Chem Res Toxicol
. 2015 Aug 17;28(8):1556-66. doi: 10.1021/acs.chemrestox.5b00132. Epub 2015 Aug 5.
Chemical Characterization of Urate Hydroperoxide, A Pro-oxidant Intermediate Generated by Urate Oxidation in Inflammatory and Photoinduced Processes
Eliziane S Patrício, Fernanda M Prado, Railmara P da Silva, Larissa A C Carvalho, Marcus V C Prates, Tony Dadamos, Mauro Bertotti, Paolo Di Mascio, Anthony J Kettle 1, Flavia C Meotti
Centre for Free Radical Research, Department of Pathology, University of Otago, Christchurch, 8140 Christchurch, New Zealand.
Abstract
Urate hydroperoxide is a strong oxidant generated by the combination of urate free radical and superoxide. The formation of urate hydroperoxide as an intermediate in urate oxidation is potentially responsible for the pro-oxidant effects of urate in inflammatory disorders, protein degradation, and food decomposition. To understand the molecular mechanisms that sustain the harmful effects of urate in inflammatory and oxidative stress related conditions, we report a detailed structural characterization and reactivity of urate hydroperoxide toward biomolecules. Urate hydroperoxide was synthesized by photo-oxidation and by a myeloperoxidase/hydrogen peroxide/superoxide system. Multiple reaction monitoring (MRM) and MS(3) ion fragmentation revealed that urate hydroperoxide from both sources has the same chemical structure. Urate hydroperoxide has a maximum absorption at 308 nm, ε308nm = 6.54 ± 0.38 × 10(3) M(-1) cm(-1). This peroxide decays spontaneously with a rate constant of k = 2.80 ± 0.18 × 10(-4) s(-1) and a half-life of 41 min at 22 °C. Urate hydroperoxide undergoes electrochemical reduction at potential values less negative than -0.5 V (versus Ag/AgCl). When incubated with taurine, histidine, tryptophan, lysine, methionine, cysteine, or glutathione, urate hydroperoxide reacted only with methionine, cysteine, and glutathione. The oxidation of these molecules occurred by a two-electron mechanism, generating the alcohol, hydroxyisourate. No adduct between cysteine or glutathione and urate hydroperoxide was detected. The second-order rate constant for the oxidation of glutathione by urate hydroperoxide was 13.7 ± 0.8 M(-1) s(-1). In conclusion, the oxidation of sulfur-containing biomolecules by urate hydroperoxide is likely to be a mechanism by which the pro-oxidant and damaging effects of urate are mediated in inflammatory and photo-oxidizing processes.
Biochimica et Biophysica Acta (BBA) - General Subjects
Urate hydroperoxide oxidizes endothelial cell surface protein disulfide isomerase-A1 and impairs adherence
Marcela FrancoMineiroaEliziane de SouzaPatricioaÁlbert SouzaPeixotoaThaís Larissa SilvaAraujoabRailmara Pereirada SilvaaAna Iochabel SoaresMorettibFilipe SilvaLimaaFrancisco Rafael MartinsLaurindobFlavia CarlaMeottia
a
Department of Biochemistry, Institute of Chemistry, University of São Paulo, São Paulo, Brazil
b
Heart Institute (Incor), University of São Paulo School of Medicine, São Paulo, Brazil
Received 17 April 2019, Revised 7 November 2019, Accepted 12 November 2019, Available online 14 November 2019.
Highlights
•
Urate hydroperoxide oxidizes PDI at a rate of constant of 6 × 103 M−1 s−1.
•
Urate hydroperoxide oxidized cell surface PDI in HUVECs
•
PDI oxidation, alkylation or inhibition decreased HUVECs adherence.
Abstract
Background
Extracellular surface protein disulfide isomerase-A1 (PDI) is involved in platelet aggregation, thrombus formation and vascular remodeling. PDI performs redox exchange with client proteins and, hence, its oxidation by extracellular molecules might alter protein function and cell response. In this study, we investigated PDI oxidation by urate hydroperoxide, a newly-described oxidant that is generated through uric acid oxidation by peroxidases, with a putative role in vascular inflammation.
