results indicated that the absorption and distribution of chitosan was significantly influenced by its Mw and water-solubility. The absorption of chitosan molecules increased with the decrease of the Mw and the increase of the water-solubility. The absorbed chitosan molecules were distributed to all tested organs such as liver, kidney, spleen, thymus, heart and lung. The chitooligomer molecules were easily absorbed and metabolized. The absorbed chitosan molecules from water-soluble WSC in all tested tissues maintained high concentration for a long period. The results suggest that different chitosan may be employed for different functional food.Absorption and distribution of chitosan in mice after oral administration - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0144861707003311
International Immunopharmacology
Volume 11, Issue 1, January 2011, Pages 121-127
International Immunopharmacology
Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress
YingQiaoabXue-FangBaiaYu-GuangDuaa
Liaoning Provincial Key Laboratory of Carbohydrates, Department of Biotechnology, Dalian Institute of Chemical Physics, Chinese Academy of Science, Dalian, Liaoning 116023, China
b
Graduate School of Chinese Academy of Science, Beijing 100049, China
Abstract
Sepsis and its derivative syndromes are major causes of morbidity and mortality in the intensive care unit. Recently, lots of studies have shown that the progression of sepsis is attributed to redox imbalance and overproduction of proinflammatory cytokines.In previous studies, we have reported the anti-oxidative and anti-inflammatory effects of chitosan oligosaccharides in vitro. In the light of these findings, we applied the model of sepsis to mice by LPS injection to investigate whether chitosan oligosaccharides have a protective effect on LPS-induced sepsis.
We found that treatment by chitosan oligosaccharides not only attenuated organ dysfunction but also improved survival rate after LPS injection.
To further understand how it works, we examined several proinflammatory markers including neutrophil infiltration in organs and TNF-α and IL-1β in serum, and found that these cytokines were significantly reduced by chitosan oligosaccharide treatment. In addition to this, anti-oxidants including glutathione (GSH) and catalase (CAT) levels were depleted and malondialdehyde (MDA) levels were increased in LPS-induced sepsis, while chitosan oligosaccharides smoothed out the redox imbalance. Furthermore, we also assessed c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase signal activation by LPS-stimulation, and found both of them were attenuated by chitosan oligosaccharide treatment.
Collectively, our data demonstrated that chitosan oligosaccharides can protect mice from the LPS challenge by virtue of anti-inflammatory effects as well as anti-oxidation properties, which might offer beneficial effects for patients with sepsis.
Abbreviations
ALTAlanine transferaseASTAspartate transaminaseCATCatalaseCLPCecal ligation and punctureCOSChitosan oligosaccharidesCRECreatinineGSHGlutathioneLPSLipopolysaccharideMDAMalondialdehydeMPOMyeloperoxidase
Keywords
SepsisLipopolysaccharideChitosan oligosaccharidesTNF-αLipid peroxidationhttps://www.sciencedirect.com/science/article/pii/S1567576910003450
Chitosan decreases total cholesterol in women: a randomized, double-blind, placebo-controlled trial
H Bokura & S Kobayashi
European Journal of Clinical Nutrition volume 57, pages721–725(2003)
Abstract
Background: Hypercholesterolemia is an important risk factor for cardiovascular disease. Orally administered chitosan binds lipids in the small intestine and reduces their absorption. Chitosan has been shown to decrease serum cholesterol in animal and human studies. This study investigated the effectiveness of chitosan in reducing serum cholesterol without concomitant diet therapy.
Methods: Ninety female volunteers (age 34–70 y) with confirmed mild to moderate hypercholesterolemia were enrolled into the study. They were randomly assigned to receive chitosan (1.2 g per day) or placebo in a double-blind manner. Serum lipids, body weight and adverse events were assessed at baseline and after 28 and 56 days of treatment. Subjects maintained their usual diet and documented the type and gross amount of food consumed.
Results: Eighty-four subjects (41 chitosan, 43 placebo) were included in the analysis. Chitosan significantly (F=3.19, P=0.04) reduced total cholesterol compared to placebo. In a subgroup of subjects with over 60 y of age, chitosan group significantly reduced total and LDL cholesterol (F=4.21, P=0.02, and F=3.46, P=0.04, respectively) compared with placebo. Adverse effects were few; no serious events were reported.
Conclusions: Our results demonstrate that chitosan is safe and effective for lowering cholesterol. However, the effect of chitosan for decreasing cholesterol is mild.
Sponsorship: Shimane Institute of Health Science, Izumo, Japan.Chitosan decreases total cholesterol in women: a randomized, double-blind, placebo-controlled trial | European Journal of Clinical Nutrition
https://www.nature.com/articles/1601603
A rapid metal free synthesis of 5-substituted-1H-tetrazoles using cuttlebone as a natural high effective and low cost heterogeneous catalyst†
Sara S. E. Ghodsiniaa and Batool Akhlaghinia*a
Author affiliations
* Corresponding authors
a Department of Chemistry, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad 9177948974, Iran
Abstract
A convenient, rapid and metal free synthesis of 5-substituted-1H-tetrazoles is described by [3 + 2] cycloaddition reaction of nitriles with sodium azide. The reaction was catalyzed by cuttlebone in DMSO at 110 °C. Cycloaddition reaction of nitriles with sodium azide happened in the presence of mesoporous cuttlebone by “electrophilic activation” of nitriles through hydrogen bond formation between the cuttlebone and nitrile. Cuttlebone as a natural low cost heterogeneous catalyst with high porosity, high flexural stiffness, high compressive strength and high thermal stability affords 5-substituted-1H-tetrazoles rapidly with high efficiency.
A rapid metal free synthesis of 5-substituted-1H-tetrazoles using cuttlebone as a natural high effective and low cost heterogeneous catalyst - RSC Advances (RSC Publishing)
https://pubs.rsc.org/en/Content/ArticleLanding/2015/RA/C5RA08147E#!divAbstract
New Applications of Cuttlebone (CB) as a Natural Marine Compound
Department of Medicinal Chemistry, School of Pharmacy, Ahvaz Jundishahpur University of Medical Sciences, Ahvaz, IranThe Persian Gulf is an important source of sea animals such as different types of fish (food and decorative), crabs, corals, sponges, shrimps, dolphins, turtles etc.
A main class of marine shellfish is Cephalopod. Cephalopods include cuttlefish, squid and octopus that are important marine resources since they are rich in polyunsaturated fatty acids (FA), particularly omega-3 FA and good sources of essential fatty acids that are not produced in the human body (1). The most common species of cuttlefish in the Persian Gulf are Sepia Pharaonis (2, 3). Cuttlefish have an internal structure called cuttlebone (CB). In the native dialect, they are called seabed (Figures 1 and 2). Cuttlebone is the hard bone tissue in the back of the cuttlefish with high porosity, oval shape and spongy form, which functions as a rigid buoyant tank in the animal; the framework of cuttlebone is an inorganic-organic composite. The organic fraction is composed of protein and β-chitin (1% - 2% by weight) that has effect on solid physical properties and control of crystal sizes (4). Inorganic fraction is composed of calcium carbonate and calcium phosphate (5). On the other hand, cuttlebone is a natural product, containing no toxins or contaminants.
Cuttlebone
Figure 1. Cuttlebone
Live Sepia Pharaonis
Figure 2. Live Sepia Pharaonis
Cuttlebone has many pharmaceutical properties. Cuttlebone powder is used for the treatment of bleeding and external infections. It is added to birdseed for adjusting the function of the liver and kidney and decreasing stomach acid. Improvements have been achieved by application of natural and marine compounds and catalysts (6) in synthetic bioactive compounds and chemical reactions (7).
However, nowadays, new researches have been done about the application of CB in chemical reactivity, which are classified in three groups:
1- In chemical reactions
Cuttlebone was used in condensation reactions, such as crossed-aldol condensation and benzoin condensation. As a base (pH ~ 8, in aqueous solution) it facilitates the elimination of α-hydrogen and formation of carbanion. For example in crossed-aldol condensation of acetone with benzaldehyde by calcinated CB/ NaNO3 with water-ethanol solvents and one hour reflux, yields of 75% have been obtained. In benzoin condensation, benzaldehyde with calcinated CB/NaNO3 and thiamine hydrochloride as a catalyst, water-ethanol solvents, two-hour in a water bath at 60°C was converted to the corresponding benzoin with the yield of 51%.
In alkali hydrolysis reaction of esters, calcinated CB/NaNO3, as a base instead of hydroxide ion, caused alkalinity of the medium and hence, facilitated hydrolysis of esters with good to excellent yield. For example methyl benzoate was hydrolyzed to benzoic acid in water-ethanol solvents with 94% yields under reflux conditions for 1.15 hour. Under these conditions, p-nitro methyl benzoate during 90 minutes with 95% yield, and methyl stearate in seven hours with 90% yield (saponification reaction) were converted to the salts of corresponding acids.
2- Removal of dyes from water and industrial waste water
Colored waste from various industries and discharge to the surface water is one of the most important environmental problems that have harmful effects for humans, sea animals and the environment. Alizarin Red S as an acidic dye and Malachite green as a basic dye were properly adsorbed on the raw powder of CB. The obtained optimal conditions for Alizarin Red S were pH = 2, initial concentration of 1 - 20 mg/L, amount of adsorbent of 500 mg, contact time of 10 minutes, and the temperature of 25°C. The achieved results showed the capability of endoskeleton powder of Sepia for adsorption of Alizarin Red S and Malachite green. The method was successfully applied for the removal of Alizarin Red S and Malachite green in real water samples.
3- Adsorption of toxic elements such as copper, lead and cadmium from aqueous solutions
Elimination of toxic heavy metals from water and wastewater is very important for the health of humans and the environment.
Cuttlebone powder as a low cost and nontoxic adsorbent was investigated for adsorption of toxic heavy metals. The optimal conditions for adsorption of Cr (III) were identified as pH of 5, initial concentration of chromium ions of 20 - 400 mg/L, and amount of damped adsorbent of 0.5 g. The maximum percentage of Cr (III) removal was found to be 98% (8).
The optimal conditions for Pb2+ removal were: pH = 5, initial concentration of metal ion of 20 mg/L, amount of adsorbent of 0.5 g and contact time of two minutes. The studied parameters for Cd removal were: pH of 7, initial concentration of cadmium ions of 5 mg/L, amount of adsorbent of 0.5 g and contact time of 20 minutes. The highest removal percentage for Pb, Cd and Cu ions were 100%, 94% and 98%, respectively. Thus, CB powder can be used in purification of water and wastewater resources from toxic heavy metals.
Of course, other new researches at the Research Center of Marine Pharmaceutical Science are being done on the use of CB in drugs and its pharmaceutical effects.
ReferencesJundishapur Journal of Natural Pharmaceutical Products | New Applications of Cuttlebone (CB) as a Natural Marine Compound
http://jjnpp.com/articles/13734.html
Original Article
Published: 03 April 2018
Cuttlebone as a Marine-Derived Material for Preparing Bone Grafts
Alisa Palaveniene, Volodymyr Harkavenko, Vitalina Kharchenko, Povilas Daugela, Mindaugas Pranskunas, Gintaras Juodzbalys, Nataliya Babenko & Jolanta Liesiene
Marine Biotechnology volume 20, pages363–374(2018)Cite this article
Abstract
The use of synthetic materials for biomedical applications still presents issues owing to the potential for unfavourable safety characteristics. Currently, there is increasing interest in using natural, marine-derived raw materials for bone tissue engineering. In our study, the endoskeleton of the mollusc Sepia, i.e. cuttlebone (CB), was used with regenerated cellulose (RC) to prepare three-dimensional composite bone grafts. CB microparticles were mechanically immobilised within a cellulose gel, resulting in a macroporous structure upon lyophilisation. The interconnected porous structure of the regenerated cellulose/cuttlebone (RC/CB) composite was evaluated by micro-computed tomography. The porosity of the composite was 80%, and the pore size predominantly ranged from 200 to 500 μm. The addition of CB microparticles increased the specific scaffold surface by almost threefold and was found to be approximately 40 mm−1. The modulus of elasticity and compressive strength of the RC/CB composite were 4.0 ± 0.6 and 22.0 ± 0.9 MPa, respectively.The biocompatibility of the prepared RC/CB composite with rat hepatocytes and extensor digitorum longus muscle tissue was evaluated. The obtained data demonstrated that both the composite and cellulose matrix samples were non-cytotoxic and had no damaging effects. These results indicate that this RC/CB composite is a novel material suitable for bone tissue-engineering applications.
