贫血与心脏病 Anemia and Heart Disease
慢性疾病性贫血-Anemia of inflammation/infection/chronic diseases
iron deficiency
inflammation-IL6-Hepcidin-reduction of iron release (by macrophages) and iron uptake

http://www.medscape.org/viewarticle/727476_transcript
.png)
.png)
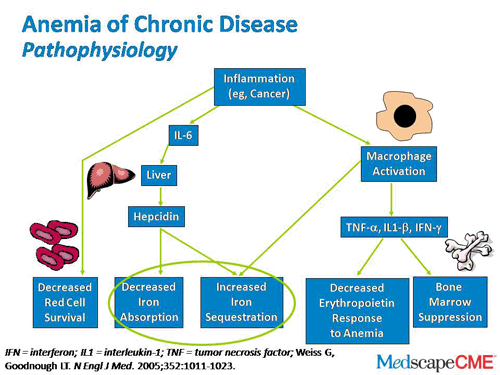
炎症会增加IL-6的产生。因此,铁调素的增加会阻止巨噬细胞铁的释放以及铁的肠道吸收,从而导致低铁血症。缩写:TF,转铁蛋白;铁,铁DMT1,二价金属转运蛋白1。
Inflammation increases interleukin-6 production. The consequent increase in hepcidin blocks macrophage iron release as well as the intestinal absorption of iron, resulting in hypoferremia. Abbreviations: TF, transferrin; Fe, iron; DMT1, divalent metal transporter 1.
Inflammation increases interleukin-6 production. The consequent... | Download
Scientific Diagram
https://www.researchgate.net/figure/Inflammation-increases-interleukin-6-production-The-consequent-increase-in-hepcidin_fig1_236186370
斯蒂芬·辛纳屈(Stephen T.Sinatra),医学博士,F.A.C.C.,F.A.C.N.,C.N.S.,C.B.T.
令人惊奇的是,看似与心脏无关的疾病实际上如何影响心脏健康。它显示了每个身体部位与系统之间的真正联系。
贫血就是一个很好的例子。当您没有足够的红血球或红血球没有足够的血红蛋白(一种使血液呈红色的富含铁的蛋白质)时,就会发生贫血。血红蛋白可帮助红细胞将氧气从肺部运送到其余器官和组织。
贫血症状
想知道您是否贫血吗?症状可能会有所不同。最常见的是虚弱,疲劳和/或疲倦。贫血的其他症状可能包括呼吸急促,头晕,头痛,心跳加快,易怒和精神错乱。
最有趣和最不寻常的贫血症状之一称为异食癖。这是渴望和吃没有营养价值的不寻常物质的习惯,例如冰,污垢,粘土,甚至纸张或纸板!我永远不会忘记和我一起上医学院、喜欢吃冰块的女生。我的意思是,她严重沉迷于冰!后来我们发现她很贫血。
什么原因导致贫血症状?
贫血发作有多种原因。最常见的原因是铁缺乏症。当人体缺乏产生血红蛋白所需的铁时,贫血症状通常会迅速发作。这通常发生在您的饮食中铁摄入不足或失血的情况下。月经过多的妇女以及患有溃疡、胃炎、结肠息肉、肠道疾病或任何其他引发出血的疾病的人都有铁缺乏的风险。贫血在孕妇中也很常见,因为女性的身体需要补充更多的血液以支持胎儿的成长。
叶酸或维生素B12缺乏症是另一种形式的贫血,巨幼细胞性贫血。这些维生素的含量不足会导致非典型的巨大红细胞,其寿命比正常红细胞短。
其他引起贫血的原因包括药物治疗,例如化学疗法和放射疗法,以及某些药物的使用,例如抗生素,癫痫发作药物,免疫抑制剂和抗凝血药。
慢性疾病性贫血
作为心脏病专家,最引起我关注的贫血是所谓的“慢性疾病性贫血”。有这种贫血的人,人体无法有效地回收血细胞中的铁(或者细胞不能有效摄取储存的铁)。人体也停止对促红细胞生成素(EPO)做出适当的反应(或肾脏不能产生足够的促红细胞生成素),促红细胞生成素是肾脏产生的一种激素,可促进红细胞的产生。
具有某些涉及炎症的长期医疗条件的人,包括某些癌症和自身免疫性疾病,肝炎,肾脏疾病和充血性心力衰竭(CHF),通常会贫血。实际上,一项对34项研究的荟萃分析和超过153,000例CHF患者发现其中37.2%患有贫血。经过六个月的随访,贫血患者中有46.8%死亡,而无贫血的则为29.5%。
贫血还会导致流向肾脏的血液减少,从而导致体液滞留,从而进一步给心脏带来压力。此外,慢性贫血还可能导致左心室肥大,左心室(心脏的主要泵腔)壁增大和增厚。这会加剧充血性心力衰竭,并建立研究人员所谓的恶性循环“其中充血性心力衰竭引起贫血,而贫血导致更多的充血性心力衰竭,并且都损害肾脏,进一步加剧贫血和充血性心力衰竭。”
所以,是的,我担心心力衰竭,心脏病发作和其他心脏病患者的贫血。氧气输送量减少导致心输出量增加。简而言之,这意味着心脏必须付出更多,更大的努力才能完成工作。不仅将近一半的因心脏病发作入院的患者患有贫血,而且贫血患者的心脏病发作风险也更高。
如何治疗贫血
如果您患有贫血症状,请去看医生。他/她可以进行适当的测试,检查潜在的健康问题,并制定正确的治疗方案。
通过这些心脏健康测试保护自己
您应该期待什么?在大多数情况下,贫血治疗需要补充铁(和/或叶酸或B12,如有需要)。如果引起贫血的根本原因是失血(除了月经),那么出血的来源也应该找到并停止。通常,这就是解决问题的全部方法。 但是,对于慢性病贫血,可能需要更广泛的治疗。一些研究表明,在严重的情况下,输血,静脉输注铁和注射促红细胞生成素可能非常有帮助。 一个警告……重要的是要记住,铁可能是一种促氧化剂,这意味着如果使用不当,它会导致体内更大的氧化应激。因此,唯一应该补充铁的人当然是月经期和孕妇。补充铁通常对儿童来说是安全的,但请事先与您的孩子的儿科医生讨论剂量。男性,绝经后的女性以及所有其他人只能在医生的照料和监督下服用铁剂。
Anemia and the Heart
By Stephen T. Sinatra, M.D., F.A.C.C., F.A.C.N., C.N.S., C.B.T.
It’s truly amazing how conditions that seemingly have nothing to do with the
heart actually do impact heart health. It shows just how interconnected every
single body part and system really is.
