Methemoglobinemia is main clinical conditions of sodium nitrite intoxification.
Dozens of drugs that have oxidizing effects can cause methemoglobinemia.
Methylene blue is the first line treatment, but with risks to worsen the condition.
Vitamin C is an effective treatment.
Vitamin is a strong reducing agent, can convert ferric iron back to ferrous state.
Glucose, also as a reducing agent, is helpful.
vitamin c is the outstanding antioxidant in the plasma. UC Berkely, 1989
Methemoglobinemia高铁血红素血症 is a hemoglobinopathy caused by high levels of methemoglobin that result from oxidation of ferrrous (Fe2+) to the ferric state (Fe3+) in hemoglogin. Methemoglobin cannot carry oxygen, leading to tissue hypoxia.
Anesthetic experience of methemoglobinemia detected during general anesthesia for gastrectomy of advanced gastric cancer -A case report-
We present a 68-year-old female patient who underwent gastrectomy for advanced gastric cancer with bleeding.
...
Ascorbic acid 600 mg mixed in fluid was administered, and methylene blue 1 mg/kg was intravenously injected slowly over 3 minutes. The methemoglobin level reduced to 11.2% after the administration of ascorbic acid and reduced to 4.3% and then 2.3% after methylene administration.
Dapsone is a sulfone antibiotic used for the treatment of Hansen's disease and other dermatologic diseases, as well as in immunosuppressed patients to prevent pneumocystitis carinii pneumonia or organ transplant patients, especially those intolerant to sulfonamide. Methemoglobinemia is a known side-effect of dapsone [4-6] and the patients of most cases had a history of dapsone-intake. The patient here had a 30-year history of dapsone-intake, although this information was not obtained initially. Dapsone is almost completely absorbed in the gastrointestinal track and passes the enterohepatic circulation. Only 20% of the dosage-intake is excreted into the urine. The plasma half-life varies from 10 to 80 hours and is dose-dependent.
Anesthetic experience of methemoglobinemia detected during general anesthesia for gastrectomy of advanced gastric cancer -A case report-
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2998655/
Vitamin C: Why we need it, dietary sources, and how we absorb and metabolize it - YouTube
https://www.youtube.com/watch?v=vcLzhCPuAmw
American Journal of Therapeutics: July/August 2014
Ascorbic Acid for the Treatment of Methemoglobinemia: The Experience of a Large Tertiary Care Pediatric Hospital
Rino, Pedro Bonifacio MD; Scolnik, Dennis MBChB; Fustiñana, Ana MD; Mitelpunkt, Alexis MD; Glatstein, Miguel MDAuthor Information
1Division of Pediatric Emergency Medicine, Hospital de Pediatría Prof. Dr. Juan P. Garrahan, Buenos Aires, Argentina;
2Division of Pediatric Emergency Medicine, Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Ontario, Canada;
3Division of Clinical Pharmacology and Toxicology, The Hospital for Sick Children, University of Toronto, Ontario, Canada;
4Division of Pediatric Emergency Medicine, Department of Pediatrics, Dana-Dwek Children Hospital, University of Tel Aviv, Tel Aviv, Israel; and
5Division of Clinical Pharmacology and Toxicology, Ichilov Hospital, University of Tel Aviv, Tel Aviv, Israel.