Cuttlebone as a Marine-Derived Material for Preparing Bone Grafts | SpringerLink
https://link.springer.com/article/10.1007/s10126-018-9816-6
Cuttlefish bone is a natural biologically-active material that possesses great bone and wound healing properties
09/17/2018 / By Rhonda Johansson
Researchers at the Kaunas University of Technology (KTU) have solved the main challenge facing the contemporary use of cuttlefish bone in modern medicine. The hard, brittle internal structure of the squid-like cephalopod — while used often in traditional Chinese and Indian medicine — has been observed to trigger certain allergic reactions in various individuals. KTU researchers found that removing a protein from cuttlebone made the material safer, all while maintaining its inherent medicinal uses.
Lead investigator and KTU researcher Alisa Palavenien? said that cuttlebone is a natural, biologically-active material that should be utilized more. Chinese remedies use the marine-derived material to promote bone and wound healing. However, cuttlebone is also notorious for triggering rashes and allergies in some patients.
This may be due, in part, to potential heavy metal contamination and contamination by other sea pollutants.
“In my research, one of the tasks was removal of the traces of the protein tropomyosin found in cuttlefish muscle tissue, which amino sequence is slightly different from human. Some people have hypersensitivity to this protein and therefore can develop allergic reactions in contact with it,” explains Palavenien?.
However, removing the protein was easier said than done. Traditional methods used alkaline hydrolysis in a degenerative process that is applied in the disposal of human remains. Invariably, the method, though effective, changes the composition of the cuttlebone.
The KTU scientists were able to develop an alternative method that removed tropomyosin using cutting-edge equipment. Their strategy, the researchers observed, was able to remove traces of tropomyosin from cuttlebone without ridding it of its therapeutic properties.
Mother Nature's micronutrient secret: Organic Broccoli Sprout Capsules now available, delivering 280mg of high-density nutrition, including the extraordinary "sulforaphane" and "glucosinolate" nutrients found only in cruciferous healing foods. Every lot laboratory tested. See availability here.
Health applications
This discovery opens the door to possible medical uses of cuttlefish bone, particularly in the field of bone tissue engineering. Compounds in the material are able to naturally bind to human bones and successfully integrate themselves during the healing process. In particular, cuttlebone is abundant in aragonite, beta-chitin, magnesium, strontium, iron, copper, and zinc. The synergistic qualities of these compounds increase the bioactivity of any biomedical product containing cuttlebone.
Scaffolds or capsules that contain A 17-year-old invented VetiGel, stops bleeding instantly ...
https://www.businessinsider.com/a-17-year-old-invented-vetigel-stops-bleeding...
VetiGel isn't the only wound-healing invention of its kind. Another product, named — oddly enough — Vitagel, also helps the body stop bleeding quickly using similar methods., which are the cells needed for bone growth. Researchers have noted that cuttlebone contains different elements that take the place of the cavity in the bone where an injury had occurred. Because cuttlebone is natural, it degrades easily and is harmless to the body.
The KTU researchers developed two pharmaceutical products using their protein-free cuttlebone. The first was a cellulose-based scaffold and the other was a calcium alginate capsule. Both products were designed to be used for small-sized bone defects in oral cavities.
Its use in traditional Chinese medicine
Chinese folk healers refer to cuttlefish bone as hai piao xiao. It is said to have “warm” and “salty” properties and is associated with the kidney, liver, and stomach meridians. It is primarily used to stop bleeding and harmonize the stomach. It is usually boiled, dried, and then prepared as a powder. A typical dose of cuttlefish bone is between six to 12 grams boiled in water.
Applied topically, hai piao xiao is used as a poultice to treat skin rashes and lesions.
Other uses of the material include treatment for gastric and duodenal ulcer, asthma, bleeding of the uterus, and premature ejaculation.Cuttlefish bone is a natural biologically-active material that possesses great bone and wound healing properties
https://health.news/2018-09-17-cuttlefish-bone-is-a-natural-biologically-active-material-that-possesses-great-bone-and-wound-healing-properties.html
How WoundSeal Works
Powder + Pressure = Instant Scab.
WoundSeal® Powder is composed of a hydrophilic, or water-loving, polymer and potassium ferrate.
When the powder is poured onto a bleeding wound, the hydrophilic polymer instantly dehydrates the blood by absorbing only the plasma or liquid portion of the blood stacking the blood solids beneath the powder. Simultaneously, the potassium ferrate dissolves, releasing iron that agglomerates (binds together) the blood solids to create an occlusive seal.
As manual pressure is applied to the powder, the seal is pushed into contact with the wound. The natural glue-like nature of drying blood adheres the seal to the wound and surrounding skin. The occlusive seal, that has formed in seconds, stops further bleeding or oozing. Blood solids continue to stack beneath the seal, strengthening it. The natural clotting process proceeds below the seal.
How WoundSeal Works | WoundSeal.com – Stop Bleeding Instantly
http://woundseal.com/how-it-works/
Potassium Ferrate | NANOIRON
https://nanoiron.cz/en/products/potassium-ferrate
Potassium ferrate containing product (ferrate = iron in high oxidation state) having powerful oxidation properties coupled with coagulation and disinfection ability. A brand new dry product Potassium ferrate is usually produced through a wet synthesis or using an electrosynthesis where the equipment is exposed to aggressive conditions.
A 17-year-old invented VetiGel, stops bleeding instantly ...
https://www.businessinsider.com/a-17-year-old-invented-vetigel-stops-bleeding...
VetiGel isn't the only wound-healing invention of its kind. Another product, named — oddly enough — Vitagel, also helps the body stop bleeding quickly using similar methods.
A simple injection could one day stop people from bleeding to death
100 percent of the rats treated with the polymer survived' a traumatic injury
By Arielle Duhaime-Ross@adrs Mar 4, 2015, 3:00pm EST
Leslie Chan / University of Washington
Blood loss kills a lot of people; one-third of deaths related to traumatic injuries are caused by bleeding. But a lab-made polymer could change that, as it was able to stop bleeding in rats whose femoral artery was cut, according to a study published today in Science Translational Medicine — the procedure essentially saved their lives.
"We designed a polymer that we can inject into the bloodstream and that’s able to integrate in the forming of clots, and it stabilizes them," says Suzie Pun, a bioengineer at The University of Washington and a co-author of the study. "I think this has real power to save people in the battlefield."
Doctors and paramedics often use tourniquets to stop someone from bleeding out, but when injuries are internal, its hard to know where to apply pressure without making things worse. In those situations, a strong blood clot can be life-saving.
FORMING BLOOD CLOTS ISN’T EASY WHEN BLOOD IS POURING OUT OF AN ORGAN
Clots are formed when platelets, a type of blood cell, group together to make a "platelet plug." This plug is reinforced thanks to a fibrous protein called fibrin. But forming blood clots isn’t easy when blood is pouring out of an organ, because the components that are needed to strengthen clots are located in the blood that spills out. Thus, when major blood loss occurs internally, clots become weak — so weak that they break down when doctors supply patients with blood transfusions. To solve this problem, researchers decided to make a polymer that could strengthen clots inside the body, even as the patient suffers massive amounts of internal bleeding.
In the study, researchers made a 3-millimeter cut in the femoral artery of 40 rats. Of those rats, about half were given an injection of the polymer solution as they were bleeding out. The rats that didn't receive the drug didn't do very well — over 50 percent of them died. But "100 percent of the rats treated with the polymer survived," Pun says.
Chan et al. 2015
The polymer, called PolySTAT, looks like a "white fluffy powder" before it’s dissolved in saline solution, Pun explains. It was engineered using the same polymer that makes up contact lenses, in addition to a peptide that’s built to seek out and attach to fibrin. When it’s injected, PolySTAT detects fibrin and weaves itself throughout the forming clot. This strengthens it, and could help the clot stay in place in humans when doctors inject blood or saline solution to increase a patient’s blood pressure. Moreover, the fact that it only binds to fibrin means that it shouldn’t suddenly start forming clots all over the place. "We designed this to only work after the initial clot has formed," says Nathan White, an emergency physician at the University of Washington and a co-author of the study.
"100 PERCENT OF THE RATS TREATED WITH THE POLYMER SURVIVED."
The method is completely novel, Pun says. "People have tried to make artificial platelets, but in terms of a synthetic polymer like this, we think it’s the first."
The researchers think the polymer could be used in the emergency room, as well as by paramedics in the field. "Most people who die of bleeding die very quickly, before they make it to the medical facility," White says. Being able to use PolySTAT in the field could make a big difference in those cases. It may also help people who have blood disorders that prevent them from forming clots. And, because it only binds to fibrin, it might one day be possible to employ this strategy to break down clots that form during a stroke, by adding other drugs to the formula that can break down clots, White says.
"PolySTAT reduced blood loss, abolished rebleeding at the injury site, and increased survival," writes Karim Brohi, a trauma science researcher at Queen Mary University in London who didn't participate in the study, in a Science news article about the experiment. But "drugs need to survive the rigors of the emergency environment," and questions about the drug’s safety remain.
The polymer doesn’t appear to be toxic — "it doesn’t cause organ damage," White says — but researchers still need to perform long-term safety tests to see what happens after the clot is formed. Using PolyStat will probably slow the natural breakdown of clots, but that still needs to be determined. The components that make up PolyStat have been used in humans before, but "what we don’t know is what effect the combination will have," White says.
"UNLESS IT ACTUALLY SAVES A LIFE, MAKING CLOTS STRONGER IS MEANINGLESS."
The researchers plan to try it on larger animals soon. They will also have to try it on different types of wounds. A nice clean cut to the femoral artery doesn’t necessarily represent what doctors see in the emergency room — or what soldiers see in the field. "Some laceration models take out a quarter of the liver," so researchers will have to test PolySTAT on bigger wounds, Pun says.
Still, the polymer looks promising. Unlike blood and plasma, the powder can be stored in a simple container; it doesn’t need to be refrigerated. That means that it might one day make its way into the kinds of first-aid kits that paramedics and soldiers employ. Until then, the increased survival rate seen in the rats in very exciting, Pun says — "it’s new paradigm for treating trauma." But the polymer still has a long way to go. "Unless it actually saves a life, making clots stronger is meaningless," she says.A simple injection could one day stop people from bleeding to death - The Verge
https://www.theverge.com/2015/3/4/8149413/injection-stops-bleeding-death-polystat
Plant-based gel can stop traumatic bleeding in seconds
Biotech startup Suneris has developed a plant-based polymer it says functions like "Lego building blocks for the body," drastically reducing the amount of time it takes to stop a wound from bleeding.
Anthony Domanico mugshot
Anthony Domanico
November 20, 2014 4:58 PM PST
When bleeding from a traumatic injury begins, time is of the essence. VetiGel, a new plant-based gel that can be applied either to skin or soft organs, halts bleeding rapidly and could thus save lives on the battlefield and elsewhere. For now, it's starting to roll out to veterinary clinics.
Joe Landolina is the CEO and co-founder of Brooklyn biotech startup Suneris. He created the plant-based polymer gel, which can be placed on open wounds to stop bleeding in just 20 seconds.
On the surface, VetiGel seems like some other liquid-bandage products on the market, but it goes beyond superficial injuries to work on both skin and organs. Imagine a surgeon being able to quickly close an organ she is operating on to minimize blood loss, or a police officer being able to stop the bleeding from a bullet wound in the field.
Landolina got the idea for VetiGel in 2011, while a freshmen at NYU. He created the first version of VetiGel with fellow NYU student Kenny Mai, and their project won top honors in the NYU Time Warner Cable Inno/Vention Competition in 2011. Landolina told the Huffington Post in 2013 that Mai left the company to focus on his studies.
In an interview with Bloomberg, which you can watch at the top of this post, Landolina describes VetiGel as being like Lego building blocks for the body. The building blocks in this case are plant-based polymers pulled from the cell walls of a plant that basically reassemble onto whatever you put them next to (skin, for example), which helps clot blood in seconds.
Seconds can sometimes mean the difference between life and death. In the Bloomberg interview, Omar Ahmad, Suneris vice president of engineering, gave a theoretical example of a soldier on a battlefield who was shot, severing his femoral artery. Existing products can stop the bleeding in 5 to 10 minutes. But VetiGel is supposed to be capable of stopping the bleeding in under a minute, giving that soldier a better chance of living.
VetiGel isn't ready for humans, yet, and needs more testing and refining before it becomes an FDA-approved, market-ready product. But it's getting closer to that point, and will soon be commercially available as a product veterinarians can use to help stop bleeding during animal surgeries. VetiGel has undergone animal testing under the supervision of a cardiovascular surgeon, and was determined to be safe enough for use in animals.
Vets and vet clinics interested in testing out VetiGel can sign up on the Suneris site.
If the product gets approved for humans, the primary initial market will likely be the armed services, and Suneris is already in talks with military officials who are interested in such a product.