Anemia is a perfect example. Anemia is a condition that occurs when you don’t
have enough red blood cells, or when your red blood cells don’t contain enough
hemoglobin—the iron-rich protein that gives blood its red color. Hemoglobin
helps red blood cells carry oxygen from the lungs to the rest of your organs and
tissues.
Anemia Symptoms
Wondering if you’re anemic? Symptoms can vary. The most common is weakness,
fatigue, and/or tiredness. Other symptoms of anemia may include shortness of
breath, dizziness, headache, rapid heartbeat, irritability, and mental
confusion.
One of the most interesting and unusual anemia symptoms is called pica. This is
the habit of craving and eating unusual substances that have no nutritional
value, such as ice, dirt, clay, even paper or cardboard! I’ll never forget a
woman I went to medical school with who was a chronic ice chewer. I mean, she
was seriously addicted to ice! We later found out she was anemic.
What Causes Anemic Symptoms?
Anemia strikes for a variety of reasons. The most common cause is iron
deficiency. When the body lacks the iron needed to produce hemoglobin, anemia
symptoms often kick in promptly. This usually occurs when you don’t get enough
iron in the diet or, much more commonly, with blood loss. Women who have heavy
menstrual periods run the risk of iron deficiency, as well as anyone who suffers
from ulcers, gastritis, colon polyps, intestinal diseases, or any other
condition that triggers bleeding. Anemia is also common in pregnancy, as a
woman’s body needs to make additional blood to support a growing fetus.
Another form of anemia, megaloblastic anemia, results from folate or vitamin B12
deficiencies. Inadequate levels of these vitamins cause atypically large red
blood cells that have a shorter-than-normal lifespan.
Other anemia causes include medical treatments such as chemotherapy and
radiation, as well as the use of certain pharmaceuticals like antibiotics,
seizure medications, immunosuppressants, and anticlotting drugs.
Anemia of Chronic Disease
The anemia cause that concerns me most as a cardiologist is what’s called
“anemia of chronic disease.” With this form of anemia, the body does not
efficiently recycle iron from blood cells, so they do not live as long as they
should. The body also stops responding properly to erythropoietin, a hormone
produced by the kidneys that’s responsible for boosting red blood cell
production.
People with certain long-term medical conditions that involve inflammation,
including some cancers and autoimmune disorders, hepatitis, kidney disease, and
congestive heart failure (CHF) often become anemic. In fact, a meta-analysis of
34 studies and more than 153,000 CHF patients found that 37.2 percent of them
had anemia. After a six-month follow-up, 46.8 percent of the anemic patients
died compared with 29.5 percent without anemia.