Methods
Amino acids specificity and kinetics of PDI oxidation by urate hydroperoxide was evaluated by LC-MS/MS and by stopped-flow. Oxidation of cell surface PDI and other thiol-proteins from HUVECs was identified using impermeable alkylating reagents. Oxidation of intracellular GSH and GSSG was evaluated with specific LC-MS/MS techniques. Cell adherence, detachment and viability were assessed using crystal violet staining, cellular microscopy and LDH activity, respectively.
Results
Urate hydroperoxide specifically oxidized cysteine residues from catalytic sites of recombinant PDI with a rate constant of 6 × 103 M−1 s−1. Incubation of HUVECs with urate hydroperoxide led to oxidation of cell surface PDI and other unidentified cell surface thiol-proteins. Cell adherence to fibronectin coated plates was impaired by urate hydroperoxide, as well as by other oxidants, thiol alkylating agents and PDI inhibitors. Urate hydroperoxide did not affect cell viability but significantly decreased GSH/GSSG ratio.
Conclusions
Our results demonstrated that urate hydroperoxide affects thiol-oxidation of PDI and other cell surface proteins, impairing cellular adherence.
General significance
These findings could contribute to a better understanding of the mechanism by which uric acid affects endothelial cell function and vascular homeostasis.Urate hydroperoxide oxidizes endothelial cell surface protein disulfide isomerase-A1 and impairs adherence - ScienceDirect
https://www.sciencedirect.com/science/article/abs/pii/S0304416519302703
Inactivation of a Thiol-Dependent Enzyme by Urate Hydroperoxide
Activated white blood cells called neutrophils, and xanthine oxidase along with myeloperoxidase/lactoperoxidase, can produce urate hydroperoxide. Previous studies characterized the formation of urate hydroperoxide and its oxidation of small biomolecules.
Urate hydroperoxide oxidized exposed thiols on GAPDH and fully inactivated the enzyme at a ratio of about 5:1. Half of its activity was recovered by reduction with DTT. In comparison, taurine chloramine inactivated GAPDH at approximately 10:1 and DTT reduction recovered all activity.
Inactivation of a Thiol-Dependent Enzyme by Urate Hydroperoxide
https://ourarchive.otago.ac.nz/handle/10523/6064
Urate hydroperoxide oxidizes human peroxiredoxin 1 and peroxiredoxin 2
Larissa A. C. Carvalho‡1, Daniela R. Truzzi‡1, Thamiris S. Fallani‡1, Simone V. Alves§, José Carlos Toledo Jr.¶, Ohara Augusto‡, Luís E. S. Netto§ and Flavia C. Meotti‡2
+Author Affiliations
From the ‡Departamento de Bioquímica, Instituto de Química (IQUSP),
§Departamento de Genética e Biologia Evolutiva, Instituto de Biociências (IB-USP), and
¶Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, São Paulo-SP CEP 05508-000, Brazil
↵2 To whom correspondence should be addressed: Instituto de Química (IQUSP), Avenida Prof. Lineu Prestes, 748, Bloco 10, Sala 1001, Universidade de São Paulo, São Paulo-SP CEP 05508-000, Brazil. Tel.: 55-11-3091-9069; E-mail: flaviam@iq.usp.br or fcmeotti@gmail.com.
Edited by F. Peter Guengerich
Abstract
Urate hydroperoxide is a product of the oxidation of uric acid by inflammatory heme peroxidases. The formation of urate hydroperoxide might be a key event in vascular inflammation, where there is large amount of uric acid and inflammatory peroxidases. Urate hydroperoxide oxidizes glutathione and sulfur-containing amino acids and is expected to react fast toward reactive thiols from peroxiredoxins (Prxs). The kinetics for the oxidation of the cytosolic 2-Cys Prx1 and Prx2 revealed that urate hydroperoxide oxidizes these enzymes at rates comparable with hydrogen peroxide. The second-order rate constants of these reactions were 4.9 × 105 and 2.3 × 106 M−1 s−1 for Prx1 and Prx2, respectively. Kinetic and simulation data suggest that the oxidation of Prx2 by urate hydroperoxide occurs by a three-step mechanism, where the peroxide reversibly associates with the enzyme; then it oxidizes the peroxidatic cysteine, and finally, the rate-limiting disulfide bond is formed. Of relevance, the disulfide bond formation was much slower in Prx2 (k3 = 0.31 s−1) than Prx1 (k3 = 14.9 s−1). In addition, Prx2 was more sensitive than Prx1 to hyperoxidation caused by both urate hydroperoxide and hydrogen peroxide. Urate hydroperoxide oxidized Prx2 from intact erythrocytes to the same extent as hydrogen peroxide. Therefore, Prx1 and Prx2 are likely targets of urate hydroperoxide in cells. Oxidation of Prxs by urate hydroperoxide might affect cell function and be partially responsible for the pro-oxidant and pro-inflammatory effects of uric acid.Urate hydroperoxide oxidizes human peroxiredoxin 1 and peroxiredoxin 2
https://www.jbc.org/content/292/21/8705.abstract
Mol Clin Oncol
. 2017 Feb;6(2):139-153. doi: 10.3892/mco.2017.1129. Epub 2017 Jan 10.