(Via Laughing Squid)Plant-based gel can stop traumatic bleeding in seconds - CNET
https://www.cnet.com/news/vetigel-plant-based-gel-can-stop-traumatic-bleeding-in-seconds/
STOPPING BLEEDING WITH BIOLOGIC GLUE - The Washington Post
https://www.washingtonpost.com/archive/lifestyle/wellness/1996/08/06/stopping-bleeding...
STOPPING BLEEDING WITH BIOLOGIC GLUE By Caryle ... has been making his blood-stopping spray for more than a decade because he cannot buy it commercially in the United States. ... "we use large ...Plant-based gel can seal bleeding wounds instantaneously ...
https://www.rt.com/news/208027-wound-sealing-gel-blood
The gel uses natural polymers to coordinate with the body’s natural cell clotting and accelerate hemostasis. It is applied directly to the source of bleeding and …
The Downside of Taking Aspirin: When Bleeding Won’t Stop
The Downside of Taking Aspirin: When Bleeding Won’t Stop | The Survival Doctorby James Hubbard, MD, MPH
I’m constantly amazed by the effect even the lowest dose of aspirin has on bleeding. I’ll be putting pressure on a cut, and the bleeding will just not be stopping. The person with the cut will have already told me they have no known bleeding disorders and haven’t taken any aspirin, and I’ll ask again.
“Well, I did take something a few days ago. It may have had aspirin in it.” Or, “Oh, I do take a baby aspirin.” Voila. I have my answer. I’ll just have to apply pressure for ten minutes instead of five, Or twenty minutes instead of ten. Aspirin doesn’t stay in the system that long, but its effect on bleeding does.
Here’s how.
If a blood vessel is injured, platelets are the first line of defense to stop bleeding. Almost immediately they start gathering at the injured site. And the bleeding triggers them to become stickier. They form a plug at the site. This gives time for a second line of clotting formation (fibrin) to build a stronger clot.
Aspirin decreases the chemical in the blood that makes platelets stickier. And, although the aspirin leaves the system within hours, its effect extends the whole lifetime of the platelet—five to ten days.
The Downside of Taking Aspirin: When Bleeding Won’t Stop | The Survival Doctor
This is a blood smear under a microscope. The fuchsia dots are platelets clotting. The reddish circles are blood cells.
Now usually, this is easily remedied by applying pressure longer to give the platelets longer to clot. (They don’t lose all their stickiness.) But, if the bleeding is internal—say, from a stomach ulcer or in a blood vessel in the head—putting pressure on it is not an option. Hence, the person is at significantly greater danger to have a larger amount of bleeding.
The only thing to do for that is get the person to a medical facility ASAP. Meantime, stop the aspirin. Eating foods high in vitamin K (essential in clotting) might help a little (unless it’s an abdomen wound and it’s dangerous to eat or drink).
What to Do For Cuts if You’re Taking Aspirin
Expect to hold pressure longer.
Apply a pressure dressing to continue the pressure.
Stop the Aspirin?
If you’re taking aspirin daily to prevent a heart attack, and the bleeding is not internal, this is a tricky question best answered by your doctor.
Remember the aspirin already has a permanent effect on the platelets in your system. It’s true new, unaffected platelets are being formed all the time, as your older platelets die off, so stopping the aspirin will gradually help.
If you stop aspirin for five or more days, it can have a rebound effect on your platelets, making them stickier and increasing your risk for a heart attack.
The Survival Doctor's Guides to Wounds and Burns
Bottom line: If the cut is small, don’t worry. If it’s larger and it’s harder to stop bleeding and you can’t get to a doctor, stop the aspirin for a day or two.
>> Bleeding tips always at the ready: The Survival Doctor’s Guide to Wounds.
What If It’s Not the Aspirin?
Other medicine and foods than can increase bleeding time include:
Any nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen, and others, which can prolong bleeding a bit, but not as much as aspirin
Other blood thinners such as Coumadin and Plavix
Certain herbs such as garlic, ginger, ginkgo, and feverfew
Fish oil
Vitamin E
Things that can improve blood clotting include:
Food rich in vitamin K (which intestinal bacteria also makes)
Green, leafy vegetables
Dried basil, sage, and thyme
Hot and bell peppers
Olive oil, soybean oil
Beans
Whole wheat
Oats
So what about you? Have you ever had bleeding that was hard to stop? What did you do?
You MayAspirin and Bleeding: How Much It Takes, How Long It Lasts
http://thesurvivaldoctor.com/2013/03/12/aspirin-and-bleeding/
Chemical Engineering Journal
Volume 230, 15 August 2013, Pages 567-572
Esterification of free fatty acids over chitosan with sulfonic acid groups
panelC.S.CaetanoaM.CaiadoaJ.FarinhaaI.M.FonsecabA.M.RamosbJ.VitalbJ.E.Castanheiroa
a
Centro de Química de Évora, Departamento de Química, Universidade de Évora, 7000-671 Évora, Portugal
b
REQUIMTE/CQFB, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal
Highlights
•
Sulfonic acid catalysts supported on chitosan were prepared.
•
Esterification of fatty acids was carried out over chitosan with sulfonic groups.
•
High catalytic activity was observed.
Abstract
Chitosan, which is an abundant biopolymer, with sulfonic acid groups was used as an efficient, environmentally friendly heterogeneous catalyst for the esterification of free fatty acids with methanol into their more fatty acid methyl ester.Sulfonic acid catalysts supported on chitosan have been studied in the esterification of palmitic acid with methanol at 60 °C. The sulfonic acid groups were introduced onto chitosan (CT) through cross-linking with sulfosuccinic acid (SSA). The catalytic activity increased as the amount of sulfonic acid groups present in chitosan was increased. However, with large amounts of sulfonic acid groups, the catalytic activity decreased.
This behaviour can be explained by the factors that limit the diffusion. The catalytic stability of the CT4 (2.08 mmol sulfonic acid groups/g) sample was evaluated through consecutive batch runs performed with the same catalyst sample. After the second batch, the catalytic activity stabilised. The CT4 catalyst was also used as a catalyst in the esterification of oleic and stearic acids with methanol. A good catalytic activity of CT4 for the different substrates used in the esterifications was observed.
Esterification of free fatty acids over chitosan with sulfonic acid groups - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S1385894713008231
Can chitosan affect the absorption of unsaturated fatty acid?
https://biology.stackexchange.com/questions/22038/can-chitosan-affect-the-absorption...
The second paper suggests that chitosan forms an emulsion with fatty acids under the acidic conditions of the stomach. This emulsion is stable and later excreted with the feces. References: Selective in vivo effect of chitosan on fatty acid, neutral sterol and bile acid excretion: a longitudinal study.
Evaluation of mixed matrices of chitosan and fatty-acids ...
https://www.sciencedirect.com/science/article/pii/S1773224719304071
The aim of this study was to utilize electrostatic interaction between chitosan (CS) and fatty-acids (FA) in designing mixed matrices filled into hard gelatin capsules as hydrodynamically balanced systems with slow drug-release.
Author: Bilal H. Alrimawi, Ahmad Bani-Jaber, Muhammad Al-Zweiri
Publish Year: 2019
Chitosan: A Versatile Platform for Pharmaceutical Applications
Raphael Riva and Christine Jerome*
Center for Education and Research on Macromolecules University of Liege, Chemistry Department, Sart-Tilman, Building B6a, 4000 Liege, Belgium
*Email: c.jerome@ulg.ac.be
Introduction
The development of new medical devices and pharmaceuticals plays an integral role in the medical industry. Both natural and synthetic polymers possess benefits that make them valuable components in therapeutics. In fact, methods to encapsulate drugs in a polymer matrix have demonstrated improved therapeutic efficiency and bioavailability while preventing the drug degredation. In nanomedicine, the development of polymer-based nanocarriers was first initiated in response to the immunologic side reactions encountered with viral-based nanocarriers.1,2 In this context, we will discuss the recent emergence of methods that use natural polymers and their derivatives as tools to achieve a high degree of biocompatibility with controlled biodegradability.3
Chitosan for Biomedical Applications
There are several families of natural polymers available on the market. Chitosan is one particular example of a polymer that has been thoroughly studied during the last few decades and shown to be a non-toxic, semi-crystalline,4 biodegradable,5 and biocompatible6 polysaccharide. Chitosan is a random copolymer of N-acetyl-glucosamine and glucosamine units obtained by the deacetylation of natural chitin, generally under alkali conditions at relatively high temperature (Figure 1).4 Natural chitin is a renewable resource that can be extracted from the exoskeleton of crustaceans or insects. Chitin can also be obtained from non-animal sources, namely from the cell walls of mushrooms.7,8 Generally, mushroom-derived chitosan displays a narrower molar mass distribution, and better traceability and reproducibility compared to chitosan prepared from animal sources.
Chemical structure of chitosan
Figure 1. Chemical structure of chitosan.
The demonstrated biocompatibility and biodegradability of chitosan has rapidly paved the way for advancements in a number of biomedical applications9 including scaffolds for tissue engineering.10 For example, the three-dimensional porous structure of chitosan can be seeded with sensitive bioactive agents, including growth factors.11 Additionally, the combined hemostatic12 and antimicrobial13 properties of chitosan make it an outstanding candidate for use in wound dressings.14–15 In particular, these two intrinsic properties limit the risk of infection which, in turn, improves skin regeneration. The hydrophilic nature of chitosan makes it a suitable starting material for use in biodegradable and biocompatible hydrogels. The mechanical properties of pH-sensitive hydrogels can be adjusted by combining chitosan with simple additives like hydroxyapatite in order to meet specific application requirements.16
Chitosan for Pharmaceutical Applications
In pharmaceutical applications, chitosan17 has been successfully applied in the development of drug carriers18–19 for controlled drug delivery. The presence of positive charges in chitosan has been shown to increase adhesion to mucosa and as a result, increase the retention time.20 The positively charged chitosan backbone also allows for the formation of stable electrostatic complexes with polyanionic macromolecules, such as polyphosphate21 or nucleic acids.22 As a result, the application of chitosan for DNA or RNA encapsulation in gene therapy is an area of significant research.23 Chitosan solubility is of critical importance and is pH dependent. Chitosan is water soluble below pH 6.5 due to the protonation of the primary amine group. When soluble chitosan is required at neutral pH, two possibilities are available; either the use of chitosan oligomers (Prod. No. 523682), which are known to be highly soluble in water in a wide range of pH values, or the use of a chemically modified chitosan derivative. Particularly, the ethoxylation of the primary alcohol of both glucosamine and acetyl-glucosamine units leads to O-glycol-chitosan, a fully water soluble chitosan derivative.
Chitosan Derivatives for Gene Delivery
Effective non-viral delivery of nucleic acids has many challenges, including degradation via nucleases and a decrease in efficiency due to negative charges accumulated while crossing over cellular membranes. One approach to address these challenges is the formation of polymer complexes, known as polyplexes formed via electrostatic interactions between a polycation and the negatively charged nucleic acid, such as the well-known synthetic non-degradable polyethyleneimine. When forming a polyplex with chitosan, the presence of a primary amine group on the glucosamine repeating unit enables control over the charge density. This control is dependent on both the degree of acetylation and the pH. These properties have led to the successful application of chitosan in non-viral gene delivery24 for purposes such as (1) gene silencing (siRNA, shRNA), (2) compensating for defective genes, and (3) producing beneficial proteins or vaccines (DNA). Chitosan under slightly acidic conditions interacts with nucleic acids such as DNA or siRNA, leading to condensation of the nucleic acids into nanoparticles. This technique was successfully used for the formulation of a siRNA drug delivery system according to an ionic gelation process.25 Additionally, the biocompatibility and low toxicity of chitosan allow it to be used in vivo.26 To afford charge permanency and solubility27 in a wider pH range similar to other cationic synthetic polymers such as poly-l-lysine, quaternization of the chitosan primary amine was investigated.28 The quaternary amine was typically generated by reaction with methyl iodide followed by the substitution of the iodide counter-ion with a chloride ion by an ionexchange process. The resulting N,N,N-trimethyl chitosan chloride (TMC) is the most frequently reported quaternized chitosan in the literature used for transfection in gene therapy applications.29 Quaternization of chitosan improved the stability of ionic complexes relative to those based on pure chitosan. Further chitosan derivatizations allowed improvement of the TMC properties. For example, a combination of quaternization and grafting of thiol on TMC yields mucoadhesive properties of TMC by disulfide formation with mucin proteins of the cell membrane.30 Another example is the conversion of some primary amines in chitosan into carboxylic acids by reaction with succinic anhydride, which leads to improved solubility in neutral aqueous media.31 The collected acidbearing chitosan shows a higher solubility into water when at least 20 mol% of the primary amines are converted into carboxylic acids. Despite the fact the stability of the complex of carboxylated chitosan with DNA was shown to be weaker than with pure chitosan, a better transfection efficiency was observed.