Anemia also causes reduced blood flow to the kidneys, which leads to fluid
retention, which places even further stress on the heart. Additionally, chronic
anemia can result in left ventricular hypertrophy, the enlargement and
thickening of the walls of the left ventricle—the heart’s main pumping chamber.
This can worsen congestive heart failure and set up what researchers call a
vicious cycle “wherein CHF causes anemia, and the anemia causes more CHF, and
both damage the kidneys, worsening the anemia and the CHF further.”
So, yeah, I get concerned about anemia in patients with heart failure…and heart
attack and other heart diseases. Decreased oxygen delivery leads to increased
cardiac output. Simply put, this means the heart has to work much, much harder
to do its job. Not only do nearly half of patients hospitalized for heart attack
have anemia, people with anemia have a much higher risk of having a heart
attack.
How to Treat Anemia
If you’re suffering from symptoms of anemia, see your doctor. He/she can run the
appropriate tests, check for underlying health problems, and prescribe the right
course of treatment.
Protect Yourself With These Heart Health Tests
What should you expect? In most cases, anemia treatment involves supplementing
with iron (and/or folate or B12, if needed). If the underlying cause of anemia
is blood loss (other than menstruation), then the source of the bleeding should
also be located and stopped. Usually, that’s all it takes to solve the problem.
With anemia of chronic disease, though, more extensive treatment may be needed.
Some research has shown that, in severe cases, blood transfusions, intravenous
delivery of iron, and injections of erythropoietin can be very helpful.
One caveat…It’s important to keep in mind that iron can be a pro-oxidant,
meaning it can lead to greater oxidative stress in the body if not used
correctly. For this reason, the only people who should supplement with iron as a
matter of course are menstruating and pregnant women. Iron supplementation is
usually safe for children, but discuss dosing with your child’s pediatrician
beforehand. Men, postmenopausal women, and all others should only take iron
under the care and supervision of their doctors.
References:
Khan Y and Tisman G. Pica in iron deficiency: a case series. J Med Case Reports.
2010;4:86.
Groenveld HF, Januzzi JL, et al. Anemia and mortality in heart failure patients:
a systematic review and meta-analysis. J Am Coll Cardiol. 2008 Sep
2;52(10):818-27.
Silverberg DS, Wexler D, Iaina A. The role of anemia in the progression of
congestive heart failure. Is there a place for erythropoietin and intravenous
iron? J Nephrol. 2004 Nov-Dec;17(6):749-61.
Anemia and the Heart | Dr. Sinatra's HeartMD Institute