The purpose of reporting this series of patients is to illustrate the role of ascorbic acid in the treatment of severe acquired methemoglobinemia (metHb), especially when methylene blue is not available. Medical records of affected patients were reviewed to collect history of exposures, food ingestion, physical examination, pulse oximetry, blood gas, and co-oximetry results, and outcomes. Five cases of acquired metHb are presented here, all of whom received treatment with ascorbic acid and fully recovered after 24 hours of treatment. Our series emphasizes that ascorbic acid is an effective alternative in the management of acquired metHb if methylene blue is unavailable and suggests that ascorbic acid infusion may be indicated in patients with glucose-6-phosphatase dehydrogenase deficiency.抗坏血酸治疗高铁血红素血症:一家三级儿科医院的经验
Pedro Bonifacio医学博士;丹尼斯MBChB Scolnik;Fustinana,安娜医学博士;Mitelpunkt Alexis医学博士;Miguel MDAuthor信息
1阿根廷布宜诺斯艾利斯圣母医院儿科急诊部Juan P. Garrahan教授;
2加拿大安大略省多伦多大学患儿医院儿科儿科急诊医学部;
3加拿大安大略省多伦多大学患儿医院临床药理学和毒理学科;
以色列特拉维夫大学达纳-德鲁克儿童医院儿科急诊科;和
以色列特拉维夫大学伊奇洛夫医院临床药理学和毒理学科。
报道这一系列患者的目的是为了说明抗坏血酸在治疗严重获得性高铁红蛋白血症(metHb)中的作用,特别是当没有亚甲蓝时。回顾受影响患者的医疗记录,收集暴露史、食物摄食史、体格检查史、脉搏血氧测量史、血气、共血氧测量结果和结果。本文报告5例获得性甲氧基丙肝患者,均接受抗坏血酸治疗,24小时后完全康复。我们的系列强调,如果没有亚甲基蓝,抗坏血酸是治疗获得性高铁红蛋白血症(metHb)的有效替代品,并建议,抗坏血酸输注可用于葡萄糖-6磷酸酶脱氢酶缺乏症患者。Ascorbic Acid for the Treatment of Methemoglobinemia: The E... : American Journal of Therapeutics
https://journals.lww.com/americantherapeutics/Abstract/2014/07000/Ascorbic_Acid_for_the_Treatment_of.3.aspx
抗坏血酸亚铁对其他形式铁盐的优点
Advantages of Ferrous Ascorbate on other forms of iron salts
2016.8.21 GUPTA先生0评论
世卫组织和联合国儿童基金会建议,在40%的贫血症流行国家的青少年和幼儿服用口服铁补充剂。大多数口服铁配方以硫酸亚铁的形式提供,也以由多麦芽糖铁复合物组成的铁化合物的形式提供。这些铁化合物在安全性、生物有效性、成本和副作用方面存在差异。除了在市场上可获得的不同化学状态的铁配方,它们也存在于不同的galenic形式。
临床上,硫酸亚铁、富马酸亚铁、葡萄糖酸亚铁等二价铁盐的应用比铁盐更为广泛。含铁形式的铁的生物利用度比含铁形式的低3 - 4倍(生物利用度为10 - 15%)。含铁的铁在碱性溶液中难溶,因此在被吸收之前必须转化为含铁的铁。
口服铁制剂遵循传统的“延长释放”配方,提高了胃肠道的耐受能力,提高了生物利用度。在亚铁形式吸收后,铁在血液中达到最大值约7小时,并保持24小时。
抗坏血酸亚铁是抗坏血酸与铁反应的产物。在抗坏血酸存在的情况下,铁被很好地吸收,因为这种化合物被认为能把铁转化为亚铁。亚铁型的铁在中性pH值下可溶,可被三倍于铁型的铁吸收。抗坏血酸可以防止氧化,因此它可以作为还原剂,并保持铁的黑色形式。由于铁与抗坏血酸的稳定螯合,抗坏血酸亚铁被认为是完整地存在于胃肠道内。这种化合物不会由于任何食物抑制剂而解离。体内抗坏血铁比硫酸亚铁更易吸收铁。研究发现,抗坏血酸亚铁在水溶液中解离为抗坏血酸离子和亚铁离子,其中抗坏血酸离子以单盐形式存在。抗坏血铁已知在pH5解离。在pH6 ~ 8时,抗坏血酸增强了溶解度,有利于抗坏血酸亚铁的吸收。
对18名健康志愿者进行了临床研究,比较了抗坏血铁和硫酸亚铁的配方。21天后测量肠道吸收无差异。但是,两组的血红蛋白含量都达到了基线值。该研究小组还进行了另一项研究,将三价铁(FeIII氢氧化物聚麦芽糖)的生物利用度与二价铁(抗坏血酸亚铁)的生物利用度进行了比较。通过估计血浆铁耐受曲线和全身铁保留值来评估空腹状态下肠道中的铁吸收。血浆铁的FeIII含量(1.2+/- 0.1%)低于抗坏血铁的43.7+/- 7.1%。进食后,二价铁的吸收变化大于三价铁的吸收变化。
在开出100mg铁28天的处方后,二价配方的血红蛋白水平比三价配方的高。其他一些类似的研究已经证明,二价形式的铁或抗坏血酸亚铁比三价形式的铁具有更大的生物利用度。因此,在制备药物时,亚铁盐特别是抗坏血酸亚铁盐比其他铁形式更有效,成本效益高,耐受性好。Advantages of Ferrous Ascorbate on other forms of iron salts | PHARMA FRANCHISE
http://pharmaceuticalcompanyindia.in/advantages-of-ferrous-ascorbate-on-other-forms-of-iron-salts/
Clin Exp Emerg Med. 2018 Sep; 5(3): 192–198.