The Role of Peroxiredoxins in Cancer
Arianna Nicolussi 1, Sonia D'Inzeo 1, Carlo Capalbo 2, Giuseppe Giannini 2, Anna Coppa 1
Affiliations collapse
Affiliations
1Department of Experimental Medicine, Sapienza University of Rome, I-00161 Rome, Italy.
2Department of Molecular Medicine, Sapienza University of Rome, I-00161 Rome, Italy.
Abstract
Peroxiredoxins (PRDXs) are a ubiquitously expressed family of small (22-27 kDa) non-seleno peroxidases that catalyze the peroxide reduction of H2O2, organic hydroperoxides and peroxynitrite. They are highly involved in the control of various physiological functions, including cell growth, differentiation, apoptosis, embryonic development, lipid metabolism, the immune response, as well as cellular homeostasis. Although the protective role of PRDXs in cardiovascular and neurological diseases is well established, their role in cancer remains controversial. Increasing evidence suggests the involvement of PRDXs in carcinogenesis and in the development of drug resistance. Numerous types of cancer cells, in fact, are characterized by an increase in reactive oxygen species (ROS) production, and often exhibit an altered redox environment compared with normal cells. The present review focuses on the complex association between oxidant balance and cancer, and it provides a brief account of the involvement of PRDXs in tumorigenesis and in the development of chemoresistance.
Keywords: chemoresistance; chemosensitization; oxidative stress; peroxiredoxins; tumorigenesis.The Role of Peroxiredoxins in Cancer - PubMed
https://pubmed.ncbi.nlm.nih.gov/28357082/
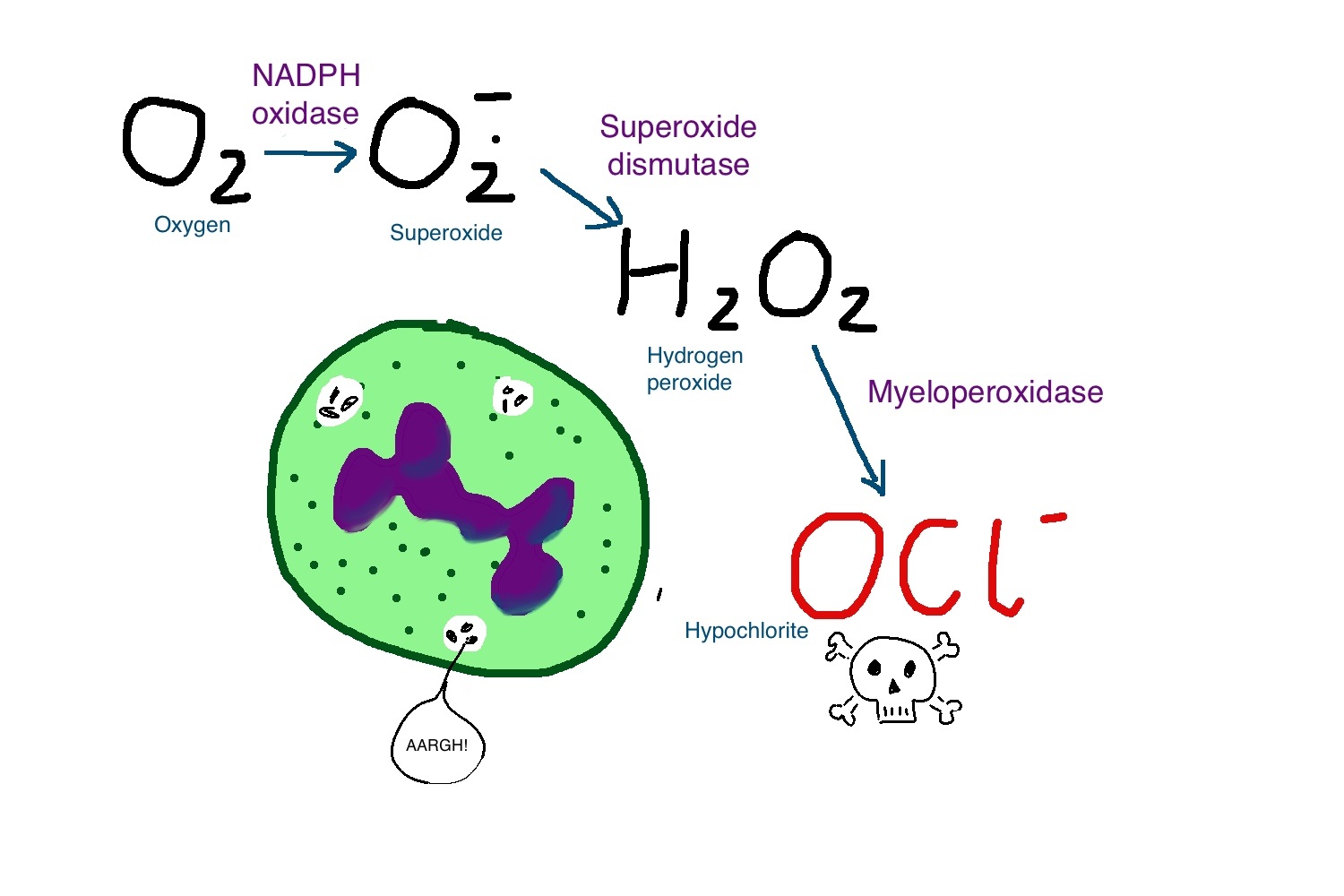