The grafting of polymers or functional groups to chitosan is another option to improve gene delivery. The grafting of additional polymers or functional groups can lead to a better solubility and improved buffering capacity compared to unmodified chitosan. For example, the introduction of secondary and tertiary amino groups was shown to improve the transfection efficiency of chitosan.34 This one-step synthesis was based on the grafting of a carboxylic acid-bearing imidazole onto chitosan by amide formation, mediated by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). This simple and reproducible process improved both the solubility and the buffering capacity of the synthesized chitosan derivatives.
Chitosan Derivatives for Drug Delivery
Recent development efforts within the pharmaceutical industry have resulted in a number of highly hydrophobic, and thus poorly water soluble active pharmaceutical ingredient candidates (i.e., BCS Class II and IV drug candidates). This lack of solubility considerably reduces the bioavailability of a drug candidate and complicates its development, preventing commercialization. Targeted nanocarriers can be used to encapsulate hydrophobic drug candidates, allowing for intravenous administration.35 Derivatized chitosan has been shown to be an attractive candidate for hydrophobic drug encapsulation. The grafting of hydrophobic moieties onto the chitosan backbone gave the synthesized polymer amphiphilic properties. This amphiphilic copolymer was shown to self-organize into micelles, with the drug solubilized in the hydrophobic core. Alkyl chains were initially grafted to the chitosan by reacting aliphatic aldehydes with the primary amine in the chitosan backbone, to form the corresponding Schiff base. This was followed by reduction with NaBH4.36–37 This strategy successfully enabled the grafting of C3, C5, C6, C8, and C12 alkyl chains to chitosan.38–40 Amphiphilic properties can be more precisely tuned by grafting both hydrophobic and hydrophilic components on a single chitosan backbone. Sequential grafting of octyl chains by reductive amination followed by the grafting of sulfate was also successfully achieved.41 The grafting of aromatic rings, namely 2-carboxybenzoyl groups or phtalimide groups, has also been investigated.42–43
The use of renewable materials for the modification of chitosan has also been demonstrated. Here, fatty acids were grafted to chitosan using a coupling reaction between the primary amine of chitosan and the carboxylic acid of the fatty acid mediated by EDC in a methanol and water solution.44–45 Using this technique, saturated stearic acid and unsaturated lineoic acid were successfully grafted onto oligochitosan. These derivatives were then modified with cholanic acid and cholesterol. Modified glycol-chitosan with 5-β-cholanic acid has been extensively studied both in vivo and in vitro as a carrier for docetaxel and paclitaxel.46,47 Similarly, tocopherol-PEG-carboxylic acid has successfully been grafted onto chitosan.48
Chitosan can also be modified with synthetic side-chains, particularly with biocompatible and hydrophobic aliphatic polyesters. The grafting of poly(ε-caprolactone) (PCL) was largely investigated for the synthesis of amphiphilic biocompatible chitosan based copolymers by both “grafting from” and “grafting to” techniques (Figure 2).49–50 In the “grafting from” technique, the polymerization of ε-caprolactone was initiated directly by the primary amine, or the hydroxyl groups, present on the chitosan chain. In the case of the “grafting to” technique, polymer chains bearing an appropriate functional group at one chain-end were grafted onto the primary amine or hydroxyl groups of chitosan.51
Illustration of the “grafting from” and “grafting to” techniques
Figure 2. Illustration of the “grafting from” and “grafting to” techniques.
A selective initiation of the polymerization or grafting of a preformed polymer chain, exclusively by the hydroxyl groups can be reached if the primary amines are protected before reaction and deprotected afterwards.50,52–53 Remarkably, the chitosan primary amines can be protected by formation of a stable electrostatic complex with methylsulfonic acid, which is easily removed by precipitation in a phosphate buffer after polymerization. The reaction of chitosan with phthalic anhydride is another way to efficiently protect the primary amine groups. Such protection also improves the solubility of chitosan in organic solvents, namely dimethylformamide.54 Ester or urethane links are two examples of organic functions used for the grafting of PCL terminated by a carboxylic acid55 or an isocyanate group,54 respectively, onto hydroxyl groups of phthalimide-chitosan (Figure 3).
Grafting PCL onto phtalimide-protected chitosan
Figure 3. Grafting PCL onto phtalimide-protected chitosan.
Compared to the “grafting from” technique, the “grafting to” technique was shown to demonstrate better control of the number and molecular weight of the PCL grafts onto chitosan. The grafting of polymer chains onto chitosan is not limited to PCL. The grafting of PEG chains onto chitosan is widely described in the literature.51,56 Recently, carboxylic acid-terminated PEG chains were grafted onto the primary amines of chitosan. The resulting grafted copolymer showed a reduced cytotoxicity compared to unaltered chitosan.51 Heterografted chitosan containing both PCL and PEG pendant chains was synthesized by Liu, et al. by simultaneous grafting of carboxylic acid-terminated PEG and PCL onto the hydroxyl group of phthalimide-chitosan leading to finely tuned amphiphilic properties.57
Finally, glucosamine58 (GlcNH2), the deacetylated monomer unit of chitosan, can be advantageously used to decorate nanoparticles for delivery of antibacterial and anticancer drugs.59–60 Indeed, GlcNH2 is known to be toxic to several malignant cell lines like human hepatoma, prostate, leukemia and breast cancer cells.61–64 Hence, GlcNH2 might be a promising target for the treatment of malignant cancer due to its inhibitory effect on transglutaminase 2 (TGase2), which contributes to drug resistance.62 GlcNH2 has also been used as a ligand in a kidneytargeted drug delivery system for delivery of prednisolone leading to an increase in concentration of prednisolone in vivo.65
Conclusions
Chitosan has received considerable attention as a functional biopolymer for diverse pharmaceutical and biomedical applications. Chitosan is a nontoxic, biocompatible, and biodegradable polymer. It can be formulated as a nanocarrier using ionic interactions, leading to drugloaded colloidal systems with mucoadhesive and remarkable permeationenhancing properties. Additionally, this pH-sensitive polysaccharide can also be formulated as a hydrogel.
The cationic properties of chitosan have enabled its extensive use for gene delivery. While nucleic acid-loaded chitosan nanoparticles fabricated from native chitosan have shown low buffering capacity and limited stability, chemically modified chitosan can help to improve in vivo transfection efficiency via: (1) quaternization to improve nanoparticle solubility and stability, (2) grafting of polymer chains to improve endosomal escape, or (3) grafting of ligands for specific cell targeting. When a hydrophobic moiety is conjugated to chitosan, the resulting polymer can self-assemble and encapsulate a poorly soluble drug. Grafting a steroid, fatty acid, or PCL onto chitosan or glycol chitosan can also lead to nanocarriers that are useful for drug delivery, in particular passive or active targeting of anticancer drugs to tumors.Chitosan: A Versatile Platform for Pharmaceutical Applications
https://www.sigmaaldrich.com/technical-documents/articles/materials-science/chitosan-a...
Here, fatty acids were grafted to chitosan using a coupling reaction between the primary amine of chitosan and the carboxylic acid of the fatty acid mediated by EDC in a methanol and water solution. 44–45 Using this technique, saturated stearic acid and unsaturated lineoic acid were successfully grafted onto oligochitosan. These derivatives were then modified with cholanic acid and cholesterol.
J Zhejiang Univ Sci B. 2006 Aug; 7(8): 608–614.
Antitumor activities of D-glucosamine and its derivatives*
Li Zhang,†‡,1,2 Wan-shun Liu,2 Bao-qin Han,2 Yan-fei Peng,2 and Dong-feng Wang†‡,1
Author information Article notes Copyright and License information Disclaimer
1College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China
2College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China
‡Corresponding Author
†E-mail:nc.ude.cuo@ilgnahzdq, nc.ude.cuo@fdgnaw
ABSTRACT
The growth inhibitory effects of D-glucosamine hydrochloride (GlcNH2·HCl), D-glucosamine (GlcNH2) and N-acetyl glucosamine (NAG) on human hepatoma SMMC-7721 cells in vitro were investigated. The results showed that GlcNH2·HCl and GlcNH2 resulted in a concentration-dependent reduction in hepatoma cell growth as measured by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. This effect was accompanied by a marked increase in the proportion of S cells as analyzed by flow cytometry. In addition, human hepatoma SMMC-7721 cells treated with GlcNH2·HCl resulted in the induction of apoptosis as assayed qualitatively by agarose gel electrophoresis. NAG could not inhibit the proliferation of SMMC-7721 cells. GlcNH2·HCl exhibited antitumor activity against Sarcoma 180 in Kunming mice at dosage of 125~500 mg/kg, dose of 250 mg/kg being the best. GlcNH2·HCl at dose of 250 mg/kg could enhance significantly the thymus index, and spleen index and could promote T lymphocyte proliferation induced by ConA. The antitumor effect of GlcNH2·HCl is probably host-mediated and cytocidal.
Keywords: D-glucosamine hydrochloride, D-glucosamine, N-acetyl glucosamine, Antitumor, Human hepatoma cell, Sarcoma 180
INTRODUCTION
Chitin, a kind of poly-β(1,4)-N-acetyl-D-glucosamine, is a natural biopolymer present in the exoskeleton of crustaceans and in cell walls of fungi, insects and yeast. A series of oligomers and monosaccharides, such as GlcNH2 and NAG, can be obtained by either chemical or enzymatic hydrolysis of chitin and chitosan (Akiyama et al., 1995). Besides hydroxyl, there are other functional groups in GlcNH2 and NAG, for example, amido and acetylamino. Many derivatives can thus be obtained from them, most of which are known to have various biological activities including antitumor activities (Chen et al., 2005), increased protective effects against infection by some pathogens (Martin et al., 2003; Wang and Chen, 2005) and antimicrobial activities (Chen, 2001).
Wang et al.(2003a; 2003b) reported that NAG, GlcNH2·HCl and GlcNH2 could induce proliferation of leukemia K562 cells and make them differentiate toward macrophage. GlcNH2 at concentration of certain range could kill tumor cells without influencing normal cells (Friedman and Skehan, 1980). It is therefore postulated that combination of GlcNH2 with membrane-active drugs may have the potential to kill tumor cells, especially for neuro-oncology. Dong et al.(2004) reported that glucosamine sulphate could inhibit proliferation of leukemia HL60 cells and induce the differentiation of HL60 cells toward the granulocytic or monocytic lineage. In addition, glucosamine sulphate has been proved to be useful in the therapy of osteoarthritis (Reginster et al., 2001).
However, there is no report on the direct cytotoxicity of D-glucosamine and its derivatives to human hepatoma cell. Moreover, there are only a few reports on the antitumor activity of D-glucosamine and its derivatives in murine models. Much controversy on its mechanism still exists. This study was aimed at investigating the inhibitory effects of GlcNH2·HCl, GlcNH2 and NAG on human hepatoma SMMC-7721 cells and the antitumor activity of GlcNH2·HCl in Kunming mice implanted with solid tumor Sarcoma 180.
Antitumor activities of D-glucosamine and its derivatives
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1533750/
Journal of Drug Delivery Science and Technology 2019
Evaluation of mixed matrices of chitosan and fatty-acids filled into hard gelatin capsules as sustained-release hydrodynamically balanced systems
Author links open overlay panelBilal H.AlrimawiaAhmadBani-JaberaMuhammadAl-Zweirib
a
Department of Pharmaceutics and Pharmaceutical Technology, School of Pharmacy, The University of Jordan, Amman, Jordan
b
Department of Pharmaceutical Sciences, School of Pharmacy, The University of Jordan, Amman, Jordan
Received 27 March 2019, Revised 19 July 2019, Accepted 22 July 2019, Available online 23 July 2019.
Abstract
The aim of this study was to utilize electrostatic interaction between chitosan (CS) and fatty-acids (FA) in designing mixed matrices filled into hard gelatin capsules as hydrodynamically balanced systems with slow drug-release. CS of low and high molecular weight (Mwt), lauric acid (LA), stearic acid (SA) and palmitic acid (PA) were used for this purpose. Riboflavin, known to have narrow absorption window in the upper gastrointestinal tract, was used as a model drug.
The interaction of CS with each FA was evaluated by kneading effervescent (Eff.) or non-effervescent (non-Eff.) CS-FA mixtures using 0.1 M HCl. The mixtures differed with respect to CS Mwt, FA type and CS:FA weight ratio. Upon drying, the mixtures were evaluated for CS-FA interaction using Fourier transform infrared spectroscopy (FT-IR). Eff. and non-Eff. mixtures of optimal CS:FA ratio for the interaction containing riboflavin were prepared as granulations and filled into hard gelatin capsules. The capsules were observed for flotation of their matrices and studied for drug-release in 0.1 M HCl.
Evident interaction between CS and each FA was demonstrated in 0.1 M HCl, irrespective of CS Mwt. However, drug-release was highly affected by CS Mwt and FA type and was the slowest for low Mwt CS and LA as Eff. matrix or PA as non-Eff. matrix. The flotation of these matrices was rapid and prolonged (>6 h).