Therapeutic effect of ascorbic acid on dapsone-induced methemoglobinemia in rats
Changwoo Kang,1,2 Dong Hoon Kim,1,2 Taeyun Kim,1 Soo Hoon Lee,1,2 Jin Hee Jeong,1 Sang Bong Lee,1 Jin Hyun Kim,2,3 Myeong Hee Jung,3 Kyung-woo Lee,4 and In Sung Park2,5
Abstract
Objective
Dapsone (diaminodiphenyl sulfone, DDS) is currently used to treat leprosy, malaria, dermatitis herpetiformis, and other diseases. It is also used to treat pneumocystis pneumonia and Toxoplasma gondii infection in HIV-positive patients. The most common adverse effect of DDS is methemoglobinemia from oxidative stress. Ascorbic acid is an antioxidant and reducing agent that scavenges the free radicals produced by oxidative stress. The present study aimed to investigate the effect of ascorbic acid in the treatment of DDS induced methemoglobinemia.
Methods
Male Sprague-Dawley rats were divided into three groups: an ascorbic acid group, a methylene blue (MB) group, and a control group. After DDS (40 mg/kg) treatment via oral gavage, ascorbic acid (15 mg/kg), MB (1 mg/kg), or normal saline were administered via tail vein injection. Depending on the duration of the DDS treatment, blood methemoglobin levels, as well as the nitric oxide levels and catalase activity, were measured at 60, 120, or 180 minutes after DDS administration.
Results
Methemoglobin concentrations in the ascorbic acid and MB groups were significantly lower compared to those in the control group across multiple time points. The plasma nitric oxide levels and catalase activity were not different among the groups or time points.
Conclusion
Intravenous ascorbic acid administration is effective in treating DDS-induced methemoglobinemia in a murine model.In this study, we demonstrated that intravenous ascorbic acid administration was effective at reducing methemoglobin levels. However, ascorbic acid was slightly less effective than methylene blue administration. At 120 minutes, the methemoglobin concentration of the ascorbic acid group was not significantly different from that of the control group, whereas the methylene blue group showed a more marked difference. In actual clinical cases, because the therapeutic response to ascorbic acid is less marked, repeated doses of intravenous ascorbic acid or an oral preparation have been prescribed, whereas we administered a single intravenous dose of ascorbic acid [20,26]. Furthermore, we administered 15 mg/kg of ascorbic acid in doses determined in a pilot study. Therefore, a higher dose of ascorbic acid might be needed to achieve better therapeutic results.
Keywords: Methemoglobin, Ascorbic acid, Methylene blue, Anti-oxidationTherapeutic effect of ascorbic acid on dapsone-induced methemoglobinemia in rats
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6166037/
Effective role of ascorbic acid as an alternative treatment of methemoglobinemia: A case report
Introduction: Methemoglobinemia is a rare
clinical disorder characterized by increase in the
blood level of methemoglobin (MetHb) which
leads to tissue hypoxia. Methemoglobinemia
may be congenital, but acquired type is more
common and occurs after exposure to oxidizing
agents. Treatment of choice is methylene blue
(MB). The side effects of MB restrict its usage
in special conditions. Ascorbic acid is a good
alternative drug with limited experience in
methemoglobinemia. Case Report: Eight years
old male patient presented with cyanosis
after dapsone exposure and diagnosis of
methemoglobinemia was confirmed. The
patient was managed successfully with
ascorbic acid. Conclusion: Ascorbic acid
could be used as an alternative treatment for
methemoglobinemia when methylene blue is
not available or contraindicated.
Keywords: Ascorbic acid, Dapsone, Methemoglobinemia
INTRODUCTION
Methemoglobinemia is a medical emergency requiring
immediate treatment. Methemoglobinemia is a condition
in which iron atom in hemoglobin is transformed form
a ferrous (Fe2+) state to a ferric (Fe3+) state due to effect
of oxidizing agent exposure. Thus, beside the inability
of methemoglobin (MetHb) to carry O2
, MetHb shifts
the oxygen - hemoglobin dissociation curve to the left,
hindering the release of O2
to the tissues [1]. There
are many drugs and toxins such as nitrate, dapsone,
prilocaine, antimalarial drugs and sulphonamides
responsible for acquired methemoglobinemia [2].