Urate as a physiological substrate for myeloperoxidase: Implications for hyperuricemia and inflammation
Apr 2011
Flavia Meotti
Guy N L Jameson
Rufus Turner[...]
Anthony J Kettle
Abstract
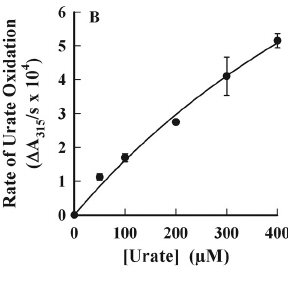
Urate and myeloperoxidase (MPO) are associated with adverse outcomes in cardiovascular disease. In this study, we assessed whether urate is a likely physiological substrate for MPO and if the products of their interaction have the potential to exacerbate inflammation. Urate was readily oxidized by MPO and hydrogen peroxide to 5-hydroxyisourate, which decayed to predominantly allantoin.The redox intermediates of MPO were reduced by urate with rate constants of 4.6 × 10(5) M(-1) s(-1) for compound I and 1.7 × 10(4) M(-1) s(-1) for compound II. Urate competed with chloride for oxidation by MPO and at hyperuricemic levels is expected to be a substantive substrate for the enzyme.
Oxidation of urate promoted super-stoichiometric consumption of glutathione, which indicates that it is converted to a free radical intermediate. In combination with superoxide and hydrogen peroxide, MPO oxidized urate to a reactive hydroperoxide. This would form by addition of superoxide to the urate radical. Urate also enhanced MPO-dependent consumption of nitric oxide.
In human plasma, stimulated neutrophils produced allantoin in a reaction dependent on the NADPH oxidase, MPO and superoxide. We propose that urate is a physiological substrate for MPO that is oxidized to the urate radical. The reactions of this radical with superoxide and nitric oxide provide a plausible link between urate and MPO in cardiovascular disease.