https://heartmdinstitute.com/health-and-wellness/anemia-and-the-heart/
慢性疾病性贫血的原因
一般讨论
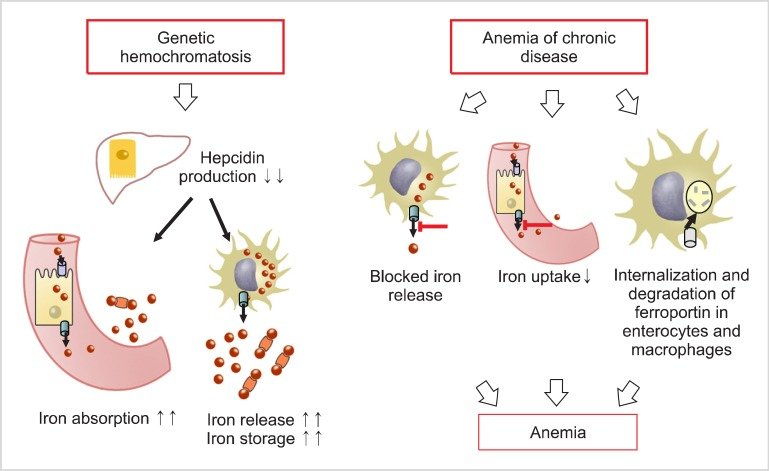
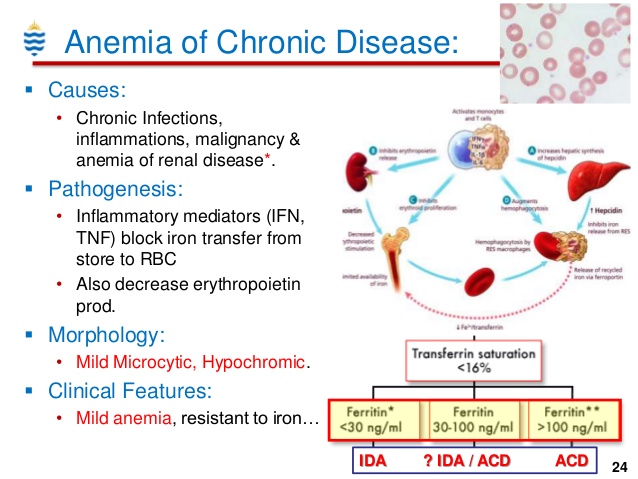
慢性疾病的贫血,也称为炎症性贫血,是一种可与许多不同的潜在疾病相关的疾病,包括慢性疾病,如癌症,某些感染以及自身免疫性疾病和炎症性疾病,如类风湿性关节炎或狼疮。贫血的特征是循环红细胞或血红蛋白(红细胞中携带氧气的部分)水平低。慢性疾病的贫血通常是轻度或中度疾病。在轻度情况下,贫血可能与任何症状无关,或可能导致疲劳,皮肤苍白(苍白)和头昏眼花。导致慢性疾病性贫血的潜在机制很复杂,尚未完全了解。
体征和症状
慢性病贫血的严重程度因人而异。在大多数情况下,贫血通常是轻度或中度的。受影响的个体可能会出现多种症状,例如疲劳,皮肤苍白(苍白),头晕,呼吸急促,心跳加快,易怒,胸痛和其他发现。这些症状可能发生在任何程度的贫血患者中。在大多数情况下,与潜在疾病相关的症状通常优先于轻度或中度贫血症状。在极少数情况下,慢性病贫血可能很严重,并且会导致更严重的并发症。
慢性疾病中贫血的确切原因可能有所不同。通常,多个进程同时发生。贫血可以由正常红细胞存活时间略微缩短引起。此外,可能会损害红细胞(促红细胞生成)或促红细胞生成素(刺激红细胞生成的激素)的产生。红血球将氧气带到身体。慢性疾病贫血的确切原因可能取决于潜在的状况。例如,癌细胞可能分泌某些损害或破坏未成熟红细胞的物质。在某些情况下,癌细胞或传染病可能会渗入骨髓,骨髓是在形成血细胞的长骨头中发现的柔软的海绵状物质。
研究人员还了解到,患有慢性疾病的贫血患者体内铁的分布也存在失衡,因此尽管组织中铁的含量充足或升高,也无法有效地利用铁来产生新的血细胞。铁是在人体所有细胞中发现的重要矿物质,对人体正常运作和正常生长至关重要。铁被发现有许多类型的食物,包括红肉,家禽,鸡蛋和蔬菜。铁水平必须在体内保持在特定范围内,否则会引起贫血(由于功能性铁水平低)或损害受影响的器官(由于某些组织中异常高的铁水平)。
需要铁来产生血红蛋白,血红蛋白是携带氧气的红血球的一部分。慢性疾病贫血的一个关键发现是某些细胞内铁的摄取和保留增加,这导致可用于产生血红蛋白的功能性铁减少。功能性铁的缺乏阻碍了血红蛋白的发展,进而降低了整个身体输送的氧气量(贫血)。
研究人员认为,免疫系统在患有慢性疾病的个体中始终保持活跃,会产生影响铁在体内的发展,存储和运输的物质。免疫系统中的细胞产生细胞因子,即刺激或抑制其他免疫系统细胞功能的特殊蛋白质。
铁调素(Hepcidin),一种在肝脏中产生的激素,有助于调节体内铁的代谢和运输,在慢性疾病性贫血的发展中起着重要作用。研究人员认为,在大多数情况下,称为白细胞介素6(IL-6)的特定细胞因子会刺激铁调素(hepcidin)的产生,尽管铁调素也可以通过不涉及IL-6的途径对炎症做出反应而产生。过量的铁调素会导致过多的铁滞留在细胞内,从而降低可用于产生血红蛋白的铁量,从而导致贫血。大多数研究人员认为,铁调素是影响慢性疾病性贫血发展的关键因素。
Causes of Anemia in Chronic Diseases
General Discussion
Anemia of chronic disease, also called the anemia of inflammation, is a
condition that can be associated with many different underlying disorders
including chronic illnesses such as cancer, certain infections, and autoimmune
and inflammatory diseases such as rheumatoid arthritis or lupus. Anemia is
characterized by low levels of circulating red blood cells or hemoglobin, the
part of red blood cells that carries oxygen. Anemia of chronic disease is
usually a mild or moderate condition. In mild cases, anemia may not be
associated with any symptoms or may cause fatigue, paleness of the skin (pallor)
and lightheadedness. The underlying mechanisms that cause anemia of chronic
disease are complex and not fully understood.
Signs & Symptoms
Anemia of chronic disease varies in severity from one person to another. In most
cases, anemia is usually mild or moderate. Affected individuals may develop a
variety of symptoms such as fatigue, paleness of the skin (pallor),
lightheadedness, shortness of breath, a fast heartbeat, irritability, chest pain
and additional findings. These symptoms may occur in any individual who has a
comparable degree of anemia. In most cases, the symptoms associated with the
underlying disease usually take precedent over the mild or moderate anemia
symptoms. In rare cases, anemia of chronic disease can be severe and cause more
serious complications.