Methylene blue (MB) is considered the commonest and
the usual treatment of methemoglobinemia which may
lead to serious complications and also it is contraindicated
in some patients like those with glucose-6-phosphate
dehydrogenase (G6PD) deficiency [3]. In this case report,
we presented a child with methemoglobinemia induced
by dapsone and the patient was treated and improved
with ascorbic acid to highlight its effectiveness as an
alternative treatment.
After confirming diagnosis, dapsone was stopped
and the child was kept on oxygen inhalation by nasal
prong, while on oxygen, the saturation only raised to
83%.Multiple dose activated charcoal 1g/kg was given
orally to the child, methylene blue was not available,
thus slow intravenous administration of 1gm ascorbic
acid was started . Injection ascorbic acid was continued
as 1gm/4hs (for 8 doses), no side effects occurred. The
child showed signs of improvement, saturation gap and
cyanosis disappeared, MetHb level was repeated after six
hours of ascorbic acid therapy, day two and day three of
admission which was 12.6%, 5.7% and 1.9% respectively,
indicating successful therapy with ascorbic acid. The
child was discharged with full recovery on the third day
of admission.
Dapsone induces a continuing oxidative stress
due to the long half-life, thus serial measurements
of methemoglobin levels should be done [4]. Initial
management of acquired methemoglobinemia inculdes
identification and discontinuation of the offending agent
and increasing tissue oxygenation by administration of
reducing agent as MB and ascorbic acid [1].
Methylene blue is the usual treatment for
methemoglobinemia, however methylene blue is
potentially hazardous. Methylene blue can conversely
cause methemoglobinemia in high doses by its oxidant
effect and induce hemolysis in cases of G6PD deficiency in
addition to turning the skin blue, which is the commonest
side effect [2, 7]. Therefore, alternative treatments are
required. Ascorbic acid is a strong reducing agent that
takes part in many oxidation–reduction reactions,
so ascorbic acid directly reduce methemoglobin and
is proven to treat cyanosis [3]. In many case reports,
ascorbic acid was used successfully in treatment of
methemoglobinemia with different doses and durations
as discussed in Table 2 [8–10]. In the present case, a
decline of 12.2% in methemoglobin levels after six hours
of administration of 2 g ascorbic acid was noticed.
On contrary, other study reported that acquired
methemoglobinemia does not respond to ascorbic
acid, because its capacity to reduce MetHb is much
inferior to that of endogenous enzymatic systems
[11]. However, failure of ascorbic acid in treatment of
methemoglobinemia could be attributed to using lower
doses or shorter durations of therapy [2].100077Z01EM.pdf
http://www.ijcasereportsandimages.com/archive/2018-pdfs/2018100077Z01EM-mohammed/100077Z01EM.pdf
stion, physical examination, pulse oximetry, blood gas, and co-oximetry results, and outcomes. Five cases of acquired metHb are presented here, all of whom received treatment with ascorbic acid and fully recovered after 24 hours of treatment. Our series emphasizes that ascorbic acid is an effective alternative in the management of acquired metHb if methylene blue is unavailable and suggests ...
Effective role of ascorbic acid as an alternative treatment of …
www.ijcasereportsandimages.com/archive/2018-pdfs/2018100077Z01EM... · PDF 文件ascorbic acid. Conclusion: Ascorbic acid could be used as an alternative treatment for methemoglobinemia when methylene blue is not available or contraindicated. Keywords: Ascorbic acid, Dapsone, Methemo-globinemia Eman Abdelfath Mohammed 12 1
Toxicology and Applied Pharmacology
Volume 21, Issue 2, February 1972, Pages 176-185
Toxicology and Applied Pharmacology
Ascorbic acid and chemically induced methemoglobinemias☆
John Z.BolyaiabRoger P.Smithab2Clarke T.Grayab
a
Department of Pharmacology and Toxicology, Dartmouth Medical School, Hanover, New Hampshire 03755 USA
b
Department of Microbiology, Dartmouth Medical School, Hanover, New Hampshire 03755 USA
Received 18 March 1971, Available online 24 September 2004.