尿酸盐和髓过氧化物酶(MPO)与心血管疾病的不良结局有关。
在这项研究中,我们评估了尿酸盐是否可能是MPO的生理底物,以及它们相互作用的产物是否具有加剧炎症的潜能。尿酸盐很容易被MPO和过氧化氢氧化成5-羟基异羟乙酸(5-hydroxyisourate),后者分解成尿囊素(allantoin)。尿酸盐还原MPO的氧化还原中间体,化合物I的速率常数为4.6×10(5)M(-1)s(-1),而化合物I的速率常数为1.7×10(4)M(-1)s(-1)化合物II。
尿酸盐与氯化物竞争被MPO氧化,并且在高尿酸水平下被认为是该酶的实质底物。尿酸盐的氧化促进了谷胱甘肽的超化学计量消耗,这表明它被转化为自由基中间体。与超氧化物和过氧化氢结合,MPO将尿酸盐氧化为反应性氢过氧化物。这将通过在尿酸盐自由基中添加超氧化物而形成。尿酸盐还增加了MPO依赖的一氧化氮消耗量。
在人血浆中,受刺激的中性粒细胞在依赖于NADPH氧化酶,MPO和超氧化物的反应中产生尿囊素。我们建议尿酸盐是被氧化成尿酸盐自由基的MPO的生理底物。该自由基与超氧化物和一氧化氮的反应在心血管疾病中提供了尿酸盐和MPO之间的合理联系。
Urate as a Physiological Substrate for Myeloperoxidase
https://www.jbc.org/content/286/15/12901
Biochimica et Biophysica Acta (BBA) - General Subjects
Volume 1864, Issue 3, March 2020, 129481
Biochimica et Biophysica Acta (BBA) - General Subjects
J Agric Food Chem. 2010 Jan 13;58(1):481-7. doi: 10.1021/jf903470p.
Characterization of Peroxides Formed by Riboflavin and Light Exposure of Milk. Detection of Urate Hydroperoxide as a Novel Oxidation Product
Morten R Clausen 1, Kevin Huvaere, Leif H Skibsted, Jan Stagsted
1Department of Food Science, Faculty of Agricultural Sciences, Aarhus University, DK-8830 Tjele, Denmark.
Abstract
Characterization of peroxides by size exclusion chromatography (SEC) of milk following exposure to riboflavin and light showed that hydrogen peroxide was the most abundant peroxide formed since it could be removed by catalase. Formation of peroxides after separation by SEC showed that hydrogen peroxide formation was primarily increased in the presence of caseins and ascorbate, although whey proteins also were found to contribute. Caseins and beta-lactoglobulin also formed catalase-resistant peroxides, presumably protein hydroperoxides.A catalase-resistant and unstable peroxide was observed in fractions containing urate. Experiments performed with pure urate suggested that urate radicals reacted further with superoxide leading to a urate hydroperoxide. Electron paramagnetic resonance spectroscopy using spin-traps showed that the presence of oxygen was required for urate radical formation, which could be assigned as nitrogen-centered radicals. These results suggest a new route during light-induced oxidation sensitized by flavins, in effect making urate pro-oxidative.
牛奶在暴露于核黄素和光照后,通过尺寸排阻色谱法(SEC)对过氧化物进行表征,结果表明,过氧化氢是形成的最丰富的过氧化物,因为过氧化氢可以被过氧化氢酶去除。通过SEC分离后过氧化物的形成表明,在酪蛋白和抗坏血酸存在下,过氧化氢的形成主要增加,尽管也发现乳清蛋白也起作用。酪蛋白和β-乳球蛋白也形成抗过氧化氢酶的过氧化物,大概是蛋白质氢过氧化物。
在含有尿酸盐的部分中观察到抗过氧化氢酶和不稳定的过氧化物。用纯尿酸盐进行的实验表明,尿酸盐自由基与超氧化物进一步反应,生成尿酸盐氢过氧化物。使用自旋阱的电子顺磁共振波谱表明,尿酸盐自由基的形成需要氧气的存在,而尿酸盐自由基可以被指定为以氮为中心的自由基。这些结果表明在黄素增敏的光诱导氧化过程中,一条新的途径实际上使尿酸盐成为氧化前者。
Characterization of Peroxides Formed by Riboflavin and Light Exposure of Milk. Detection of Urate Hydroperoxide as a Novel Oxidation Product - PubMed
https://pubmed.ncbi.nlm.nih.gov/19994860/
J Biol Chem. 2011 Apr 15; 286(15): 12901–12911.