The exact cause of anemia of chronic disease may vary. Usually several processes
are occurring concurrently. Anemia can be caused by a slight shortening of
normal red blood cell survival. In addition, the production of red blood cells
(erythropoiesis) or erythropoietin (a hormone that stimulates red blood cell
production) may be impaired. Red blood cells carry oxygen to the body. The exact
cause of anemia of chronic disease may depend upon the underlying condition. For
example, cancer cells may secrete certain substances that damage or destroy
immature red blood cells. In some cases, cancer cells or infectious disease may
infiltrate the bone marrow, the soft spongy material found in long bones where
blood cells are formed.
Researchers have also learned that individuals with anemia of chronic disease
also have an imbalance in the distribution of iron in the body and as a result
cannot effectively use iron to create new blood cells despite having sufficient
or elevated levels of iron stored in the tissues. Iron is a critical mineral
that is found in all cells of the body and is essential for the body to function
and grow properly. Iron is found many types of food including red meat, poultry,
eggs and vegetables. Iron levels must remain in a specific range within the
body, otherwise they can cause anemia (due to low functional iron levels) or
damage to affected organs (due to abnormally high iron levels in certain
tissues).
Iron is needed to produce hemoglobin, the part of a red blood cell that carries
oxygen. A key finding in anemia of chronic disease is increased uptake and
retention of iron within certain cells, which leads to reduced amounts of
functional iron that is available for the production of hemoglobin. The lack of
functional iron hinders the development of hemoglobin, which, in turn, reduces
the amount of oxygen delivered throughout the body (anemia).
Researchers believe that the immune system, which remains constantly active in
individuals with chronic diseases, produces substances that influence the
development, storage and transport of iron within the body. Cells in the immune
system produce cytokines, specialized proteins that stimulate or inhibit the
function of other immune system cells.
Hepcidin, a hormone produced in the liver that helps regulate the metabolism and
transport of iron within the body, plays a significant role in the development
of anemia of chronic disease. Researchers believe a specific cytokine known as
interleukin-6 (IL-6) stimulates the production of hepcidin in most cases,
although hepcidin can also be produced in response to inflammation by pathways
that do not involve IL-6. Excess hepcidin causes too much iron to be trapped
within cells, lowering the amount of iron available to produce hemoglobin,
thereby resulting in anemia. Most researchers believe that hepcidin is a key
factor influencing the development of anemia of chronic disease.
Anemia of Chronic Disease - NORD (National Organization for Rare Disorders)
https://rarediseases.org/rare-diseases/anemia-of-chronic-disease/
心脏病专家:贫血导致和加重心脏病 增加死亡风险
斯蒂芬·辛纳屈(Stephen T.Sinatra)博士是美国著名的心脏病专家。
令人惊奇的是,看似与心脏无关的疾病实际上如何影响心脏健康。它显示了每个身体部位与系统之间的真正联系。
贫血就是一个很好的例子。当您没有足够的红血球或红血球没有足够的血红蛋白(一种使血液呈红色的富含铁的蛋白质)时,就会发生贫血。血红蛋白可帮助红细胞将氧气从肺部运送到其余器官和组织。
实际上,一项对34项研究的荟萃分析和超过153,000例充血性心力衰竭患者发现其中37.2%患有贫血。经过六个月的随访,贫血患者中有46.8%死亡,而无贫血的则为29.5%。
贫血是红细胞氧气输送量减少导致心输出量增加。简而言之,这意味着心脏必须付出更多,更大的努力才能完成工作。不仅将近一半的因心脏病发作入院的患者患有贫血,而且贫血患者的心脏病发作风险也更高。
缺铁性贫血是最常见贫血原因。此外,贫血也见于很多的慢性疾病中,如癌症,某些感染以及自身免疫性疾病和炎症性疾病,如类风湿性关节炎或狼疮。
研究发现,炎症会缩短红细胞的寿命,同时增加炎症因子IL-6的产生,刺激肝脏释放调控铁代谢的关键激素铁调素。铁调素的增加会阻止巨噬细胞铁的释放以及铁的肠道吸收,从而导致低铁血症和贫血。
Hepcidin expression is determined through transcriptional regulation by systemic
iron status. However, acute or chronic inflammation also increases the
expression of hepcidin, which is associated with the dysregulation of iron
metabolism in pathological conditions.