Abstract
Sodium nitrite was added to suspensions of washed guinea pig red cells in concentrations which converted about a third of the total blood pigment to methemoglobin. Various aliquots also contained added ascorbic acid in concentrations of 0.05, 0.5, or 5.0 mm (“physiological” levels are between 0.05 and 0.1 mm). Methemoglobin levels were followed for 2 hr at 37°C. None of the tested concentrations of ascorbate significantly attenuated methemoglobin formation. On the contrary, significantly higher methemoglobin levels were sometimes found with 5.0 mm ascorbate. Guinea pigs were maintained several weeks on a scorbutogenic diet with or without ascorbate supplementation in their water. When blood ascorbate levels were significantly different, both groups were given intraarterial sodium nitrite, 40 mg/kg, and methemoglobin levels were followed with time as above. At none of the sampling times was there a significant difference in the methemoglobin levels between the 2 groups. As judged by the appearance of radioactivity in blood at various times after sc injection, the two groups also showed no difference in the rates at which they absorbed KS14CN. In human red cell suspensions 5.0 mm ascorbate significantly attenuated the methemoglobinemic response to tested concentrations of sodium nitrite, hydroxylamine and phenylhydroxylamine at 1 hr, but lower concentrations of ascorbate were without effect. When human red cells were first exposed to nitrite, then washed and ascorbate added, significant acceleration of methemoglobin reduction was observed with 5.0 mm ascorbate at 12, 24, and 36 hr, and with 0.5 mm ascorbate at 12 and 24 hr. In contrast, the oxygen capacities of suspensions with 5.0 mm ascorbate were significantly higher than control only at 12 and 24 hr, whereas with 0.5 mm ascorbate they were significantly higher at 12, 24, and 36 hr. In plain buffer, 5.0 mm ascorbate produced a small but significant loss of the tested concentration of sodium nitrite within 2 hr, but not at shorter intervals or at lower ascorbate concentrations.Ascorbic acid and chemically induced methemoglobinemias - ScienceDirect
https://www.sciencedirect.com/science/article/pii/0041008X72900609
WHAT TREATMENT WORKS?
The course of hereditary methemoglobinemia type I is benign, but these patients should not be administered oxidant drugs. Treatment may be required for cosmetic reasons or for an inadvertent use of oxidant drugs. Ascorbic acid, 300 to 600 mg orally daily divided into 3 or 4 doses, is helpful.15
For methemoglobinemia due to drug exposure, traditional first-line therapy consists of an infusion of methylene blue, whose action depends on the availability of reduced nicotinamide adenine nucleotide phosphate (NADPH) within the red blood cells. After an acute exposure to an oxidizing agent, treatment should be considered when the methemoglobin is 30% in an asymptomatic patient and 20% in a symptomatic patient.16 Patients with anemia or cardiorespiratory problems should be treated at lower levels of methemoglobin. Methemoglobinemia due to hemoglobin M does not respond to ascorbic acid or methylene blue.
Dextrose should be given17 because the major source of NADH in the red blood cells is the catabolism of sugar through glycolysis. Dextrose is also necessary to form NADPH through the hexose monophosphate shunt, which is necessary for methylene blue to be effective.
Methylene blue is an oxidant; its metabolic product leukomethylene blue is the reducing agent. Therefore, large doses of methylene blue may result in higher levels of methylene blue rather than the leukomethylene blue, which will result in hemolysis and, paradoxically, methemoglobinemia in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.18 Patients with G6PD deficiency also may not produce sufficient NADPH to reduce methylene blue to leukomethylene blue; thus, methylene blue therapy may be ineffective in these patients.18
Some drugs, such as dapsone, benzocaine, and aniline, produce a rebound methemoglobinemia, in which methemoglobin levels increase 4 to 12 hours after successful methylene blue therapy.19
N-Acetylcysteine, cimetidine, and ketoconazole are experimental therapies in the treatment of methemoglobinemia that have shown some promising results.20,21,22 Exchange transfusion is reserved for patients in whom methylene blue therapy is ineffective.Drugs or toxins that can cause methemoglobinemia*
Acetanilid
Alloxan
Aniline
Arsine
Benzene derivatives
Benzocaine
Bivalent copper
Bismuth subnitrate
Bupivacaine hydrochloride
Chlorates
Chloroquine
Chromates
Clofazimine
Dapsone
Dimethyl sulfoxide
Dinitrophenol
Exhaust fumes
Ferricyanide
Flutamide
Hydroxylamine
Lidocaine hydrochloride
Metoclopramide hydrochloride
Methylene blue
Naphthalene
Nitrates
Nitric oxide
Nitrites
Nitrofuran
Nitroglycerin
Sodium nitroprusside
Paraquat
Phenacetin
Phenazopyridine hydrochloride
Phenol
Phenytoin
Prilocaine hydrochloride
Primaquine phosphate
Rifampin
Silver nitrate
Sodium valproate
Smoke inhalation
Sulfasalazine
Sulfonamides
TrinitrotolueneEvidence-Based Case Review: Methemoglobinemia
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1071541/
Sodium Nitrite
(Redirected from SN)
“All-meat regimen cured my depression.”