Keywords
ChitosanFatty-acidsHydrodynamically balanced systemFloatationSustained-release
The synthesis and characterization of fatty acid salts of chitosan as novel matrices for prolonged intragastric drug delivery
July 2012Archives of Pharmacal Research 35(7):1159-1168
DOI: 10.1007/s12272-012-0706-6
Authors:
Ahmad Bani-jaber
20.31University of Jordan
Imad Hamdan
29.12University of Jordan
Mahmoud Alkawareek
24.56University of Jordan
The aim of this study was to prepare fatty acid salts of chitosan (CS) and to evaluate the salts as matrices for sustained drug release and prolonged gastric retention.CS-laurate and CS-palmitate were formed by mixing saturated CS solution and aqueous solutions of sodium laurate and sodium palmitate, respectively, and collected by centrifugation.
They were characterized using Fourier-transform infrared spectroscopy and differential scanning calorimetry. Different matrices as effervescent tablets were prepared using each of these CS-salts, CS and the corresponding physical mixtures of CS and the fatty acids. Sodium bicarbonate as an effervescent agent and ranitidine HCl as a model drug were incorporated into these matrices.
In vitro buoyancy and drug dissolution were studied for the matrices in 0.1 M HCl. Tablets with fatty acid salts of CS showed both rapid and prolonged buoyancy (> 8 h). Comparatively, CS tablets exhibited a short floatation period (< 2 h) and tablets were completely disintegrated within 1 h of soaking. In addition, slow and prolonged drug release was achieved from tablets of fatty acid salts of CS with average drug release of 80.1 and 71.8% for CS-laurate and CS-palmitate, respectively. Rapid drug release (> 80% at 1 h) was exhibited by tablets with CS or the physical mixtures.
In vitro buoyancyAll of the matrices with no sodium bicarbonateincorporated were non-floating (Table II).
(PDF) The synthesis and characterization of fatty acid salts of chitosan as novel matrices for prolonged intragastric drug delivery
https://www.researchgate.net/publication/230613083_The_synthesis_and_characterization_of_fatty_acid_salts_of_chitosan_as_novel_matrices_for_prolonged_intragastric_drug_delivery
What happens when fatty acid reacts with NaOH? - Answers
https://www.answers.com/Q/What_happens_when_fatty_acid_reacts_with_NaOH
Saponification is a reaction involving a strong base (ex: NaOH) and a fatty acid. The reaction yields a glycerol and 3 fatty acid salts. Therefore the reaction products are: glycerol: CH2 (OH)--CH (OH)--CH2 (OH) + 3 fatty acid salts: CH3-- (CH2)14--COO (-) Na...
Chitosan Nanoparticles as Drug Carriers
Prakash#1, K. Ram Kumar#2, R. G. Rithick#3, S. Sivasankaran#4, K. Karthick Babu*5
#UG Student, *Assistant professor
#*Department of Biomedical Engineering Dhanalakshmi Srinivasan Engineering College Thuraiyur Main Road, Perambalur – 621 212
Abstract- Chitosan is a natural polymer obtained by deacetylation of chitin. After cellulose chitin is the second most abundant polysaccharide in nature. It is biologically safe, non-toxic, biocompatible and biodegradable polysaccharide. Chitosan nanoparticles have gained more attention as drug delivery carriers because of their better stability, low toxicity, simple and mild preparation method and providing versatile routes of administration. Their sub-micron size is also suitable for mucosal routes of administration i.e. oral, nasal and ocular mucosa which is non-invasive route. Chitosan nanoparticles showed to be a good adjuvant for vaccine. Therefore, the objectives of this review are to summarize the available preparation techniques and to discuss various applications of chitosan.The efficacy of many drugs is often limited by their potential to reach the site of therapeutic action. In most cases only a small amount of administered dose reaches the target site, while the majority of the drug distributes throughout the rest of the body in accordance with its physiochemical and biological properties. Therefore developing a drug delivery system that optimizes the pharmaceutical action of drug while reducing its toxic side effects in vivo is a challenging risk. One of the approaches is the use of colloidal drug carriers that can provide site specific or targeted drug delivery combined with optimal drug release profiles. Among these carriers liposomes and nanoparticles have been the most extensively investigated. Liposomes present some technological limitations including poor reproducibility and stability, low drug entrapment efficiency.
许多药物的疗效常常受到它们到达治疗作用地点的潜力的限制。在大多数情况下,只有少量的给药剂量到达靶部位,而大多数药物根据其理化和生物特性分布在身体的其他部位。因此,开发一种既能优化药物作用,又能减少药物在体内的毒副作用的给药系统是一个具有挑战性的风险。其中一种方法是使用胶体药物载体,它可以提供结合最佳药物释放特性的位点特异性或靶向药物传递。在这些载体中,脂质体和纳米颗粒的研究最为广泛。脂质体存在一些技术上的限制,包括再现性和稳定性差,药物包封效率低。
Catharanthus roseus, commonly known as the Madagascar periwinkle, rose periwinkle, or rosy periwinkle, is a species of flowering plant in the dogbane family Apocynaceae. It is native and endemic to Madagascar, but grown elsewhere as an ornamental and medicinal plant, a source of the drugs vincristine and vinblastine, used to treat cancer. Other English names include 'Cape periwinkle and "old-maid". It was formerly included in the genus Vinca as Vinca rosea.
Fig. Synthesis of chitosan nanoparticle
potential to be utilized in a number of medical and industrial applications. Formation of nanoparticles can be achieved under extremely mild conditions particle size and surface charge was modulated by changing the proportion and specification of raw materials. The results demonstrated that nanoparticle size was obtained from the lower molecular weight of chitosan and CuSo4 loading. The incorporation of catharanthus roseus is a more protective method in opposition to the cancer activities. The biodegradable nature and anti- cancer activity acts as a best carrier for the cancer therapy when compared to other natural polymers available. The further studies should be perform to increase the nanoparticles efficiency. Commercial production of the prepared nanoparticles is in progress.Chitosan Nanoparticles as Drug Carriers – IJERT
https://www.ijert.org/chitosan-nanoparticles-as-drug-carriers
Drug delivery with nanostructured porous silicon nanoparticles
Mesoporous silicon nanomaterials can be fabricated and biofunctionalized for in vitro and in vivo controlled drug delivery and theranostic applications.
01 July 2013 Hélder A. Santos
Nanomedicines have great potential to address some of the big problems in cancer therapy, such as how to get enough of the right drug to the right place without causing side effects or inducing drug resistance. Traditional chemotherapies can be toxic, but the current research advances in nanotechnology enable the design and manufacture of nanoparticles that carry drugs to tumor sites and release them in situ in a controlled manner. Multifunctional nanoparticles with carefully controlled chemistry, size, surface charge, and other properties can carry drugs and give them new functions, providing a safer and more effective therapy than conventional chemotherapy. Despite intensive research in the development of efficient nanomedicines, there are still several hurdles that need to be overcome before the efficient clinical application of nanodrugs.
Nanostructured mesoporous silicon (PSi) has received considerable attention in the past few years in the field of biomedical nanotechnology. PSi-based materials are fabricated by electrochemical etching. This top-down approach enables the pore sizes to be tailored within the nanometer range, with different surface chemistries (hydrophilic or hydrophobic) and a wide range of porosities (up to 90%) to enable high drug loading levels. Furthermore, the nanoparticles can be engineered to be highly biodegradable and biocompatible. Thus, nanostructured PSi-based nanomaterials can be strictly designed for specific applications (see Figure 1).1–6 For example, the surface chemistry provides a suitable platform for covalent conjugation and electrostatic attachment of fluorophores—fluorescent and radioactive molecules2, 3—that have considerable promise as the next generation of nanomedicines for the early detection, simultaneous monitoring, and treatment of diseases with minimal toxicity.
Figure 1. Schematic representation of (i) spherical-shaped nano- structured mesoporous silicon (PSi) drug carriers (ii) that can be biofunctionalized with different biological ligands and polymers (iii) to allow travel through the bloodstream and release the therapeutic compounds in the vicinity of tumor sites, (iv) as well as enabling simultaneous, real-time monitoring of its actions both in vitro and in vivo.
Figure 2. The self-assembly of hydrophobin protein onto the surface of radiolabelled thermally hydrocarbonized PSi (THCPSi) nanoparticles modulates the biodistribution and clearance of the fraction of PSi nanoparticles found in the liver and spleen compared with the uncoated PSi nanoparticles.
Figure 3. Transmission electron microscope images of (a) THCPSi nanoparticles and (b) THCPSi solid-lipid nanocomposites (THCPSi-SLNCs) prepared using a solid-in-oil-in-water (S/O/W) emulsion evaporation method. (c) Dispersions of the THCPSi nanoparticles and THCPSi-SLNCs in aqueous solution, demonstrating the higher stability of the latter. (d) Impact of the human plasma on the particle size for both nanoparticles. (e) Confocal fluorescence microscopy images of macrophages cells (orange) after a 3h incubation with THCPSi nanoparticles and THCPSi-SLNCs (green), showing a reduction in cellular association to the latter particles. (f) Controlled furosemide (FUR) release from the THCPSi-SLNCs at pH 5.5 and 37°C.
To develop a bioimaging platform based on PSi nanoparticles for biomedical applications, we first successfully developed a method to radiolabel thermally hydrocarbonized PSi (THCPSi) nanoparticles of size ∼142nm with a fluorine-18 (18F) radioisotopic label.5–7 The biodistribution of the nanosized 18F-nanoparticles after oral, subcutaneous, and intravenous administration in rats confirmed that fluorine-18 was adequate for determining the blood circulation life-time of the labelled particles and for tracking them through the gastrointestinal tract.6, 7 The radiolabelled nanosystem exhibited excellent in vivo stability and was even able to trace the fate of the PSi nanoparticles after biofunctionalization with a hydrophobin protein,5, 6 which renders the PSi-based material a flexible platform for in vivo imaging purposes.
Then, to study the ‘biofate’ (distribution) of the particles after intravenous administration to rats, we coated 18F-labeled THCPSi nanoparticles with self-assembling hydrophobin protein from the fungus Trichoderma reesei and studied the intravenous biodistribution in rats. Protein coating altered the hydrophobicity of the THCPSi nanoparticles, resulting in a pronounced change in the degree of plasma protein adsorption to the nanoparticle surface in vitro. This also changed the biofate of the nanoparticles between the liver and spleen (see Figure 2),5 which can be used to modulate the immune recognition and subsequent elimination of the nanoparticles from the circulation.
Recently, we created a novel nanocomposite for controlled and targeted drug delivery based on the encapsulation of THCPSi nanoparticles—see Figure 3(a)—with solid-lipid nanoparticles in a 1 :1 ratio using a solid-in-oil-in-water emulsion solvent evaporation method.8 This approach enables us to form a nanocomposite with rather different surface smoothness and hydrophobicity compared with the uncoated PSi nanoparticles: see Figure 3(b) and (c). In addition, the method greatly improved the stability of the nanocomposite in human plasma and the cytocompatibility, and reduced cellular association: see Figure 3(d) and (e). A clear prolonged release for a model drug (furosemide) further demonstrated the capability of the nanoparticles to sustain the release of the compound: see Figure 3(f).
Overall, we anticipate the emergence of PSi-based nanodrugs to result in substantial benefits to the field of theranostics (a combination of therapy and diagnostics). PSi is an acceptable biomaterial with several advantageous features that render it an efficient drug carrier and imaging agent. The great advantages of the nanostructured PSi materials are the good biocompatibility, biodegradability, high pore volume necessary for hosting large amounts of therapeutics, tunable pore sizes for fine control of drug loads and release kinetics, high surface area for drug adsorption, easy surface chemistry modification for further biofunctionalization, and control of drug loading and release. We are now developing advanced nanostructured PSi-based theranostic platforms to improve the intracellular transport of drugs to the desired unhealthy cells or tissues with simultaneous real-time imaging, without damaging the healthy cells.
This research is supported by grants from the Academy of Finland (decision numbers 252215 and 256394), University of Helsinki and European Research Council under the European Union's Seventh Framework Programme (FP/2007–2013) grant number 310892. A special acknowledgment goes to Jouni Hirvonen (University of Helsinki), Jarno Salonen (University of Turku), and Anu Airaksinen (University of Helsinki) for their faithful collaborations throughout the years.
Figure 1. Schematic representation of (i) spherical-shaped nano- structured mesoporous silicon (PSi) drug carriers (ii) that can be biofunctionalized with different biological ligands and polymers (iii) to allow travel through the bloodstream and release the therapeutic compounds in the vicinity of tumor sites, (iv) as well as enabling simultaneous, real-time monitoring of its actions both in vitro and in vivo.