Hepcidin - an overview | ScienceDirect Topics
www.sciencedirect.com/topics/medicine-and-dentistry/hepcidin
Anemia and the Heart | Dr. Sinatra's HeartMD Institute
https://heartmdinstitute.com/health-and-wellness/anemia-and-the-heart/
Hepcidin and iron homeostasis - ScienceDirect
https://www.sciencedirect.com/science/article/pii/S016748891200016X
Hepcidin has a central role in maintenance of iron homeostasis. Hepcidin
synthesis is regulated at the transcriptional level by multiple stimuli.
Intracellular and extracellular iron concentrations increase hepcidin
transcription, as does inflammation, whereas increased erythropoietic activity
suppresses hepcidin production.
病毒感染和铁代谢
Viral infection and iron metabolism
哈尔·德拉克史密斯&安德鲁·普伦蒂斯
自然评论微生物学第6卷,第541–552页(2008年)
关键点
简要总结了铁在细胞生理学基本过程中的核心作用。这些过程必须有效地进行病毒复制,因此富含铁的细胞成为病毒的良好家园。
概述了人类体内的铁稳态,并描述了肝素铁调素的作用。铁调素维持铁平衡,其合成受许多蛋白质调节,其中一种是HFE。
铁超载是丙型肝炎病毒(HCV)感染导致严重疾病的危险因素。 HCV本身可操纵细胞中铁的转运并影响铁调素的合成。
在感染了HIV-1的个体中,铁积累与死亡率增加有关。铁在巨噬细胞中的积累可能有利于病毒复制,有益于继发性病原体并导致贫血。
HIV-1蛋白Nef和人类巨细胞病毒(HCMV)蛋白US2靶向HFE,因此调节铁的转运。
新大陆出血性沙状病毒(Arenavirus),犬和猫细小病毒和小鼠乳腺肿瘤病毒均使用宿主蛋白转铁蛋白受体1进入细胞。通过这种方式,这些病毒感染了激活的铁获取细胞,可以促进它们的复制。
铁螯合剂将铁的可用性限制在受感染的细胞内,从而抑制了HIV-1,HCMV,牛痘病毒,单纯疱疹病毒1和乙型肝炎病毒的体外生长。在感染了HCV的患者中,除铁可改善疾病。
总之,这些研究表明,病毒可直接操纵铁体内平衡,并且病毒诱导的铁运输变化与疾病状态的改变有关。
抽象
基本的细胞操作,包括DNA合成和ATP的产生,都需要铁。病毒劫持细胞以进行复制,而有效的复制则需要一个铁充足的主机。一些病毒通过在细胞进入过程中与转铁蛋白受体1结合而选择性感染感染铁的细胞。其他病毒会改变涉及铁稳态的蛋白质(例如HFE和铁调素)的表达。在HIV-1和丙型肝炎病毒感染中,铁超载与预后不良有关,可能部分由病毒本身引起。了解铁代谢与病毒感染之间如何相互作用可能会提出控制疾病的新方法。
Viral infection and iron metabolism
Hal Drakesmith & Andrew Prentice
Nature Reviews Microbiology volume 6, pages541–552(2008)Cite this article
Key Points
The central role of iron in fundamental processes of cellular physiology is
briefly summarized. These processes must be operational for efficient viral
replication, and therefore cells that are replete in iron make good homes for
viruses.
Iron homeostasis in humans is outlined, and the action of the liver hormone
hepcidin is described. Hepcidin maintains iron balance, and its synthesis is
regulated by many proteins, one of which is HFE.
Iron overload is a risk factor for severe disease in hepatitis C virus (HCV)
infection. HCV itself manipulates cellular iron transport and influences
hepcidin synthesis.
In individuals infected with HIV-1, iron accumulation is associated with
increased mortality. Iron accumulation in macrophages might favour virus
replication, benefit secondary pathogens and lead to anaemia.
The HIV-1 protein Nef and the human cytomegalovirus (HCMV) protein US2 target
HFE and therefore regulate iron transport.
New World haemorrhagic arenaviruses, canine and feline parvoviruses and mouse
mammary tumour virus all use the host protein transferrin receptor 1 to gain
entry to cells. In this way, these viruses infect activated, iron-acquiring
cells, which can facilitate their replication.
Limiting iron availability to infected cells by iron chelators curbs the growth
of HIV-1, HCMV, vaccinia virus, herpes simplex virus 1 and hepatitis B virus in
vitro. In patients who are infected with HCV, iron removal ameliorates disease.
Together, these studies indicate that viruses directly manipulate iron
homeostasis and that virally induced changes in iron transport are associated
with altered disease states.
Abstract
Fundamental cellular operations, including DNA synthesis and the generation of
ATP, require iron. Viruses hijack cells in order to replicate, and efficient
replication needs an iron-replete host. Some viruses selectively infect
iron-acquiring cells by binding to transferrin receptor 1 during cell entry.