― Jordan Bernt Peterson, Canadian psychologist and professor of psychology
Sodium nitrite (SN), mainly used in preserving processed meats and fish, has been mentioned in murder cases, suicide cases, and accidental poisoning cases. A list of such incidents in medical literature has been documented in Sodium Nitrite Poisoning Cases, as well as anecdotal experiences from forum users[Archive].
Contents
1 Simple step-by-step instructions
2 How it works
3 Sodium nitrite
4 Antiemetics
5 Antacids
6 Required time
7 Consequences of failure
8 Success stories on the news
9 Tips
10 Where to find
11 Further Reading
12 References
Simple step-by-step instructions
There are many ways to commit suicide by sodium nitrite. The following is an easy, quick, and pain-free recipe.
1. Take 30 mg metoclopramide (another editor suggested 800 mg of Tagamet, as well).
2. Wait 1 hour.
3. Dissolve 15 – 25 g sodium nitrite in 50 – 100 ml water.
4. Drink the solution and relax on a bed, a couch, or a reclining chair.
How it works
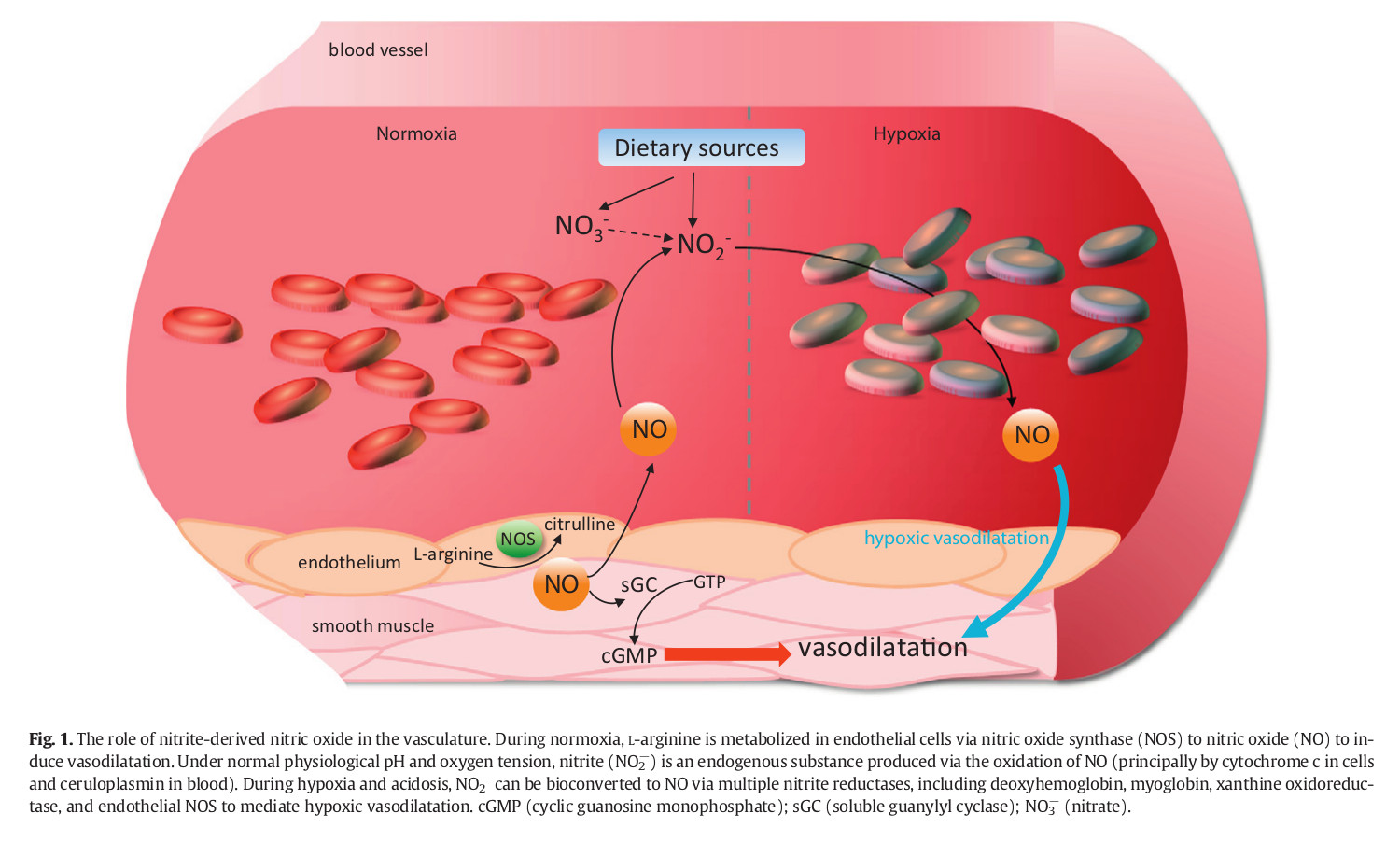
Sodium nitrite, NaNO2, is a catalyst that converts the hemoglobin in blood into methemoglobin (MetHB), a molecule with a much higher affinity with oxygen. This conversion occurs when the ferrous ions in the regular hemoglobin are converted into ferric ones. Since its affinity is so high, methemoglobin does not let the oxygen flow into other tissues that need it thus depriving them of oxygen—even while you’re breathing. Death, then, occurs due to hemolytic anemia or anemic hypoxia, not hypoxic hypoxia. The main reason for peacefulness of sodium nitrite method is that nitrate and nitrite reduce to nitric oxide, a vasodilator. Vasodilators relax hence dilate the smooth muscle in veins. Dilating veins will cause fall in blood pressure and loss of consciousness. (Bailey, Feelisch, Horowitz, Frenneaux & Madhani, 2014) illustrated the nitrite-derived NO signalling[1|2|3].