Figure 2. The self-assembly of hydrophobin protein onto the surface of radiolabelled thermally hydrocarbonized PSi (THCPSi) nanoparticles modulates the biodistribution and clearance of the fraction of PSi nanoparticles found in the liver and spleen compared with the uncoated PSi nanoparticles.
What Exactly is Chitin and Chitosan?
Chitosan-Flake-Quarter-Close-Up-Sustainable-1033201
Chitin is a high molecular weight biopolymer. When chitin is processed by the removal of acetyl groups, the chitin molecule becomes a molecule called chitosan [poly(glucosamine-co-acetylglucosamine)].
Chitosan is a cationic polyelectrolyte that is soluble in dilute organic acid. Chitosan’s long-chained and polyelectrolyte structure of chitosan molecules provides unique behavior for a variety of applications such as in water treatment, film formation, antimicrobial activity, and more.
Additionally, the chemical makeup of chitosan provides bioactive properties such as an immune elicitor in plants.
Properties
Chitin and chitosan are naturally nontoxic, biodegradable, and biocompatible while having valuable properties for a wide range of applications:
Positive electrostatic charge
Chitosan binds with heavy metals, hydrocarbons, debris, and other toxins in water treatment. Chitosan also binds to red blood cells when, used as a hemostatic agent, and to suspended particles in beverage manufacturing.
Bacteriostatic
Chitosan also binds to the cell walls of microorganisms, suppressing microbes through a nontoxic mode of action. Alternatively, most antibiotics and bactericidal agents work through a toxic mode of action.
Chitosan / Chitin: Made in the USA with eco-friendly processes
https://tidalvisionusa.com/chitosan/
VOLUME 5, ISSUE 7, E02036, JULY 01, 2019
Preparation of α-chitin-based nanocomposite as an effective biocatalyst for microwave aided domino reaction
Abstract
In this paper, chitin (Ch) was extracted by an optimized method from cuttlebone of the Persian Gulf cuttlefish (Sepiidae, Cephalopoda). The extracted chitin was characterized by Fourier-transform infrared spectroscopy (FT-IR), X-ray powder diffraction (XRD) and thermal gravimetric analysis (TGA) which showed that the extracted chitin was in alpha form. The degree of N-acetylation (DA) and degree of substitution (DS) of α-chitin were calculated using titration method and FTIR spectroscopy and found to be 80–82% and 19.57 respectively. The α-Chitin was used as biomolecules for the preparation of nanostructured Ch/ZnO via a hydrothermal method. The obtained nanocomposite was characterized using FT-IR, XRD, scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDX) analysis. The antimicrobial aspect of Ch/ZnO nanocomposite was previously proposed. In this paper, attempt was made to add the catalytic feature to these traits. For this purpose, the nanostructured Ch/ZnO was used as reusable nanocatalyst in the green and efficient synthesis of Benzo[a]pyrano(2,3-c)phenazine derivatives thru a four components microwave aided domino reaction. Eco-friendly, easy work up and separation of the nanostructured catalyst are some of the highlighted features this protocol.
The presence of the acetamide groups gives it biological functions such as biodegradability and biocompatibility, which makes chitin a functional material of great importance. So, the conversion of natural chitin resource into valuable composites is of foremost interest. As a result, in recent years, research on the development of new chitin-based functional materials has received considerable attention (Noguchi et al., 2019).
The nucleation and growth of ZnO crystals can be influenced by the application of biomolecules such as polypeptides proteins and also polysaccharides which can cause the formation of ZnO with controllable morphology and unique structural and physicochemical properties under mild conditions (Tomizaki et al., 2012) (Waltz et al., 2012). As far as we know, there are only a few publications on the combination of chitin with ZnO, (Kumar et al., 2013) and none with α-chitin. So, in the first part of this work, chitin was extracted from cuttlebone by modified method, the extracted chitin was then used to prepare Ch/ZnO via a hydrothermal method. Since the antimicrobial activity of Ch/ZnO nanocomposite was previously reported, (Wysokowski et al., 2013) the study of the catalytic activity of Ch/ZnO was the main objective of our research. For this purpose, the synthesis of Benzo[a]pyrano(2,3-c)phenazine derivatives which comprise two bio-active heterocyclic cores phenazines (I) and chromenes (II) was pursued (Fig. 2a).
2.2 Chittin extraction
Several techniques to extract chitin from different sources have been reported. The most common method is referred to as the chemical procedure. The chemical method for isolation of chitin from the waste involves various major steps: extraction of protein matter in alkaline medium (deproteinization) and it is traditionally done by treating the waste with aqueous solutions of NaOH. Elimination of inorganic matter (CaCO3) in dilute acidic medium (demineralization), which is accomplished by using HCl and finally bleaching in dilute NaOCl. The resulting chitin by our extraction method was colorless and no bleaching step with sodium hypochlorite (NaClO) was required (Fig. 3).
Table 4The comparison of Ch/ZnO nanocomposite as catalyst for the synthesis benzo[a]pyrano(2,3-c)phenazine derivatives with other catalysis.4. Conclusion
In this study chitin was extracted via an optimized method from cuttlebone of the Persian Gulf cuttlefish and characterized. The results from FT-IR, XRD and TGA showed that the extracted chitin was in alpha form. The DA and DS were determined by using titration method and FTIR spectroscopy and found to be 80–82% and 19.57 respectively. These properties are valuable for biomedical applications.
The thermo-stable α-chitin was used as a low cost natural template for the preparation of nanostructured Ch/ZnO via a hydrothermal method. The catalytic performance of Ch/ZnO was investigated for the first time in the synthesis of 3-amino-12-methyl-1-H-benzo[a]pyrano [2,3-c]phenazine-2-carbonitrile under microwave condition. The environmental sustainability, recyclability, alteration of waste to value-added catalyst, easy work up and separation of the nanostructured catalyst are some of the highlighted features this protocol.Preparation of α-chitin-based nanocomposite as an effective biocatalyst for microwave aided domino reaction: Heliyon
https://www.cell.com/heliyon/fulltext/S2405-8440(19)35696-8
Chitosan in the Light of Nanobiotechnology: A Mini Review
Tanvi Jain1,2, Sushil Kumar1, and PK Dutta2,3*
1Department of Chemical Engineering, Motilal Nehru National Institute of Technology, Allahabad 211004, U.P., India
2Department of Chemistry, Motilal Nehru National Institute of Technology, Allahabad 211004, U.P., India
3Centre for Medical Diagnostic & Research, Motilal Nehru National Institute of Technology, Allahabad 211004, U.P., India
Abstract
The field of biotechnology emerged in the fast pace of biology with the interface of science, engineering and technology and mostly known for its multidisciplinary nature. As we progress successively in this domain, the refinement of the field is quite obvious. Looking into an era of nanoscience and technology, now a days , we are using the broader term “nanobiotechnology” which includes all basic scientific research currently studying the fundamental, biologically related physicochemical properties of nanomaterials and cellular nanoscale phenomena like biopolymer-protein assemblies, molecular motors, cellular electrochemical behavior and so on. Such fantastic nature based research understanding has guided us to utilize the natural polymer obtained from crustacean shells, fungi etc.: chitin the second most naturally occurring polysaccharide just after cellulose. Chitosan, the principle derivative of chitin is much more versatile and finds curiosity-driven research in nanobiotechnology. This mini review focuses on various formulation based on chitosan utilized in clinical as well as biomedical field with special emphasis on quantum dots, nanoparticles, carbon dots and also about some of the application such biosensors and biomarkers detection in the light of biopolymer, chitosan.
Keywords: Nanobiotechnology; Quantum dots; Biosensors; Carbon dots; Chitosan
Drug Delivery
There has already been immense number of researches to elucidate polymer-based (like PEG, PLGA, Hyaluronic acid (HA) etc., drug delivery carriers which are having advantageous encapsulation properties of polymers. Presently, variety of chitosan based nanoparticles is extensively used for drug delivery applications. Chitosan has been widely utilized as drug delivery systems for low molecular drugs, peptides and genes [60-62]. Qu et al. [63] studied the effect of PEG conjugation on PTX-loaded N-octyl-sulfate chitosan nanoparticles. Also, Varshosaz et al. [64] investigated chitosan microspheres which was coated with cellulose acetate butyrate using emulsion-solvent evaporation method, for delivery of 5-ASA into the colon. A lot of studies have been earlier done on chitosan based nanocarriers for targeted drug delivery into the kidney [65-67]. In another related study, Janes et al. [68] effectively encapsulate DOX into the chitosan nanoparticles synthesized by ionotropic gelation technique. The results showed that the cytotoxicity of DOX-loaded nanoparticles in human melanoma cells (A375) and (C26) murine colorectal carcinoma cells indicated that the synthesized formulations were able to sustain cytostatic activity relative to free DOX. Also, the confocal microscopy studies revealed that DOX-loaded chitosan nanoparticles are adopted by these cells and thus also degraded to release the drug intracellularly.
Recently, Yongling D et al. [69] synthesized chitosan (CS) based carboxymethyl-β-cyclodextrin (CM-β-CD) polymer modified by using Fe3O4 magnetic nanoparticles for delivery of anticancer drug 5-fluorouracil. The results indicated that results indicated that the quantity of cross linking agent and bonding times played a vital role in analyzing the morphological characteristics of the prepared hybrid nanocarriers. Therefore, chitosan based nanocarriers for drug delivery plays a vital role in the treatment of various diseases
Conclusion
Nanobiotechnology, one of the relatively recent, promising and yet, largely untapped field of science, has its origins in the nanotechnological advances made in the last four decades. It is a marriage between the fields of technical and biological sciences and combines the complementary strengths of biological molecules and nanoelectronics for biomedical based devices. There are three specific areas of application where chitosan based formulations like quantum dots, carbon dots, biosensors, nanoparticles is being utilized in nanobiotechnology with excellent outcome. In this mini review, recent effort in the evolving field of nanobiotechnology, we have mentioned chitosan based formulations like nanovehicles for DNA/SiRNA delivery, quantum dots and nanocomposites. Due to the excellent biological properties of chitosan, a lot of work can be done in the field of medical diagnosis for various disease treatments as well as in biomedical research like in drug delivery, tissue engineering etc. Last five years this area has been witnessed a proliferation of high-resolution devices (biosensors) for in-vivo imaging of diseases in animal models using quantum dots; and the detection of biomarkers for generating new diagnostic and therapeutic techniques and also for utilized for drug delivery treatment of diseases like cancer. Apart from this nanobiotechnology raises many issues as with any introduction of new modality, including concerns about the toxicity and environmental impact of nanomaterials, and their capability on the world economy. Therefore, chitosan based nanobiotechnology is capable of creating several novel nanomaterials and devices with an infinite range of applications, such as in medicine, theranostics, biomedical and clinical applications for the benefit of mankind.
https://www.elynsgroup.com/journal/article/chitosan-in-the-light-of-nanobiotechnology-a-mini-review
Top 9 Benefits Of Chitosan Supplement | Inlifehealthcare
https://www.inlifehealthcare.com/2015/10/01/top-9-benefits-of-chitosan-supplement/
http://www.aspiera.com/technology/
As most bacteria carry a net negative surface charge, 3 adhesion of bacteria is discouraged on negatively charged surfaces, while it is promoted on positively charged surfaces. 4 – 6 Adhesion, however, is only one of the first steps in the formation of a biofilm infection 7 and in order for a biofilm to develop fully, the adhering bacteria have to grow. 8 Surface growth of the initially adhering bacteria was found by Harkes et al. 9 to be absent on positively charged poly(methacrylates) for Escherichia coli.
Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria | Journal of Antimicrobial Chemotherapy | Oxford Academic
https://academic.oup.com/jac/article/48/1/7/924030
Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria
Bart Gottenbos, Dirk W. Grijpma, Henny C. van der Mei, Jan Feijen, Henk J. Busscher
Journal of Antimicrobial Chemotherapy, Volume 48, Issue 1, July 2001, Pages 7–13,
Abstract
The infection of biomaterials is determined by an interplay of adhesion and surface growth of the infecting organisms.In this study, the antimicrobial effects on adhering bacteria of a positively charged poly(methacrylate) surface (ξ potential +12 mV) were compared with those of negatively charged poly(methyl methacrylate) (–12 mV) and a highly negatively charged poly(methacrylate) (–18 mV) surface.
Initial adhesion of Staphylococcus aureus ATCC 12600, Staphylococcus epidermidis HBH2 102, Escherichia coli O2K2 and Pseudomonas aeruginosa AK1 to these surfaces was measured in a parallel plate flow chamber in phosphate-buffered saline.
Adhering bacteria were allowed to multiply by perfusing the flow chamber with growth medium. All bacteria adhered most rapidly to the positively charged surface, but there was no subsequent surface growth of the Gram-negative strains.