Urate as a Physiological Substrate for Myeloperoxidase
IMPLICATIONS FOR HYPERURICEMIA AND INFLAMMATION*
Flavia C. Meotti,‡,1 Guy N. L. Jameson,§ Rufus Turner,‡ D. Tim Harwood,‡ Samantha Stockwell,‡ Martin D. Rees,¶ Shane R. Thomas,¶,2 and Anthony J. Kettle‡,3
Author information Article notes Copyright and License information Disclaimer
From the ‡Free Radical Research Group, Department of Pathology, University of Otago, P. O. Box 4345, 8140 Christchurch, New Zealand,
the §Department of Chemistry, University of Otago, Dunedin, New Zealand, and
the ¶Centre for Vascular Research, School of Medical Sciences, University of New South Wales, Sydney, New South Wales 2052, Australia
3 To whom correspondence should be addressed: Dept. of Pathology, University of Otago Christchurch, PO Box 4345 Christchurch, New Zealand., E-mail: zn.ca.ogato@elttek.ynot.
1Present address: Dept. de Farmacologia, Universidade Federal de Santa Catarina, 88040-900 Florianópolis-SC, Brazil.
2Supported by National Health and Medical Research Council RD Wright Career Development Award 401113.
Abstract
Urate and myeloperoxidase (MPO) are associated with adverse outcomes in cardiovascular disease. In this study, we assessed whether urate is a likely physiological substrate for MPO and if the products of their interaction have the potential to exacerbate inflammation. Urate was readily oxidized by MPO and hydrogen peroxide to 5-hydroxyisourate, which decayed to predominantly allantoin. The redox intermediates of MPO were reduced by urate with rate constants of 4.6 × 105 m−1 s−1 for compound I and 1.7 × 104 m−1 s−1 for compound II. Urate competed with chloride for oxidation by MPO and at hyperuricemic levels is expected to be a substantive substrate for the enzyme.Oxidation of urate promoted super-stoichiometric consumption of glutathione, which indicates that it is converted to a free radical intermediate. In combination with superoxide and hydrogen peroxide, MPO oxidized urate to a reactive hydroperoxide. This would form by addition of superoxide to the urate radical. Urate also enhanced MPO-dependent consumption of nitric oxide.
In human plasma, stimulated neutrophils produced allantoin in a reaction dependent on the NADPH oxidase, MPO and superoxide. We propose that urate is a physiological substrate for MPO that is oxidized to the urate radical. The reactions of this radical with superoxide and nitric oxide provide a plausible link between urate and MPO in cardiovascular disease.
MPO对一氧化氮的尿酸盐依赖性消耗
MPO可通过直接氧化NO或通过生成与NO反应的自由基中间体来调节血管炎性反应(32,33)。因此,我们还测试了由MPO生成的尿酸根自由基与一氧化氮(NO)以与酪氨酰基自由基类似的方式反应的可能性(32,33)。 NO供体NOC-9生成NO。单独的MPO和过氧化氢的组合促进了NO的消耗,但是这通过添加尿酸盐来增强(图7A)。单独使用尿酸盐并与MPO(数据未显示)或过氧化氢(图7A)结合使用不会促进NO的消耗(数据未显示)。 NO的消耗取决于其生理范围内尿酸盐的浓度(图7B)。在促进NO的消耗方面,尿酸盐与酪氨酸相当。从这些结果,我们得出结论,尿酸盐可促进MPO的NO氧化酶活性。
Keywords: Antioxidant, Inflammation, Neutrophil, Nitric Oxide, Oxidative Stress, Oxygen Radicals, Peroxidase, Superoxide Ion, Uric Acid, MyeloperoxidaseUrate as a Physiological Substrate for Myeloperoxidase
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3075637/
Biochem J. 1995 Sep 15; 310(Pt 3): 745–749.
Nitric oxide rapidly scavenges tyrosine and tryptophan radicals.
J P Eiserich, J Butler, A van der Vliet, C E Cross, and B Halliwell
Department of Internal Medicine, University of California, Davis, 95616, USA
By utilizing a pulse-radiolytic technique, we demonstrate for the first time that the rate constant for the reaction of nitric oxide (.NO) with biologically relevant tyrosine and tryptophan radicals (Tyr. and Trp. respectively) in amino acids, peptides and proteins is of the order of (1-2) x 10(9) M-1.s-1. We also show that .NO effectively interferes with electron-transfer processes between tryptophan and tyrosine residues in proteins subjected to pulse radiolysis. The near diffusion-controlled rates of these reactions, coupled with the increasingly recognized role of protein radicals in enzyme catalysis and oxidative damage, suggest that Tyr. and Trp. are likely and important targets for .NO generated in vivo.Nitric oxide rapidly scavenges tyrosine and tryptophan radicals.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1135961/