Other viruses alter the expression of proteins involved in iron homeostasis,
such as HFE and hepcidin. In HIV-1 and hepatitis C virus infections, iron
overload is associated with poor prognosis and could be partly caused by the
viruses themselves. Understanding how iron metabolism and viral infection
interact might suggest new methods to control disease.
Viral infection and iron metabolism | Nature Reviews Microbiology
https://www.nature.com/articles/nrmicro1930
Cited by: 827
Publish Year: 2012
Author: Tomas Ganz, Elizabeta Nemeth
Hepcidin: inflammation's iron curtain
H. McGrath, Jr, P. G. Rigby
Rheumatology, Volume 43, Issue 11, November 2004, Pages 1323–1325,
https://doi.org/10.1093/rheumatology/keh345
Published: 27 July 2004
Issue Section: Editorials
Rheumatologists and their patients are the beneficiaries of a recently
identified peptide, hepcidin (Table 1). Isolated from human urine and plasma in
the year 2000 [1, 2], hepcidin appears to be the long-sought iron-regulatory
hormone responsible for the anaemia of chronic disease [3, 4]. It is more than
that: it is an acute-phase reactant, responding to infection and inflammation
[5]; it is an antimicrobial peptide that disrupts microbial membranes [1, 6];
and it provides an iron-restricted internal milieu inhospitable to microbes [7,
8].
TABLE 1.
Dynamics of iron and hepcidin regulation
Afferent: blood → Liver → Efferent: gut
Stimulation or down-regulation Hepcidin production Action on iron absorption in
gut and immunity
1. Inflammation/infection ↑ ↓ intake of Fe by gut
↑ IL-6 Antibacterial in innate immunity
Sequestration of Fe in macrophages reduces serum Fe
2. Body Fe ↑ ↑ ↓ intake of Fe by gut
Haemochromatosis
Haemosiderosis
3. Body Fe ↓ ↓ ↑ intake of Fe by gut
Bleeding Egress of Fe out of macrophage
4. Anaemia, hypoxaemia ↓ ↑ intake of Fe by gut
5. Erythropoiesis ↑ ↓ ↑ intake of Fe by gut
Open in new tab
Hepcidin is a 25 amino acid, 2–3 kDa, cationic peptide that has broad
antibacterial and antifungal actions [1]. In concert with other antimicrobial
peptides, known as defensins and cathelicidins [9], it provides a first line of
defence at mucosal barriers [1, 2]. However, more germane for rheumatologists is
its control of iron kinetics. Produced by hepatocytes, hepcidin inhibits the
intestinal absorption [1, 10], macrophage release [3, 7] and placental passage
[10] of iron. Hepcidin mRNA moves with the body's iron levels, increasing as
they increase and decreasing as they decrease [11]. More pertinently, hepcidin
rises with infection or inflammation and falls with hypoxia or anaemia [12].
The anaemia of chronic disease has long confounded physicians. It is generally
normocytic and normochromic, but may be hypochromic or microcytic [13]. The low
serum iron and normal-to-low iron-binding capacity, in conjunction with a
high-to-normal serum ferritin level in patients with inflammatory disease, has
been perplexing. Also notable has been the shortened red blood cell survival and
blunted erythropoietin-induced production of red blood cells. At one time known
as the anaemia of infection, it became known, after man's entry into the age of
antibiotics, as the anaemia of chronic disease, and now, perhaps more aptly, it
is the anaemia of inflammation.
Iron can be toxic. It catalyses the generation of reactive free radicals [14]
and activates NF-κB, the prototypic transcription factor for genes involved in
inflammation [15]. At high levels, iron is damaging to tissues. Humans need
little dietary iron, 1–2 mg a day sufficing for the average adult male [16].
However, mammals lack a regulated pathway for iron excretion [12], so iron
absorption has to be tightly regulated. Hepcidin acts as a negative regulator of
iron absorption: USF2 knockout mice lacking hepcidin mRNA become iron-overloaded
[17]; transgenic mice with increased hepcidin expression die at birth with
severe iron deficiency [10]; humans with hepcidin-producing adenomas develop an
iron-refractory iron deficiency anaemia [4]; and gene mutations affecting
hepcidin cause haemochromatosis in humans [18] and in mouse models [17].
