¡¡
Vitamin C: C-ing a New Way to Fight Leukemia
Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany
Metabolic cues and (epi-)genetic factors are emerging regulators of hematopoietic stem cell (HSC) potency. Two new studies in Nature and Cell, from Agathocleous et al. (2017) and Cimmino et al. (2017), respectively, show that vitamin C regulates HSC function and suppresses leukemogenesis by modulating Tet2 activity.
Main Text
DNA methylation is an epigenetic modification that plays a critical role in hematopoiesis, controlling proper hematopoietic stem cell (HSC) self-renewal, and lineage differentiation (Jeong and Goodell, 2014). Dysregulation of DNA methylation leads to aberrant stem cell function and cellular transformation. Tet proteins have been identified as key players of DNA demethylation by acting as Fe2+ and ¦Á-ketoglutarate-dependent dioxygenases (Tahiliani et al., 2009). These enzymes catalyze the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), which leads to DNA demethylation mediated by either replication-dependent dilution or base excision repair (BER). TET2 recurrently undergoes loss-of-function mutations in a wide range of myeloid and lymphoid malignancies (Jan et al., 2012). These lesions are early events in leukemogenesis and are associated with DNA hypermethylation, tumor progression, and poor patient outcome. In homeostasis, the stability and activity of Tet proteins are regulated at multiple levels. Acting as a cofactor, vitamin C (also known as ascorbate) promotes the activity of Tet enzymes (Blaschke et al., 2013). Now, two new studies published in Nature and Cell provide mechanistic insights on how vitamin C regulates HSC frequencies and leukemogenesis by augmentation and restoration of Tet2 function, respectively (Figure 1) (Agathocleous et al., 2017, Cimmino et al., 2017).¡¡
Figure thumbnail gr1
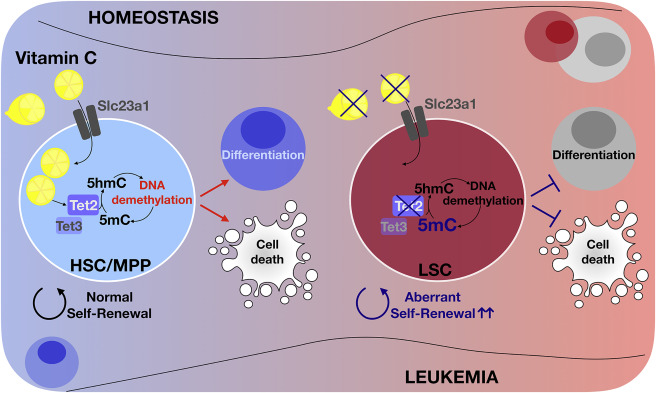
Figure 1The Role of Vitamin C in Homeostatic HSC/MPPs and Aberrant LSC Function
Show full caption
View Large ImageFigure ViewerDownload Hi-res imageDownload (PPT)
In Nature, Sean J. Morrison¡¯s laboratory reports that vitamin C limits HSC frequency and suppresses leukemogenesis by acting through Tet2-dependent and -independent mechanisms (Agathocleous et al., 2017). Agathocleous and colleagues combine extensive metabolomics analyses with a remarkable set of genetic mouse models. To unravel the metabolic signatures of distinct murine hematopoietic cell types, the authors establish a highly sensitive metabolomics workflow applicable to rare cell populations. This comprehensive analysis identified ascorbate/vitamin C to be highly enriched in HSCs and multipotent progenitor cells (MPPs) compared to more committed cell types of the blood system. In addition, the ascorbate-transporter Slc23a2 was also highly expressed in HSC/MPPs. Consistently, ascorbate and the SLC23A2 transporter were enriched in human HSCs.
To address the role of ascorbate in HSCs, the authors generated mice lacking Gulo, the enzyme responsible for its synthesis. Strikingly, ascorbate-depleted cells showed increased HSC frequencies and associated lineage reconstitution capacity in transplantation assays. These results provide genetic evidence that vitamin C is a negative regulator of HSC function. This phenotype resembles mice carrying a homozygous Tet2 deletion (Moran-Crusio et al., 2011). Indeed, ascorbate-depleted HSC/MPPs displayed decreased levels of 5hmC, predominantly mediated by reduction of Tet2 function (Agathocleous et al., 2017).
TET2 mutations together with FLT3ITD mutations can cooperatively cause acute myeloid leukemia (AML) with adverse patient outcome (Shih et al., 2015). Strikingly, ascorbate depletion in combination with Flt3ITD overexpression fostered myelopoiesis, partially mediated by reduced Tet2 activity (Agathocleous et al., 2017). This effect was reversed by repletion of dietary vitamin C. Finally, Agathocleous and colleagues showed that Flt3ITD together with Slc23a2 depletion led to the highest in vivo reconstitution capability of HSCs when compared to single mutants. The authors concluded that vitamin C is cell-autonomously metabolized by HSC/MPPs and involved in regulating Flt3ITD-driven myelopoiesis.
In an independent study published in Cell, the laboratories of Iannis Aifantis and Benjamin G. Neel find that treatment of Tet2-mutated HSCs with vitamin C blocked their aberrant self-renewal activity, mimicking the effect observed upon Tet2 restoration (Cimmino et al., 2017). To assess the role of Tet2 deficiency in the maintenance of leukemic stem cells, the authors generated an elegant, reversible, transgenic RNAi mouse model to restore endogenous Tet2 expression. As previously described, Tet2 knockdown led to a myeloid differentiation bias and a competitive advantage in transplantation experiments (Moran-Crusio et al., 2011). Strikingly, restoration of Tet2 was sufficient to reverse leukemic self-renewal capacities by inducing genome-wide DNA demethylation, differentiation, and cell death. These findings indicate that persistent Tet2 deficiency is required to maintain leukemic self-renewal.
