神奇的免疫系统 THE MAGICAL IMMUNE SYSTEM
What is a T4 lymphocyte?
It is a type of white blood cell.
T4 lymphocytes, also known as "Helper cells", are a type of white blood cell that orchestrate the immune response and enhance the activities of the killer T-cells (those that destroy pathogens) and B-cells (antibody and immunoglobulin producers).
White blood cells are released by the immune system when the body is under threat (often from bacteria or a virus). The T4 lymphocytes help the response to a threat by triggering the maturation of other types of white blood cell. They produce special proteins, called cytokines, which act like a 999/911 call and summon all of the other immune cells to the area, or it causes nearby cells to differentiate (become specialised) into mature T-cells.
It is worth noting that in AIDS, the number of T4 cells decreases drastically.
https://socratic.org/questions/what-is-a-t4-lymphocyte
Oxygen-dependent myeloperoxidase-independent intracellular killing (Figure11A)
During phagocytosis glucose is metabolized via the pentose monophosphate shunt and NADPH is formed. Cytochrome B which was part of the specific granule combines with the plasma membrane NADPH oxidase and activates it. The activated NADPH oxidase uses oxygen to oxidize the NADPH. The result is the production of superoxide anion. Some of the superoxide anion is converted to H2O2 and singlet oxygen by superoxide dismutase. In addition, superoxide anion can react with H2O2 resulting in the formation of hydroxyl radical and more singlet oxygen. The result of all of these reactions is the production of the toxic oxygen compounds superoxide anion (O2-), H2O2, singlet oxygen (1O2) and hydroxyl radical (OH•).
Oxygen-dependent myeloperoxidase-dependent intracellular killing (Figure 11B)
As the azurophilic granules fuse with the phagosome, myeloperoxidase is released into the phagolysosome. Myeloperoxidase utilizes H2O2 and halide ions (usually Cl-) to produce hypochlorite, a highly toxic substance. Some of the hypochlorite can spontaneously break down to yield singlet oxygen. The result of these reactions is the production of toxic hypochlorite (OCl-) and singlet oxygen (1O2).
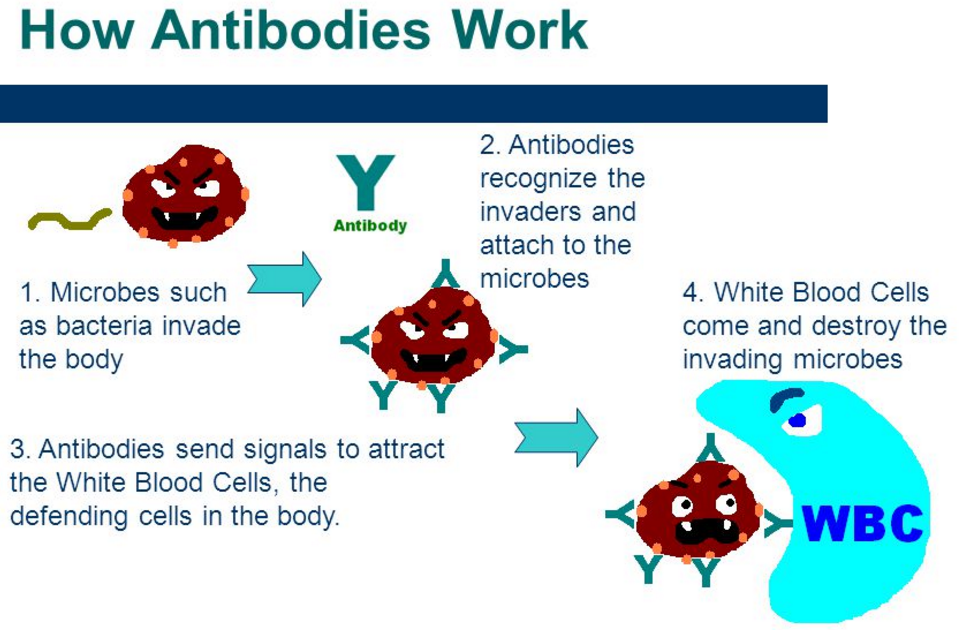
抗体由适应性免疫系统的B细胞分泌,主要由被称为浆细胞的分化B细胞分泌。抗体可以以两种物理形式出现,一种是可溶性形式,从细胞分泌到血浆中游离,另一种是膜结合形式,附着在B细胞表面,称为B细胞受体(BCR)。BCR发现只有表面上的B细胞和促进这些细胞的激活及其后续分化称为抗体工厂的浆细胞或记忆B细胞,在体内生存和记住相同抗原,以便在将来接触是可以迅速做出反应。
抗体是属于免疫球蛋白超家族的糖蛋白(Glycoprotein)。它们构成了血液蛋白质中伽玛球蛋白的大部分。免疫球蛋白多样性
几乎所有的微生物都能引发抗体(antibody)反应。成功识别和消灭许多不同类型的微生物需要抗体的多样性;它们的氨基酸组成各不相同,可以与许多不同的抗原相互作用。据估计,人类产生了大约100亿种不同的抗体,每一种都能与抗原的不同表位结合。虽然在单个个体中产生了大量不同的抗体,但是制造这些蛋白质的基因数量受到人类基因组大小的限制。一些复杂的遗传机制已经进化,允许脊椎动物B细胞从相对较少的抗体基因中产生多种抗体
天然杀手细胞
天然杀伤细胞(也称为NK细胞,K细胞和杀伤细胞)是一种淋巴细胞(白细胞),是先天免疫系统的组成部分。
NK细胞在肿瘤和病毒感染细胞的宿主排斥中起主要作用。
NK细胞具有细胞毒性;细胞质中的小颗粒含有特殊的蛋白质,例如穿孔素和称为颗粒酶的蛋白酶。
在紧邻预定要杀死的细胞释放时,穿孔素在靶细胞的细胞膜中形成孔,粒酶和相关分子可通过该孔进入,诱导凋亡。
细胞凋亡和细胞裂解之间的区别在免疫学中很重要-裂解感染了病毒的细胞只会释放病毒体,而细胞凋亡会导致病毒内部的破坏。
NK细胞响应干扰素或巨噬细胞衍生的细胞因子而被激活。
它们起着遏制病毒感染的作用,而适应性免疫反应正在产生可以清除感染的抗原特异性细胞毒性T细胞。
NK细胞不足的患者被证明对疱疹病毒感染的早期阶段高度敏感。NATURAL KILLER CELLS
Natural killer cells (also known as NK cells, K cells, and killer cells) are a type of lymphocyte (a white blood cell) and a component of innate immune system.
NK cells play a major role in the host-rejection of both tumours and virally infected cells.
NK cells are cytotoxic; small granules in their cytoplasm contain special proteins such as perforin and proteases known as granzymes.
Upon release in close proximity to a cell slated for killing, perforin forms pores in the cell membrane of the target cell through which the granzymes and associated molecules can enter, inducing apoptosis.
The distinction between apoptosis and cell lysis is important in immunology - lysing a virus-infected cell would only release the virions, whereas apoptosis leads to destruction of the virus inside.
NK cells are activated in response to interferons or macrophage-derived cytokines.
They serve to contain viral infections while the adaptive immune response is generating antigen-specific cytotoxic T cells that can clear the infection.
Patients deficient in NK cells prove to be highly susceptible to early phases of herpes virus infection.Natural killer cell
https://www.sciencedaily.com/terms/natural_killer_cell.htm
.png)

.png)
.png)
.png)
.png)
.png)
.png)
.png)