In animals and man, the anaemia of inflammation is due primarily to
hepcidin-induced sequestration of iron in the macrophage [18]. The link between
inflammation/infection and liver production of hepcidin is attributed to IL-6,
produced at sites of infection/inflammation [13]. Human hepatocytes increase
hepcidin mRNA in the presence of IL-6 or lipopolysaccharide and in the presence
of IL-6 produced by monocytes exposed to lipopolysaccharide [5]. Infection in
one human subject reportedly increased excretion of hepcidin in the urine
100-fold [5]. Mice respond to the inflammation generated by an injection of
turpentine with a six-fold increase in hepcidin mRNA and a two-fold decrease in
serum iron [10]. Remarkably, the white bass responds to infection with
Streptococcus iniae with a 4500-fold rise in hepcidin mRNA expression [19].
In addition to iron levels and inflammation/infection, there is another factor
that affects hepcidin levels: it is anaemia. Along with hypoxia, anaemia
overrides the effects of iron and inflammation/infection, reducing levels of
hepcidin mRNA [4, 12]. Were this not the case, inflammation, by maintaining high
hepcidin levels, would keep the haematocrit dropping. Instead, down-regulation
of hepcidin mRNA expression by anaemia produces a new steady state, usually with
haematocrits 3–5 points below normal.
In addition to disrupting bacterial membranes, hepcidin provides an inhospitable
internal milieu for microbes that successfully enter the bloodstream.
Micro-organisms need iron [14]. Bacteria require iron for the production of the
superoxide dismutase that protects them from host oxygen radicals [20, 21].
Hepcidin, by inducing macrophage sequestration of iron, robs bacteria of this
element. Blood and intracellular bacteria [22] may weaken; biofilms may not
develop [7]. Pertinent here is the recent report of an inverse relationship
between the incidence of tuberculosis and rheumatoid arthritis (RA) [23],
raising the possibility that that the inaccessibility of iron in RA protects
from tuberculosis [24].
Pallor, weakness and fatigue have been recognized as hallmarks of chronic
disease for millennia. Anaemia obviously contributes to the pallor. Less obvious
is whether the decrease in serum iron diminishes its availability to myoglobin
and the enzymes catalysing the redox reactions required for the generation of
energy (cytochromes) sufficiently to contribute to weakness and fatigue.
Defensins are antimicrobial peptides produced by cells of epithelial linings
[9]. Hepcidin, like defensins, is an antimicrobial peptide that kills on
contact. However, because it is produced by the liver, has not been found to
have chemotactic properties, and differs structurally from defensins [25], it
will likely be classified as an acute-phase reactant [5].
The identification of hepcidin opens the door to therapeutic approaches for
several disorders and to proscriptions regarding the use of iron. Recombinant
hepcidin may be the ideal therapeutic agent for those with some forms of
juvenile haemochromatosis and with the less severe but more common form of
haemochromatosis caused by mutations in the HFE gene [26]. Hepcidin-induced iron
deprivation may prove helpful in preventing the development of resistant
bacterial biofilms [10]. For the anaemia of inflammation, often resistant to
erythropoietin therapy [27], inhibitors of hepcidin, by releasing sequestered
iron, could restore haemoglobin levels and conceivably correct an iron lack in
myoglobin and cytochromes as well. Finally, of related interest is a recent
report that moderate alcohol intake reduces levels of C-reactive protein and
IL-6 [28], the principle chemokine for the generation of hepcidin mRNA,
extrapolating to a possible ameliorative role of alcohol in both inflammation
and the anaemia of inflammation.
As to proscriptions, iron supplements should be monitored, not only because the
resulting increase in hepcidin can fuel antimicrobial engines unnecessarily, but
because hepcidin increases macrophage iron sequestration in the synovium as
elsewhere. Synovial iron has the propensity to generate oxygen free radicals
that have been linked to the chronicity and erosiveness of joint disease in RA
[29]. In fact, intramuscular injections of iron have long ago been reported to
cause acute flares of joint inflammation in RA [30]. A broader phlogistic
potential of iron towards the joint comes from a recent report that iron
depletion by serial phlebotomies diminishes recurrences of gouty arthritis [31].
If one adds all of the above to the reported links of iron sufficiency to colon
cancer [32], diabetes mellitus [33], chronic hepatitis [34] and atherosclerosis
[35], it would seem best to phase out gratuitous iron supplementation
altogether.
The discovery of hepcidin provides a thread that ties together the perplexing
triad of decreased serum iron, increased macrophage iron and chronic
inflammation. In addition, it offers a unique opportunity for determining the
effects of iron on disease, the usefulness of hepcidin inhibitors or promoters
to control iron kinetics, and the proper means of iron administration. In the
aggregate, these will represent a step forward in the treatment of a variety of
diseases.
The authors have declared no conflicts of interest.
Hepcidin: inflammation's iron curtain | Rheumatology | Oxford Academic
https://academic.oup.com/rheumatology/article/43/11/1323/2389966