On the negatively charged surfaces, despite a slower initial adhesion, surface growth of the adhering bacteria was exponential for both Gram-positive and Gram-negative strains. These results suggest that positively charged biomaterial surfaces exert an antimicrobial effect on adhering Gram-negative bacteria, but not on Gram-positive ones.
As most bacteria carry a net negative surface charge,3 adhesion of bacteria is discouraged on negatively charged surfaces, while it is promoted on positively charged surfaces.4
Bacterial ξ potentials
All bacterial strains studied here were negatively charged in PBS and their ξ potentials were –10 mV for S. aureus ATCC 12600, –8 mV for S. epidermidis HBH2 102, –16 mV for E. coli O2K2 and –7 mV for P. aeruginosa AK1.
Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria | Journal of Antimicrobial Chemotherapy | Oxford Academic
https://academic.oup.com/jac/article/48/1/7/924030
What is Zeta Potential?
Zeta Potential is the analysis technique for determining the surface charge of nanoparticles in solution (colloids). Nanoparticles have a surface charge measured in millivolts, that attracts a thin layer of ions of opposite charge to the nanoparticle surface.
Why is Zeta Potential important for disinfectants & sanitizer?
The greater the positive charge disinfectants or sanitizing solutions has the greater killing effect against negatively charged bacteria and virus, by way of opposite pole magnetic attraction.
How does Zeta Potential improve the efficacy of disinfectants & sanitizers?
The majority of bacteria and virus are negatively charged Nano particles, with a positively charged disinfectant & or sanitizer they will be attracted to each other and agglomerate the negatively charged bacteria or virus particles around the positively charged disinfectant or sanitizer solution therefore improving the kill time and efficacy rate per volume of liquid or disinfectant or sanitizer.
Can Zeta Potential kill bacteria and virus’s magnetically?
Yes, by way of changing the magnetic charge around bacteria or virus cell, which can damage the cell wall membrane, causing osmosis imbalance to the bacteria or viruses. This causes their cell wall to rupture, lyse and explode.Our Technology | Shield US Tech
http://www.shieldustech.com/our-technology/
Preparation of highly efficient antibacterial polymeric films via the modulation of charge density and hydrophobicity
Li-Hua Yin† ab, Bin Ranb, Tian-Jiao Hub, Chen Yangb, Jun-Jie Feia and Yi-He LiORCID logo*bc
aCollege of Chemistry, Xiangtan University, Xiangtan 411100, China
bCollege of Science, National University of Defense Technology, Changsha 410073, China. E-mail: yhli@nudt.edu.cn
cState Key Library of NBC Protection for Civilian, Beijing 102205, China
Received 31st October 2016 , Accepted 2nd December 2016
First published on 18th January 2017
Highly efficient antibacterial polymeric films were prepared in a facile manner via a thiol–ene reaction assisted by ultraviolet radiation. The influence of the positive charge density and hydrophobicity on the antimicrobial activity was evaluated with Escherichia coli and Staphylococcus aureus. There was a synergetic enhancement in sterilization in the presence of a positive charge density and hydrophobicity, which provided a convenient way to design and synthesize highly effective antimicrobial polymers. The prepared films, with abundant cations and sufficient hydrophobicity, exhibited robust antibacterial effects against E. coli and S. aureus. Their excellent thermostability makes these films suitable for practical applications.
Conclusions
By varying the ratio of hydrophobicity and the charge density group, a series of polymeric films was successfully prepared via thiol–ene photopolymerization. This outcome of this convenient and concise assay was in good agreement with the XPS and ATR-FTIR analyses. The resulting films with abundant cations displayed a remarkable antimicrobial activity against both S. aureus and E. coli, which correlated with the electrostatic properties. The antimicrobial activity of the polymer films was positively correlated with the charge density and nature of the long alkyl chain. The polymeric antibacterial films had excellent stability under physiological conditions. The thermal stability was enhanced as the charge density increased. This method of synthesizing antibacterial polymeric films with a tunable charge density or hydrophobicity by introducing a cationic cross-linking agent is important in studies of the mechanisms involved in cationic antibacterial polymers and clarifies the effects of charge density and hydrophobicity.Preparation of highly efficient antibacterial polymeric films via the modulation of charge density and hydrophobicity - RSC Advances (RSC Publishing) DOI:10.1039/C6RA26071C
https://pubs.rsc.org/en/content/articlehtml/2017/ra/c6ra26071c
RESEARCHARTICLE
Adhesion Potential of Intestinal Microbes Predicted byPhysico-Chemical Characterization Methods
TomasdeWouters1☯*,ChristophJans1☯,TobiasNiederberger1,PeterFischer2*,Patrick AlbertoRühs2
1 LaboratoryofFoodBiotechnology,ETHZurich,InstituteofFood,NutritionandHealth, Schmelzbergstrasse9,8092,Zurich,Switzerland,2 LaboratoryofFoodProcessEngineering,ETHZurich, InstituteofFood,NutritionandHealth,Schmelzbergstrasse9,8092,Zurich,SwitzerlandAbstract
Bacterial adhesion to epithelial surfaces affects retention time in the human gastro-intestinal tract and therefore significantly contributes to interactions between bacteria and their hosts.Bacterial adhesion among other factors is strongly influenced byp hysico-chemicalfactors.
The accurate quantification of the sephysico-chemical factors inadhesion is however limited bythe available measuring techniques.We evaluated surface charge,interfacial rheology and tensiometry(interfacialtension) as novel approaches to quantify these interactions and evaluated the irbiological significance via an adhesion assay using intestinal epithelial surface molecules(IESM)for a set of model organisms present in the human gastrointestinal tract.
StrainpairsofLactobacillusplantarumWCFS1withitssortase knockoutmutant Lb.plantarumNZ7114 andLb.rhamnosusGGwithLb.rhamnosusDSM20021T were usedwithEnterococcusfaecalisJH2-2ascontrolorganism.
Intra-speciescomparison revealedsignificantlyhigherabilitiesforLb.plantarumWCSF1andLb.rhamnosusGGvs. Lb.plantarumNZ7114 andLb.rhamnosusDSM20021T todynamicallyincreaseinterfacial elasticity(10−2 vs.10−3 Pa*m)andreduceinterfacialtension(32vs.38mN/m).This furthercorrelated forLb.plantarumWCSF1andLb.rhamnosusGGvs.Lb.plantarum NZ7114andLb.rhamnosusDSM20021T withthedecreaseofrelative hydrophobicity(80– 85%vs.57–63%),Zetapotential(-2.9to-4.5mVvs.-8.0to-13.8mV)andhigherrelative adhesioncapacitytoIESM(3.0–5.0vs1.5–2.2).HighestadhesiontotheIESMcollagen IandfibronectinwasfoundforLb.plantarumWCFS1(5.0)andE.faecalisJH2-2(4.2) whereasLb.rhamnosusGGshowedhighestadhesiontotypeIImucus(3.8). Significantly reducedadhesion(2fold)tothetestedIESMwasobservedforLb.plantarumNZ7114and Lb.rhamnosusDSM20021T correspondingwithlowerrelativehydrophobicity,Zetapotentialandabilitiestomodifyinterfacialelasticityandtension.Conclusively,theuseofZeta potential,interfacialelasticityandinterfacialtensionareproposedassuitablenoveldescriptiveandpredictiveparameterstostudytheinteractionsofintestinalmicrobeswiththeir hosts.
Fig1. Schematicoverviewofbacterialadsorptionatinterfaces.Bacteriaareattracteddifferentiallytohydrophobicinterfacesdependingontheircharge densityandsurfacehydrophobicity.Thesephysico-chemicalcharacteristicscanbequantifiedatanoil-waterinterfaceasrepresentedintheupperpartofthe picture.Usingrheologyinterfacialelasticitycanbequantifiedasreadoutforbacterialadsorptionandnetworkdevelopmentattheinterfacesincethey increaseinterfacialelasticity.Tensiometryquantifiesbacterialadsorptionthroughitsdisruptiveeffectontheinterfacialtensionattheoil—waterinterface.The Zetapotential,ameasureofionstrengtcanbeusedasareadouttforsurfacechargeofbacteriainanaqueoussolutionandtherebygiveaquantificationofits expectedhydrophobicity.Thebiologicalsignificanceofthesevaluescanbevalidatedinvitrothroughadhesiontospecificsurfacemoleculessumarisedin thelowerpartofthepictureunderbioassays.Therebythebacteriaareappliedonacoatedsurfacesorcelllinebasedmodelstoestimatetheiradheion potentialtodifferentsurfacemoleculesrepresentedbysymmetricshapesonthelowerpartofthefigure.
Schematic overview of bacterial adsorption at interfaces. Bacteria are... | Download Scientific Diagram
https://www.researchgate.net/figure/Schematic-overview-of-bacterial-adsorption-at-interfaces-Bacteria-are-attracted_fig1_281634151
Most stains are BASIC, meaning they are positively charged. Therefore, they stick to cells, which are always negatively charged.
http://pbstatemicrobiology.pbworks.com/w/page/107813090/Simple%20stains
Drug-Loaded Polymeric Nanoparticles for Cancer Stem Cell Targeting
Binbin Li1,2†, Qinghua Li3†, Jingxin Mo4,5* and Honglian Dai1,2*
1State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, China
2Biomedical Materials and Engineering Research Center of Hubei Province, Wuhan, China
3Department of Neurology, Affiliated Hospital of Guilin Medical University, Guilin, China
4Key Laboratory for Stem Cells and Tissue Engineering (Sun Yat-sen University), Ministry of Education, Guangzhou, China
5Department of Histology and Embryology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
Cancer stem cells (CSCs) have been reported to play critical roles in tumor initiation, propagation, and regeneration of cancer. Nano-size vehicles are employed to deliver drugs to target the CSCs for cancer therapy. Polymeric nanoparticles have been considered as the most efficient vehicles for drug delivery due to their excellent pharmacokinetic properties. The CSCs specific antibodies or ligands can be conjugated onto the surface or interior of nanoparticles to successfully target and finally eliminate CSCs. In this review, we focus on the approaches of polymeric nanoparticles design for loading drug, and their potential application for CSCs targeting in cancer therapy.Interfering the CSCs Differentiation
Although the anticancer drugs for ideal targeting have being looked for decades, only few are successfully applied in clinical trial. One of them is all-trans retinoic acid (ATRA), Warrell et al. (1993) found that the differentiation of leukemic promyelocytes were blocked and failed to differentiate into mature granulocytes after treating the patients with ATRA. The rational theory of ATRA therapy is ATRA could drive the CSCs to differentiate from stem cell into non-CSCs (e.g., epithelial cells). This case indicated the therapy of interfering CSCs differentiation may be an effective way to treat other forms of cancers. Other agents such as phorbol myristate acetate (Carey et al., 1996), hexamethylamine bisacetamide (Wu et al., 1991), dimethylsulfoxide (Arcangeli et al., 1993) and vitamin D3 (Olsson et al., 1984) also were reported to have the similar function in CSCs differentiation therapy.
FIGURE 3. The usual surface modifications of polymeric nanoparticles. The usual modification includes surface charge modification, bioactive peptides graft, amphipathic compound graft, and siRNA, etc.
Surface modification of polymeric nanoparticles has been proved to be useful in offering multi-functionality, and cannot only easily optimize the nanoparticles distribution in cells and tissues, but also promote cellular uptake or reduce cellular interactions. The modified polymeric nanoparticles can accumulate the loaded-drugs within the tumor tissues accurately and avoid damaging the nearby healthy tissues.Particle Size
One of the most widely utilized mechanisms of drug delivery system for targeting is based on particle size. The enhanced EPR effect allows nanoparticles less than 500 nm to penetrate tumor vasculature and gather in the regions of tumor (Petros and Desimone, 2010). The larger nanoparticles would be prevented getting there because they are trapped in the lungs or removed out the body by the reticuloendothelial system (RES). The nanoparticles are usually modified to obtain neutral surface charge by coating hydrophilic molecules such as PEG to eliminate particle aggregation and avoid RES for the EPR effect (Torchilin, 2011).
Chitosan
Chitosan, derived from chitin, is a positive biocompatible and biodegradable natural polysaccharide. It is widely used in the field of biomedicine including drug and gene delivery, tissue engineering, wound healing, and antimicrobial (Şenel and Mcclure, 2004; Riva et al., 2011). The chitosan nanoparticles were developed for targeting the overexpressed CD44 receptors on CSLCs. The nanoparticles around 20 nm were delivered into the tumor to actively target and be internalized in CD44+ CSLCs by EPR effect. The drugs in nanoparticles then would release out and diffuse into the cytosol, eventually accumulate in the nuclei to produce cytotoxicity to kill CSLCs eventually (Chauhan et al., 2012; Sykes et al., 2014). To lower the toxicity of platinate, Nascimento et al. (2015) developed the chitosan-based polymeric nanoparticles to encapsulate hydrophobic drugs by grafting PEG and peptides. Their study reported that the modified platinate encapsulated into chitosan-based nanoparticles can obviously decreased the toxicity in cells. The chitosan also can be used as cell recognition. A doxorubicin-encapsulated polymeric nanoparticles decorated with chitosan on the surface were developed to specifically target the CD44 receptors of the cancer treatment. This nanoparticle system released the doxorubicin in acidic environments localized in the cellular endosomes/lysosomes to achieve the goal of killing the CSCs (Rao et al., 2015).