Sodium nitrite poisoning symptoms include nausea, vomiting, vertigo, headaches, and tachycardia. All of these symptoms will not emerge in everyone. These symptoms will provide low level discomfort, and you will suffer no discomfort through the process. As for the physical symptoms, the color of blood, skin, fingertips, nail beds, and lips will change. The blood will take a bluish chocolate brown color, and the tips of fingers, toes, and nose will turn slightly blue from cyanosis (PubChem). In April 2019, a kindergarten teacher in China poisoned 23 children by lacing their breakfast with sodium nitrite. The only noticeable change in appearance of kindergarten students were their lips that had turned blue[1|2|3|4]. Additionally, in September 2019, four members of an Indian family committed suicide using sodium nitrite; similarly, little change to bluish[1|2|3|4|5|6] was visible in the color of their skin, their fingertips, and blood[1|2|3|4]. Also, (Warren and Blackwood, 2019) published a case report of a 25-year-old woman who had used benzocaine. The percentage of methemoglobin in her blood was 44%. The patient appeared cyanotic and had dark arterial and venous blood[1|2|3]. She was treated with intravenous methylene blue and had considerable improvement in her breathing and reduction in skin discoloration. Putting on a proper makeup[1|2|3|4] to go out in style can conceal any changes in your appearance.Sodium Nitrite - Suicide Wiki
https://suicide.wiki/w/Sodium_Nitrite
SODIUM NITRITE AS ANTIDOTE FOR CYANIDE/ACRYLONITRITE POISONING
Indication: Cyanide/acrylonitrite
Mode of Action: Nitrites facilitate conversion of hemoglobin to methemoglobin. Methemoglobin has higher binding affinity to cyanide which further facilitates its excretion.
Dosage: 10mL of 3% sodium nitrite solution through IV for 5-20 minutes followed by sodium thiosulphateDrugs & Their Antidotes: A Nurse's Ultimate Guide - NurseBuff
https://www.nursebuff.com/common-drugs-and-their-antidotes/
.png)
.png)