To pharmacologically restore Tet2 activity, Cimmino and colleagues treated Tet2-deficient HSCs with vitamin C in vitro. This approach mimicked the effects observed upon Tet2 restoration, increasing Tet2 as well as Tet3 activity. Further, treatment of Tet2-deficient mice with high doses of vitamin C led to genome-wide increased 5hmC levels and decreased white blood cell counts and myeloid cells. Strikingly, treatment of human-patient-derived leukemic cell lines, including non-Tet2-mutated leukemia with vitamin C, caused increased 5hmC levels and decreased clonogenicity and cell viability. The authors concluded that supra-physiological vitamin C levels could prevent myeloid disease progression.
An exciting observation of this study is the effect seen upon combinatorial application of vitamin C together with a poly-(ADP-ribose) polymerase (PARP)-inhibitor. As an essential mediator of BER, PARP is involved in DNA damage repair mechanisms. Its inhibition has been demonstrated to increase tumor sensitivity to DNA damage. The authors now showed that ascorbate treatment enhanced the efficacy of PARP inhibition and suppressed leukemogenesis in vitro (Cimmino et al., 2017). It will be of great interest to investigate the efficiency of this treatment strategy in vivo.
Both groups thus identify vitamin C as a novel metabolic tumor suppressor involved in epigenetic remodeling (both in mouse and human) and highlight a putative innovative treatment strategy for leukemia (Agathocleous et al., 2017, Cimmino et al., 2017). Although previous reports did not detect positive effects of vitamin C intake in the context of leukemia, patients suffering from hematological diseases often display low ascorbate levels (Huijskens et al., 2016). These two studies point now to supra-physiological concentrations of vitamin C potentially impeding or even reversing leukemogenesis (Agathocleous et al., 2017, Cimmino et al., 2017). Hence, an adequate intake of vitamin C might be highly beneficial for patients with clonal hematopoiesis. Additional work will be needed to investigate whether this approach is also applicable to other types of leukemia, solid tumors, or even metastases harboring a partial or complete loss of TET2 function.
The apparent link between metabolic cues and epigenetic activity has become a highly active and exciting area of research offering the potential to address many unmet medical needs. It is now evident that specific vitamins are key regulators of transformed as well as normal HSCs (Agathocleous et al., 2017, Cabezas-Wallscheid et al., 2017, Cimmino et al., 2017), highlighting the relevance of dietary habits to maintain a healthy stem cell pool. For instance, metabolites from the retinoic acid pathway (vitamin A) have recently been shown to be involved in the in vivo modulation of stem cell features (Cabezas-Wallscheid et al., 2017). The great advances in metabolomics (Agathocleous et al., 2017) and other -omics analyses significantly extend the possibilities in the field of stem cell research and other rare cell types for prevention, diagnosis, and treatment of diseases. This may represent the start of a new era of innovative treatment strategies that combine genome-wide epigenetic analyses and specific metabolites or diets with standard therapies to fight hematological diseases and possibly other types of cancer.
Vitamin C: C-ing a New Way to Fight Leukemia: Cell Stem Cell
https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(17)30383-1#%20¡¡
SLC23A1 Gene (Protein Coding)
Solute Carrier Family 23 Member 1Entrez Gene Summary for SLC23A1 Gene
The absorption of vitamin C into the body and its distribution to organs requires two sodium-dependent vitamin C transporters. This gene encodes one of the two transporters. The encoded protein is active in bulk vitamin C transport involving epithelial surfaces. Previously, this gene had an official symbol of SLC23A2. Alternatively spliced transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Dec 2008]
GeneCards Summary for SLC23A1 Gene
SLC23A1 (Solute Carrier Family 23 Member 1) is a Protein Coding gene. Diseases associated with SLC23A1 include Hypoascorbemia. Among its related pathways are Metabolism of water-soluble vitamins and cofactors and Metabolism. Gene Ontology (GO) annotations related to this gene include transporter activity and dehydroascorbic acid transmembrane transporter activity. An important paralog of this gene is SLC23A2.SLC23A1 Gene - GeneCards | S23A1 Protein | S23A1 Antibody
https://www.genecards.org/cgi-bin/carddisp.pl?gene=SLC23A1¡¡
SLC23A1 Gene (Protein Coding)
Solute Carrier Family 23 Member 1
The Solute Carrier Family 23 Member 1 (SLC23A1) gene is associated with the synthesis of Solute Carrier Family 23 Member 1(SLC23A1) protein, a transporter which is found to be associated with the absorption of vitamin C and distribution to the rest of the body.
Most mammals synthesize ascorbic acid (vitamin C) on their own, however, humans cannot produce this vitamin and depend on dietary sources. One of the well-known historical anecdotes associated with this vitamin requirement is that of the exploration by Ferdinand Magellan. This Spanish explorer was the first to travel around the world with his crew, showing that the world was indeed round and not flat as was commonly believed. Most of his crew are believed to have died during the expedition due to scurvy, a condition caused due to the lack of this nutrient. However, cats that were taken as pets during the expedition survived as they could produce this vitamin. Some people are shown to be associated with an increased requirement for vitamin C, based on the variant of the SLC23A1 gene that they carry.
This vitamin is necessary for the biosynthesis of collagen, catecholamine and carnitine, non-heme iron absorption and in the synthesis of anti-oxidants. Its deficiency can lead to scurvy, leading to fatigue and weakness, reduction in bone and muscle strength and poor immunity.Know Your Genes: SLC23A1 ¡°Vitamin C Gene¡± - Xcode Life
https://www.xcode.life/dna-and-health/know-genes-slc23a1-vitamin-c-gene/¡¡