In summary, a large number of polymeric nanoparticles with different physicochemical characteristics for multiple drugs loading have been developed to control the drugs release via responding internal or external stimuli to significantly improve therapeutic efficacy by CSCs targeting.
http://journal.frontiersin.org/article/10.3389/fphar.2017.00051
Structure of the Neutrophil-activating Protein from Helicobacter pylori
Article in Journal of Molecular Biology 323(1):125-130 · November 2002
Giuseppe Zanotti
University of Padova
Elena Papinutto
+ 4
William G Dundon
34.61International Atomic Energy Agency (IAEA)
Roberto Battistutta
36.68University of Padova
Helicobacter pylori is a major human pathogen associated with severe gastroduodenal diseases, including ulcers and cancers. An H. pylori protein that is highly immunogenic in humans and mice has been identified recently. This protein has been termed HP-NAP, due to its ability of activating neutrophils. In order to achieve a molecular understanding of its unique immunogenic and pro-inflammatory properties, we have determined its three-dimensional structure. Its quaternary structure is similar to that of the dodecameric bacterial ferritins (Dps-like family), but it has a different surface potential charge distribution. This is due to the presence of a large number of positively charged residues, which could well account for its unique ability in activating human leukocytes.Structure of the Neutrophil-activating Protein from Helicobacter pylori | Request PDF
https://www.researchgate.net/publication/222517826_Structure_of_the_Neutrophil-activating_Protein_from_Helicobacter_pylori
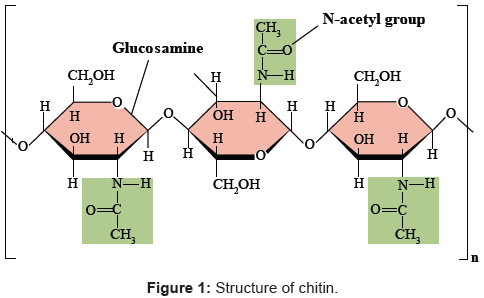
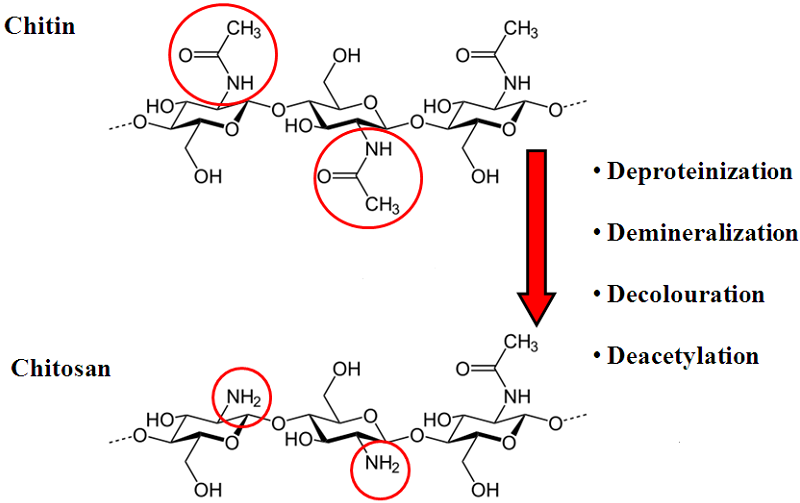
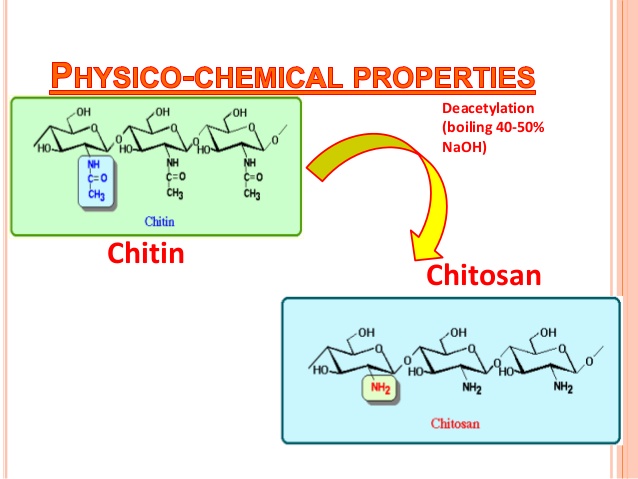
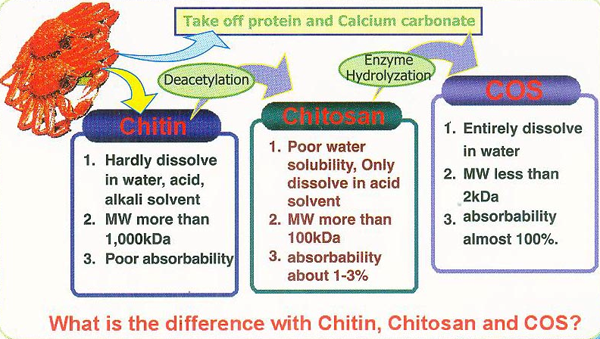

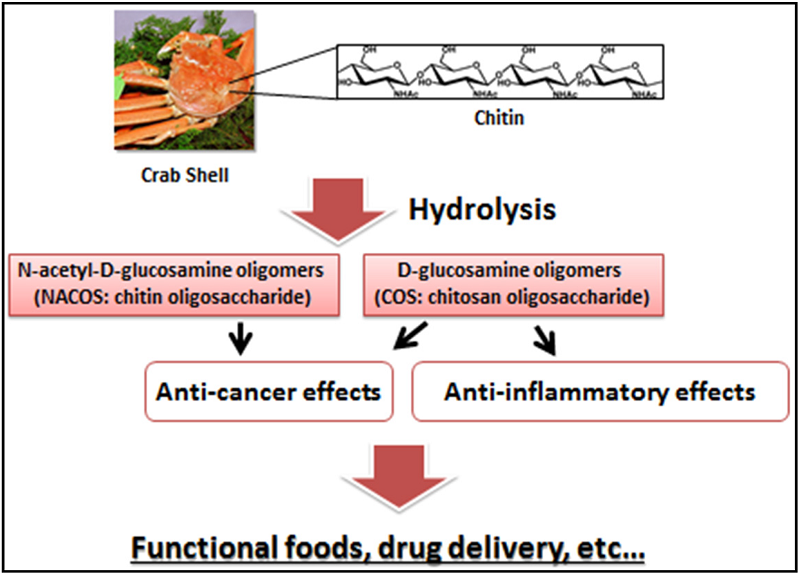
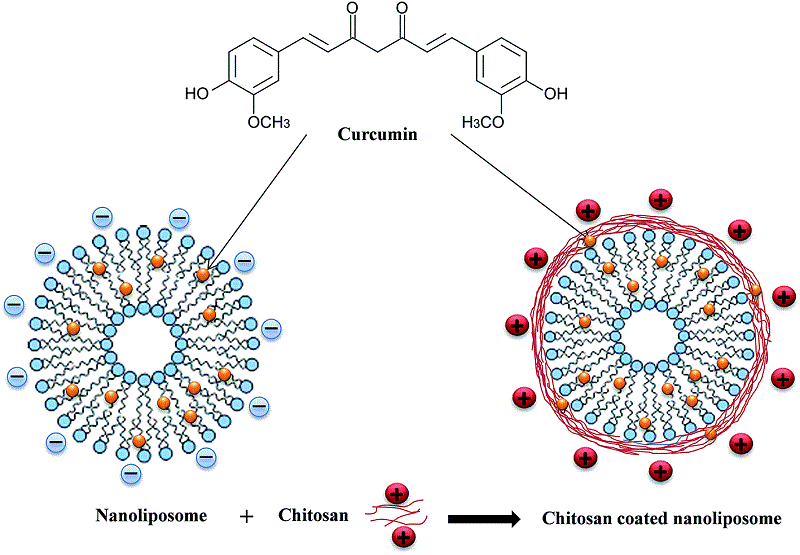
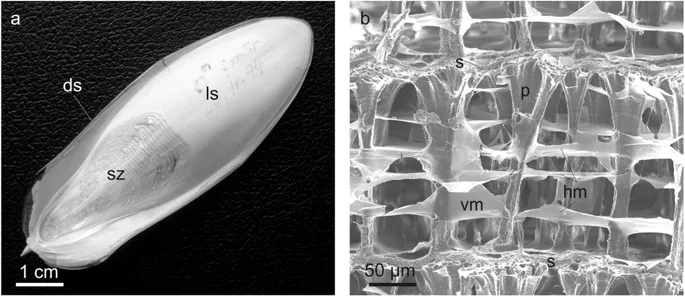
.png)
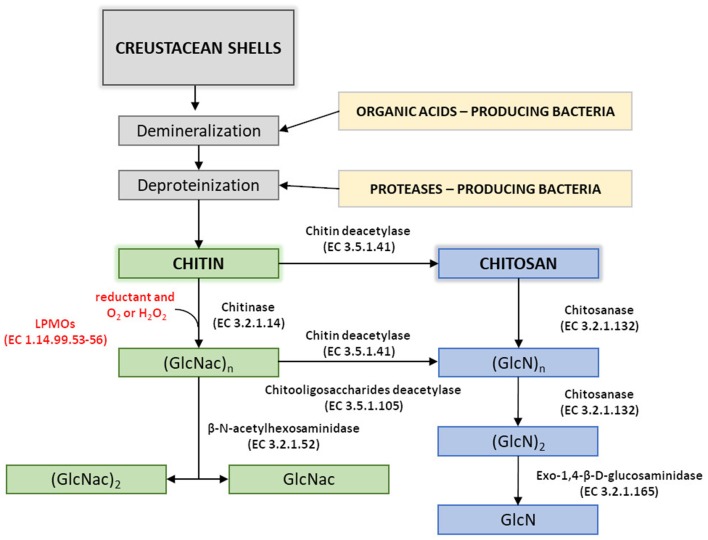
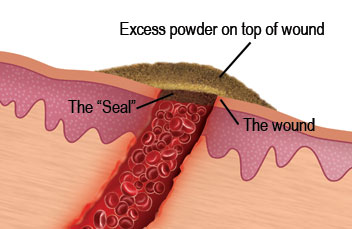
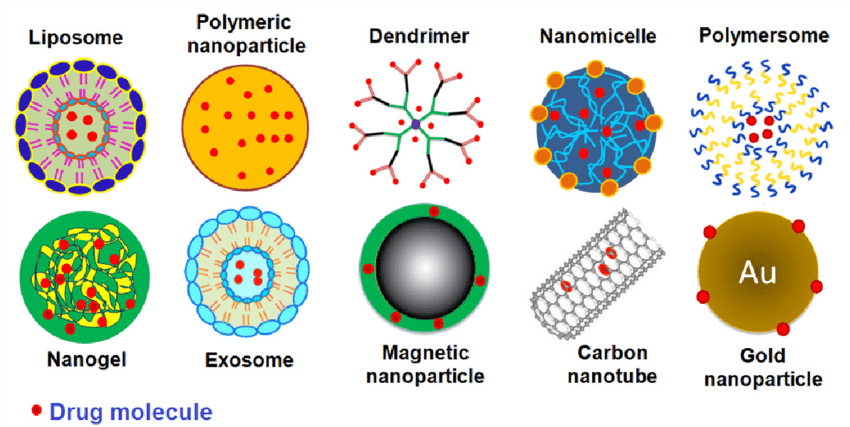

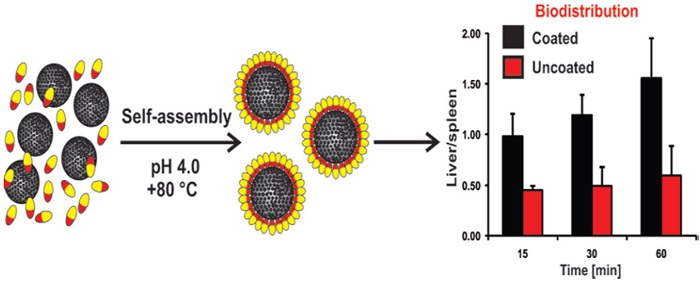

.jpg)